Abstract
OBJECTIVE
Vitamin D insufficiency occurs commonly in HIV-infected youth in the United States. In light of the importance of vitamin D for skeletal and nonskeletal health, including innate immunity, developing methods for improving vitamin D status in HIV-infected children and adolescents is an important area of clinical research. The objective of this study was to evaluate the effect of administration of oral cholecalciferol, 100 000 IU every 2 months, and 1 g/day calcium on serum 25-hydroxyvitamin D concentrations, serum and urine calcium, and HIV disease progression during a 12-month period.
METHODS
HIV-infected children and adolescents who were aged 6 to 16 years were randomly assigned to receive vitamin D (100 000 IU bimonthly) and calcium (1 g/day; n = 29) or double placebo (n = 27). Serum 25-hydroxyvitamin D concentrations as measured by radioimmunoassay, albumin-corrected calcium concentrations, and spot urinary calcium-creatinine ratios were determined monthly.
RESULTS
No abnormalities in serum calcium concentration were observed. One participant who received placebo developed hypercalciuria. No group differences were seen in the change in CD4 count or CD4% or viral load during 12 months. The overall mean monthly serum 25-hydroxyvitamin D concentrations were higher in the group that received vitamin D and calcium than in the placebo group, as was the monthly serum 25-hydroxyvitamin D area under the curve. After completing 12 months of study, 2 (6.7%) participants in the group that received vitamin D and calcium had a trough serum 25-hydroxyvitamin D concentration <20 ng/mL compared with 14 (50%) in the placebo group. Twelve (44.4%) in the group that received vitamin D and calcium had a trough serum 25-hydroxyvitamin D concentration of ≥30 ng/mL compared with 3 (11.1%) in the placebo group.
CONCLUSIONS
Administration of oral cholecalciferol to HIV-infected children and adolescents at a dosage of 100 000 IU every 2 months, together with 1 g/day calcium, is safe and results in significant increases in serum 25-hydroxyvitamin D concentrations
Keywords: HIV, cholecalciferol, vitamin D, 25-hydroxyvitamin D, calcium, randomized, controlled trial
Vitamin D, A regulatory hormone involved in bone and mineral metabolism as well as immune function, is reported as insufficient in as many as 87% of HIV-infected adolescents and young adults who live in the United States.1 In light of the importance of vitamin D to normal immune function and calcium homeostasis, development of interventions to improve vitamin D status in HIV-infected children and adolescents is an important research priority. The objective of this study was to evaluate the effect of oral cholecalciferol, 100 000 IU given every 2 months, and 1 g/day calcium on monthly serum 25- hydroxyvitamin D (25-OHD) concentrations, serum and urine calcium, and the course of disease in HIV-infected children and adolescents.
METHODS
Participants were recruited during 2004–2005 from among children and adolescents who were enrolled in 4 hospital-based pediatric HIV treatment programs at St Luke’s-Roosevelt Hospital Center, Harlem Hospital Center, Bronx-Lebanon Hospital Center, and Metropolitan Hospital Center, which are located in New York City, NY.
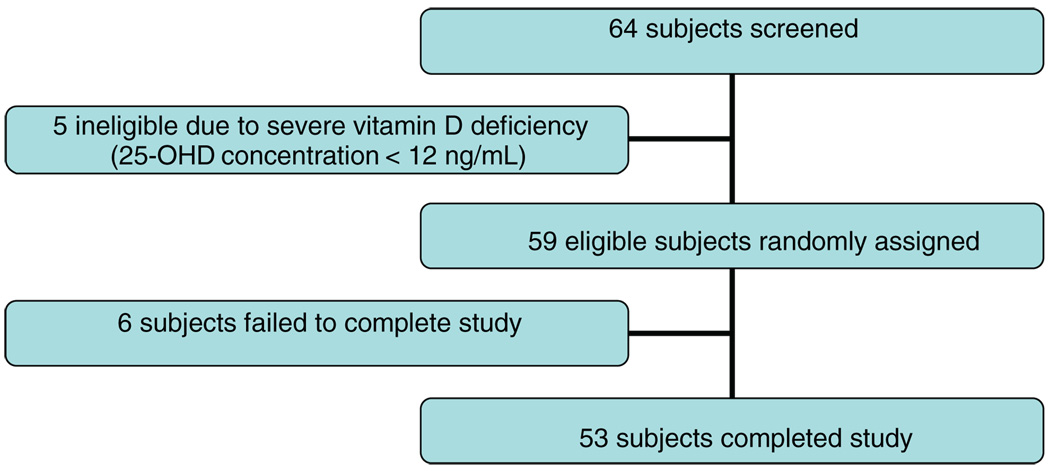
The flow of study participants through the study stages is presented in Fig 1. Sixty-four perinatally HIV-infected children and adolescents who were aged 6 to 16 years underwent screening. Five (7.8%) participants with severe vitamin D deficiency defined as initial 25-OHD concentration <12 ng/mL were deemed ineligible to avoid possible randomization to placebo and were referred for treatment. Fifty-nine with screening serum 25-OHD concentrations ≥12 ng/mL were randomly assigned to receive vitamin D and calcium (VD+) or double placebo (VD−). Randomization was performed by the study statistician (Mr McMahon) by using computer-generated random numbers (SAS 8.2 for Windows [SAS Institute, Cary, NC]) stratified by gender, age (> or ≤12 years), and study site. Allocation was communicated to the research coordinator who initiated and dispensed study medications (active or placebo). Fifty-six participants (VD+: n = 29; VD−: n = 27) completed 12 months of study and are included in the analysis.
FIGURE 1.
Study participant flow.
Oral cholecalciferol 100 000 IU (Tishcon Laboratories Inc, Salisbury, MD) or placebo was administered by study personnel every 2 months during study visits. Content of active ingredient was analyzed in Tishcon Laboratories by high-performance liquid chromatography by using United States Pharmacopeia methods.2 In addition, calcium as the carbonate salt, in the form of chocolate-flavored chews that each contained 500 mg of calcium, or placebo was dispensed monthly by study personnel (Lifesmart Nutrition Technologies, Salt Lake City, UT). Study personnel and participants were blinded to treatment allocation. Study participants were instructed to take 2 chews daily. Dietary intake of vitamin D and calcium was assessed by food frequency questionnaire (Block Kids Questionnaire [Block Dietary Data Systems, Berkeley, CA]). Estimated weekly sunlight exposure in hours was determined by self-report. Pubertal status was determined by study investigators (Drs Arpadi and Horlick) by using the method of Tanner,3,4 and HIV disease was classified by using Centers for Disease Control and Prevention criteria.5 Serum albumin–corrected calcium and spot urinary calcium-creatinine ratios were measured monthly.6 Participants with spot urinary calcium-creatinine ratios >0.25 were evaluated further with 24-hour urinary calcium excretion (Uca). Hypercalciuria was defined as Uca >4 mg/kg per day. Serum 25-OHD concentrations were obtained monthly, immediately before administration of cholecalciferol dose or placebo (trough) and 1 month after dosing. Specimens for each individual were analyzed in the same assay in the Irving Center for Clinical Research (Columbia University, New York, NY) by radioimmunoassay (Diasorin, Stillwater, MN). The coefficients of variation for normal and elevated interassay values were 9.7% and 9.1%, respectively. Adverse events were assessed at each visit by means of a questionnaire and a symptom checklist.
Group differences in continuous measures at baseline were compared with independent t tests, whereas group differences in dichotomous categorical measures at base-line were estimated with Fisher’s exact test. Data are reported as means ± SD or counts and percentages. Viral load results (RNA copies per mL) were log-transformed before analysis. Differences in changes between baseline and 1-year measurements were compared by analysis of covariance with fixed effects of study group and baseline values of dependent variables. Group differences in the frequency of rating of ordinal severity of safety symptoms was estimated with Jonckheere-Terpstra test for trend.7
Analysis of group differences in change over time used mixed linear model analysis with fixed effects for group, time, and group by time interaction; random effects for subject and error; and a compound symmetry covariance structure for the within-subject correlation between times. To adjust for possible seasonal variations in vitamin D status, we coded a dummy variable, seasonal month, such that January and February = 1, March and December = 2, April and November = 3, etc, and last, July and August = 6.
This study was approved by the institutional review boards of all participating institutions. Informed consent and assent were obtained before enrollment of participants.
RESULTS
The characteristics of the study sample are presented in Table 1. At baseline, the mean serum 25-OHD was 24.5 ± 10.0 ng/mL. VD+ and VD− groups did not differ with respect to age, race, gender, anthropometrics, dietary intake of vitamin D and calcium, or percentage of participants with baseline serum 25-OHD concentrations between 12 and 20 ng/mL. The participants in both study groups had mean daily vitamin D and calcium dietary intakes that were considerably below the recommendation for adequate dietary calcium intake for age (800 mg/day and 1300 mg/day for ages 4–8 and 9–13 years, respectively).8
TABLE 1.
Baseline Characteristics of VD+ and VD− Patients
| Characteristic | VD+ (N = 29) | VD− (N = 27) | P |
|---|---|---|---|
| Age, mean ± SD, y | 10.2 ± 2.9 | 10.6 ± 2.4 | .60 |
| Weight, mean ± SD, kg | 38.4 ± 10.9 | 43.2 ± 11.9 | .12 |
| Weight-for-age z score, mean ± SD | 0.37 ± 0.96 | 0.56 ± 1.07 | .48 |
| Height, mean ± SD, m | 1.42 ± 0.16 | 1.45 ± 0.13 | .36 |
| Height-for-age z score, mean ± SD | 0.01 ± 0.29 | 0.00 ± 1.03 | .97 |
| Male, n (%) | 14 (48) | 12 (44) | .77 |
| Black, n (%) | 17 (59) | 18 (67) | |
| Hispanic, n (%) | 12 (41) | 9 (33) | .53 |
| Tanner stage, n (%) | |||
| 1 | 13 (45) | 10 (37) | |
| 2 | 2 (7) | 2 (7) | |
| 3 | 6 (21) | 2 (7) | |
| 4 | 4 (14) | 7 (26) | |
| 5 | 4 (14) | 6 (22) | .50 |
| CD4%, mean ± SD | 30.6 ± 10.5 | 29.4 ± 10.1 | .65 |
| CD4 count, mean ± SD, cells/mL | 769 ± 343 | 724 ± 369 | .64 |
| CDC class, n (%) | |||
| A | 12 (41) | 9 (33) | |
| B | 12 (41) | 12 (44) | |
| C | 5 (17) | 6 (22) | .80 |
| Viral load Log10, mean ± SD, RNA copies/mL | 3.39 ± 0.91 | 3.46 ± 0.81 | |
| Viral load more than level of detection, n (%) | 18 (62) | 18 (67) | .72 |
| Sunlight exposure, mean ± SD, h/wk | 6.5 ± 4.4 | 5.8 ± 4.3 | .58 |
| Dietary calcium, mean ± SD, mg/d | 684 ± 305 | 675 ± 362 | .92 |
| Dietary vitamin D, mean ± SD, IU/d | 158 ± 93 | 150 ± 94 | .75 |
| Serum 25-OHD, mean ± SD, ng/mL | 24.1 ± 9.1 | 23.6 ± 10.3 | .84 |
| Serum 25-OHD between 12 and 20 ng/mL, n (%) | 12 (41.4) | 12 (44.4) | .91 |
There were no differences between the VD+ and VD− groups with respect to the frequencies of possible adverse symptoms, including gastrointestinal (nausea, vomiting, diarrhea, constipation, stomachache), genitourinary (polyuria, dysuria), or neuromuscular (headache, confusion, drowsiness, weakness, fatigue) symptoms (3.3% vs 4.1% [P = .62], 3.0% vs 4.1% [P = .48], and 0.3% vs 0.6% [P = .62], respectively).
During the course of the study, a single study participant in the placebo group was found to have transient hypercalciuria (UCa of 6.2 mg/kg per 24 hours). There were no episodes of hypercalcemia. The mean monthly corrected serum calcium concentrations did not differ between the 2 study groups.
There were no differences between study groups with respect to adverse effects of cholecalciferol and calcium on the course of HIV disease or treatment during the study period. The mean viral load, CD4 number, and CD4% measurements did not differ between study groups at either entry or 12 months (Table 2); neither did the groups differ in the incidence of virologic treatment failure during the study among those who entered the study on antiretroviral treatment and had well-suppressed HIV replication (ie, treatment failure defined as an increase in HIV RNA concentration during the study among those with undetectable HIV RNA concentration at study entry). The study groups did not differ with respect to the percentage of participants who required a change in antiretroviral medications as a result of clinical or immunologic deterioration as determined by the participants’ usual medical provider.
TABLE 2.
Growth and Disease Status Between VD+ and VD− HIV-Infected Children and Adolescents at Time of Enrollment and After 1 Year
| Parameter | VD+ (N = 29) | VD− (N = 27) | Pa | ||
|---|---|---|---|---|---|
| Baseline | 1 y | Baseline | 1 y | ||
| Weight, kg | 38.2 ± 11.0 | 42.2 ± 11.3 | 43.0 ± 11.9 | 47.9 ± 12.3 | .63 |
| Height, cm | 141.7 ± 16.4 | 145.2 ± 15.5 | 144.8 ± 12.4 | 148.3 ± 11.4 | .59 |
| Weight z score | 0.27 ± 1.03 | 0.39 ± 0.96 | 0.61 ± 1.11 | 0.71 ± 0.98 | .92 |
| Height z score | −0.10 ± 0.78 | −0.07 ± 0.79 | 0.11 ± 1.25 | −0.04 ± 1.30 | .92 |
| CD4 count, cells/mL | 771 ± 328 | 776 ± 359 | 719 ± 382 | 661 ± 363 | .18 |
| CD4% | 29.6 ± 10.3 | 30.8 ± 9.1 | 29.4 ± 10.5 | 27.0 ± 9.9 | .09 |
| Viral load log 10, copies/mL | 2.8 ± 0.9 | 2.4 ± 0.9 | 2.9 ± 1.0 | 2.5 ± 1.1 | .66 |
Data are means ± SD.
Analysis of covariance with fixed effect of treatment group and baseline value of dependent variable entered as continuous covariate.
The overall mean monthly serum 25-OHD concentrations during the 12 months of the study (Fig 2) were significantly higher in the VD+ group than in the VD− group (32.4 ± 9.0 vs 21.9 ± 9.4 ng/mL; P < .0001). After completing 12 months of study, 2 (6.7%) participants in the VD+ group had a trough serum 25-OHD concentration <20 ng/mL compared with 14 (50%) in the VD− group (P < .001), and 12 (44.4%) in the VD+ group had a trough serum 25-OHD concentration ≥30 ng/mL compared with 3 (11.1%) in the VD− group (P < .02). In addition, the monthly serum 25-OHD area under the curve for the 12-month period was higher in the VD+ compared with the VD− group (P < .0001). In a multiple regression model predicting serum 25-OHD concentration, statistically significant treatment group differences persisted (P < .0001) after adjustment for number of months on study (1–12; P < .90) and seasonal month (1–6; P < .02).
FIGURE 2.
Mean monthly serum 25-OHD ± SEM in HIV-infected children who received bimonthly oral cholecalciferol 100 000 IU (solid line; n = 29) or double placebo (dashed line; n = 27).
DISCUSSION
The results of this study demonstrate that supplementation of HIV-infected children and adolescents with orally administered bimonthly doses of 100 000 IU of cholecalciferol together with 1 g/day calcium during a 12-month period is well tolerated and safe and results in significant increases of serum 25-OHD concentrations. In addition, this regimen results in serum concentrations of 25-OHD that are >30 ng/mL on average, the level now considered optimal for intestinal calcium absorption, suppressing parathyroid activity, and reducing osteoporotic fractures in adults.9
We elected to use bimonthly instead of daily or other dosing schedules because this schedule has been used effectively to prevent wintertime vitamin D deficiency in otherwise healthy adolescents10 and also to minimize disruption of other medications that many of the study participants take as part of treatment of their HIV infection. In addition, this dosing schedule allows for direct administration during study visits, thereby ensuring adherence. To our knowledge, assessment of the effect of 1 year of this treatment regimen on serum 25-OHD has not been performed previously; neither have studies of vitamin D supplementation in children and adolescents with HIV infection been reported.
The bimonthly doses of 100 000 IU of cholecalciferol together with daily calcium supplementation did not significantly affect Uca or serum calcium during the 12-month period of observation. In addition, we observed no difference in progression of HIV disease as measured by CD4 count, increase in plasma HIV viral load, or rate of failure of antiretroviral treatments. Thus, there seems to be no short-term adverse effect of this regimen on the course or treatment of HIV infection. Caution is warranted in interpreting our study results. Because of the small sample size, our capacity to detect infrequent events is limited. For example, although no hypercalcemia occurred among the vitamin D–treated participants, the calculated 95% confidence interval for this estimate indicates that the true underlying prevalence of hypercalcemia could be as high as 12%.
The average increase in 25-OHD resulting from the cholecalciferol used in this study was less than previously reported in healthy male adults.11 This may reflect underlying alterations in intestinal absorption or vitamin D metabolism, both of which are documented in HIV.12,13 Studies of higher dosages or shorter (eg, monthly) interval dosing seem to be justified given that 75% of the participants in the vitamin D treatment group had at least 1 monthly 25-OHD concentration <30 ng/mL.
Although there is no consensus on the optimal definition, by using a serum 25-OHD concentration <20 ng/mL as recommended by a number of experts, the prevalence of vitamin D deficiency among all participants who underwent initial screening was 45%.14,15 This is lower than results from a larger multicenter study of HIV-infected adolescents and young adults that reported vitamin D insufficiency in 87%.1 The higher prevalence may be accounted for by differences in racial composition of the study participants, time of year of sampling, dietary practices, or amount of sunlight exposure; however, because different assay methods (eg, competitive protein binding, radioimmunoassay) and criteria (eg, 25-OH D <15 ng/mL) were used, direct comparisons cannot be made. Inclusion of measurement of parathyroid hormone in our study would have improved ascertainment of vitamin D deficiency.
Our data suggest that inadequate dietary intake of vitamin D contributes to the low vitamin D levels that we observed. This study’s findings of low dietary intake of vitamin D are similar to those reported in other studies of children, adolescents, and young adults with HIV infection.1,16 Poor dietary intake of vitamin D and low vitamin D concentrations do not seem to be related to HIV infection per se. Vitamin D deficiency is highly prevalent among urban youth and is reported equally among both HIV-infected and healthy urban-dwelling black and Hispanic adolescents.1,17
Insufficient exposure to sunlight could also be a factor in the low vitamin D levels in our study sample. Sunlight exposure results in vitamin D3 synthesis in skin, and when exposure is adequate, vitamin D is released from body fat stores during winter months, when vitamin D cannot be produced. Exposure of arms and legs for 5 to 30 minutes between 10 am and 3 pm twice a week can be sufficient and is the equivalent to ingestion of 10 000 IU of vitamin D.18–21 Despite reported sunlight exposure many times this level, we observed a high prevalence of vitamin D deficiency. Our study is limited, however, because we did not estimate a number of important factors that are known to affect solar irradiation–induced cutaneous synthesis of vitamin D3, such as the amount of sun-exposed skin, time of year of sun exposure, and skin color.
Although unlikely to be the sole factor causing low bone mass in HIV-infected children, suboptimal vitamin D status together with inadequate calcium intake could be an important contributor to the low bone mass that is reported in HIV-infected children.22–24 Treatment with vitamin D and calcium supplementation results in improvements in bone mass density in HIV-infected adults with osteopenia and osteoporosis.25 Whether bimonthly supplementation with vitamin D in doses that result in the modest increases in serum 25-OHD concentrations observed in our participants, together with daily calcium supplementation, is sufficient for meaningful gains in bone mass accrual in children and adolescents with HIV is important to determine.
A number of additional potential benefits to vitamin D supplementation may be of particular importance to HIV-infected children and adolescents. Vitamin D is a potent immunomodulator, and reduced levels adversely affect immune function.26 Vitamin D insufficiency (eg, 25-OHD concentrations <20 ng/mL) results in reduced monocyte and macrophage innate immunity to infectious agents such as Mycobacterium tuberculosis, the leading cause of AIDS death in many parts of the world.27,28 In addition, vitamin D increases insulin production and myocardial contractility, both of which can be abnormal in HIV-infected children and adolescents.29,30 The possible relationship of vitamin D and alterations in insulin metabolism and myocardial and immune function reported in pediatric HIV also warrants additional study.31–34
What’s Known on This Subject.
Vitamin D insufficiency, which increases risk for osteoporosis, is reported in as many as 87% of HIV-infected adolescents and young adults in the United States; however, no published studies have addressed the management of vitamin D insufficiency in this population.
What This Study Adds.
This study reports that supplementation of HIV-infected children and adolescents with orally administered bimonthly doses of 100 000 IU of cholecalciferol together with 1 g/day calcium is well tolerated and safe and results in significant increases of serum 25-OHD concentrations.
ACKNOWLEDGMENTS
This study was supported by funding from the National Institutes of Health (DK63666 and RR00645).
We thank Mr Robert Warford and Drs Emma Stuard and Savita Manwani for assistance on this study.
Abbreviations
- 25-OHD
25-hydroxyvitamin D
- VD+
cholecalciferol and calcium supplementation group
- VD−
placebo group
- Uca
urinary calcium excretion
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Stephensen CB, Maquis GS, Kruzich LA, Douglas SD, Altrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83(5):1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 2.United States Pharmacopeia. Rockville, MD: United States Pharmacopeial Convention, Inc; 2002. [Google Scholar]
- 3.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43(RR-12):1–19. [Google Scholar]
- 6.Kruse K, Kracht U, Kruse U. Reference values for urinary calcium excretion and screening for hypercalcuria in children and adolescents. Eur J Pediatr. 1984;143(1):25–31. doi: 10.1007/BF00442743. [DOI] [PubMed] [Google Scholar]
- 7.Pirie W. Jonckheere tests for ordered alternatives. In: Kotz S, Johnson NL, editors. Encyclopedia of Statistical Sciences. Vol 4. New York, NY: John Wiley & Sons, Inc; 1983. pp. 315–318. [Google Scholar]
- 8.Institute of Medicine. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 9.Heaney R. The vitamin D requirements in health and disease. J Steroid Biochem Mol Biol. 2005;97(1–2):13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Guillemant J, Le HT, Maria A, Allemandu A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int. 2001;12(10):875–879. doi: 10.1007/s001980170040. [DOI] [PubMed] [Google Scholar]
- 11.Heaney RP, Davies M, Chen CT, Holick MF, Burger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 12.Carroccio A, Fontana M, Spagnuolo MI, et al. Pancreatic dysfunction and its association with fat malabsorption in HIV infected children. Gut. 1998;43(4):558–563. doi: 10.1136/gut.43.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madeddu G, Sapnu A, Solinas P, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48(1):39–48. [PubMed] [Google Scholar]
- 14.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson DL, Spiegelman D, Duggan C, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41(3):339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 17.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among health adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiciency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Garabedian M. Vitamin D: photobiology, metabolism, mechanism of action and clinical applications. In: Favus MH, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed. Washington, DC: American Society for Bone Mineral Research; 2006. pp. 129–137. [Google Scholar]
- 20.Reid JR, Gallagher DJ, Bosworth J. Prophylaxis against vitamin d deficiency in the elderly by regular sunlight exposure. Age Ageing. 1986;15(1):35–40. doi: 10.1093/ageing/15.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Chel VG, Oomus ME, Popp-Snijders C, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13(8):1238–1242. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Sala N, Bricalli D, et al. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001;15(14):1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien K, Razavi M, Henderson R, Caballero B, Ellis K. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr. 2001;73(4):821–826. doi: 10.1093/ajcn/73.4.821. [DOI] [PubMed] [Google Scholar]
- 24.Arpadi S, Horlick M, Thornton J, Wang J, Kotler D. Bone mineral content is lower in HIV-infected children. J Acquir Immune Defic Syndr. 2002;29(5):450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. 2005;38(4):426–431. doi: 10.1097/01.qai.0000145352.04440.1e. [DOI] [PubMed] [Google Scholar]
- 26.Penna G, Roncari A, Armuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+ Foxp3+ regulatory T-cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 27.Liu PT, Stenfer S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 28.De Cock KM. HIV infection, tuberculosis and World AIDS Day, 2006. Int J Tuberc Lung Dis. 2006;10(12):1305. [PubMed] [Google Scholar]
- 29.Chiu KC, Chu A, Go VL, Saad MF. Hypovitamin D is associated with insulin resistance and B cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 30.Zitterman A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Rondanelli M, Caselli D, Trotti R, et al. Endocrine pancreatic dysfunction in HIV-infected children: association with growth alterations. J Infect Dis. 2004;190(5):908–912. doi: 10.1086/422255. [DOI] [PubMed] [Google Scholar]
- 32.Bitnun A, Sochett E, Dick PT, et al. Insulin sensitivity and beta-cell function in protease inhibitor-treated and -naive human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2005;90(1):168–174. doi: 10.1210/jc.2004-0125. [DOI] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Circulation. 1998;97(13):1246–1256. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starc TJ, Lipshultz SE, Kaplan S, et al. Cardiac complications in children with human immunodeficiency virus infection. Pediatrics. 1999;104(2) doi: 10.1542/peds.104.2.e14. Available at: www.pediatrics.org/cgi/content/full/104/2/e14. [DOI] [PMC free article] [PubMed] [Google Scholar]