Abstract
This study aimed to evaluate the influence of insulin resistance status on weight changes in non-obese women who followed a home-based exercise program and slight caloric restriction over a period of 12 months. Middle-aged (25–45 yr), non-obese (body mass index of 23–29.9 kg/m2) women were randomly assigned to control (CG) or home-based exercise group (HB). The HB group received a booklet explaining the physical exercises to be practiced at home at least three times per week (40 min/session). Both groups were required to follow a small energy restriction of 100–300 calories per day. For the analysis, women were stratified in two groups according to baseline insulin sensitivity: NIR (non-insulin resistant; n=121) and IR (insulin resistant; n=64). Women classified as IR at baseline had greater weight loss after 12 months of follow-up (−1.6 kg vs. −1.1 kg; p=0.01), and HB exercise helped to reduce weight only among NIR women (−1.5 vs. −0.7; p=0.04); no differences were observed between intervention groups for IR women (−1.5 vs. −1.7; p=0.24). There were no differences between IR and NIR groups for lipid profile after adjustment for weight changes. Insulin resistance facilitated weight loss, and home-based exercise promoted greater weight loss only in non-insulin resistance women.
Keywords: Diabetes, Prevention, Weight change, Obesity, Physical activity
Introduction
Lifestyle interventions focused on diet and physical activity remain cornerstones of the treatment and prevention of diabetes and obesity [1–3], although the results regarding the role of physical activity on prevention of weight regain are still controversial [4].
A meta-analysis by Wu et al. [5] reinforced the long-term role of physical activity on weight control, although other studies have reported that the inclusion of physical activity in addition to an energy restriction did not facilitate weight loss [6,7].
It has been postulated that insulin resistance (IR) is a physiological adaptation to obesity that limits fat deposition and leads to weight stabilization [8,9]. Swinburn et al. [10] followed weight changes over 3 years for 192-non diabetic Pima Indians, and they found that insulin-resistant subjects gained less weight than insulin sensitive subjects (3.1 vs. 7.6 kg). Similar results were found in the Rancho Bernardo Study [11] in which insulin-resistant individuals had a threefold increased likelihood of losing 10 kg or more compared with those without insulin resistance during the 8 years of follow-up.
Other studies have found a different relationship between IR and weight change, including a study conducted by Houmard et al. [12]. This study, which included 3389 postmenopausal women of diverse ethnic groups, demonstrated that IR was a significant predictor of weight gain. Additionally, Johnson et al. [13] examined the influence of baseline insulin sensitivity on longitudinal changes in body fat mass during growth in children, and they showed that insulin sensitivity was negatively associated with the increase in fat mass over time.
The identification of people who are at risk of gaining body weight or who are less able to lose weight during treatment is important for the development of successful strategies for the prevention of obesity. IR is one of the potential factors that could be used to identify these individuals. However, the results from studies of the relationship between IR and weight change have been primarily observational and are still inconclusive. Only few small experimental studies of short duration have been conducted, and their results were controversial [14–16]. Therefore, we analyzed the data from an experimental study on diet and exercise among non-obese women to evaluate the influence of insulin resistance status on weight changes during 12 months of follow-up.
Materials and methods
The present study was a secondary analysis of a randomized controlled trial primarily designed to compare the effects of low and high glycemic index diets on weight changes in non-obese women. The full description and results of the dietary intervention have been published elsewhere [17]. The trial had a factorial design, with half of the dietary intervention group also receiving instruction on a home-based exercise program to follow for 12 months; and the other half received only the dietary intervention.
In short, 644 women were screened, and 230 were ineligible based on the criteria of eligibility. To be eligible, the women had to be between 25 and 45 years of age with a body mass index (BMI) of 23–29.9 kg/m2, not pregnant, and not breastfeeding. Women with physician-diagnosed thyroid disease or diabetes or who were menopausal were not eligible to participate. Recruitment was conducted at two primary health care centers affiliated with Rio de Janeiro State University, in Brazil. Of the 644 women screened, 414 were eligible and 203 completed the 2 run-in periods and were randomly assigned to the intervention or control groups. All participants received information about the goal of the study, which was to promote weight loss during the follow-up period. The study was approved by the Institutional Review Boards of the Harvard School of Public Health and of Rio de Janeiro State University.
Intervention
Women were randomly assigned to one of two groups for the exercise intervention: the control group (CG) or the home-based exercise group (HB). The HB group received a booklet explaining the physical exercises to be practiced at home at least three times per week, 40 minutes per session, at a low to moderate intensity (available at www.nebin.org). The exercise program consisted of stretching exercises and an aerobic circuit with continuous movements involving large arm and leg muscles as well as exercises using a ball or ropes, stair climbing, and standing up from a chair (balls and ropes were given to the participants). A more detailed description of the exercise intervention can be found in Mediano et al. [7].
Compliance with the exercise program was assessed once per month by having the women mark the days on which they exercised on a specific card printed with the days of the week. Both groups received dietary counseling aimed to reduce caloric intake by 100–300 calories per day.
Measurements
Weight was measured monthly. Circumferences and fasting blood samples were collected at baseline and after 3, 6, and 12 months of follow-up. Height was measured to the nearest 0.5 cm with a wall-mounted stadiometer, and body weight was measured by using the same calibrated digital scale for all participants. Circumferences were determined with the participants standing, taken at the largest girth of the hip and smallest girth of the waist. All measurements were performed in the morning, and blood samples were collected after a 10-hour fast. Aliquots of plasma and serum were isolated from the blood samples and frozen at −70°C within 2 h of being drawn.
Plasma lipids and glucose were measured using Gold Analisa kits with an intra-assay coefficient of variation (CV) varying from 0.9% to 1.2% and an inter-assay CV from 1.9% to 2.7%. The LDL and VLDL cholesterol concentrations were calculated based on the triacylglycerol measurements according to the Friedewald equation [18]. Serum insulin concentrations were determined by radioimmunoassay using an ImmuChem™ 125/RIA kit with an intra-assay CV varying from 4.2% to 8.2% and an inter-assay CV from 6.4% to 8.8%. Relative insulin resistance (HOMA-IR) was estimated according to the formula [(glucose in mmol/L × insulin μU/ml)/22.5].
Data analysis
Women were stratified in one of two groups (NIR: non-insulin resistant; and IR: insulin resistant) according to the Brazilian criteria for insulin resistance, which state a cut-off value of 2.71 [19]. The baseline characteristics of the groups were compared using Student's t-test. Temporal changes according to exercise groups and insulin resistance were evaluated by repeated random regression analysis using PROC MIXED in SAS (version 9.1; SAS Institute Inc, Cary, NC), including all subjects regardless of loss to follow-up or compliance. To evaluate the differences between the insulin resistance groups, the models included time, IR and the time × IR interaction adjusted for baseline values and dietary and exercise interventions. The term of interest was time × IR. The effects of exercise stratified by insulin resistance included time, exercise and the time × exercise interaction, adjusted for baseline values and type of diet. In this case, the term of interest was the time × exercise interaction. Residual plots of all models were examined, and their distribution did not show major deviations from the regression assumptions. Statistical significance was set at p<0.05 for all analyses.
Results
A total of 185 of the 203 women had HOMA-IR baseline values and were included in the analysis (NIR=121; IR=64). There were no major differences between the IR groups at baseline, except for the expected differences in waist circumference, waist-to-hip ratio, and measures of glucose metabolism (glucose, insulin and HOMA-IR) (Table 1). When the NIR and IR groups were further stratified by exercise intervention, there were differences according to exercise with respect to height, BMI and waist-to-hip ratio in the IR group (Table 2).
Table 1.
Means and (standard deviation) of baseline characteristics of participants by insulin resistance.
| Variable | NIR (n=121) | IR (n=64) | p-valuea |
|---|---|---|---|
| Age (years) | 37.4 (5.4) | 37.7 (5.3) | 0.70 |
| Body weight (kg) | 67.9 (6.9) | 68.6 (7.2) | 0.53 |
| Height (m) | 160.5 (6.0) | 160.1 (6.6) | 0.72 |
| Waist circumference (cm) | 80.8 (5.4) | 82.7 (4.9) | 0.02 |
| Hip circumference (cm) | 104.5 (5.7) | 103.9 (5.7) | 0.52 |
| Body mass index (kg/m2) | 26.3 (2.0) | 26.7 (1.8) | 0.21 |
| Waist-to-hip ratio | 0.77 (0.05) | 0.80 (0.06) | 0.004 |
| Total cholesterol (mg/dl) | 190.5 (33.1) | 193.8 (41.7) | 0.56 |
| HDL cholesterol (mg/dl) | 43.1 (15.2) | 43.7 (16.6) | 0.78 |
| LDL cholesterol (mg/dl) | 130.1 (33.5) | 131.8 (38.1) | 0.76 |
| VLDL cholesterol (mg/dl) | 17.4 (7.9) | 18.3 (11.0) | 0.51 |
| Triacylglycerol (mg/dl) | 86.0 (38.7) | 91.3 (55.0) | 0.45 |
| Glucose (mg/dl) | 82.0 (9.8) | 93.3 (20.0) | > 0.001 |
| Insulin (μU/ml) | 9.5 (2.0) | 15.9 (4.3) | > 0.001 |
| HOMA -IR | 1.90 (0.41) | 3.57 (0.94) | > 0.001 |
NIR - non-insulin resistant IR - insulin resistant
Student's t test
Table 2.
Means and (standard deviation) of baseline characteristics of participants by insulin resistance and physical activity.
| Variable | NIR (n=121) |
p-valuea | IR (n=64) |
p-valuea | ||
|---|---|---|---|---|---|---|
| HB (n=57) | CG (n=64) | HB (n=33) | CG (n=31) | |||
| Age (years) | 36.7 (5.3) | 38.0 (5.4) | 0.16 | 36.5 (5.4) | 39.0 (5.0) | 0.07 |
| Body weight (kg) | 68.5 (6.0) | 67.4 (7.7) | 0.38 | 68.3 (7.3) | 68.9 (7.2) | 0.74 |
| Height (m) | 160.8 (5.7) | 160.1 (6.3) | 0.50 | 158.4 (7.1) | 161.9 (5.6) | 0.04 |
| Waist circumference (cm) | 80.4 (5.1) | 81.1 (5.6) | 0.51 | 83.6 (4.8) | 81.7 (5.0) | 0.14 |
| Hip circumference (cm) | 104.5 (5.5) | 104.2 (6.0) | 0.65 | 103.3 (5.4) | 104.5 (6.1) | 0.38 |
| Body mass index (kg/m2) | 26.5 (1.8) | 26.2 (2.1) | 0.55 | 27.2 (1.8) | 26.2 (1.6) | 0.03 |
| Waist-to-hip ratio | 0.77 (0.05) | 0.78 (0.05) | 0.25 | 0.81 (0.06) | 0.78 (0.05) | 0.05 |
| Total cholesterol (mg/dl) | 189.9 (33.0) | 191.0 (33.4) | 0.85 | 194.0 (43.6) | 193.6 (40.2) | 0.97 |
| HDL cholesterol (mg/dl) | 40.4 (12.6) | 45.4 (16.9) | 0.07 | 42.5 (14.4) | 45.0 (18.8) | 0.55 |
| LDL cholesterol (mg/dl) | 132.7 (34.3) | 127.8 (32.8) | 0.42 | 132.0 (39.3) | 131.5 (37.4) | 0.96 |
| VLDL cholesterol (mg/dl) | 16.8 (8.0) | 17.9 (7.8) | 0.44 | 19.5 (14.2) | 17.0 (6.0) | 0.38 |
| Triacylglycerol (mg/dl) | 82.0 (38.5) | 89.6 (38.8) | 0.28 | 97.5 (70.7) | 84.8 (30.5) | 0.36 |
| Glucose (mg/dl) | 81.6 (7.78) | 82.4 (11.3) | 0.66 | 97.2 (26.0) | 89.1 (11.8) | 0.10 |
| Insulin (μU/ml) | 9.6 (2.0) | 9.3 (2.1) | 0.44 | 16.3 (5.2) | 15.5 (13.2) | 0.48 |
| HOMA -IR | 1.93 (0.40) | 1.88 (0.43) | 0.57 | 3.74 (1.01) | 3.39 (0.82) | 0.13 |
NIR - non-insulin resistant IR - insulin resistant
Student's t test
Women classified as IR at baseline experienced greater weight loss after 12 months of follow-up in comparison with women in the NIR group (−1.6 vs. −1.1 kg; p=0.01), independent of the interventions (Table 3). Changes in BMI showed a similar pattern, with greater reduction for IR women in comparison with NIR women after 12 months (−0.6 vs. −0.4 kg/m2; p=0.007). No statistically significant differences were found between the groups with respect to changes in the waist circumference and the waist-to-hip ratio (Table 3).
Table 3.
Crude means (standard deviation) and adjusted changes from baseline (Δ) for anthropometric characteristics during the follow-up by insulin resistance.
| 3 months (NIR=72 IR=39) | 6 months (NIR=51 IR=28) | 12 months (NIR=65 IR=41) | p valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean (sd) | Δ a | Mean (sd) | Δ a | Mean (sd) | Δ a | ||
| Body Weight (kg) | |||||||
| NIR | 67.1 (7.7) | −0.5 | 66.7 (7.7) | −0.7 | 66.9 (7.4) | −1.1 | 0.01 |
| IR | 67.8 (6.9) | −0.5 | 67.7 (7.6) | −0.8 | 66.4 (5.8) | −1.6 | |
| Body Mass Index (kg/m2) | |||||||
| NIR | 26.0 (2.1) | −0.2 | 25.9 (2.2) | −0.3 | 25.8 (2.2) | −0.4 | 0.007 |
| IR | 26.5 (2.0) | −0.2 | 26.3 (1.6) | −0.3 | 25.7 (1.8) | −0.6 | |
| Waist Circumference (cm) | |||||||
| NIR | 80.2 (6.0) | −0.4 | 80.2 (5.8) | −0.7 | 78.8 (5.6) | −1.3 | 0.41 |
| IR | 81.8 (5.0) | −0.8 | 82.0 (4.4) | −1.2 | 80.3 (5.0) | −2.0 | |
| Waist -to- hip ratio | |||||||
| NIR | 0.78 (0.05) | 0.0007 | 0.78 (0.05) | 0.0007 | 0.77 (0.05) | 0.0007 | 0.47 |
| IR | 0.80 (0.06) | −0.003 | 0.81 (0.06) | −0.004 | 0.79 (0.05) | −0.006 | |
= Model based on repeated measures include time, insulin resistance and time × insulin resistance interaction adjusted for baseline values and intervention
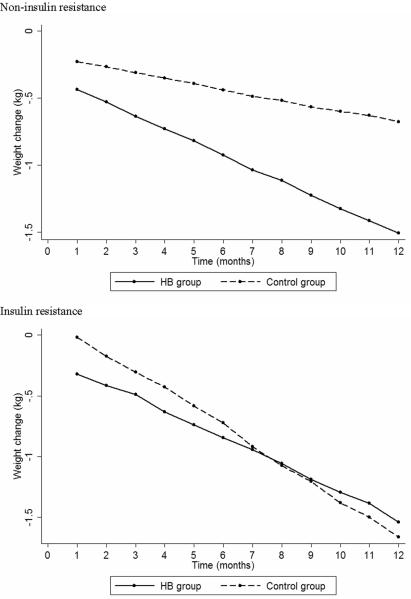
When women were further stratified by exercise groups, the HB group showed a higher reduction in body weight only for NIR women (−1.5 vs. −0.7; p=0.04), with no differences observed between physical activity groups for IR women (−1.5 vs. −1.7; p=0.24) (Figure 1). Changes in BMI showed a similar pattern, with a greater reduction for the HB women in the NIR group (−0.6 vs. −0.2; p=0.03), with no differences observed between exercise groups for IR women (−0.6 vs. −0.7; p=0.22). There was no difference between exercise groups with respect to waist circumference or waist-to-hip ratio changes (Table 4).
Figure 1.
Weight changes according to physical activity intervention stratified by insulin resistance.
Table 4.
Crude means (standard deviation) and adjusted changes from baseline (Δ) for anthropometric characteristics during the follow-up by insulin resistance and physical activity.
| 3 months (NIR=72 IR=39) | 6 months (NIR=51 IR=28) | 12 months (NIR=65 IR=41) | p valuea | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (sd) | Δ a | Mean (sd) | Δ a | Mean (sd) | Δ a | |||
| Body Weight (kg) | ||||||||
| NIR | ||||||||
| HB | 67.9 (6.8) | −0.6 | 67.4 (7.5) | −0.9 | 67.8 (7.3) | −1.5 | 0.04 | |
| Control | 66.4 (8.4) | −0.3 | 65.8 (8.0) | −0.4 | 66.0 (7.4) | −0.7 | ||
| IR | ||||||||
| HB | 66.6 (4.9) | −0.5 | 67.6 (8.4) | −0.9 | 67.0 (5.0) | −1.5 | 0.24 | |
| Control | 69.5 (9.0) | −0.3 | 67.8 (6.9) | −0.7 | 66.0 (6.8) | −1.7 | ||
| Body Mass Index (kg/m2) | ||||||||
| NIR | ||||||||
| HB | 26.1 (2.1) | −0.3 | 26.0 (2.2) | −0.4 | 25.8 (2.4) | −0.6 | 0.03 | |
| Control | 25.9 (2.1) | −0.1 | 25.8 (2.2) | −0.2 | 25.9 (2.2) | −0.2 | ||
| IR | ||||||||
| HB | 26.6 (2.1) | −0.2 | 26.6 (1.8) | −0.3 | 26.2 (1.8) | −0.6 | 0.22 | |
| Control | 26.4 (2.0) | −0.1 | 25.9 (1.5) | −0.3 | 25.0 (1.6) | −0.7 | ||
| Waist Circumference (cm) | ||||||||
| NIR | ||||||||
| HB | 79.5 (5.2) | −0.4 | 80.7 (5.8) | −0.7 | 79.3 (6.4) | −1.1 | 0.42 | |
| Control | 80.8 (6.8) | −0.4 | 79.5 (5.8) | −0.7 | 78.4 (4.9) | −1.4 | ||
| IR | ||||||||
| HB | 82.3 (5.2) | −0.9 | 83.6 (3.8) | −1.5 | 82.3 (2.9) | −2.4 | 0.93 | |
| Control | 81.3 (4.9) | −0.6 | 80.4 (4.5) | −1.0 | 79.1 (5.6) | −1.8 | ||
| Waist- to- hip ratio | ||||||||
| NIR | ||||||||
| HB | 0.77 (0.04) | 0.0002 | 0.78 (0.05) | −0.00006 | 0.77 (0.06) | −0.0004 | 0.77 | |
| Control | 0.79 (0.05) | 0.001 | 0.78 (0.05) | 0.002 | 0.77 (0.04) | 0.002 | ||
| IR | ||||||||
| HB | 0.82 (0.06) | −0.006 | 0.83 (0.06) | −0.008 | 0.80 (0.06) | −0.012 | 0.77 | |
| Control | 0.78 (0.05) | −0.0003 | 0.78 (0.06) | −0.0006 | 0.78 (0.05) | −0.003 | ||
HB - Home-based exercise group
= Model based on repeated measures include time, treatment and time × treatment interaction adjusted for baseline values and type of diet stratified by insulin resistance
The lipid profile changes during the follow-up stratified by IR groups are shown in Table 5. The IR group had a greater reduction in VLDL-c (−1.7 vs. 1.9 mg/dl; p=0.008) and triacylglycerols (−8.3 vs. 7.6 mg/dl; p=0.008) in comparison with the NIR group. However, when these results were further adjusted for weight change, there was no difference between the groups (p>0.10).
Table 5.
Crude means (standard deviation) and adjusted changes from baseline (Δ) for lipid profile during the follow-up by insulin resistance.
| 3 months (NIR=78 IR=43) | 6 months (NIR=68 IR=41) | 12 months (NIR=51 IR=23) | p valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean (sd) | Δ a | Mean(sd) | Δ a | Mean(sd) | Δ a | ||
| Total Cholesterol (mg/dl) | |||||||
| NIR | 191.1 (38.3) | −1.5 | 184.7 (33.6) | −2.4 | 187.1 (37.4) | −4.1 | 0.75 |
| IR | 196.5 (39.5) | −0.4 | 186.9 (41.0) | −0.7 | 203.7 (36.3) | −2.9 | |
| HDL-cholesterol (mg/dl) | |||||||
| NIR | 46.9 (10.6) | 4.1 | 51.4 (11.4) | 7.6 | 55.3 (15.5) | 14.3 | 0.96 |
| IR | 44.2 (10.8) | 2.2 | 50.5 (13.4) | 6.6 | 55.9 (11.3) | 12.4 | |
| LDL-cholesterol (mg/dl) | |||||||
| NIR | 127.5 (38.0) | −5.4 | 117.4 (34.0) | −10.4 | 111.4 (34.8) | −19.6 | 0.25 |
| IR | 133.8 (39.2) | −2.2 | 124.1 (38.2) | −5.5 | 131.0 (37.3) | −12.5 | |
| VLDL-cholesterol (mg/dl) | |||||||
| NIR | 16.7 (8.6) | 0.01 | 15.9 (7.7) | 0.8 | 20.5 (10.9) | 1.9 | 0.008 |
| IR | 18.4 (11.0) | −0.5 | 16.0 (7.5) | −0.4 | 16.7 (8.8) | −1.7 | |
| Triacylglycerol (mg/dl) | |||||||
| NIR | 78.9 (40.0) | −1.4 | 75.9 (35.8) | 2.0 | 100.4 (55.3) | 7.6 | 0.008 |
| IR | 92.0 (55.2) | −2.4 | 79.9 (37.6) | −1.9 | 83.7 (43.7) | −8.3 | |
= Model based on repeated measures include time, insulin resistance and time × insulin resistance interaction adjusted for baseline values and intervention
The compliance with the exercise protocol was 78.2%, 79.5% and 86.1% for months 3, 6 and 12, respectively. The HB exercise group had almost the same percentage of loss to follow-up as the non-exercise group.
Discussion
The main finding of the present study was that women with baseline IR lost more weight and BMI during 12 months of follow-up than women without IR, independent of the type of intervention. In some observational studies, baseline insulin resistance, measured in a variety of ways, has been associated with greater weight loss [20,21], and a reverse association, that is, that insulin resistance is associated with future weight gain, was seen in others [13,22]. No association has also been reported [14,23].
One of the large studies was conducted by Meyer-Davis et al. [24], who studied 1194 adults aged 39–69 years and showed that baseline fasting insulin levels (a surrogate marker for insulin sensitivity) were inversely associated with 5-year weight changes even after the data were adjusted for potential confounders. In another study, Travers et al. [25] evaluated the relationship between insulin resistance and future fat accumulation in a 3-year prospective study of 111 healthy children. In this study, baseline insulin sensitivity was divided into tertiles for each gender, with the lowest tertile representing the most insulin-resistant children. For girls, those in the lowest tertile maintained their body fat percentage over 3 years, while girls in the middle and upper tertiles increased in their body fat percentage. For boys, those in the lowest tertile showed a decrease in their body fat percentage, whereas boys in the middle and upper tertiles maintained their body fat percentage.
It has been postulated that obesity mediates the association between insulin resistance and weight changes [20]. Howard et al. [12] showed that insulin resistance predicted weight gain only among leaner women and that the reverse association was observed among heavier women, in which insulin resistance was a predictor of weight loss. Nonetheless, in the Rancho Bernardo Study [11], IR predicted weight loss even among normal weight individuals, in agreement with our findings.
These inconsistencies among studies may be explained by the fact that, in most studies, participants with large girths, which are associated with insulin resistance, also have greater adiposity. This problem was overcome in our study because the IR group and NIR group had similar BMIs and were not obese, although the IR group had greater average waist circumference and waist-to-hip ratio. Studies have shown that visceral adipose tissue is more resistant to the antilipolytic effects of insulin than subcutaneous fat [26]. Conversely, catecholamines have a lipolytic effect that acts predominately on the adipocytes of visceral tissue [27]. Therefore, it could be postulated that insulin-resistant individuals, who have greater visceral fat mass, are more prone to greater weight loss than other individuals with the same adiposity without insulin resistance.
In addition, among IR individuals, insulin is a determinant of reduced weight gain through the direct effect of insulin on the central nervous system, resulting in satiety and reduced food intake over the time [8,9]. Insulin resistance may also have peripheral effects that lead to decreased carbohydrate oxidation, which would, in turn, increase fat oxidation, thus limiting fat storage and leading to weight loss or attenuation of weight gain [28].
Another important finding of the present study was that physical activity enhanced weight loss only among women without baseline insulin resistance, who had a difficult time losing weight. In the IR group, the effect of the small amount of energy expenditure due to physical activity may have been blunted by the higher lipolytic effect of high insulin levels. However, in the NIR group, physical activity may have contributed to the energy deficit and to an increase in fat metabolism, thus promoting greater weight loss [29–32]. Since all analyses were adjusted for type of diet, the factorial design of the study (diet and exercise), and the fact that all participants were advised to reduce energy intake, we could attribute most of this weight change differences to the home-based exercise.
The greater weight loss among women with baseline insulin resistance was also associated with a greater reduction of VLDL-c and triacylglycerols in comparison with those without insulin resistance. Weight loss has been recognized to promote improvements in the lipid profile, thus reducing the cardiovascular risk associated with dyslipidemia commonly observed in overweight and obese individuals [33,34].
A possible limitation of the present study was the use of the HOMA-IR index to classify women according to baseline insulin resistance, but HOMA-IR is feasible and has been validated by several studies [35,36]. In addition, although the compliance with the exercise protocol was high, a lack of a direct measurement to evaluate physical activity could be considered a limitation. Our previous results showed increased HDL cholesterol in the HB group, a marker of greater physical activity levels [7].
To conclude, our findings indicate that baseline insulin resistance facilitated weight loss among non-obese women and that home-based exercise promoted greater weight loss only among non-insulin-resistant women, who had greater difficulty losing weight. The major changes observed in the lipid profiles for the IR group were associated with greater weight loss observed in this group than in the NIR group.
Acknowledgments
Research related to this study was funded by grant R03 TW005773-03 from the National Institutes of Health - NIH and Grant 500404/2003-8 from the Brazilian National Research Council - CNPq.
Grant support: Research related to this abstract was funded by grant R03 TW005773-03 from the National Institutes of Health - NIH and Grant 500404/2003-8 from Brazilian National Research Council - CNPq
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- 1.Horton ES. Effects of lifestyle changes to reduce risks of diabetes and associated cardiovascular risks: results from large scale efficacy trials. Obesity. 2009;17(Suppl 3):S43–48. doi: 10.1038/oby.2009.388. [DOI] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.Blonde L. Current antihyperglycemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am J Med. 2010;123:S12–18. doi: 10.1016/j.amjmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Barte JC, Ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mediano MF, Barbosa JS, Moura AS, Willett WC, Sichieri R. A randomized clinical trial of home-based exercise combined with a slight caloric restriction on obesity prevention among women. Prev Med. 2010 doi: 10.1016/j.ypmed.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckel RH. Insulin resistance: an adaptation for weight maintenance. Lancet. 1992;340:1452–1453. doi: 10.1016/0140-6736(92)92633-q. [DOI] [PubMed] [Google Scholar]
- 9.Porte D, Seeley RJ, Woods SC, Baskin DG, Figlewicz DP, Schwartz MW. Obesity, diabetes and the central nervous system. Diabetologia. 1998;41:863–881. doi: 10.1007/s001250051002. [DOI] [PubMed] [Google Scholar]
- 10.Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedick NM, Mayer-Davis EJ, Wingard DL, Addy CL, Barrett-Connor E. Insulin resistance precedes weight loss in adults without diabetes: the Rancho Bernardo Study. Am J Epidemiol. 2001;153:1199–1205. doi: 10.1093/aje/153.12.1199. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Adams-Campbell L, Allen C, Black H, Passaro M, Rodabough RJ, et al. Insulin resistance and weight gain in postmenopausal women of diverse ethnic groups. Int J Obes Relat Metab Disord. 2004;28:1039–1047. doi: 10.1038/sj.ijo.0802645. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001;86:3182–3187. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin T, Abbasi F, Carantoni M, Schaaf P, Reaven G. Differences in insulin resistance do not predict weight loss in response to hypocaloric diets in healthy obese women. J Clin Endocrinol Metab. 1999;84:578–581. doi: 10.1210/jcem.84.2.5441. [DOI] [PubMed] [Google Scholar]
- 15.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28:2939–2941. doi: 10.2337/diacare.28.12.2939. [DOI] [PubMed] [Google Scholar]
- 16.Evangelou P, Tzotzas T, Christou G, Elisaf MS, Kiortsis DN. Does the presence of metabolic syndome influence weight loss in obese and overweight women? Metab Syndr Relat Disord. 2010;8:173–178. doi: 10.1089/met.2009.0066. [DOI] [PubMed] [Google Scholar]
- 17.Sichieri R, Moura AS, Genelhu V, Hu F, Willett WC. An 18-mo randomized trial of a low-glycemic-index diet and weight change in Brazilian women. Am J Clin Nutr. 2007;86:707–713. doi: 10.1093/ajcn/86.3.707. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Geloneze B, Repetto EM, Geloneze SR, Tambascia MA, Ermetice MN. The threshold value for insulin resistance (HOMA-IR) in an admixtured population IR in the Brazilian Metabolic Syndrome Study. Diabetes Res Clin Pract. 2006;72:219–220. doi: 10.1016/j.diabres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Pittas AG, Roberts SB. Dietary composition and weight loss: can we individualize dietary prescriptions according to insulin sensitivity or secretion status? Nutr Rev. 2006;64:435–448. doi: 10.1301/nr.2006.oct.435-448. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus R, Sparrow D, Weiss S. Temporal relations between obesity and insulin: longitudinal data from the Normative Aging Study. Am J Epidemiol. 1998;147:173–179. doi: 10.1093/oxfordjournals.aje.a009431. [DOI] [PubMed] [Google Scholar]
- 22.Sigal RJ, El-Hashimy M, Martin BC, Soeldner JS, Krolewski AS, Warram JH. Acute postchallenge hyperinsulinemia predicts weight gain: a prospective study. Diabetes. 1997;46:1025–1029. doi: 10.2337/diab.46.6.1025. [DOI] [PubMed] [Google Scholar]
- 23.Silver RJ, Mehta S, Soeldner JS, Martin BC, Warram JH, Goldfine AB. Acute insulin secretion as a predictor of weight gain in healthy humans. Obesity. 2006;14:67–72. doi: 10.1038/oby.2006.9. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Kirkner GJ, Karter AJ, Zaccaro DJ. Metabolic predictors of 5-year change in weight and waist circumference in a triethnic population: the insulin resistance atherosclerosis study. Am J Epidemiol. 2003;157:592–601. doi: 10.1093/aje/kwg022. [DOI] [PubMed] [Google Scholar]
- 25.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. 2002;87:3814–3818. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 28.Woods SC, Benoit SC, Clegg DJ, Seeley RJ. Clinical endocrinology and metabolism. Regulation of energy homeostasis by peripheral signals. Best Pract Res Clin Endocrinol Metab. 2004;18:497–515. doi: 10.1016/j.beem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr. 2005;82:226S–229S. doi: 10.1093/ajcn/82.1.226S. [DOI] [PubMed] [Google Scholar]
- 30.Hansen D, Dendale P, Berger J, van Loon LJ, Meeusen R. The effects of exercise training on fat-mass loss in obese patients during energy intake restriction. Sports Med. 2007;37:31–46. doi: 10.2165/00007256-200737010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 32.Mediano MF, Barbosa JS, Sichieri R, Pereira RA. Effects of exercise on insulin sensitivity in obese women submitted to a weight loss program: a clinical trial. Arq Bras Endocrinol Metabol. 2007;51:993–999. doi: 10.1590/s0004-27302007000600015. [DOI] [PubMed] [Google Scholar]
- 33.Andersen RE, Wadden TA, Bartlett SJ, Vogt RA, Weinstock RS. Relation of weight loss to changes in serum lipids and lipoproteins in obese women. Am J Clin Nutr. 1995;62:350–357. doi: 10.1093/ajcn/62.2.350. [DOI] [PubMed] [Google Scholar]
- 34.Poobalan A, Aucott L, Smith WC, Avenell A, Jung R, Broom J, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]