Abstract
Colorectal cancer survivorship begins at diagnosis and continues throughout life. After diagnosis, survivors face the possibility of second cancers, long-term effects of cancer treatment, and comorbid conditions. Interventions that can provide primary, secondary, and tertiary prevention in this population are important. Physical activity has been demonstrated to lower colon cancer incidence and recurrence risk as well as improve quality of life and non-cancer health outcomes including cardiovascular fitness in colon cancer survivors. The data are less robust for rectal cancer incidence and recurrence, although improvements in quality of life and health outcomes in rectal cancer survivors are also seen. Potential mechanisms for this benefit may occur through inflammatory or insulin-like growth factor pathways. The issues of colorectal cancer survivorship and the impact of physical activity on these issues are reviewed, with discussion of possible biologic mechanisms, barriers to physical activity intervention studies, and future research directions for physical activity in this burgeoning survivor population.
Keywords: Colorectal cancer, colon cancer, survivorship, physical activity, exercise
Introduction
Cancer survivorship begins at diagnosis and continues throughout life. Essential care includes prevention of recurrent and new cancers, surveillance for recurrence and medical or psychological effects of cancer, intervention for consequences of cancer and treatment, and health promotion (1). Research in this burgeoning population has primarily focused on early survivorship and quality of life rather than long-term health outcomes and disease prevention. Therefore, evaluation of interventions targeting long-term effects of cancer and preventive care are warranted. Physical activity represents an intervention that may provide primary (protect against development of new disease), secondary (detect disease at earliest stages), and tertiary (limit complications or progression of disease while restoring function) preventive benefits to cancer survivors with regard to the cancer itself and/or comorbid conditions. In this article, we review the disease-specific benefits and possible preventive mechanisms of physical activity as it relates to the population of colon and rectal cancer survivors.
Issues of Colorectal Cancer Survivorship
Colon and rectal cancers are the third most common cause of cancer in the U.S., accounting for 10% of the cancer survivor population (2, 3). Improvements in screening and systemic therapy have increased the overall five-year survival rate for colorectal cancer from 51% in 1975 to 66% in 2006, which increases to 90.4% for localized disease and 69.5% for regional disease (4). A significant proportion of patients will go on to become long-term survivors with the potential to develop second colorectal and non-colorectal cancers, comorbid conditions, and long-term effects of treatment. Survivors are at risk for second primary colorectal cancers, with 43% of second primary colorectal cancers occurring more than two years from diagnosis (5). Non-colorectal second primary cancers may also occur, most commonly breast, prostate, genitourinary, skin, and lung cancers (6, 7). Last, the burden of comorbidity is high, with up to 80% of colorectal cancer survivors reporting at least one comorbidity (8, 9). The most common comorbidities include cardiovascular disease, musculoskeletal problems, lung or breathing problems, and depression (10–12). The combination of a cancer diagnosis and comorbidity can result in lower health-related quality of life, lower productivity, and more health and functional limitations, even in long-term (more than five years from diagnosis) survivors (10).
Although colorectal cancer survivors generally report an excellent quality of life, a cancer diagnosis can negatively affect functional and social well-being (8, 13, 14). Sequelae of the cancer and treatment such as fatigue, physical discomfort, negative body image, depression, and physical limitations may persist for years after diagnosis. These issues may be more prevalent with radiation therapy, with surgery involving a stoma, in elderly or female survivors, or in overweight, obese or low-income survivors (9, 15, 16). Permanent sensory neuropathy associated with oxaliplatin-based adjuvant therapy has been reported in up to 15% of survivors four years after completion of this treatment (17). Age, body weight, and comorbidities may affect long-term physical health and quality of life more than did the cancer diagnosis itself, with 32% of long-term survivors reporting limitations in usual activities due to comorbid conditions (11, 18). Therefore although overall quality of life is high in this survivor population, physical quality of life may be low because of cancer- and comorbidity-related issues (8, 11). Interventions that augment the impact of a colon or rectal cancer diagnosis on quality of life and survival outcomes, prevent the development of second colorectal and non-colorectal cancers, and modify or ameliorate the development of comorbidities warrant investigation to further improve the survivorship phase of the colorectal cancer continuum.
Physical Activity: Current Recommendations
Physical activity has been proposed as a non-pharmacological means to improve quality of life and health-related outcomes (disease-specific and general), and has been endorsed as an important part of cancer therapy. The National Comprehensive Cancer Network recommends activity enhancement,, including initiating a program encompassing endurance and resistance exercise during active treatment and post-treatment, to combat cancer-related fatigue (19). The American Cancer Society encourages physical activity, citing feasibility, safety, enhancement of quality of life and physical or functional well-being, and primary prevention for comorbid conditions (20). Most recently, the American College of Sport Medicine published a consensus statement and recommendations for cancer survivors endorsing the U.S. Department of Health and Human Services general population recommendations for a minimum of 150 minutes of moderate or 75 minutes of vigorous exercise per week, stretching, and two or three weekly strength training sessions for major muscle groups. Development of a physical activity program must consider prediagnosis fitness level and activities, requires an understanding of the cancer diagnosis and sequelae of treatment, and must incorporate an evaluation of comorbid conditions (21, 22). Recommendations should be tailored to an individual survivor’s needs, abilities, and medical condition (21).
The Benefits of Moving: Physical Activity in General and Cancer Populations
In general, physical activity lowers the risk of premature death, cardiovascular disease, diabetes, falls, and metabolic syndrome; improves cardiopulmonary fitness, cognitive function in older adults, sleep quality, and bone density; and contributes to weight loss and weight maintenance when combined with calorie restriction (22). A meta-analysis of physical activity intervention trials in various cancer survivor populations concluded that physical activity was safe during and after cancer treatment. Physical activity improved aerobic fitness, upper and lower body strength, functional quality of life, mood, anxiety, self-esteem, body image, and fatigue in both the on-treatment and post-treatment phases (23). In postmenopausal breast cancer survivors enrolled in the Women’s Health Initiative, participation in moderate- to vigorous-intensity recreational physical activity (i.e. brisk walking, bicycling, swimming) approximately 3 hours per week before or after a breast cancer diagnosis lowered the risk of all-cause and breast cancer-specific mortality. The benefit of physical activity after diagnosis was independent of stage of disease at diagnosis. Further, those women who increased or maintained physical activity levels at or above 3 hours per week, including those inactive at baseline, experienced 33% lower risk of all cause mortality compared to inactive women (24). These findings suggest that adopting an active lifestyle can improve quality of life, physical function, overall longevity, and prognosis from a cancer diagnosis.
Supervised and home-based exercise interventions, as well as strength training, appear to be beneficial. The Reach out to Enhance Wellness (RENEW) study evaluated a telephone-counseling and print-material diet and exercise intervention (versus usual activity) in survivors of breast, colorectal, and prostate cancers who were overweight and aged 65–91 years. Physical function and quality of life declined less and strength training and exercise endurance improved with the exercise intervention (25). Another study found improvements in cardiopulmonary function, muscular strength, physical functioning, fatigue, and health-related quality of life in survivors of various cancers enrolled in an 18-week strength training program initiated within 6 weeks of completing chemotherapy; these effects persisted after one year of follow-up (26, 27). Additional studies of physical activity in colorectal cancer survivors have demonstrated higher overall quality of life and physical functioning and less fatigue among physically more-active survivors (28–30). These modest lifestyle changes result in clinically meaningful improvements that can combat physical, functional, and psychological decline in colorectal cancer survivors, including those at high risk such as older or overweight survivors.
Physical Activity and Colorectal Cancer: Preventive Across the Disease Spectrum
Epidemiological evidence supports the role of physical activity in cancer prevention (31–33). A National Institutes of Health (NIH)–AARP study found an 18% reduction in colon cancer risk (relative risk 0.82; 95% confidence interval [CI], 0.73–0.82) among people ages 50–71 years who exercised at least five times per week compared with like-aged people who never or rarely exercised (34). Other studies in European and Asian populations found similar associations (35–39). Convincing evidence of a preventive effect exists for colon cancer, where meta-analyses of over 60 studies suggest a reduction of 20%–25% in colon cancer risk in people with the highest level compared with people with the lowest level of physical activity. The source of physical activity does not matter, as occupational and leisure-time activities confer similar reductions in risk, and a dose-response effect exists (31, 33). Despite these convincing data, public awareness of the role of physical activity in the prevention of colon cancer is low (40). Similar effects are not seen in the epidemiology of rectal cancer risk, where physical activity does not appear to have a protective effect (41).
Modeling the effect of physical activity suggests that population compliance with recommended levels of activity would reduce the population incidence of colon cancer by up to 21% (33, 42). Associations between physical activity and risk of cancer are also probable for breast and endometrial cancer and possible for lung, ovarian, and prostate cancer. Between 9% and 19% of breast, lung, colon, endometrial, prostate, and ovarian cancers in Europe were estimated to have been preventable in 2008 if the population had maintained sufficient levels of activity. Sufficient levels in this analysis were defined as 30 minutes of moderate-intensity activity for 5 days or 20 minutes of vigorous-intensity activity for 3 days above a base level of 60 minutes of moderate-intensity activity every day. No association for rectal cancer or other cancer sites and physical activity definitively exist (33). Therefore, one could speculate that physical activity may be a non-pharmacologic intervention not only to decrease an initial colon cancer, but perhaps also could decrease the risk of second colon and non-colorectal cancers by modifying underlying risk factors that predisposed a survivor to the initial colon cancer.
Physical activity also appears to affect disease outcome and recurrence after diagnosis. Although epidemiological evidence suggests physical activity has the greatest effect on colon cancer incidence, cohort studies in both colon and rectal cancer survivors suggest that physical activity also may affect outcomes after diagnosis and treatment although the literature does not always distinguish between these two populations (Table 1). Haydon et al. evaluated general physical activity and walking in a cohort of 526 colon and rectal cancer survivors (all stages) prospectively enrolled in the Melbourne Collaborative Cohort Study. In the overall study population, regular physical activity, even as little as once per week, was associated with an absolute improvement of 14% in overall survival and 12% in disease-specific survival at five years compared with no regular activity. This effect was greatest in survivors with right colon tumors and in survivors with stage II or III disease, who had a 39% reduction in all-cause mortality and 51% reduction in disease-specific mortality. No effect on survival was noted, however, in the subgroup with rectal cancer or when regular walking was evaluated alone, suggesting that this benefit may be confined to colon cancer and requires a more vigorous physical activity routine than walking alone (43).
Table 1.
Studies evaluating impact of physical activity on outcomes after a diagnosis of colon or rectal cancer
| Author | Population | Activity Level | Median Follow-up | Survival HR (95% CI; P-value) | |
|---|---|---|---|---|---|
| Disease-specific | Overall | ||||
| Haydon et al 2006 (43) | Colorectal cancer patients (all stages and both sexes) | Any regular weekly exercise vs. none at all | 5.5 years from diagnosis | 0.73 (0.54–1.00; P = 0.05) | 0.77 (0.58–1.03; P = 0.08) |
| Meyerhardt et al 2006 (44) | Women with colorectal cancer (stages I to III) | 18 MET-hours/week vs. < 3 MET-hours/week | 9.6 years from diagnosis | 0.39 (0.18–0.82; P = 0.008) | 0.43 (0.35–0.74; P = 0.003) |
| Meyerhardt et al 2009 (45) | Men with colorectal cancer (stages I to III) | 27 MET-hours/week vs. < 3 MET-hours/week | 8.6 years from diagnosis | 0.47 (0.24–0.92; P = 0.002) | 0.59 (0.41–0.86; P <0.001) |
| Meyerhardt et al 2006 (46) | Colon Cancer (stage III and both sexes) receiving adjuvant therapy | 18–26.9 MET-hours/week vs. < 3 MET-hours/week | 3.8 years from trial entry | 0.51 (0.26–1.01) | 0.71 (0.32–1.59) |
| ≥ 27 MET-hours/week vs. < 3 MET-hours/week | 0.60 (0.036–1.01; P for trend = 0.03) | 0.37 (0.16–0.82; P for trend = 0.01) | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; MET, metabolic equivalent task.
Meyerhardt et al. also examined the role of physical activity, as measured in metabolic equivalent task (MET)-hours per week (described in Table 2), after colon or rectal cancer diagnosis and treatment in three cohorts of patients. Physical activity of at least 18 MET-hours per week was associated with a lower rate of colorectal cancer–specific death and overall mortality, compared with less than 18 MET-hours per week in 573 women diagnosed with stages I to III colon and rectal cancers enrolled on the Nurses Health Study. An increase in physical activity after diagnosis also improved disease-specific and overall survival in this population, regardless of prediagnosis activity level (44). Similar findings were seen in 668 men with stages-I-to-III colon and rectal cancers enrolled in the Health Professionals Follow-up Study. The amount of activity required to achieve survival benefits, however, was higher in the Health Professionals Follow-up Study (≥ 27 MET-hours per week) (45). This difference may be due to a larger number of men (50.4%) engaging in high levels of physical activity at study entry than did women (25.9%) in the Nurses Health Study (44, 45). In both studies, the benefit of physical activity remained after adjusting for stage, age, body mass index, year of diagnosis, tumor location including rectum, and exclusion of people who died within two years of questionnaire response. Pre-diagnosis physical activity levels, however, did not have an effect on post-diagnosis outcomes (44, 45).
Table 2.
| MET-hour | Activity level as measured in walking at an average pace |
|---|---|
| 1 | Sitting quietly for 1 hour |
| < 3 | < 1 hour of walking per week |
| 3–8.9 | 1 to < 3 hours of walking per week |
| 9–17.9 | 3 to < 6 hours of walking per week |
| 18–26.9 | 6 to < 9 hours of walking per week |
| ≥ 27 | ≥ 9 hours of walking per week |
MET (metabolic equivalent task) is defined as the ratio of the metabolic rate associated with a specific activity divided by the resting metabolic rate. MET-hours is defined by the MET score multiplied by the reported hours per week engaged in that activity.
The benefit of physical activity may be independent of other treatment, as suggested in 832 patients with stage III colon cancer receiving fluoropyrimidine-based therapy as part of a randomized adjuvant therapy trial. Patients engaging in at least 18 MET-hours per week of activity enjoyed a statistically significant 47% improvement in disease-free survival compared with inactive patients. Relapse-free and overall survival were also improved with increased physical activity, even after adjusting for other predictors of survival or excluding patients who died within six months of assessment. This benefit was independent of sex, body mass index, age, number of positive nodes, performance status, or type of chemotherapy received (46). The protective effect of physical activity can be seen with as few as 6 MET-hours per week, levels off above 30 MET-hours per week, and may differ based on gender (44–46). Physical activity may therefore provide additional benefit in recurrence and survival outcomes above the benefit seen with adjuvant chemotherapy, although the exact amount and mode of activity needs further investigation.
The Biology Behind Physical Activity: Possible Mechanisms of Action
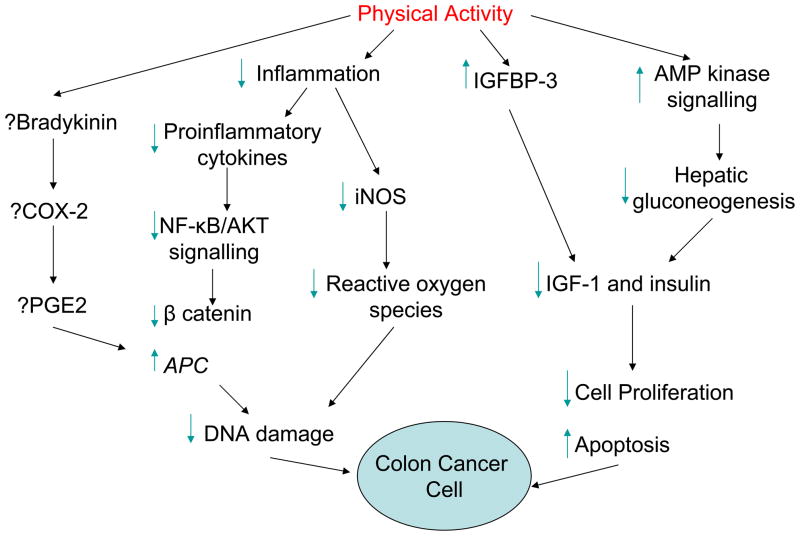
The biologic mechanisms underlying the protective effects of physical activity in patients with colon or rectal cancer are still elusive (Fig. 1; refs. (47–50)). Effects on immune function, oxidative damage, and the insulin axis have been proposed (49, 51–53). Xenograft models of colon cancer and aerobic exercise such as swimming and treadmill running suggest that exercise training exerts anti-inflammatory and anti-proliferative effects on colonic mucosa by attenuating levels of key tumorigenic factors such as cycloxygenase-2, inducible nitric oxide synthase, and tumor necrosis factor α (49, 54, 55). Rodent models of colon carcinogenesis have demonstrated that physical activity with wheel and treadmill running inhibits colon tumor formation after exposure to carcinogenic agents and decreased polyp formation in xenograft models harboring the adenomatous polyposis coli (APC) gene mutation (49). Additional mechanisms may also be involved; i.e., these effects may only attenuate the development of precancerous aberrant crypt foci without affecting tumor development (54, 56). Oxidative DNA damage and inflammatory cytokines may also be affected. Short-term moderate-intensity exercise is associated with lower levels of urinary markers of oxidative damage in colorectal cancer survivors after primary therapy. High-intensity exercise increased these markers, suggesting that oxidative damage can be influenced by physical activity and a threshold of intensity may exist between protective and damaging effects (52). Postoperative moderate-intensity exercise may also augment immunity in colorectal cancer survivors, as demonstrated by declines in interleukin-1 (IL-1) receptor antagonist levels after as few as two weeks of exercise (51).
Fig. 1.
Hypothesized mechanisms of physical activity in colon cancer. Physical activity may block inflammatory pathways, resulting in downregulating nuclear factor kappa B (NF-κB)/AKT signaling, decreased development of oxidative species, and less DNA damage. Physical activity may also affect the insulin-like growth factor (IGF) pathway and AMP kinase signaling, resulting in lower levels of cell proliferation and increased apoptosis. The effect of physical activity on cycloxygenase-2 (COX-2) and prostaglandin E2 (PGE2) levels is unclear (47–50, 54). IGFBP, IGF binding protein; iNOS, inducible nitric oxide synthase; APC, adenomatous polyposis coli.
Alterations in the insulin axis may play a role in the effect of physical activity on the development and recurrence of colon or rectal cancer. Insulin-like growth factor-1 (IGF-1) is an important activator of the phosphoinositide 3-kinase (PI3K)/AKT pathway in multiple cancers and normally complexes with IGF binding protein-3 (IGFBP-3) in the circulation, limiting its bioavailability and tumorigenic potential (57). Levels of IGF-1 are further altered by activation of AMP kinase signaling via inhibition of hepatic gluconeogenesis and IL-6 via alterations in binding protein (IGFBP) expression (47, 49). High levels of pre-diagnosis IGF-1 have been associated with an increased incidence of colorectal cancer in both the Nurses Health Study and Physicians Health Study (58, 59). However, this association was not upheld in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, a large cohort study evaluating relationships between cancer risk and various nutritional and metabolic risk factors. EPIC found no relationship between prediagnosis IGF-1 or IGFBP-3 and colorectal cancer risk in the overall population, although a suggestion of increased colon cancer risk was seen in patients under age 55 with higher baseline IGF-1 levels (60). Meta-analyses also refute this overall association. A meta-analysis of 21 studies by Renehan et al. found no association between colon cancer risk and IGF-1 and Rinaldi et al. reported a relative risk of 1.07 (95% CI 1.01–1.14) based on ten prospective studies evaluating IGF-1 and colorectal cancer risk (60, 61).
Alterations in IGF-1 and IGFBP-3 levels with physical activity have been demonstrated in breast cancer survivors, although the results have been conflicting (62, 63). These alterations have not been extensively evaluated in the colon or rectal cancer survivor population, raising the question of what influence physical activity exerts on this pathway after diagnosis. Haydon et al. reported that IGF-1 levels were not associated with outcome in colorectal cancer survivors in the Melbourne Collaborative Cohort Study. However, physically active survivors with high baseline IGFBP-3 levels did have a reduction in the likelihood of mortality (hazard ratio = 0.52; 95% CI, 0.33–0.83; P = 0.0006) compared with physically active survivors with low levels of IGFBP-3, when adjusted for IGF-1 levels. For cancer-specific survival, the hazard ratio was 0.24 (95% CI, 0.08–0.73) for physically active survivors with the highest compared with the lowest IGFBP-3 levels. Exercisers were approximately half as likely to die of colorectal cancer as non-exercisers, and physical activity appeared to exert the greatest effect in survivors with high IGFBP-3 levels. IGF-1 and IGFBP-3 levels did not affect outcomes in physically inactive survivors (57). These data suggest a link between circulating IGFBP-3 and cancer outcomes, and may define a subset of survivors with high IGFBP-3 levels who will derive the greatest benefit from physical activity interventions. Prediagnosis levels of C-peptide have also been correlated with mortality outcomes in colorectal cancer survivors, with higher levels of C-peptide associated with increased risk of mortality (64). Further study is warranted in evaluating the insulin pathway, immune modulation, and oxidative stress as they relate to cancer-specific outcomes in colon and rectal cancers, second cancers, and other health outcomes. Biomarkers within these pathways may define subpopulations of survivors who may benefit greatest from physical activity and subpopulations for whom additional activity will not modify cancer outcomes.
Barriers and Behaviors: Overcoming Inertia in the Survivor Population
Although colorectal cancer survivors express high interest in exercise intervention studies (65, 66), previous such attempts have demonstrated significant barriers to participation in physical activity interventions, further highlighting the complexity of modifying long-term health behaviors in this population. Cancer survivors report uncertainty about the type of activity they should be performing, do not routinely ask their health care providers for recommendations, and would prefer not to spend additional time at the hospital to participate in physical activity programs (67). In colorectal cancer survivors, barriers to physical activity include the effects of cancer treatment (e.g., stoma, chronic diarrhea, neuropathy), difficulty with sleeping or fatigue, digestion issues, lack of time, work responsibilities, and the belief that they get enough activity on their own (65, 68). Lower education level, lack of choices for activity participation, and increased number of patient-reported barriers also lower the likelihood of physical activity engagement and adherence (28, 68, 69). Adherence may also be affected by a colorectal cancer survivor’s attitude toward adopting exercise as a lifestyle behavior, with those in a precontemplative state (not exercising nor thinking about starting in the near future) less likely than those in the action or maintenance state (exercising regularly within the previous six or more months) to engage in or adhere to physical activity interventions (68, 70).
Health care providers may also provide inadvertent barriers, as many cancer survivors report conflicting messages from their providers regarding their ability to exercise (65). Colorectal cancer survivors may be especially vulnerable, as physician recommendations for exercise are less likely to be made in these survivors (16%) than in adults without cancer (26.9%; P = 0.003), regardless of weight, age, current physical activity level, provider contact, or current health behaviors (71). Furthermore, many providers admit to a lack of knowledge of the benefits of physical activity, do not feel comfortable making physical activity recommendations, and are not convinced that cancer survivors are capable of exercising during treatment or would follow an exercise recommendation (65, 72). Providers also report concern about a lack of time during the office visit to provide physical activity counseling (67).
Colorectal cancer survivors tend to decrease their physical activity after participation in exercise intervention studies (65); given this tendency and the myriad of barriers to adopting a physically active life after colorectal cancer, long-term behavioral change strategies are needed in this population. While a cancer diagnosis by itself is a strong motivator for change, the oncology provider plays an important role in providing recommendations during what may be an important teachable moment (72, 73). Based on the Theory of Planned Behavior and Self-Determination Theory, survivors will engage in a behavior if they perceive that the benefit outweighs the risk or their lack of interest in performing the behavior, that authority figures value and encourage the behavior, and that the behavior is under their control (74, 75). As authority figures, physicians can support these beliefs, as demonstrated in a randomized study finding that 77% of breast cancer survivors receiving a physical activity recommendation from an oncologist recalled the recommendation. Furthermore, the oncologist’s recommendation resulted in perceived approval and support, a belief that exercise is an important behavior, and greater motivation for exercise, although intention did not directly translate into behavior (74). The oncologist’s recurrent endorsement of existing health guidelines and encouragement of survivors to take an active role in health preventive strategies may be the greatest catalyst for change that results in long-term adoption of physical activity behaviors in this population (76).
Learning from Experience: Intervention Strategies to Get Survivors Moving
Randomized controlled trials of exercise interventions have been designed to assess both quality of life and disease-specific outcomes in this population, with implications for future intervention study designs. Courneya et al. evaluated a home-based exercise intervention on quality of life in colorectal cancer survivors. Although no difference was seen between the intervention and usual-care groups in the intent-to-treat analysis, an unplanned analysis suggested an improvement in quality of life in survivors whose fitness level improved with the intervention (77). Further evaluation demonstrated that contamination in the control group and adherence in the experimental group may have affected the study results. Control group participants who were actively exercising continued their exercise pattern despite being assigned to the control arm, and participants in the experimental arm who had not actively thought about changing their exercise pattern were less likely to adhere to the intervention. The use of self-reported activity may also have biased results, as participants may have felt compelled to over-or under-report their activity depending upon their group assignment (70). The LiveWell program, a three-month personalized lifestyle intervention that includes an exercise component, has been shown to be feasible and of high interest to colorectal cancer survivors (65). Interview data from LiveWell participants suggest that the provision of tailored advice responsive to individual circumstances, supportive delivery of the intervention, and the ability to address an individual’s feedback on capabilities and lifestyle in one-to-one interactions enhanced the intervention experience. Participants further felt that starting a physical activity intervention between three and five months after the completion of treatment allowed time to recover from cancer therapy without losing motivation to make lifestyle changes (65).
Results of physical activity interventions in various other cancer populations suggest a preference for home-based interventions with individualized programs and consultation with exercise specialists who can evaluate the proposed physical activity in the context of a survivor’s cancer, medical, and previous fitness history (67). The use of goal setting, print and/or mailed materials, and other behavioral change strategies such as telephone counseling are also preferred methods of physical activity interventions (76, 78). Survivors and their health care providers desire adequate information on the risks and benefits of an intervention and guidance on what is appropriate for a given survivor (67). The provision of choices of physical activities may also increase adherence and participation by responding to a survivor’s level of fitness, comfort, and interest (69). Last, survivors indicate that they wish for lifestyle information early in the course of diagnosis and treatment, although chemotherapy, radiation, and surgical sequelae may hinder initial uptake of exercise behaviors and necessitate reiteration of the importance of physical activity throughout the cancer survivorship journey (68, 76).
Ongoing studies may address some of these barriers and learned lessons while incorporating behavioral change techniques and biologic correlates. The randomized I’m Physically Active After Cancer Treatment (ImPACT) and Colon Health and Life-long Exercise Change (CHALLENGE) trials in colorectal and colon cancer survivors, respectively, will address the impact of structured physical activity interventions on outcomes including cardiopulmonary fitness, biomarkers, fatigue, quality of life, disease-specific outcomes, and cost effectiveness (79, 80). The CanChange study will evaluate the effect of a lifestyle intervention program on cost effectiveness and healthy behaviors including physical activity in colorectal cancer survivors (81). The results of these studies have the potential to help guide clinicians in their recommendations for post-treatment activity and provide a better understanding of the mechanisms behind the preventive effect.
Moving Toward Future Directions for Research
Despite clear recommendations regarding physical activity requirements, further research is needed to answer the multitude of questions surrounding the biology and logistics of physical activity in colorectal cancer survivors. To further elucidate some of the biological mechanisms of physical activity, colon and rectal cancer xenograft models engaged in various MET-hour equivalents of aerobic activities (walking, treadmill running, swimming, etcetera) could be evaluated for changes in the insulin pathway, inflammatory pathways, and development or regression of metastatic disease after tumor implantation. In the clinic, colon and rectal cancer survivor populations need to be clearly defined and investigated separately in order to determine the true effects of physical activity in these potentially distinct populations. Randomized clinical trials of interventions in uniform populations defined by stage of exercise contemplation and baseline exercise behavior, with endpoints of disease-free and overall survival, may be the ideal, most-informative way to assess the effect of physical activity on disease outcomes in the post-adjuvant treatment setting. The most appropriate modalities and the amount of activity needed to prevent colon cancer development or recurrence require definition, and further work regarding the timing of initiation and duration of physical activity is needed. Other needed directions of future research include elucidation of the beneficial impact, if any, of physical activity on rectal cancer incidence and recurrence; evaluation of the benefits of physical activity in survivors treated with contemporary adjuvant therapy regimens including oxaliplatin, including the potential role of physical activity in prevention or treatment of oxaliplatin-induced sensory neuropathy; further elucidation of patient and physician preferences and educational needs, which is imperative to help increase awareness among the public, survivors, and their oncologists; trials evaluating interventions aimed at increasing health care providers’ awareness of the benefits of physical activity and at increasing their encouragement of survivors’ adoption of routine physical activity, with endpoints including patient awareness, quality of life, and disease outcomes; and definition of biomarkers and potential biologically important pathways in a correlative fashion including in further investigations of the inflammatory-response and insulin-pathway alterations associated with physically active and sedentary behavior. Although randomized clinical trials provide the most unbiased data, prospective cohort studies can also provide information regarding the impact of physical activity and educational interventions in these arenas. These studies should define colon and rectal populations separately, account for stage of exercise behavior comtemplation and barriers to participation, and incorporate correlative biomarker, psychosocial, and patient/provider-preference studies to shed further light on these important questions.
Conclusions
Physical activity is an important factor in the prevention of colon cancer in the general population, and has been suggested to be an emerging approach for lowering the risk of recurrence after a colon cancer diagnosis and improving both disease-specific and all-cause prognosis. The benefit in rectal cancer is less clear. Physical activity also may reduce the development of comorbidities and attenuate the decline in function, physical well-being, and (temporarily) quality of life associated with cancer treatment and toxicity. The beneficial effects of physical activity as primary, secondary, and tertiary prevention have prompted multiple national organizations to recommend or endorse exercise in the general population and, more recently, in the cancer survivor population. Health care providers should encourage their colon and rectal cancer survivors to engage in routine physical activity as part of their rehabilitation process. Further investigation of physical activity interventions in colon and rectal cancer survivor populations with correlative studies evaluating biologic mechanisms and impacts on quality of life are warranted to better define subpopulations that might best be served by these interventions.
Acknowledgments
The authors would like to thank Andrea Barsevick and Michael Hall for their insightful comments and suggestions during preparation of the manuscript for this article.
Footnotes
Disclosure of Potential Conflicts of Interest
Crystal Denlinger has no financial interests to disclose relevant to this publication.
Paul Engstrom has no financial interests to disclose relevant to this publication
References
- 1.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C: The National Academies Press; 2006. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Robison LL. Cancer survivorship research: opportunities and future needs for expanding the research base. Cancer Epidemiol Biomarkers Prev. 2008;17:1551–7. doi: 10.1158/1055-9965.EPI-08-0490. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, Kosary CF, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review: 1975–2007. 2010 [cited 2010 September 16]; Available from: http://seer.cancer.gov/statfacts/html/colorect.html.
- 5.Green RJ, Metlay JP, Propert K, Catalano PJ, Macdonald JS, Mayer RJ, et al. Surveillance for Second Primary Colorectal Cancer after Adjuvant Chemotherapy: An Analysis of Intergroup 0089. Ann Intern Med. 2002;136:261–9. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- 6.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J Clin Oncol. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 7.Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23:6126–31. doi: 10.1200/JCO.2005.02.543. [DOI] [PubMed] [Google Scholar]
- 8.Jansen L, Koch L, Brenner H, Ardnt V. Quality of life among long-term (≥ 5 years) colorectal cancer survivors--systematic review. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.06.010. in press. [DOI] [PubMed] [Google Scholar]
- 9.Phipps E, Braitman LE, Stites S, Leighton JC. Quality of life and symptom distribution in long-term colon cancer survivors. Journal of Evaluation in Clinical Practice. 2008;14:254–8. doi: 10.1111/j.1365-2753.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of Illness in Cancer Survivors: Findings From a Population-Based National Sample. Journal of the National Cancer Institute. 2004;96:1322–30. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 11.Trentham-Dietz A, Remington PL, moinpour CM, Hampton JM, Sapp AL, Newcomb PA. Health-related quality of life in female long-term colorectal cancer survivors. The Oncologist. 2003;8:342–9. doi: 10.1634/theoncologist.8-4-342. [DOI] [PubMed] [Google Scholar]
- 12.Brown BW, Brauner C, Minnotte MC. Noncancer Deaths in White Adult Cancer Patients. Journal of the National Cancer Institute. 1993;85:979–87. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–303. [PubMed] [Google Scholar]
- 14.Denlinger CS, Barsevick AM. The Challenges of Colorectal Cancer Survivorship. Journal of the National Comprehensive Cancer Network (JNCCN) 2009;7:883–94. doi: 10.6004/jnccn.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. Quality of Life Among Disease-Free Survivors of Rectal Cancer. J Clin Oncol. 2004;22:354–60. doi: 10.1200/JCO.2004.03.137. [DOI] [PubMed] [Google Scholar]
- 16.Schneider EC, Malin JL, Kahn KL, Ko CY, Adams J, Epstein AM. Surviving colorectal cancer: patient-reported symptoms 4 years after diagnosis. Cancer. 2007;110:2075–82. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]
- 17.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97:1228–34. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 19.NCCN Practice Guidelines in Oncology. Cancer-related fatigue. 2010 [cited 2010 September 22]; Available from: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 20.Brown JK, Byers T, Doyle C, Courneya KS, Demark-Wahnefried W, Kushi LH, et al. Nutrition and Physical Activity During and After Cancer Treatment: An American Cancer Society Guide for Informed Choices. CA Cancer J Clin. 2003;53:268–91. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, GALVÃO DA, Pinto BM, et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Medicine & Science in Sports & Exercise. 2010;42:1409–26. doi: 10.249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008 [cited 2010 September 15]; Available from: http://www.health.gov/paguidelines/
- 23.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 24.Irwin ML, McTiernan A, Manson JE, Thompson C, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women’s Health Initiative. Cancer Prev Res. 2011;4:522–9. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of Home-Based Diet and Exercise on Functional Outcomes Among Older, Overweight Long-term Cancer Survivors: RENEW: A Randomized Controlled Trial. JAMA. 2009;301:1883–91. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Backer IC, Van Breda E, Vreugdenhil A, Nijziel MR, Kester AD, Schep G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncologica. 2007;46:1143–51. doi: 10.1080/02841860701418838. [DOI] [PubMed] [Google Scholar]
- 27.De Backer IC, Vreugdenhil G, Nijziel MR, Kester AD, van Breda E, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: persistent improvement of physical performance and quality of life. Br J Cancer. 2008;99:30–6. doi: 10.1038/sj.bjc.6604433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peddle C, Au H-J, Courneya K. Associations Between Exercise, Quality of Life, and Fatigue in Colorectal Cancer Survivors. Diseases of the Colon & Rectum. 2008;51:1242–8. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 29.Lynch BM, Cerin E, Newman B, Owen N. Physical activity, activity change, and their correlates in a population-based sample of colorectal cancer survivors. Ann Behav Med. 2007;34:135–43. doi: 10.1007/BF02872668. [DOI] [PubMed] [Google Scholar]
- 30.Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer. 2009;115:4001–9. doi: 10.1002/cncr.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedenreich CM. Physical Activity and Cancer Prevention. Cancer Epidemiology Biomarkers & Prevention. 2001;10:287–301. [PubMed] [Google Scholar]
- 33.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. European Journal of Cancer. 2010;46:2593–604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Howard R, Freedman D, Park Y, Hollenbeck A, Schatzkin A, Leitzmann M. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes and Control. 2008;19:939–53. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsen TIL, Romundstad PlR, Petersen H, Gunnell D, Vatten LJ. Recreational Physical Activity and Cancer Risk in Subsites of the Colon (the Nord-Trøndelag Health Study) Cancer Epidemiology Biomarkers & Prevention. 2008;17:183–8. doi: 10.1158/1055-9965.EPI-07-0746. [DOI] [PubMed] [Google Scholar]
- 36.Isomura K, Kono S, Moore MA, Toyomura K, Nagano J, Mizoue T, et al. Physical activity and colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Science. 2006;97:1099–104. doi: 10.1111/j.1349-7006.2006.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. European journal of cancer (Oxford, England : 1990) 2006;42:2590–7. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Cantor KP, Dosemeci M, Lynch CF, Zhu Y, Zheng T. Occupational and Leisure-Time Physical Activity and Risk of Colon Cancer by Subsite. Journal of Occupational and Environmental Medicine. 2006;48:236–43. doi: 10.1097/01.jom.0000199521.72764.26. [DOI] [PubMed] [Google Scholar]
- 39.Larsen I, Grotmol T, Almendingen K, Hoff G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterology. 2006;6:5. doi: 10.1186/1471-230X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coups EJ, Hay J, Ford JS. Awareness of the role of physical activity in colon cancer prevention. Patient Education and Counseling. 2008;72:246–51. doi: 10.1016/j.pec.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedenreich C. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 42.de Vries E, Soerjomataram I, Lemmens VEPP, Coebergh JWW, Barendregt JJ, Oenema A, et al. Lifestyle changes and reduction of colon cancer incidence in Europe: A scenario study of physical activity promotion and weight reduction. European journal of cancer (Oxford, England : 1990) 2010;46:2605–16. doi: 10.1016/j.ejca.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 43.Haydon AMM, MacInnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical Activity and Survival After Colorectal Cancer Diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 45.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical Activity and Male Colorectal Cancer Survival. Arch Intern Med. 2009;169:2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of Physical Activity on Cancer Recurrence and Survival in Patients With Stage III Colon Cancer: Findings From CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 47.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 48.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 49.Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer: a systematic review of potential mechanisms. J Sports Med Phys Fitness. 2003;43:121–38. [PubMed] [Google Scholar]
- 50.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and Colon Cancer. Gastroenterology. 2010;138:2101–14.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 51.Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detection and Prevention. 2004;28:208–13. doi: 10.1016/j.cdp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scandinavian Journal of Gastroenterology. 2008;43:971–8. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- 53.Hursting SD, Berger NA. Energy Balance, Host-Related Factors, and Cancer Progression. Journal of Clinical Oncology. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aoi W, Naito Y, Takagi T, Kokura S, Mizushima K, Takanami Y, et al. Regular exercise reduces colon tumorigenesis associated with suppression of iNOS. Biochemical and Biophysical Research Communications. 2010;399:14–9. doi: 10.1016/j.bbrc.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 55.Demarzo MM, Martins LV, Fernandes CR, Herrero FA, Perez SE, Turatti A, et al. Exercise Reduces Inflammation and Cell Proliferation in Rat Colon Carcinogenesis. Medicine & Science in Sports & Exercise. 2008;40:618–21. doi: 10.1249/MSS.0b013e318163274d. [DOI] [PubMed] [Google Scholar]
- 56.Lunz W, Peluzio MCG, Dias CMGC, Moreira APB, Natali AJ. Long-term aerobic swimming training by rats reduces the number of aberrant crypt foci in 1,2-dimethylhydrazine-induced colon cancer. Brazilian Journal of Medical and Biological Research. 2008;41:1000–4. doi: 10.1590/s0100-879x2008001100009. [DOI] [PubMed] [Google Scholar]
- 57.Haydon AMM, MacInnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–94. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giovannucci EL, Pollack MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–9. [PubMed] [Google Scholar]
- 59.Ma J, Pollack MN, Giovannucci EL, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor-1 and IGF-binding protein-3. Journal of the National Cancer Institute. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 60.Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. International Journal of Cancer. 2010;126:1702–15. doi: 10.1002/ijc.24927. [DOI] [PubMed] [Google Scholar]
- 61.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 62.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of Exercise Training on Fasting Insulin, Insulin Resistance, Insulin-like Growth Factors, and Insulin-like Growth Factor Binding Proteins in Postmenopausal Breast Cancer Survivors. Cancer Epidemiology Biomarkers & Prevention. 2003;12:721–7. [PubMed] [Google Scholar]
- 63.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized Controlled Trial of Aerobic Exercise on Insulin and Insulin-like Growth Factors in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiology Biomarkers & Prevention. 2009;18:306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the Insulin-Like Growth Factor Axis, and Mortality in Patients With Nonmetastatic Colorectal Cancer. Journal of Clinical Oncology. 2009;27:176–85. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson A, Caswell S, Wells M, Steele R, MacAskill S. “It makes you feel so full of life” LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Supportive Care in Cancer. 2010;18:409–15. doi: 10.1007/s00520-009-0677-4. [DOI] [PubMed] [Google Scholar]
- 66.Stull V, Snyder D, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137:243S–8S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 67.Peeters C, Stewart AK, Segal R, Wouterloot E, Scott CG, Aubry T. Evaluation of a cancer exercise program: patient and physician beliefs. Psycho-Oncology. 2009;18:898–902. doi: 10.1002/pon.1406. [DOI] [PubMed] [Google Scholar]
- 68.Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Vallance JKH, et al. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005;29:147–53. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- 69.Carter CL, Onicescu G, Cartmell KB, Sterba KR, Tomsic J, Fox T, et al. Factors associated with cancer survivors’ selection between two group physical activity programs. J Cancer Surviv. 2010;4:388–98. doi: 10.1007/s11764-010-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Fairey AS. Predictors of adherence and contamination in a randomized trial of exercise in colorectal cancer survivors. Psycho-Oncology. 2004;13:857–66. doi: 10.1002/pon.802. [DOI] [PubMed] [Google Scholar]
- 71.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider Counseling About Health Behaviors Among Cancer Survivors in the United States. J Clin Oncol. 2007;25:2100–6. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- 72.Jones LW, Courneya KS, Peddle C, Mackey JR. Oncologists’ opinions towards recommending exercise to patients with cancer: a Canadian national survey. Support Care Cancer. 2005;13:929–37. doi: 10.1007/s00520-005-0805-8. [DOI] [PubMed] [Google Scholar]
- 73.Demark-Wahnefried W, Aziz N, Rowland J, Pinto B. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23:5814–30. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones LW, Courneya KS, Fairey AS, Mackey JR. Does the Theory of Planned Behavior mediate the effects of an oncologist’s recommendation to exercise in newly diagnosed breast cancer survivors? Results from a randomized controlled trial. Health Psychology. 2005;24:189–97. doi: 10.1037/0278-6133.24.2.189. [DOI] [PubMed] [Google Scholar]
- 75.Peddle CJ, Plotnikoff RC, Wild TC, Au HJ, Courneya KS. Medical, demographic, and psychosocial correlates of exercise in colorectal cancer survivors: an application of self-determination theory. Support Care Cancer. 2008;16:9–17. doi: 10.1007/s00520-007-0272-5. [DOI] [PubMed] [Google Scholar]
- 76.Demark-Wahnefried W, Jones L. Promoting a Healthy Lifestyle Among Cancer Survivors. Hematology/Oncology Clinics of North America. 2008;22:319–42. doi: 10.1016/j.hoc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care. 2003;12:347–57. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 78.Knols R, de Bruin E, Shirato K, Uebelhart D, Aaronson N. Physical activity interventions to improve daily walking activity in cancer survivors. BMC Cancer. 2010;10:406. doi: 10.1186/1471-2407-10-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spence R, Heesch K, Eakin E, Brown W. Randomised controlled trial of a supervised exercise rehabilitation program for colorectal cancer survivors immediately after chemotherapy: study protocol. BMC Cancer. 2007;7:154. doi: 10.1186/1471-2407-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change (CHALLENGE) trial (CO.21) 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hawkes A, Pakenham K, Courneya K, Gollschewski S, Baade P, Gordon L, et al. A randomised controlled trial of a tele-based lifestyle intervention for colorectal cancer survivors (‘CanChange’): study protocol. BMC Cancer. 2009;9:286. doi: 10.1186/1471-2407-9-286. [DOI] [PMC free article] [PubMed] [Google Scholar]