INTRODUCTION
In the United States the rate of cesarean delivery (CD) has risen 48% since 1996, reaching a level of 31.8% in 2007. 1 This trend is reflected in many parts of the world, with the most populous country in the world, China, approaching 50%2 and some private clinics in Brazil approaching 80% 3. While a significant number of CD are preformed for obstetrical indications, some are simply due to maternal request and may incur several risks for the child. Well known among these risks are neonatal depression due to general anesthesia, fetal injury during hysterotomy and/or delivery, increased likelihood of respiratory distress even at term, and breastfeeding complications. Concurrent with the trend of increasing CD, there has been an epidemic of both autoimmune diseases such as type 1 diabetes, Crohn's disease, and multiple sclerosis and allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis4, 5. The occurrence of these diseases is higher in more affluent, Western, industrialized countries. Several theories have emerged that suggest environmental influences are contributing to this phenomenon. Most notably, the “hygiene hypothesis” suggests that an overly clean environment, especially in early childhood, may contribute to the development of several childhood diseases. It was first proposed by Strachan, who observed an inverse correlation between hay fever and the number of older siblings. 6 This was subsequently extended by others from the allergies to autoimmune diseases such as type 1 diabetes. 5 Whether the increase in CD incidence is also causally related will be addressed in this review.
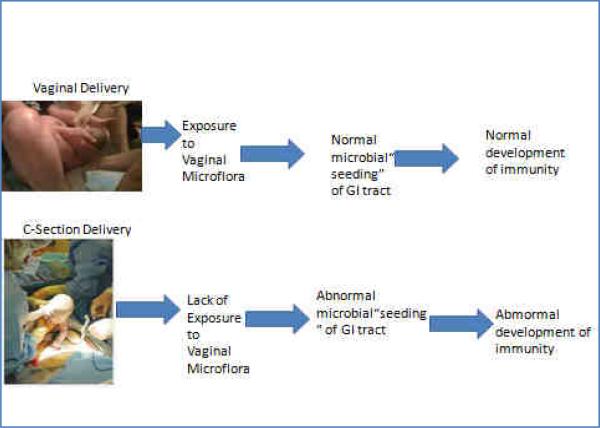
The interplay between the emerging microbial ecology of the gastrointestinal tract and the developing mucosal immune system serves as a backdrop for a relationship between CD and the emergence of some of these diseases. With the highly immunoreactive intestine serving as the largest surface area of the body that is exposed to the environment, especially a vast array of luminal microbes and antigens, it is intriguing to speculate that the intestinal environmental interaction during early development of the immune system may relate to these diseases. One intriguing component of this relates to the early development of the intestinal microbiota, the developing immune system and the early influence of cesarean versus vaginal delivery (VD) on these phenomena. The immune system undergoes major development during infancy and is highly related to the microbes that colonize the intestinal tract.7-9 It has been suggested that different initial exposures depend on mode of delivery (VD vs. CD). The microbes that “seed’ the intestine during either CD or VD may lead to changes in long term colonization and subsequent altering of immune development (Fig. 1). Here we will provide background about the human microbiota, its relationship to the developing immune system, and the relationship of mode of delivery on the colonization of the infant intestine, development of the immune system, and subsequent childhood allergies, asthma and autoimmune diseases.
Figure 1.
THE HUMAN MICROBIOTA
The human body, consisting of about 100 trillion cells, carries about ten times as many microorganisms in the intestines. 10-12 It is estimated that these gut flora have around 100 times as many genes in aggregate as there are in the human genome.13 The metabolic activities performed by these bacteria resemble those of an organ, leading some to liken gut bacteria to a “forgotten” organ. 12 Microorganisms perform a host of useful functions, such as fermenting unused energy substrates, training the immune system, preventing growth of harmful, pathogenic bacteria, regulating the development of the gut, and producing vitamins for the host (such as biotin and vitamin K). 14 Excitement about the potential of harnessing the intestinal microbiota for therapeutic purposes and health is reflected by the popularity of pro- and prebiotics and even such seemingly esoteric therapies as human fecal transplantation. 15
Not all the species in the gut have been identified because most cannot be cultured, 10 and identification is difficult. An effort to better describe the microflora of the gut and other body locations using newly developed non-culture based technologies16 has been initiated and termed the “Human Microbiome Project”17. This project has a mission of generating resources enabling comprehensive characterization of the human microbiota and analysis of its role in human health and disease. Although the human intestine is the site where most studies are being focused, other sites such as the skin, bladder, mouth and vagina harbor distinct microbial populations and are likely to also play major roles in health and disease16.
INTESTINAL MICROECOLOGY OF THE FETUS AND NEWBORN
Most current literature suggests that the gastrointestinal tract of a normal fetus is sterile. During birth and rapidly thereafter, bacteria from the mother and the surrounding environment colonize the infant's gut. It is obvious that exposure at birth would differ by mode of delivery. What long term sequelae or impact this difference in exposure may have on the child has yet to be determined.
Some recent research work suggests colonization may begin even earlier. While the paradigm has been that babies’ intestines are sterile until birth, recent work found a microbial community already dwelling in the meconium of some babies born prematurely.18 It has also been shown that amniotic fluid of mothers with preterm labor contains a large and diverse spectrum of bacterial rDNA. 20 While a baby is in utero, it typically swallows 400 to 500 milliliters of amniotic fluid per day at term, and the hypothesis that intra-amniotic infection is the driving force behind preterm labor is one being widely studied in obstetrics.19 Whether the microbes or microbial components swallowed in the amniotic fluid stimulate an inflammatory response driving preterm birth remains to be evaluated. The effect these organisms have on the developing immune system, aside from their role in preterm labor, also raises interesting questions.
Currently, very few studies have investigated the development of the human microbiota after birth using non-culture based techniques. In a step toward greater systematic investigation of babies born at term, Palmer et al.21 evaluated the developing microbiota of infants during the first year after birth using microarray techniques to detect and quantify the small subunit ribosomal RNA (SSU rRNA) gene sequences of most currently recognized species and taxonomic groups of bacteria; this was done along with sequencing of cloned libraries of PCR-amplified SSU rDNAto profile the microbial communities in 14 healthy full-term infants during the first year after birth. To investigate possible origins of the infant microbiota, the researchers also profiled vaginal and milk samples from most of the mothers as well as stool samples from all of the mothers, most of the fathers, and two siblings. The investigators found that the composition and temporal patterns of the microbial communities varied widely from baby to baby, but the distinct features of each baby's microbial community were recognizable for intervals of weeks to months. The strikingly parallel temporal patterns from a set of dizygotic twins suggested that incidental environmental exposures play a major role in determining the distinctive characteristics of the microbial community in each baby. By the end of the first year of life, microbial ecosystems in each baby, although still distinct, had converged toward a profile characteristic of the adult gastrointestinal tract. Of interest, Bifidobacteria were not found in these infants using these techniques. This could be highly significant in that it may debunk the large amount of attention this microbe has received as a potentially important microbe that may be harnessed as a probiotic. On the other hand, this could be a technical problem that still needs to be solved using these newly developed methodologies.
Although a few studies have monitored the bacterial communities in preterm infants, our picture of the intestinal microbiota still remains limited. To determine whether noncultured bacteria represent an important part of the community in premature babies’ intestinal ecosystems, Magne et al.22 used 16S rRNA genes and PCR-based electrophoretic profiling of 288 clones obtained from the fecal samples of 16 preterm infants. These were classified into 25 molecular species. The mean number of molecular species per infant was 3.25 and ranged from one to eight. The researchers found high interindividual variability. The main bacterial groups encountered belonged to the Enterobacteriaceae family and the genera Enterococcus, Streptococcus, and Staphylococcus. The preterm infants were colonized by anaerobes and only four bifidobacteria (again seeming to minimize these taxa during development). The researchers did not determine the relative impacts of delivery mode, sex, gestational age, birth weight, age at sampling, feeding modes, and antibiotic therapies. They concluded that species diversity was low and interindividual variability was high in the feces of preterm infants, as revealed by sequences of 16S rRNA genes and PCR-temporal temperature gradient gel electrophoresis profiles (TGGE). The intestinal ecosystem of these preterm infants had no typical characteristic.
In summary, whether the fetal intestinal ecosystem is sterile at the time of birth remains a question. This may be the case in some infants, but not necessarily in others, especially preterms. This may in turn play a role in the initiation of preterm labor. Nevertheless, the species diversity does appear to be low in most infants shortly after birth, but this increases with environmental exposure. Very little is currently known about the specific emergence of the microbial ecology of infants during the first year after birth and how this specifically relates to development of immunity and subsequent health and disease.
FUNCTIONS OF THE INTESTINAL MICROBIOTA
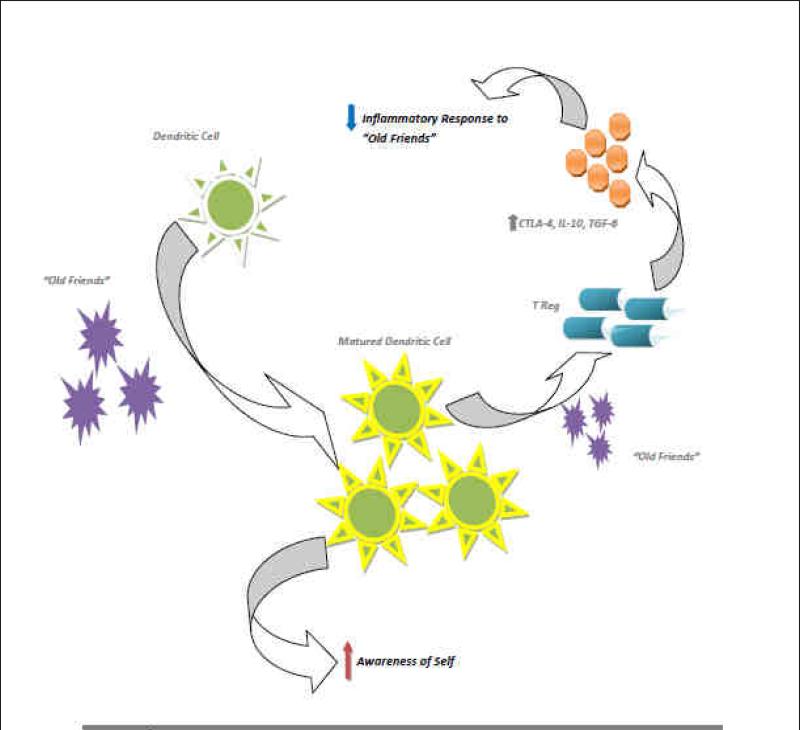
A comprehensive review of the functions of the intestinal microbiota is beyond the scope of this review, but here we wish to focus on the immunologic functions because of their importance in development of the immune system and possible pathogenesis of several known allergic and autoimmune diseases. Intestinal bacteria are key in promoting the early development of the gut's mucosal immune system, both in terms of its physical components and function and continue to play a role later in life in its operation. The bacteria stimulate the lymphoid tissue associated with the gut mucosa to produce antibodies to pathogens. The immune system recognizes and fights harmful bacteria, but leaves the helpful species alone, a tolerance developed in infancy, and sometimes termed the “old friends” hypothesis. 23 (Figure 2) This hypothesis appears to be a synthesis of the hygiene hypothesis that proposes that the role of these microorganisms that have evolve with humans provide an essential role in the establishment of the immune system wherein the microorganisms and the host have evolved a co-dependence: the most relevant organisms are those that co-evolved with mammals. These microorganisms are interacting with other modern environmental changes that also lead to enhanced inflammatory responses such as inappropriate diet, obesity, psychological stress, vitamin D deficiency, pollution (dioxins), and perhaps even cesarean delivery. The range of chronic inflammatory disorders that is affected is potentially larger than usually assumed and include allergies, autoimmunity, inflammatory bowel disease, but also vascular disease, some cancers, depression/anxiety and perhaps neurodegenerative disorders and type 2 diabetes.
Fig 2. “The Old Friends Hypothesis”.
Common organisms interact with dendritic cells in the GI tract, leading to increased maturation of dendritic cells. When there is interaction with these organisms again, the dendritic cells increase Treg maturation; not Th1 or Th2. This increases the baseline amount of anti-inflammatory cytokines, producing a Bystander Suppression. Another consequence of the increased number of mature dendritic cells is as they interact with self antigens, they increase the number Treg specific to these antigens. This is referred to as Specific Suppression. Together these two arms lead to tolerance of both self antigens as well as those of helpful gut organisms.
Basic laboratory based research is supplementing the epidemiologic studies. Recent findings have shown that gut bacteria play a role in the expression of Toll-like receptors (TLRs) in the intestines. TLRs are one of the two classes of pattern recognition receptors (PRR) that provide the intestine the ability to discriminate between pathogenic and commensal bacteria. These PRRs identify the pathogens that have crossed the mucosal barrier and trigger a set of responses that take action against the pathogen, involving 3 main immunosensory cells: surface enterocytes, M cells and dendritic cells.24 The other class of PRRs are known as the nucleotide-binding oligomerization domain/caspase recruitment domain isoforms (NOD/CARD), which are cytoplasmic proteins that recognize endogenous or microbial molecules or stress responses and form oligomers that activate inflammatory caspases. This would result in the cleavage and activation of important inflammatory cytokines and/or activate the NF-κB signaling pathway to induce the production of inflammatory molecules. 24
Bacteria can influence the phenomenon known as oral tolerance, in which the immune system is less sensitive to an antigen (including those produced by gut bacteria) once it has been ingested. This tolerance, mediated in part by the gastrointestinal immune system and in part by the liver, can reduce overreactive immune responses like those found in allergies and auto-immune disease.25
There are several antenatal and perinatal events that might also affect the development of the intestinal microbial ecology. Therapy with broad-spectrum antibiotics is a common practice for mothers who go into premature labor or who have a CD. This treatment can reduce the biodiversity of the fecal microbiota and may be a factor in the cause of necrotizing enterocolitis. 26, 27 Studies in mice show that intestinal commensal microbiota have an influence on early postnatal immune development via interactions with intestinal Toll like receptors, which in turn are likely to influence the development of the mucosal immune system and mucosal-related diseases.28 Other studies suggest that specific microbes may induce regulatory T-cell development. For example, a prominent human commensal, Bacteroides fragilis, directs the development of Foxp3(+) regulatory T cells (Tregs) with a unique “inducible” genetic signature. 29 Monocolonization of germ-free animals with B. fragilis increases the suppressive capacity of Tregs and induces anti-inflammatory cytokine production exclusively from Foxp3(+) T cells in the gut. This effect appears to be mediated by an immunomodulatory molecule, polysaccharide A (PSA), of B. fragilis, which mediates the conversion of CD4(+) T cells into Foxp3(+) Treg cells that produce IL-10 during commensal colonization. Functional Foxp3(+) Treg cells are also produced by PSA during intestinal inflammation, and Toll-like receptor 2 signaling is required for both Treg induction and IL-10 expression. These studies also show that PSA is not only able to prevent, but also cure experimental colitis in animals and therefore demonstrate that B. fragilis Treg lineage differentiation pathway in the gut to actively induce mucosal tolerance.29
VAGINAL VS. CESAREAN DELIVERY
During vaginal delivery, the contact with the maternal vaginal and intestinal flora is an important source for the start of the infant's colonization. During CD, this direct contact is absent, and non-maternally derived environmental bacteria play an important role for infants’ intestinal colonization.31 Some authors have suggested that the composition of the very first human microbiota could have long lasting effects on the intestine in breast fed infants. For example, Gronlund, et al 32 showed that the primary gut flora in infants born by cesarean delivery may be disturbed for up to 6 months after birth. Another study using culture based techniques showed that the mode of delivery was associated with differences in intestinal microbes 7 years after delivery. 33 The clinical relevance of these changes is unknown, and even longer follow-up is needed to establish how long-lasting these alterations of the primary gut flora can be.
Nevertheless, there is accumulating evidence that intestinal bacteria play an important role in the postnatal development of the immune system. 30 Thus, if the intestinal flora develops differently depending on the mode of delivery, the postnatal development of the immune system might also be different. Available epidemiological data show that atopic diseases appear more often in infants after cesarean delivery than after vaginal delivery.34-37 The composition of enteric microbiota in early days of life seems, therefore, to be a very important factor for achieving and maintaining good health in the years to come. It follows that it is fundamental to identify more thoroughly the intestinal ecosystem of the newborn.
Although there is an increasing body of evidence that the intestinal microbiota play an essential role in the postnatal development of the immune system, the mechanisms remain poorly understood. Malamitsi-Puchner et al.38 found that only vaginal delivery promotes the production of various cytokines implicated in neonatal immunity. Hallstrom et al. 39 found a link between cesarean delivery, disturbed intestinal colonization, and, possibly, occurrence of necrotizing enterocolitis (NEC) in preterm infants. Although the epidemiological studies demonstrated that elective cesarean delivery provides an increased risk for allergic diseases in later childhood, confounding factors could also play intermediate roles. Data available from several studies indicate a delayed onset of lactation with cesarean section.40, 41 Thus, many infants born by cesarean delivery also lacked the early support of breast milk as stimulator for a physiological intestinal flora. Both the nonphysiological start of colonization and the missing early dietary support by delayed start of lactation might result in these long-lasting effects.
Babies are born with immunological tolerance that is instructed by the mother by preferential induction of regulatory T lymphocytes42, which might allow the baby to become colonized by this first inoculum. The mechanism is via substantial numbers of maternal cells crossing the placenta to reside in fetal lymph nodes, inducing the development of CD4+CD25highFoxP3+ Tregs that suppress fetal anti-maternal immunity and persist at least until early adulthood. However, only a subset (if any) of the microbes to which the newborn is initially exposed will permanently colonize available niches and contribute to the distinctive microbiota harbored by the body habitats of adults. 21 As more and more deliveries bypass the vagina, babies may not be exposed to these microbes at birth. Differences in delivery mode have been linked with differences in the intestinal microbiota of babies.31, 32, 43, 44 Initial communities may serve as a direct source of protective or pathogenic bacteria very early in life.
Another recent study45, offers a detailed look at the early stages of the body's colonization by microbes. Babies born vaginally were colonized predominantly by Lactobacillus, whereas cesarean delivery babies were colonized by a mixture of potentially pathogenic bacteria typically found on the skin and in hospitals, such as Staphylococcus and Acinetobacter, suggesting babies born by CD were colonized with skin flora in lieu of traditionally vaginal type of bacterium.
The effect of mode of delivery on development of childhood disease has just recently begun to be explored (Table 1). The effect appears to be most robust in the area of immune mediated diseases. CD has been associated with a significant increased rate of asthma, especially in females, and allergic rhinitis, but not atopic dermatitis.46 This increase was even more apparent when accounting for the factors surrounding the CD. The risk of asthma was increased by 60% in females who underwent a repeat cesarean without ruptured membranes versus those babies with ruptured membranes and/or labor prior to CD.46
Table 1.
| Cesarean Delivery Associated Childhood Diseases1,2 | |
|---|---|
| Allergic Rhinitis | |
| All Cesareans | 1.37 (1.14-1.63) |
| Repeat Cesareans Only | 1.78 (1.34-2.37) |
| Asthma | |
| All Cesareans | 1.24 (1.01-1.53) |
| Female | 1.53 (1.10-2.10) |
| Female & Repeat Cesarean 3 | 1.83 (1.13-2.97) |
| Celiac Disease | 1.80 (1.13-2.88) |
| Diabetes Mellitus (Type 1) | 1.19 (1.04-1.36) |
| Gastroenteritis 4 | 1.31 (1.24-1.38) |
| Gastroenteritis AND Asthma | 1.74 (1.36-2.23) |
Children born by CD are also significantly more likely to suffer from celiac disease and to be hospitalized for gastroenteritis.47 No association has been found between CD and Crohn's disease or ulcerative colitis. However, while preterm birth has been implicated in the development of inflammatory bowel disease, mode of delivery has not 48
Type I Diabetes Mellitus has been on the rise in recent decades, mirroring the rise in CD.49 Meta-analysis found a 19% increase in Type I DM in cesarean children when controlling for confounders such as gestational age, maternal age, and birth weight. 50 A recent retrospective study of children in Scotland failed to show such an association. 51 However, it is important to point out that the Scotland study had a very small number of subjects (n=361) compared to the meta-analysis (n=9938) and the rate of CD was only 14% in the Scottish study (much below the US average).
SUMMARY AND CONCLUSIONS
While CD is necessary in modern obstetrics, the procedure appears to shift a baby's first bacterial community. A better understanding of this early colonization, which is also influenced by events such as breast-feeding, may lead to medical practices for establishing healthy bacterial colonization. The causal relationship between CD, the shift in microbiota and many childhood diseases continues to be studied. However, there are several problems with the studies we have reviewed here.
It is impossible to lump CD into one category without delineating the indication for CD. It stands to reason that a fetus delivered after arrest at 8 centimeters dilation after a long labor would be exposed to a much different microbial environment than a fetus that undergoes CD for maternal request prior to rupture of membranes. It is naïve to think that the fetus is only exposed to microbes as the head passes through the vaginal introitus onto the perineum and to ignore the constant exposure to vaginal flora after rupture of membranes. Sonntag et al 48 failed to show a relationship between mode of delivery and inflammatory bowel disease. However, the average age of a subject in this study was 42 years old. Indication for CD in the late 1960's, prior to common use of external fetal monitoring, is strikingly different than modern obstetrical indications. The intrapartum exposures of these subjects is most likely vastly different than a more contemporary cohort. Future studies must be more meticulous in categorizing CD to fully understand the effect of CD on colonization and childhood disease.
The role of antepartum and intrapartum antibiotics must also be accounted for in future studies. What effect, if any, these have on the microbiota of the fetus and/or subsequent development of disease is unknown. Nearly 20% of women in the US are colonized with Group B Streptococcus and will subsequently receive intrapartum antibiotics. Standard of care also dictates that antibiotics be administered prior to cesarean delivery and to mothers in preterm labor and/or with premature prolonged rupture of membranes. Given all of this, the exposure to antenatal antibiotics is significant. Dominguez-Bello 45 noted a difference in fetal colonization based on mode of delivery. However, none of their vaginally delivered patients received antibiotics and the cesarean cases received cephalosporin “several hours” prior to incision which is not the recommended course in the US. Whether this exposure accounts for the difference, or if fetuses who receive antibiotics per standard guidelines in the US show a different colonization pattern, is an important research area to explore.
The link between mode of delivery and subsequent childhood pathology is an important one. This becomes even more important as maternal desire for primary cesarean delivery is on the rise and rates of vaginal birth after cesarean (VBAC) are declining in this country. This new information about colonization differences with differing modes of delivery seems to be taking the hygiene hypothesis to an entirely new level.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Neu is an Advisory Board Member for Mead Johnson and Medela.
~ The journey of a thousand miles begins with one step. ~
Lao Tsu
REFERENCES
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary Data for 2007. National Vital Statistics Reports. 2009;57(12):1–21. [PubMed] [Google Scholar]
- 2.Lumbiganon P, Laopaiboon M, Gülmezoglu M, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007—08. The Lancet. 2010;375(9713):490–9. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- 3.Rebelo F, da Rocha CM, Cortes TR, et al. High cesarean prevalence in a national population-based study in Brazil: the role of private practice. Acta Obstet Gynecol Scand. 2010;89(7):903–8. doi: 10.3109/00016349.2010.484044. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caicedo RA, Schanler RJ, Li N, Neu J. The developing intestinal ecosystem: implications for the neonate. Pediatr Res. 2005;58(4):625–8. doi: 10.1203/01.PDR.0000180533.09295.84. [DOI] [PubMed] [Google Scholar]
- 8.Rautava S, Walker WA. Commensal bacteria and epithelial cross talk in the developing intestine. Curr Gastroenterol Rep. 2007;9(5):385–92. doi: 10.1007/s11894-007-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2(6):478–85. doi: 10.1038/mi.2009.114. [DOI] [PubMed] [Google Scholar]
- 10.Sears CL. A dynamic partnership: celebrating our gut flora. Anaerobe. 2005;11(5):247–51. doi: 10.1016/j.anaerobe.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Steinhoff U. Who controls the crowd? New findings and old questions about the intestinal microflora. Immunol Lett. 2006;99(1):12–6. doi: 10.1016/j.imlet.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 12.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 15.Khoruts A, Dicksved J, Jansson JK, et al. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44(5):354–60. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 16.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–8. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group NHW, Peterson J, Garges S, et al. The NIH Human Microbiome Project. Genome Res. 2009;19(12):2317–23. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mshvildadze M, Neu J, Schuster J, et al. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(1):20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer C, Bik EM, Digiulio DB, et al. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magne F, Abély M, Boyer F, et al. Low species diversity and high interindividual variability in faeces of preterm infants as revealed by sequences of 16S rRNA genes and PCR-temporal temperature gradient gel electrophoresis profiles. FEMS Microbiol Ecol. 2006;57(1):128–38. doi: 10.1111/j.1574-6941.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 23.Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160(1):70–90. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Round JL, O'Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34(3):J220–5. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimmitt RA, Staley EM, Chuang G, et al. Role of Postnatal Acquisition of the Intestinal Microbiome in the Early Development of Immune Function. J Pediatr Gastroenterol Nutr. 2010 doi: 10.1097/MPG.0b013e3181e1a114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(21):12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björkstén B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25(3-4):257–70. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 31.Biasucci G, Benenati B, Morelli L, et al. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138(9):1796S–800S. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 32.Grönlund MM, Lehtonen OP, Eerola E, et al. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Salminen S, Gibson GR, McCartney AL, et al. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388–9. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negele K, Heinrich J, Borte M, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15(1):48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 35.Debley JS, Smith JM, Redding GJ, et al. Childhood asthma hospitalization risk after cesarean delivery in former term and premature infants. Ann Allergy Asthma Immunol. 2005;94(2):228–33. doi: 10.1016/S1081-1206(10)61300-2. [DOI] [PubMed] [Google Scholar]
- 36.Laubereau B, Filipiak-Pittroff B, von Berg A, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch Dis Child. 2004;89(11):993–7. doi: 10.1136/adc.2003.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggesbø M, Botten G, Stigum H, et al. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112(2):420–6. doi: 10.1067/mai.2003.1610. [DOI] [PubMed] [Google Scholar]
- 38.Malamitsi-Puchner A, Protonotariou E, Boutsikou T, et al. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum Dev. 2005;81(4):387–92. doi: 10.1016/j.earlhumdev.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Hällström M, Eerola E, Vuento R, et al. Effects of mode of delivery and necrotising enterocolitis on the intestinal microflora in preterm infants. Eur J Clin Microbiol Infect Dis. 2004;23(6):463–70. doi: 10.1007/s10096-004-1146-0. [DOI] [PubMed] [Google Scholar]
- 40.Dewey KG, Nommsen-Rivers LA, Heinig MJ, et al. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3Pt1):607–19. doi: 10.1542/peds.112.3.607. [DOI] [PubMed] [Google Scholar]
- 41.Evans KC, Evans RG, Royal R, et al. Effect of caesarean section on breast milk transfer to the normal term newborn over the first week of life. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F380–2. doi: 10.1136/fn.88.5.F380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mold JE, Michaëlsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–45S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 44.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–2. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renz-Polster H, David MR, Buist AS, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35(11):1466–72. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 47.Decker E, Engelmann G, Findeisen A, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125(6):e1433–40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 48.Sonntag B, Stolze B, Heinecke A, et al. Preterm birth but not mode of delivery is associated with an increased risk of developing inflammatory bowel disease later in life. Inflamm Bowel Dis. 2007;13(11):1385–90. doi: 10.1002/ibd.20206. [DOI] [PubMed] [Google Scholar]
- 49.Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 50.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–35. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 51.Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case-control study. BMC Public Health. 2010;27(10):281. doi: 10.1186/1471-2458-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]