Abstract
Objective
Minimal information on physical activity is available for non-Western populations undergoing the transition to a market economy. This is unfortunate given the importance of these data for understanding health issues such as the global obesity epidemic. We consider the utility of using accelerometry technology to examine activity patterns and energy use regulation among indigenous Shuar, an Ecuadorian forager-horticulturalist population undergoing economic and lifestyle change. We investigate sex differences in Shuar activity patterns and the effects of reproductive status on activity. Finally, we discuss the potential of accelerometry use in human biology research.
Methods
Physical activity levels were measured using Actical accelerometers in 49 indigenous Shuar adults (23 males, 26 females) from a rural Ecuadorian community. Female participants were in various reproductive states including pregnant, lactating, and non-pregnant/non-lactating.
Results
Activity counts (AC), activity energy expenditure (AEE), and physical activity levels (PAL) were significantly higher (P < 0.05) in males than females. Significant differences in energy expenditure were found among pregnant or lactating females and males with pregnant or lactating partners (P < 0.001). Males with pregnant or lactating partners also had significantly higher activity levels than did other men (P < 0.01).
Conclusions
Shuar activity levels are relatively low compared to other non-Western populations. Despite increasing market integration, pregnant and lactating females seem to be adopting a strategy noted in other subsistence populations where male participation in subsistence activities increases to compensate for their partners’ elevated reproductive costs. Despite certain limitations, use of accelerometry in human biology research shows promise.
Keywords: energetics, physical activity, reproductive ecology, market integration, Shuar
INTRODUCTION
In indigenous societies, increased market integration, defined as the commoditization of material, food, and labor (Lu, 2007), has been linked to changes in health outcomes such as obesity, type 2 diabetes, and hypertension (Baker et al., 1986; Friedlander et al., 1987; Huss-Ashmore et al., 1992; Snodgrass et al., 2007). Changes in energy dynamics, or more specifically, increased energy intake and reductions in energy expenditure, have been implicated in this health shift as populations transition from traditional subsistence economies to increasingly sedentary occupations. Numerous studies have documented dietary transitions (e.g., Monteiro et al., 1995; Popkin, 2006), yet relatively few studies have systematically measured physical activity in populations undergoing economic development and lifestyle change. This is unfortunate given the importance of physical activity data for understanding the health effects of market integration and the increasing global prevalence of overweight and obesity.
The relative lack of available data on activity patterns in subsistence-level populations partially reflects the difficulty of accurately quantifying habitual or “free-living” physical activity under field conditions. Physical activity is typically measured using time allocation, doubly-labeled water (DLW), or heart rate (HR) monitors, but all these methods have distinct advantages and disadvantages (Snodgrass, 2011; Westerterp, 2009). Most population-level studies of subsistence groups have used time allocation data, but this technique can underestimate energy costs, especially at high levels of physical activity (Leonard et al., 1997; Spurr et al., 1996). DLW, generally considered the “gold standard” for measuring free-living energy expenditure, estimates activity over a relatively long period of time (typically 7–10 days), is expensive and its high cost has limited its use in non-Western contexts (Coward, 1998). HR monitoring, on the other hand, is far less costly, but requires individual calibration of heart rate/energy expenditure relationships, which is time consuming and adds a significant measurement burden for participants.
Recently, technological advances in accelerometry have given researchers a new tool for estimating total daily energy expenditure (TDEE) and physical activity level (PAL) under field conditions although, as with all methods, there are advantages and disadvantages to their use (Chen and Bassett, 2005; Plasqui and Westerterp, 2007; Snodgrass, 2011; Swartz et al., 2000). Accelerometers are electronic motion sensors typically worn at the waist that can objectively measure the intensity, frequency, and duration of body movement. The devices are also durable, fairly non-intrusive, reasonably affordable, and allow for the estimation of activity variation on shorter timescales than possible using time allocation studies or DLW. Several models of accelerometers have been extensively validated (Blanton et al., 2005; Chen and Bassett, 2005; Heil, 2006; Hoos et al., 2003; Plasqui and Westerterp, 2007), with data quality from accelerometers generally considered second only to DLW (Westerterp, 2009). While accelerometry has been widely applied in Western research contexts, particularly in clinical studies and among athletes, this technology has rarely been used in non-Western field settings.
An area of research where accelerometery could be particularly useful is in determining the effects of increasing market integration on patterns of energy expenditure and sexual division of labor. Most research to date suggests that a transition from a subsistence-based to market economy typically leads to reductions in female activity levels. For example, among Inuit from Igloolik, Canada, who were experiencing the initial stages of economic development, both males and females demonstrated a reduction in subsistence activities, although males maintained relatively higher activity levels than females (Shephard and Rode, 1996). In the Yakut (Sakha) of Siberia, a herding population that has experienced rapid economic development over the past decade, overall population activity levels were generally low, but they were considerably lower among females than males (Snodgrass et al., 2006). These sex differences in physical activity patterns are shaped by variation in subsistence participation and by other cultural factors. Even subsistence groups living in broadly comparable environments and with similar subsistence economies can have distinct physical activity levels. For example, the Tukanoan women of Colombia have significantly higher activity levels than Brazilian Ribeirinha women (Dufour and Piperata, 2008) despite both populations living in Amazonian neo-tropical forest environments. This difference reflects inter-cultural variability in household sexual divisions of labor (i.e., male participation in harvesting activities), settlement patterns (i.e., proximity to gardens), and minor dietary differences (i.e., amount of processing of the cassava staple).
Since energy is a fundamental limiting resource that must be allocated to different somatic functions, an accelerometry-based energetics approach can provide a useful tool to investigate life history trade-offs such as the energy available for reproduction. An evolutionary life history framework has been useful in understanding the socio-behavioral strategies adopted by subsistence populations for economizing energy expenditure during costly reproductive states such as pregnancy and lactation (Hill and Hurtado, 1996; Stearns, 1992). A small number of studies have addressed the various strategies used by rural women in subsistence-based economies, whose energy conserving options are more restricted than women from urban settings (e.g., Guillermo-Tuazon et al., 1992; Piperata and Dufour, 2007). Among subsistence-based populations in Nepal (Panter-Brick, 1993) and Brazil (Piperata and Dufour, 2007), women decreased energy expenditure for subsistence activities, presumably as a means of offsetting their elevated energy needs for reproduction, even at the cost of reducing contributions to food production. In some contexts, a decrease in female economic contribution has been shown to prompt a compensatory increase in familial support or, more specifically, male provisioning. For example, Marlowe (2003) observed greater food returns from Hadza hunter-gatherer men with pregnant and lactating wives, a socio-behavioral strategy that extends until the time of weaning.
The present study was designed as a preliminary step towards addressing the data gaps outlined above by using accelerometry to document energetic patterns in the Shuar, an indigenous Ecuadorian forager-horticulturalist population. The goals of this study are to: 1) compare physical activity levels among the Shuar with other populations for which data are available; 2) investigate potential sex differences in physical activity and examine the effects of reproductive status on activity; and 3) discuss the advantages and disadvantages of accelerometer use in human biology research.
METHODS
Ethnographic Context
The Shuar are an indigenous forager-horticulturalist population concentrated in the southeastern neo-tropical forest of Ecuador. Approximately 50,000 Shuar primarily reside in the Morona-Santiago and Zamora provinces of Ecuador. Traditionally, Shuar lived in scattered households across the Paute and Upano River Valley between the eastern Andean foothills and the Cordillera de Cutucu mountain range, and in the Yaupi and Morona drainages east of the Cutucu (Harner, 1984; Rubenstein, 2001). Subsistence was based on hunting, fishing, and swidden horticulture. Although these subsistence activities remain common, infrastructural development has led to an increasingly rapid lifestyle change for many Shuar, especially in the Upano Valley. In the past ten years, dirt roads have been extended to many Shuar villages in this region leading to increased access to market goods and a style of life associated with market integration (e.g., electricity). Despite these changes, Shuar health in more acculturated areas appears to be, in some respects, worse than in less integrated areas, as measured by factors such as body fat, blood lipids, and growth (Blackwell et al., 2009; Lindgarde et al., 2004).
Participants
This study was conducted as part of the Shuar Health and Life History Project (http://www.bonesandbehavior.org/shuar) in an Ecuadorian Upano River Valley community located approximately forty minutes by truck from Sucua, the nearest major town center.
Participants included 49 Shuar adult (14–66 years of age; 23 males, 26 females) volunteers, representing approximately 50% of the adult residents in the study community. Among the female participants, five were pregnant (P), ten were lactating (L), and eleven were non-pregnant/non-lactating (NPNL). Among the male participants, 16 did not have pregnant or lactating wives while the remaining seven males did.
All participants gave individual informed verbal consent, with both parental consent and child consent for subjects under 15 years old. Research was conducted from a Ministerio de Salud health center, and the study design was approved by village leaders, the Federación Interprovincial de Centro Shuar (FISCH), and the Office for Protection of Human Subjects at the University of Oregon.
Seasonality
Data were collected over the course of three field seasons: August-September 2008, February 2009, and August-September 2009. Activity data were recorded for each participant for one of the three field seasons. The months of data collection are all considered “dry” months (i.e., periods of moderate rainfall of approximately 300–400mm) (Sirén, 2007). Furthermore, although neo-tropical in flora and fauna, the Upano River Valley region (located 1000 meters above sea level at the base of the Andes) experiences less rainfall and temperature variation across the year than in the Amazonian lowlands east of the Cutucu and, thus, our expectation is that activity will not vary as much seasonally as in some subsistence populations in other regions.
Anthropometry
Participant stature (measured to the nearest mm) and weight (measured to the nearest 0.1 kilogram [kg]) were recorded using a field stadiometer (Seca, Hanover, MD) and digital scale (Tanita BF-558 electronic scale, Tokyo, Japan) respectively, according to established procedures (Lohman et al., 1988). Body mass index (BMI) was calculated by dividing body mass (in kg) by height squared (in meters).
Physical Activity
Physical activity was estimated using Actical accelerometers (Respironics, Bend, OR), one of the more widely validated monitoring devices (Plasqui and Westerterp, 2007). Each monitor was initialized before placing it on the participant, which involves entering a start date and time into the Actical 2.1 software platform. During this initialization process, the participant’s sex, age, height, and weight were also entered. The Actical begins recording automatically at the designated start time and continues to record activity until the data are downloaded or the device memory reaches its limit (ranging from 11–45 days, depending on the user-defined epoch length selected). Each participant wore an Actical at the waist, positioned over their right iliac crest for at least two consecutive days irrespective of weekend or weekday. Participants engaged in their normal activities or those already planned, and kept the monitor on while bathing and sleeping. Following the activity recording period, the data from the Actical were downloaded to a computer.
A shortcoming of the present study is that the number of days the device was worn, the time the device was initialized, and when the device was removed varied between participants. This limitation in the study design reflects the constraints of standardizing the collection of activity data from free-living populations living in remote environments. Participants lived various distances from the health center and although all individuals wore the device for at least two days, a few individuals were not available to remove the device at a precise time and continued to wear the monitor for several additional days. No participant wore the device for more than four days. The average number of days in which a device was worn was 2.51 days (SD = 0.681). Data were averaged over standardized 24-hour periods, so the specific time the device was initialized or removed is unlikely to enter systematic bias in the data.
The accelerometer devices record Activity Counts (AC), which represent the frequency and intensity of acceleration events that occur during user-defined epochs. The epoch length is the period of time the device will accumulate and record ACs, and then reset the counter to zero. Depending on the user preference, Actical epoch lengths may vary between 15 seconds and one minute; this option does not affect the actual accumulation of activity counts, only the detail of the data output. The significance of accelerometer epoch lengths will vary depending on the type of research being implemented. For example, shorter epochs are useful for studies examining short, sporadic bouts of activity as with research on children (e.g., Stone et al., 2009). Since the purpose of this study was to examine activity patterns over a period of a few days, the precise length of the epoch is not critical and therefore, the default setting, a 25-second epoch, was used.
Although the Actical software converts ACs into caloric energy expenditure, two additional techniques are employed to estimate energetic parameters from raw accelerometry data: 1) a two-regression model by Heil (2006) to determine activity energy expenditure (AEE) based on ACs (averaged for the recorded days); and 2) total daily energy expenditure (TDEE) calculated as AEE + basal metabolic rate (BMR). BMRs for males and NPNL women were estimated using the Oxford predictive equations according to weight and age (Henry, 2005). BMR among pregnant participants was determined using an appropriate multiplication factor depending on trimester (Prentice et al., 1996). Using FAO/UNU/WHO (2004) guidelines, BMR changes in lactating women were determined as approximately 675 kcal above their non-lactating metabolic requirements.
Currently, there is no consensus on the best method for adjusting for the effects of body size and composition in energetics, although physical activity level (PAL; TDEE/BMR) is the most commonly used method of comparing activity data in human biology research. However, using PALs as a measure for pregnant women may be complicated by a progressively increasing BMR that occurs throughout the course of pregnancy. Thus, a woman participating in the same activities with similar duration and intensity would show a decline in PAL throughout her pregnant state. For this reason, we also present data for the activity variables AEE and AC in order to examine population-level differences in activity.
Statistical Analyses
Pairwise comparisons were conducted using independent samples t-tests, with two-tailed P-values and equal variance assumed. Variance components were assessed in R using the lme and VarCorr procedures from the package nlme. Age patterns were fit with thin-plate splines using generalized additive modeling (GAM) by using the gam procedure in the R package mgcv. ANOVAs were used to assess interactions between sex and the reproductive state of females in a household (i.e., pregnant, lactating, NPNL). Following ANOVAs, post-hoc comparisons were done using two-tailed t-tests. Statistical analyses were performed using a combination of SPSS 17.0 (SPSS, Inc.) and R 2.10.1 (www.r-project.org).
RESULTS
Descriptive statistics for age, anthropometric, and activity data are presented in Table 1. Men had significantly higher average AEEs (762 kcal/d vs. 573 kcal/d, t = 2.56, P = 0.01), PALs (1.54 vs. 1.42, t = 2.24, P = 0.03), and ACs (290,064 vs. 224,900, t = 1.97, P = 0.05) than women. However, differences between men and women in TDEE (2176 kcal/d for men vs. 2033 kcal/d for women) and estimated BMR were non-significant (1403 kcal/d for men vs. 1444 kcal/d for women). Controlling for sex, we tested for differences between activity parameters collected during August/September field seasons compared to the field season in February, and between activity levels from weekends (two day averages starting on Friday, Saturday, or Sunday) compared to weekdays. Activity levels from February were lower than activity levels in August/September (marginal means: AEE: 494 vs. 694 kcal/day, F1,45 = 6.32, P = 0.02; PAL: 1.37 vs. 1.46, F1,45 = 5.06, P = 0.03; AC: 199,052 vs. 265,847, F1,45 = 3.23, P = 0.08). Activity levels from weekends were lower than weekdays, but this difference was not statistically significant (AEE: 525 vs. 662 kcal/day, F1,45 = 2.97, P = 0.09; PAL: 1.38 vs. 1.48, F F1,45 = 2.62, P = 0.11; AC: 207,741 vs. 257,159, F1,45 = 1.78, P = 0.19).
Table 1.
| Measure | Males (n = 23) Mean (SD) |
Females (n = 26) Mean (SD) |
P | Combined (n = 49) Mean (SD) |
|---|---|---|---|---|
| Age (years) | 32.7 (12.9) | 27.8 (10.7) | ns | 30.1 (11.9) |
| Height (cm) | 155.6 (8.6) | 146.0 (4.3) | *** | 149.5 (8.2) |
| Weight (kg) | 58.2 (10.5) | 50.0 (8.2) | *** | 53.7 (9.9) |
| BMI (kg/m2)c | 23.8 (2.8) | 23.4 (2.8) | ns | 23.6 (2.8) |
| TDEE (kcal/d) | 2176 (396) | 2033 (374) | ns | 2100 (387) |
| BMR (kcal/d)d | 1403 (170) | 1444 (318) | ns | 1425 (257) |
| ACe | 290,064 (128,589) | 224,900 (102,905) | * | 255,000 (119) |
| AEE (kcal/d)f | 762 (290) | 573 (227) | * | 661 (273) |
| PALg | 1.54 (0.18) | 1.42 (0.19) | * | 1.48 (0.19) |
TDEE, total daily energy expenditure; BMR, basal metabolic rate; AEE, activity energy expenditure; PAL, physical activity level.
Differences between females and males are statistically significant at *P < 0.05; ***P < 0.001.
Weight divided by height in meters squared (kg/m2).
Calculated using the Oxford equations (Henry, 2005).
Activity counts (AC) represent the frequency and intensity of acceleration events that occur during a user-defined epoch.
AEE from activity counts based on two-regression equation (Heil, 2006).
TDEE/BMR.
We used a random effects model to examine the variance in PALs between participants, and found that 22% of the variance in PALs was attributable to participant identity (variance participant = 0.013, SD = 0.11, Residual = 0.145, SD = 0.21). Similarly, a participant’s day one activity level was significantly correlated with his or her day two activity level (r = 0.37, P = 0.01). Although correlation coefficients between day 1 and day 2 activity levels are modest, Shuar in the study community engage in mixed subsistence and agricultural production, so day to day variability in activity levels is expected, just as it would be under the traditional foraging economy.
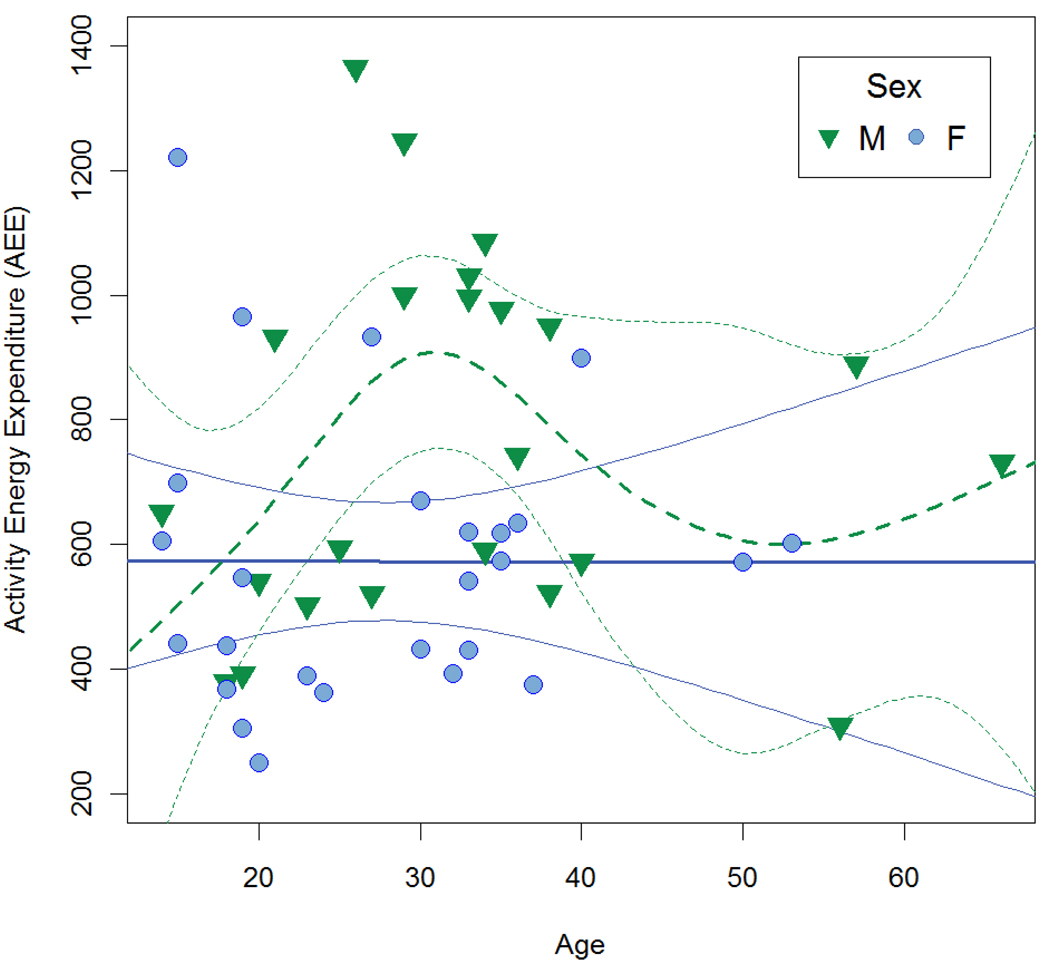
We examined the age and sex patterning of activity levels using GAM, and fit thin plate splines to the age pattern (Table 2; Fig. 1). GAM determines the optimum degrees of freedom for the spline fit through GCV minimization. In the GAM models the overall effect of sex was significant (t = 2.29, P = 0.03). For females the optimum model included no age effect. For males, the overall effect of age was also non-significant, but did include additional degrees of freedom (F = 1.61, estimated df = 3.44, P = 0.19). Examining a plot of the splines and individual data points (Fig. 1) reveals that the AEE difference between males and females is driven largely by males in the 25 to 35 year age category and that the only obvious age effect is within this age group. Examining only individuals age 25–35 years shows that males and females differed significantly in AEE (t = 3.33, df = 17, P < 0.01), PAL (t = 3.05, df = 17, P < 0.01), and AC (t = 2.05, df = 17, P = 0.05), whereas sex differences in activity measures were non-significant for other ages.
Table 2.
GAM models for age, sex, and reproductive status effects on AEE
| Without Reproductive Status | With Reproductive Status | |||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | t | P | B | SE | t | P | |
| Intercept | 572.60 | 49.49 | 11.57 | <.01 | 653.83 | 74.68 | 8.76 | <.01 |
| Sex (Male) | 166.62 | 72.93 | 2.29 | .03 | 0.73 | 96.62 | 0.01 | .99 |
| PL | −136.84 | 96.27 | −1.42 | .16 | ||||
| Sex (Male) x PL | 473.82 | 145.41 | 3.26 | <.01 | ||||
| edf | Ref.df | F | P | edf | Ref.df | F | P | |
| S(Age) Females | 1.00 | 1.00 | 0.00 | .99 | 1.00 | 1.00 | 0.05 | .83 |
| S(Age) Males | 3.44 | 4.24 | 1.61 | .19 | 1.00 | 1.00 | 0.21 | .65 |
| Model R2 (adj) | .18 | .23 | ||||||
Figure 1.
Activity energy expenditure (kcal/d) by sex and age. Lines are thin plate splines from the generalized additive model in Table 2 that does not control for reproductive status. Solid lines and circles are females, while dashed lines and triangles are males. Lighter colored lines are 95% confidence intervals for the spline value. Note that confidence intervals for males and females overlap except for ages from ~25 to ~35.
When the effect of pregnancy and lactation on activity levels was examined, a clearer picture of the sex differences in activity profiles emerged. We coded a variable indicating whether the individual (for females) or the individual’s partner (for males) was pregnant or lactating (PL) and included this in our GAM (Table 2). Including this variable eliminated the age effect and revealed a significant interaction between having a PL female in the household and sex differences in AEE (B = 473 kcal/d, t = 3.26, P < 0.01). Removing the age terms from the model, we ran two-way ANOVAs on AEE, AC, and PAL with sex and the PL variable. The interaction between sex and family reproductive status was highly significant for each activity measure (PAL: F1,45 = 18.8, P < 0.01; AEE: F1,45 = 10.9, P < 0.01; ACs: F1,45 = 6.0, P = 0.02). Including variables for season of collection and whether the data was collected on a weekend did not significantly alter these results (PAL: F1,43 = 15.96, P < 0.01; AEE: F1,43 = 8.45, P < 0.01; ACs: F1,43 = 4.46, P = 0.04). Moreover, neither season nor weekend remained significant in any model.
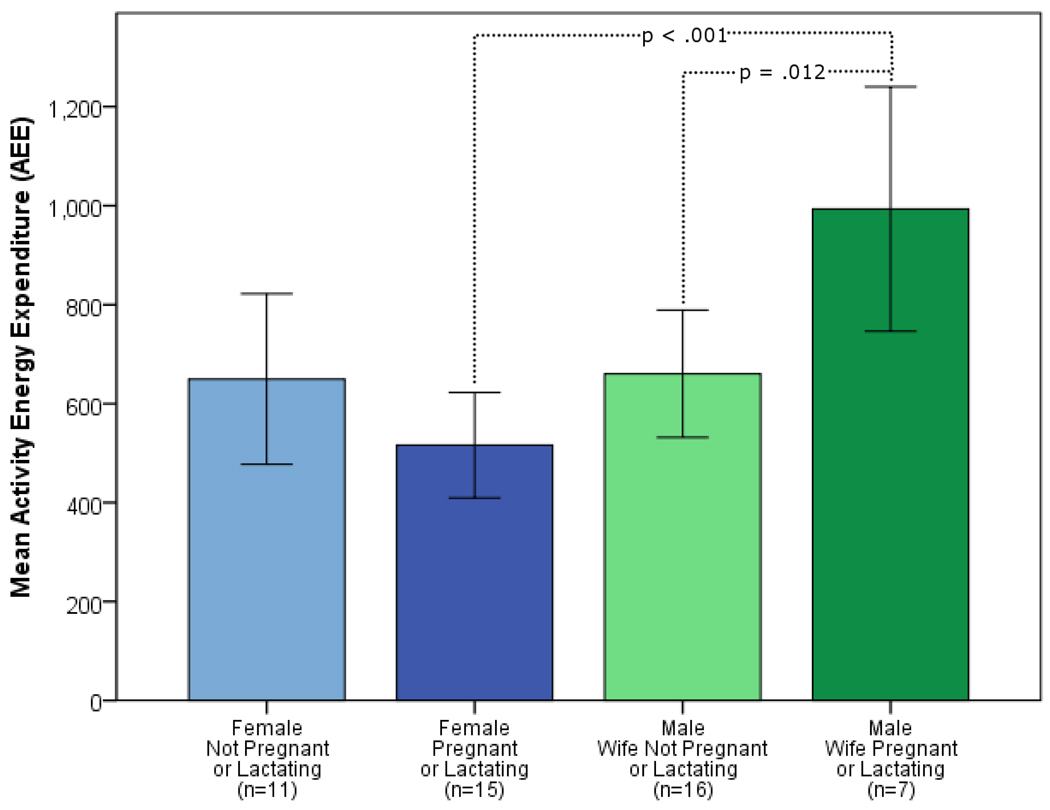
We next ran post-hoc pairwise comparisons between groups (Fig. 2). In households without a pregnant or lactating female there were no significant sex differences in activity levels. In families where the female was either pregnant or lactating, males had significantly higher activity levels than females (AEE: t = 4.79, P < 0.01; PAL: t = 5.80, P < 0.01; AC: t = 3.28, Bonferroni corrected P < 0.01). Compared to males without PL partners, males with PL partners had significantly higher AEEs (t = 2.95, Bonferroni corrected P = 0.01), and PALs (t = 2.42, Bonferroni corrected P = 0.05), but not higher ACs (Table 3). PL females had lower PALs than non-PL females (t = 3.86, P < 0.01) but not AEEs or ACs. Table 4 presents descriptive statistics for females based on reproductive status and illustrates that group differences are not the result of activity levels but rather the higher BMRs found in the PL sub-group.
Figure 2.
Mean activity energy expenditure (AEE) by sex and reproductive status. Error bars are 95% confidence intervals for the mean. Two-tailed t-tests were used for pairwise comparisons between males and females in each reproductive category and between reproductive status within each sex. A Bonferroni correction was applied to P-values to account for multiple testing. Non-significant P-values are not shown.
Table 3.
Descriptive statistics for males with pregnant or lactating partners (PL) compared to males without PL partnersa,b
| Measure | Males without PL partners (n=16) Mean (SD) |
Males with PL wives (n=7) Mean (SD) |
P |
|---|---|---|---|
| Age (years) | 33.4 (15.4) | 31.0 (4.4) | ns |
| Height (cm) | 154.2 (9.7) | 158.8 (4.8) | ns |
| Weight (kg) | 55.1 (10.7) | 65.4 (5.0) | * |
| BMI (kg/m2)c | 22.9 (2.6) | 26.0 (1.8) | * |
| TDEE (kcal/d) | 2035 (347) | 2498 (313) | * |
| BMR (kcal/d)d | 1360 (174) | 1505 (112) | * |
| ACe | 263,493 (116,902) | 350,798 (142,398) | ns |
| AEE (kcal/d)f | 661 (242) | 993 (267) | * |
| PALg | 1.48 (0.16) | 1.66 (0.17) | * |
TDEE, total daily energy expenditure; BMR, basal metabolic rate; AEE, activity energy expenditure; PAL, physical activity level.
Differences between the groups indicated are significant at p < 0.05 in an independent samples t-test with equal variance assumed.
Weight divided by height in meters squared (kg/m2).
Calculated using the Oxford equations (Henry, 2005).
Activity counts (AC) represent the frequency and intensity of acceleration events that occur during a user-defined epoch.
AEE from activity counts based on two-regression equation (Heil, 2006).
TDEE/BMR.
Table 4.
| Measure | Pregnant (n=5) Mean (SD) |
Lactating (n=10) Mean (SD) |
NPNL (n=11) Mean (SD) |
P |
|---|---|---|---|---|
| Age (years) | 24.4 (4.5) | 31.6 (7.3) | 25.9 (14.3) | ns |
| Height (cm) | 148.2 (4) | 146.7 (4.4) | 144.3 (4.0) | ns |
| Weight (kg) | 54.1 (7.3) | 50.6 (8.4) | 47.6 (8.2) | ns |
| BMI (kg/m2)c | 24.6 (2.8) | 23.4 (2.7) | 22.8 (2.9) | ns |
| BMR (kcal/d)d | 1416 (193) | 1769 (194) | 1161 (99) | *** |
| TDEE (kcal/d) | 1927 (340) | 2331 (234) | 1811 (325) | *** |
| ACe | 191,847 (121,662) | 193,014 (67,560) | 268,912 (112,910) | ns |
| PALg | 1.36 (0.15) | 1.3 (0.08) | 1.55 (0.2) | * |
| AEE (kcal/d)f | 511 (238) | 519 (180) | 650 (257) | ns |
TDEE, total daily energy expenditure; BMR, basal metabolic rate; AEE, activity energy expenditure; PAL, physical activity level.
Differences between females are statistically significant at *P < 0.05; ***P < 0.001.
Weight divided by height in meters squared (kg/m2).
Calculated using the Oxford equations (Henry, 2005).
Activity counts (AC) represent the frequency and intensity of acceleration events that occur during a user-defined epoch.
AEE from activity counts based on two-regression equation (Heil, 2006).
TDEE/BMR.
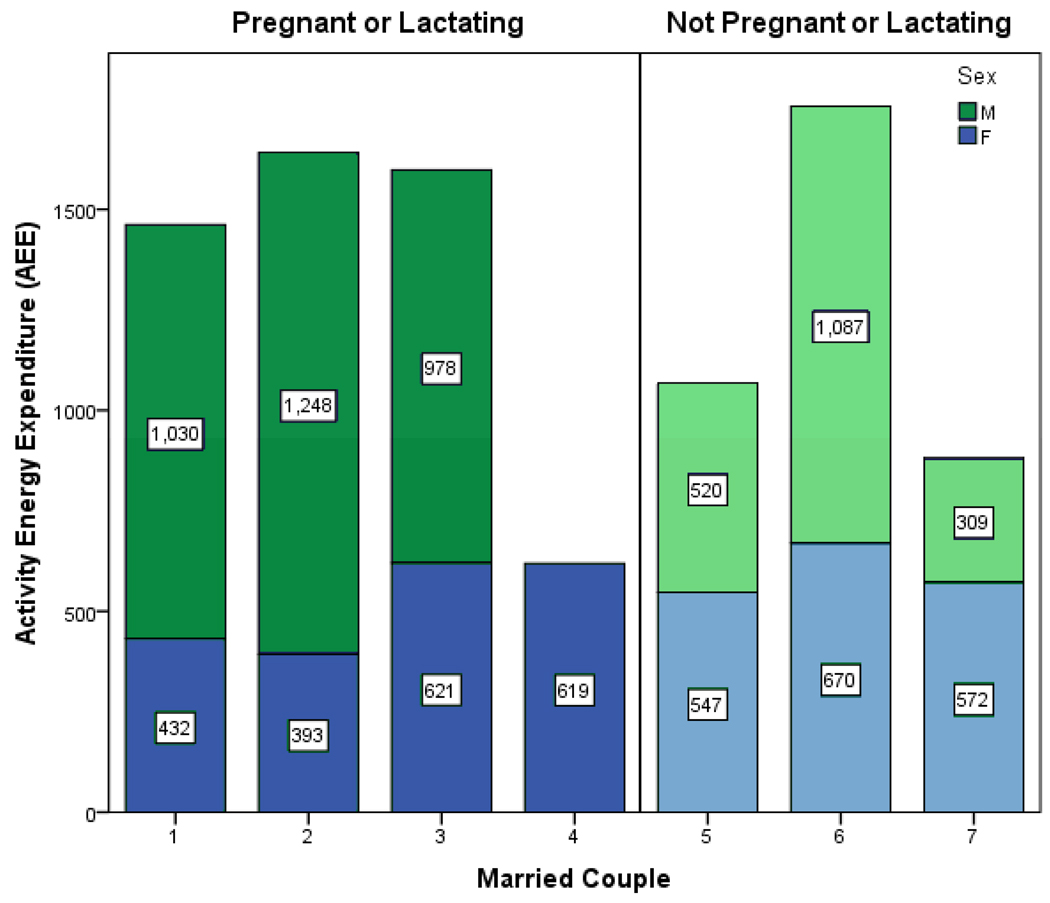
Of the 49 study participants, 13 had partners who were also in the study. These included five monogamous couples and one male with two wives. Of these, four women were pregnant or lactating and three were not (Fig. 3). Note that the male in couple 3 is polygynous: he lives primarily with female 3, but is also married to female 4, who lives in a house a short distance away. Both females were lactating at the time of the study. Couple 6 is a young NPNL pair and appears to follow the pattern of PL couples; this characteristic may be explained by their ownership of a small store where they sell a small amount of basic market goods including noodles, rice, eggs, soap, and oil. The NPNL wife often tends the store while caring for her two young children, which may account for why her activity level is relatively low compared to that of her husband. Although the sample size is small, a trend towards higher activity output by males with PL counterparts is evident.
Figure 3.
Activity energy expenditure (AEE) in kcal/d for married couples for which both partners participated in this study.
DISCUSSION
The present study used accelerometry to investigate physical activity patterns in Upano River Valley Shuar who are currently undergoing rapid economic development and lifestyle change. Despite the shift towards increasing market integration, Shuar from the region continue to be dependent on subsistence activities, particularly horticultural production. Most people in the study community actively engage in subsistence activities, spending most mornings and afternoons at their chacras (gardens) cultivating the land and harvesting cassava (yuca), plantains, papa china (a local tuber), maize, legumes, and other staple foods.
Physical activity levels for Shuar males are approximately 1.54, while Shuar females exhibit significantly lower PALs of 1.42. Based on FAO/WHO/UNU (2004) standards, average PALs among the Shuar are within the light (PAL ~1.4) to moderate (PAL ~1.75) range. Other measures of physical activity, including AC and AEE, were also significantly lower among females than males.
It is difficult to compare activity profiles between the Shuar and other subsistence populations since other energetics studies have used a variety of different techniques to assess physical activity (e.g., DLW, HR monitors, and time allocation). Few studies to date have used accelerometry to estimate energy expenditure in remote field settings. Further, because various types of accelerometers generate different output (e.g., uni-axial versus tri-axial accelerometers), comparing activity levels between studies using different types of accelerometers can also be problematic. Converting activity counts into common energetic parameters using published regression equations is one means of overcoming the obstacle of comparing between accelerometer types. However, comparisons between populations using different activity techniques will always introduce error so results must be interpreted with caution. Nevertheless, these problems of inter-study comparison are not limited to this study. Until researchers employ a standardized means of recording activity data, or systematize analytic procedures for cross-methodological comparisons, such approaches remain essential in order to address important issues in human biology, including those related to global health.
Shuar activity values are low when compared to other subsistence populations (Table 5). Using the DLW method, Snodgrass et al. (2006) found that among the indigenous Yakut of Siberia, individuals who participated in fewer subsistence activities had significantly lower activity levels and consumed a greater percentage of market foods than individuals with more traditional lifestyles. The Shuar participant community is in the process of transitioning towards a market economy, so low activity levels might be similarly related to decreased participation in subsistence behaviors and increased market good consumption. However, among Gambian (Lawrence and Whitehead, 1988) and Andean Aymara (Kashiwazaki et al., 1995) rates of energy expenditure were attributed not to short bursts of intensive activity, but to extended periods of activities with moderate energy cost. In fact, in a comparison of activity levels across populations in developing countries, Dufour and Piperata (2008) show that subsistence activities may not necessarily involve high energy expenditure, and therefore they caution researchers from making generalizations about the energy needs of subsistence-based lifestyles. Lower than expected activity values among the Shuar may therefore be explained by a focus on habitual tasks of long duration requiring relatively low energy output (e.g., gathering legumes, clearing weeds from chacras, or processing yuca for the production of chicha [a locally-made fermented beverage]). Alternately, the low values could be due to the fact that Shuar activity was measured only during dry season months. Although we documented variation in activity levels across the dry season, it seems unlikely that wet season activity levels would be higher, given that Shuar devote less time to hunting, fishing, and garden clearing activities during this rainier period. Future data collection is necessary to test this proposition.
Table 5.
Activity profile comparison between the Shuar and published data setsa
| Population | Source | Methodb | Sex | n | Age (years) |
Weight (kg) |
TDEE (kcal/d) |
AEE (kcal/d) |
BMR (kcal/d) |
PAL | Activity Levelc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shuar | This study | ACC | M | 23 | 32.7 (12.9) | 58.2 (10.5) | 2175.6 (395.5) |
761.8 (289.5) |
1403.3 (169.6) |
1.54 | L |
| F | 26 | 27.8 (10.7) | 50.0 (8.2) | 2033.2 (374.4) |
572.7 (227.3) |
1443.7 (317.8) |
1.42 | L | |||
|
Coastal Ecuadoreans |
Leonard et al., 1995 | HRM | M | 5 | 49 (19) | 61.3 (9.2) | 2414.7 (353.5) |
885.7 | 1529 | 1.58 | L-M |
| F | 5 | 34 (16) | 55.7 (11.5) | 1992.0 (138.5) |
767 | 1225 | 1.63 | L-M | |||
|
Highland Ecuadoreans |
Leonard et al., 1995 | HRM | M | 11 | 32 (12) | 61.3 (9.2) | 3807.2 (759.5) |
2207.2 | 1600 | 2.39 | H |
| F | 11 | 40 (13) | 55.7 (11.5) | 2457.7 (707.0) |
1205.7 | 1252 | 1.97 | H | |||
|
Ache (Paraguay) |
Hill et al., 1984; Leonard and Robertson, 1992 |
TIME | M | n/a | n/a | 59.6 | 3327 | 1796 | 1531 | 2.17 | H |
| F | n/a | n/a | 51.8 | 2626 | 1232 | 1394 | 1.88 | H | |||
|
Yakut (Siberia) |
Snodgrass et al., 2006 | DLW | M | 14 | 33.3 (9.8) | 72.2 (14.5) | 3100.9 (1939.0) |
1254.3 (168.7) |
1848.7 (69.1) |
1.68 | L-M |
| F | 14 | 31.4 (9.6) | 65.2 (19.5) | 2297.7 (124.7) |
765.5 (84.8) |
1533.9 (65.0) |
1.5 | L | |||
| Burkina Faso | Bleiberg et al., 1981 | TIME | M | 11 | 45 (3.32) | 56.5 (1.75) | 2261 (67.4) |
n/a | n/a | 1.36 | L |
| F | 14 | 30.6 (2.63) | 49.9 (0.92) | 2144 (49.1) |
n/a | n/a | 1.54 | L | |||
|
!Kung (Botswana) |
Lee, 1979; Leonard and Robertson, 1992 | TIME | M | n/a | n/a | 46 | 2319 | 936 | 1383 | 1.68 | L-M |
| F | n/a | n/a | 41 | 1712 | 613 | 1099 | 1.56 | L | |||
| Huli (PNG) | Yamauchi et al., 2001 | HRM | M | 15 | n/a | 63.6 (7.3) | 3138 (506.4) |
1434 | 1704 | 1.84 | M-H |
| F | 12 | n/a | 53.3 (7.6) | 2639 (621) |
1248 | 1391 | 1.88 | H |
Means (SD),
ACC, accelerometry; HRM, heart rate monitors; TIME, time allocation; DLW, doubly labeled water,
Activity level is determined using the FAO/WHO/UNU (2004) classification system of light (L), moderate (M), and heavy (H) activity levels.
Sex Differences in Activity Patterns
Energetics provides a powerful tool with which to study life history trade-offs under different ecological conditions (Snodgrass, 2011). Life history theory examines the age- and context-dependent resource allocations that organisms make between competing demands such as growth, reproduction, and somatic maintenance (Charnov and Schaffer, 1973; Gadgil and Bossert, 1970; Hill and Hurtado, 1996; Hill and Kaplan, 1999; Lessels, 1991; Stearns, 1976). For successful reproduction, women in subsistence-level societies must allocate sufficient resources to energy intensive pregnancy and lactation for extended periods of time, while simultaneously maintaining essential metabolic function and the physical activity levels necessary to acquire food, care for offspring, and engage in other critical daily activities (Bogin, 1999; Ellison, 1994; Hrdy, 1999). Biological and behavioral strategies regulating the balance of energy allocation to costly reproductive effort (e.g., pregnancy and lactation) and the energetic costs of food acquisition, somatic maintenance, and parental investment are therefore expected to have evolved. Strategies may involve increasing dietary intake or minimizing energy expenditure, such as through adjusting metabolic efficiency, drawing on fat stores, or reducing activity costs (Piperata, 2009; Piperata and Dufour, 2007; Ulijaszek, 1995). Adoption of any of these energetic strategies may be dependent upon the degree of social support available to the woman, including from husbands or older non-reproductive aged offspring who contribute substantially to the high costs of child-rearing. This investment from other community members would support heightened energy demands during critical reproductive periods by way of provisioning for young infants and mothers themselves (Hill and Hurtado, 2009; Hrdy, 2005; Marlowe, 2003; Meehan, 2009; Reiches et al., 2009). From this perspective, female reproduction and energy dynamics may be better understood within the dynamics of a larger cooperative reproductive effort, which would take into account familial energetics or, at the very least, those of mating partners.
The present study was a preliminary investigation of the utility of accelerometry to study potential sex differences in energy expenditure and considered activity patterns based on reproductive status. Males had significantly higher PALs than females, but as over half of the women in the study were either pregnant or lactating, using PALs are problematic when comparing between sexes. When AC and AEE were used as the energy parameters, significant sex differences were maintained.
Male AEE was more variable than female AEE, with female activity values generally clustering under 600 kcal/d. Male activity peaked between the ages of 25–35 years. Almost all pregnant and lactating females fell into this age category; they also produced the lowest total energetic output in the study population. There were no significant differences in energetic output, as measured by AEE and AC, between females who were pregnant or lactating and those who were not. PALs did vary significantly between PL and NPNL females, a difference attributable to the higher BMR costs of energetically costly reproductive states. Interestingly, while BMR requirements are higher in PL women, their work output is fairly constant, comparable to that of NPNL female participants. A more critical observation is the significantly higher activity levels among males with PL partners compared to other males. Although the sample size is small, the data suggest that while the higher basal metabolic costs for PL females do not cause a decline in their energy expenditure, it appears to incite a compensatory increase in the activity output of male partners.
In some subsistence populations, women reduce energy expenditure during pregnancy and lactation, which appears to at least partially offset elevated energy needs (Panter-Brick, 1993; Piperata and Dufour, 2007). A decrease in female economic contribution can present costs to household production and, therefore, may prompt a compensatory increase in male provisioning as noted among the Ache and Hiwi foragers (Hurtado et al., 1992) and the Hadza hunter-gatherers (Marlowe, 2003). Our data suggest that the increased energetic needs of pregnant or lactating females initiates behavioral changes, not by a reduction in activity output by females themselves, but rather through an increase in the activity levels of other family members, more specifically, their male partners. The necessity to contribute to work output may demand women to maintain their baseline activity patterns while men are increasing their own economic contribution as a means of offsetting their partner’s up-regulated metabolic costs.
Although the sample size in this preliminary study is small, the low PALs, AEEs and ACs among pregnant and lactating women coupled with significantly higher activity levels among men with pregnant or lactating partners suggest a cooperative effort to cope with high maternal energy demands. Despite the rapid shift towards increasing market integration among Shuar in the Upano Valley, the results of the present study are consistent with data from other subsistence-based populations in which male and children’s subsistence activities appear to be critical for supplementing women’s energetic needs during pregnancy and lactation (e.g., Ivey, 2000; Marlowe, 2003; Reiches et al., 2009). Although these data logically fit well with a cooperative breeding model of human reproduction (Hill and Hurtado, 2009; Hrdy, 2000), it should be emphasized that our results are based on a small sample, and further research is needed to confirm them. Nevertheless, the results do indicate the potential utility of accelerometry for investigating such issues.
Field Methods of Measuring Physical Activity
Measurement of physical activity outside of a laboratory setting is notoriously difficult and no method is without problems (Snodgrass, 2011). A variety of methods are available for estimating physical activity levels and TDEE, the most accurate of which is the doubly labeled water (DLW) technique. DLW yields a measure of TDEE over the course of 1–2 weeks, based on the elimination rates of two labeled stable isotopes from the body. This technique is generally accepted as the most accurate measure of energy expenditure among free-living humans, and has been used extensively in clinical studies in industrial nations (Black et al., 1996; FNB/IOM, 2002; Speakman, 1997). DLW provides an excellent basis for analysis of average TDEE across longer timescales and is particularly useful for characterizing and comparing general activity levels between men and women, or between populations. Unfortunately, the high cost of DLW has limited its use in populations in the developing world (Coward, 1998). When data are available from non-Western groups, sample sizes are generally small (less than 50 individuals), which makes comparisons between populations problematic. Further, DLW does not provide information on daily or hourly fluctuations in energy use, or the energy costs associated with specific activities.
The time allocation technique is the most commonly used method for estimating activity levels in subsistence populations and can provide a record of daily or hourly changes in activity. This method estimates physical activity by either observing or using interview techniques to estimate the amount of time an individual spends in different activities, and combining this with published information on energy costs of each activity (e.g., FAO/WHO/UNU, 2004; James and Schofield, 1990; Ulijaszek, 1995). Summing the results for one day thereby provides an estimate of TDEE. However, published values for the energy costs of different activities are typically based on only a few individuals and no data exist for many common activities researchers observe in field settings. In addition to being time and labor-intensive, the time allocation technique often substantially underestimates energetic parameters, since it does not record many involuntary activities (e.g., fidgeting). This method is most inaccurate at moderate to high activity levels, often underestimating TDEE by at least 15% (Kashiwazaki et al., 2009; Leonard et al., 1997; Spurr et al., 1996).
Heart rate (HR) monitoring can also be used to estimate physical activity, based on the known relationship of HR to energy expenditure. The HR monitor records HR at a designated interval (e.g., every minute) while participants wear the monitors during waking hours over the course of several days. At the end of the measurement period, HR data is downloaded from the instrument and energy expenditure is calculated. Despite proven accuracy of the measurement compared to DLW (e.g., Kashiwazaki, 1999), there are also limitations to this technique. For example, in order to obtain accurate results, one key issue involves the need for individual calibration of HR with energy expenditure. This process requires the relationship of HR to energy expenditure to be established for each individual both at rest and during graded sub-maximal exercise (Leonard, 2003).
Technological advances in accelerometry promise to help overcome some of the challenges of affordably quantifying physical activity under field conditions. Accelerometers can objectively measure the movement of the body by detecting and recording acceleration in one or multiple planes (Chen and Bassett, 2005; Gerdhem et al., 2008; Heil, 2006). The devices are durable, non-intrusive, relatively inexpensive, and can provide detailed information on physical activity patterns, including the duration and intensity of activity. Several accelerometer devices, including the Actical, have been validated and show a high correlation with oxygen consumption and DLW (Heil, 2006; Hoos et al., 2003; Plasqui and Westerterp, 2007). However, it is important to note that most accelerometer validation studies to date have been conducted in laboratory-based samples of participants from industrialized nations.
The Actical is one of the more extensively validated commercially available accelerometer devices (Plasqui and Westerterp, 2007). Acticals are small, rugged data loggers, equipped with a highly sensitive multi-directional accelerometer. The ability to sense motion in more than a single plane is an advantage for measuring complex human movements (Heil, 2006). The Actical accelerometer generates a variable voltage based on amplitude and frequency of motion and produces an electrical current that varies in magnitude. An increase in the intensity of motion will result in an increase in voltage; this information is integrated over a user-selected epoch and recorded in onboard memory in the form of “Activity Counts.” An activity count (AC) is an arbitrary dimensionless unit that varies between different brands of accelerometer devices, so direct comparisons of raw activity counts from assorted devices are not inherently meaningful. However, ACs can be used to calculate AEE or TDEE using the device software or through accelerometer-specific published regression equations allowing for cross-methodological comparisons yet, as noted earlier, with limitations.
As with other means of activity recording there are restrictions to using accelerometry to determine activity levels and energy expenditure. These limitations vary with the type of device used and how it is utilized. For example, placement at the hip captures gross body movement but may be less sensitive to upper body movements or the energetic costs associated with load-carrying, which may result in underestimations of physical activity output (Swartz et al., 2000). Nevertheless, several studies have documented the validity of hip-placed activity monitors for estimating whole-body energy expenditure when compared to alternative sites such as the wrist and ankle (Freedson et al., 1998; Hendelman et al., 2000; Swartz et al., 2000). Another limitation is that accelerometers do not provide the context of activity, although this may be obtained by simultaneously incorporating other techniques (e.g., daily recall or direct observation). Further, as mentioned earlier, an issue with accelerometer devices that can be extended to all types of activity monitors is that comparison of activity across populations using different models of accelerometers will entail greater measurement error than will studies that use the same device, thus small but statistically significant differences must be interpreted cautiously. However, if one is interested in documenting relative activity differences within a population using identical devices and methods, these are minor limitations.
The current study represents a preliminary step towards greater use of accelerometry in non-clinical, non-Westernized contexts. The results presented here are consistent with predictions based on a life history theory approach to understanding energetics, thus illustrating the potential usefulness of accelerometer devices in addressing such questions. Clearly, at this preliminary stage, comparisons of activity patterns with other populations must be interpreted with caution. However, given the importance of recognizing how shifts in energy expenditure can inform emerging global health issues, and the scant nature of these data from subsistence-level populations, researchers must overcome the urge to wait until a perfect dataset is available prior to reporting their findings.
CONCLUSIONS
The present study investigates energetic patterns in an Ecuadorian Shuar community undergoing rapid economic development and lifestyle change. It provides preliminary evidence that activity levels in this transitioning Shuar community are modest, especially when compared with other subsistence populations. This study also investigated life history trade-offs related to female reproductive status. With regard to the Shuar participant community, it suggests that female reproduction and energy dynamics may be better understood within the dynamics of a larger cooperative reproductive effort, including that of mating partners. Despite a shift towards market integration, Shuar pregnant and lactating females may adopt a cooperative strategy noted in other subsistence-based populations where male participation in subsistence activities is higher in order to compensate for their partners’ elevated reproductive costs. Finally, this study demonstrates the promise of accelerometry use under field conditions and argues that despite some limitations, this technique offers useful information regarding population-level activity patterns.
ACKNOWLEDGMENTS
We thank Tara J. Cepon, Tiffany R. Gandolfo, Ruby Fried, Cesar Kayap, and Oswaldo Mankash for assistance with data collection. We also thank Bill Leonard and two anonymous reviewers for their comments on an earlier version of this manuscript. Finally, we wish to express our gratitude to the participants in this study.
Contract grant sponsor: Wenner-Gren Foundation for Anthropological Research, Contract grant number: 7970; Contract grant sponsor: NSF, Contract grant number: BCS-0824602; Contract grant sponsors: Evonuk Foundation, Leakey Foundation, Ryoichi Sasakawa Young Leaders Fellowship, NIH grant number 5DP1OD000516-5 via UCSB Center for Evolutionary Psychology, University of Oregon
LITERATURE CITED
- Baker PT, Hanna JM, Baker TS. The changing Samoans: behavior and health in transition. Oxford: Oxford University Press; 1986. [Google Scholar]
- Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: analysis of 574 doubly-labeled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- Blackwell AD, Pryor G, III, Pozo J, Tiwia W, Sugiyama LS. Growth and market integration in Amazonia: a comparison of growth indicators between Shuar, Shiwiar, and nonindigenous school children. Am J Hum Biol. 2009;21:161–171. doi: 10.1002/ajhb.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiberg FM, Brun TA, Goihman S, Lippman D. Food intake and energy expenditure of male and female farmers from Upper-Volta. Br J Nutr. 1981;45:505–515. doi: 10.1079/bjn19810129. [DOI] [PubMed] [Google Scholar]
- Bogin B. Evolutionary perspective on human growth. Annu Rev Anthropol. 1999;28:109–153. doi: 10.1146/annurev.anthro.28.1.109. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Schaffer WM. Life history consequences of natural selection: Cole´s result revisited. Am Nat. 1973;107:791–793. [Google Scholar]
- Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37:S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- Coward WA. Contributions of the doubly labeled water method to studies of energy balance in the Third World. Am J Clin Nutr. 1998;68:962S–969S. doi: 10.1093/ajcn/68.4.962S. [DOI] [PubMed] [Google Scholar]
- Dufour DL, Piperata BA. Energy expenditure among farmers in developing countries: what do we know? Am J Hum Biol. 2008;20:249–258. doi: 10.1002/ajhb.20764. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Advances in human reproductive ecology. Annu Rev Anthropol. 1994;23:255–275. doi: 10.1146/annurev.an.23.100194.001351. [DOI] [PubMed] [Google Scholar]
- FAO/UNU/WHO. Report of a joint FAO/WHO/UNU expert consultation. Rome: United Nations University, World Health Organization, Food and Agricultural Organization of the United Nations; 2004. Human energy requirements. [Google Scholar]
- Food and Nutrition Board (FNB)/Institute of Medicine (IOM) Dietary reference intakes of energy, carbohydrates, fiber, fat, protein, and amino acids (Macronutrients) National Academies Press; 2002. [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sport Exer. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Friedlaender JS, Howells WW, Rhoads JG, editors. The Solomon Islands Project: a long- term study of health, human biology, and culture change. Oxford: Oxford University press; 1987. [Google Scholar]
- Gadgil M, Bossert W. Life history consequences of natural selection. Am Nat. 1970;104:1–24. [Google Scholar]
- Gerdhem P, Dencker M, Ringsberg K, Akesson K. Accelerometer-measured daily physical activity among octogenerians: results and associations to other indices of physical performance and bone density. Eur J Appl Physiol. 2008;102:173–180. doi: 10.1007/s00421-007-0571-z. [DOI] [PubMed] [Google Scholar]
- Guillermo-Tuazon MA, Barba CVC, van Raaij JMA, Hautvast J. Energy intake, energy expenditure, and body composition of poor rural Philippine women throughout the first 6 months of lactation. Am J Clin Nutr. 1992;56:874–880. doi: 10.1093/ajcn/56.5.874. [DOI] [PubMed] [Google Scholar]
- Harner MJ. The Jívaro, people of the sacred waterfalls. Berkeley: University of California Press; 1984. [Google Scholar]
- Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77:64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- Hendelman D, Miller K, Baggett C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sport Exer. 2000;32:S442–S449. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8:1133–1152. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- Hill KR, Hawkes K, Hurtado M, Kaplan H. Seasonal variance in the diet of Ache hunter-gatherers in Eastern Paraguay. Hum Ecol. 1984;12:101–135. [Google Scholar]
- Hill KR, Hurtado AM. The evolution of premature reproductive senescence and menopause in human females: an evaluation of the grandmother hypothesis. In: Betzig L, editor. Human nature: a critical reader. New York: Oxford University Press; 1996. pp. 313–350. [DOI] [PubMed] [Google Scholar]
- Hill KR, Hurtado AM. Cooperative breeding in South American hunter-gatherers. Proc R Soc B. 2009;276:3863–3870. doi: 10.1098/rspb.2009.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Kaplan H. Life history traits in humans: theory and empirical studies. Annu Rev Anthropol. 1999;28:397–438. doi: 10.1146/annurev.anthro.28.1.397. [DOI] [PubMed] [Google Scholar]
- Hoos MB, Plasqui G, Gerver WJM, Westerterp KR. Physical activity level measured by doubly labeled water and accelerometry in children. Eur J Appl Physiol. 2003;89:624–626. doi: 10.1007/s00421-003-0891-6. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Mother nature: a history of mothers, infants, and natural selection. New York: Pantheon Books; 1999. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. The optimal number of fathers: evolution, demography and history in the shaping of female mate preferences. Ann NY Acad Sci. 2000;907:75–96. doi: 10.1111/j.1749-6632.2000.tb06617.x. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Comes the child before the man: how cooperative breeding and prolonged postweaning dependence shaped human potentials. In: Hewlett B, Lamb M, editors. Hunter-gatherer childhoods: evolutionary, developmental, and cultural perspectives. New York, NY: Aldine; 2005. pp. 65–91. [Google Scholar]
- Hurtado AM, Hill K, Kaplan H, Hurtado I. Trade-offs between female food acquisition and child care among Hiwi and Ache foragers. Human Nat. 1992;3:185–216. doi: 10.1007/BF02692239. [DOI] [PubMed] [Google Scholar]
- Huss-Ashmore R, Schall J, Hediger M, editors. Health and lifestyle change. Philadelphia: University of Pennsylvania; 1992. [Google Scholar]
- Ivey PK. Cooperative reproduction in Ituri Forest hunter-gatherers: who cares for Efe infants? Curr Anthropol. 2000;41:856–866. [Google Scholar]
- James WPT, Schofield EC. Human energy requirements. New York: Food and Agriculture Organization of the United Nations/Oxford University Press; 1990. [Google Scholar]
- Kashiwazaki H, Dehima Y, Orias-Rivera J, Coward WA. Energy expenditure determined by the doubly labeled water method in Bolivian Aymara living in a high altitude agropastoral community. Am J Clin Nutr. 1995;62:901–910. doi: 10.1093/ajcn/62.5.901. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki H. Heart rate monitoring as a field method for estimating energy expenditure as evaluated by the doubly labeled water method. J Nutr Vitaminol. 1999;45:79–94. doi: 10.3177/jnsv.45.79. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki H, Uenishi K, Kobayashi T, Rivera JO, Coward WA, Wright A. Year-round high physical activity levels in agropastoralists of Bolivian Andes: results from repeated measurements of DLW method in peak and slack seasons of agricultural activities. Am J Hum Biol. 2009;21:337–345. doi: 10.1002/ajhb.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, Whitehead RG. Physical activity and total energy expenditure of child-bearing Gambian village women. Eur J Clin Nutr. 1988;42:145–160. [PubMed] [Google Scholar]
- Lee RB. The !Kung San: men, women and work in a foraging society. Cambridge: Cambridge University Press; 1979. p. 526. [Google Scholar]
- Leonard WR. Measuring human energy expenditure: what have we learned from the flex-heart rate method? Am J Hum Biol. 2003;15:479–489. doi: 10.1002/ajhb.10187. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Robertson ML. Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Biol. 1992;4:179–195. doi: 10.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- Leonard WR, VA Galloway, Ivakine E. Underestimation of daily energy expenditure with the factorial method: implications for anthropological research. Am J Phys Anthropol. 1997;103:443–454. doi: 10.1002/(SICI)1096-8644(199708)103:4<443::AID-AJPA2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Katzmarzyk PT, Stephen MA, Ross AGP. Comparison of the heart rate-monitoring and factorial methods: assessment of energy-expenditure in highland and coastal Ecuadorians. Am J Clin Nutr. 1995;61:1146–1152. doi: 10.1093/ajcn/61.4.1146. [DOI] [PubMed] [Google Scholar]
- Lessels CM. The evolution of life histories. In: Krebs JR, Davies NB, editors. Behavioural ecology. London: Blackwell Scientific Publications; 1991. pp. 32–68. [Google Scholar]
- Lindgarde F, Widen I, Gebb M, Ahren B. Traditional versus agricultural lifestyle among Shuar women of the Ecuadorian Amazon: effects on leptin levels. Metabolism. 2004;53:1355–1358. doi: 10.1016/j.metabol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization: reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- Lu F. Integration in the market among indigenous peoples: a cross-cultural perspective from the Ecuadorian Amazon. Curr Anthropol. 2007;48:593–602. [Google Scholar]
- Marlowe F. A critical period for provisioning by Hadza men: implications for pair bonding. Evol Hum Behav. 2003;24:217–229. [Google Scholar]
- Meehan CL. Maternal time allocation in two cooperative childrearing societies. Hum Nature. 2009;20:375–393. [Google Scholar]
- Monteiro CA, Mondini L, Medeiros de Souza AL, Popkin BM. The nutrition transition in Brazil. Eur J Clin Nutr. 1995;49:105–113. [PubMed] [Google Scholar]
- Panter-Brick C. Seasonality of energy expenditure during pregnancy and lactation for rural Nepali women. Am J Clin Nutr. 1993;57:620–628. doi: 10.1093/ajcn/57.5.620. [DOI] [PubMed] [Google Scholar]
- Piperata BA. Variation in maternal strategies during lactation: the role of the biosocial context. Am J Hum Biol. 2009;21:817–827. doi: 10.1002/ajhb.20898. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Dufour DL. Diet, energy expenditure, and body composition of lactating Ribeirinha women in the Brazilian Amazon. Am J Hum Biol. 2007;19:722–734. doi: 10.1002/ajhb.20628. [DOI] [PubMed] [Google Scholar]
- Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obes. 2007;15:2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- Popkin BM. Technology, transport, globalization and the nutrition transition food policy. Food Policy. 2006;31:554–569. [Google Scholar]
- Prentice AM, Spaaij CJK, Goldberg GR, Poppitt SD, van Raaij JMA, Totton M, Swann D, Black AE. Energy requirements of pregnant and lactating women. Eur J Clin Nutr. 1996;50 Suppl 1:82–111. [PubMed] [Google Scholar]
- Reiches MW, Ellison PT, Lipson SF, Sharrock KC, Gardiner E, Duncan LG. Pooled energy budget and human life history. Am J Hum Biol. 2009;21:421–429. doi: 10.1002/ajhb.20906. [DOI] [PubMed] [Google Scholar]
- Rubenstein S. Colonialism, the Shuar Federation, and the Ecuadorian state. Environ Plann D. 2001;19:263–293. [Google Scholar]
- Shephard RJ, Rode A. The health consequences of ‘modernization’: evidence from circumpolar peoples. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Sirén AH. Population growth and land use intensification in a subsistence-based indigenous community in the Amazon. Hum Ecol. 2007;35:669–680. [Google Scholar]
- Snodgrass JJ, Leonard WR, Tarskaia LA, Schoeller DA. Total energy expenditure in the Yakut (Sakha) of Siberia as measured by the doubly labeled water method. Am J Clin Nutr. 2006;84:798–806. doi: 10.1093/ajcn/84.4.798. [DOI] [PubMed] [Google Scholar]
- Snodgrass JJ, Sorensen MV, Tarskaia LA, Leonard WR. Adaptive dimensions of health research among indigenous Siberians. Am J Hum Biol. 2007;19:165–180. doi: 10.1002/ajhb.20624. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Doubly labeled water: theory and practice. London: Chapman & Hall; 1997. [Google Scholar]
- Spurr GB, Dufour DL, Reina JC. Energy expenditure of urban Colombian women: a comparison of patterns and total daily expenditure by the heart rate and factorial methods. Am J Clin Nutr. 1996;63:870–878. doi: 10.1093/ajcn/63.6.870. [DOI] [PubMed] [Google Scholar]
- Snodgrass JJ. Human energetic. In: Stinson S, Bogin B, O’Rourke D, editors. Human biology: and evolutionary and biocultural approach. 2nd edition. New York: Wiley-Blackwell; 2011. in press. [Google Scholar]
- Stearns SC. Life history tactics: a review of the ideas. Q Rev Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Stone MR, Rowlands AV, Eston RG. Relationships between accelerometer-assessed physical activity and health in children: impact of the activity-intensity classification method. J Sports Sci and Med. 2009;8:136–143. [PMC free article] [PubMed] [Google Scholar]
- Swartz AM, Strath SJ, Bassett DR, O"Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sport Exer. 2000;32:S450–S456. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]
- Ulijaszek SJ. Human energetics in biological anthropology. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–828. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Umezaki M, Ohtsuka R. Physical activity and subsistence pattern of the Huli, a Papua New Guinea highland population. Am J Phys Anthropol. 2001;114:258–268. doi: 10.1002/1096-8644(200103)114:3<258::AID-AJPA1024>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]