Abstract
Synthetic soluble Aβ oligomers are often used as a surrogate for biologic material in a number of model systems. We compared the activity of Aβ oligomers (synthetic and cell culture media derived) on the human SH-SY5Y neuroblastoma and C2C12 mouse myoblast cell lines in a novel, modified MTT assay. Separating oligomers from monomeric peptide by size-exclusion chromatography produced effects at peptide concentrations approaching physiologic levels (10–100 nM). Purified oligomers, but not monomers or fibrils, elicited an increase of a detergent-insoluble form of MTT formazan within two hours as opposed to a control toxin (H2O2). This effect was comparable for biological and synthetic peptide in both cell types. Monomeric Aβ attenuated the effect of soluble oligomers. This study suggests that the activities of biological and synthetic oligomers are indistinguishable during early stages of Aβ oligomer-cell interaction.
Keywords: H4 neuroglioma, C2C12 myoblast, SH-SY5Y neuroblastoma, size exclusion chromatography, ELISA
Introduction
Recent studies have focused on soluble multimeric forms of Aβ as potential etiologic factors of Alzheimer’s disease (AD) initiation and progression. Familial forms of AD involving mutations in the presenilin component of γ-secretase result in the production of an increased proportion of the longer Aβ(x-42) peptide at the expense of Aβ(x-40) [1]. The longer 42-amino acid form of Aβ readily forms soluble oligomeric structures with potent biological activity toward a variety of cell types including neurons [3]. Multiple forms of Aβ(1-42) oligomers can be produced from the synthetic peptide depending on the method of solubilization of the peptide and the conditions of incubation in aqueous media [2, 3, 10]. Size exclusion chromatography of brain tissue from AD cases or APP/PS1 knock-in mice reveals a profile of apparent oligomer sizes of 100 kDa to 600 kDa and a large peak of monomer with essentially no intermediate size Aβ-containing species detectable by immunoassay [17]. A similar size distribution of oligomers is produced following incubation of disaggregated Aβ(1-42) at room temperature for 1–2 hours.
Synthetic Aβ(1-42) is frequently used to prepare oligomers as a surrogate for biologically-derived peptide, as a means to reproducibly generate easily characterized material. Biologically derived Aβ oligomers are heterogeneous, and starting concentrations are low in tissue culture media. However, isolation from human or transgenic mouse brain requires a large investment of effort compared to cell culture. Thus, many studies are carried out with more readily available synthetic peptide. Synthetic oligomers are prepared under a variety of conditions that produce different populations of oligomeric and protofibrillar structures that elicit different responses from cells [3]. Despite this, the equivalence of biological and synthetic oligomers in eliciting cellular responses is rarely demonstrated.
A common assay used to detect cellular effects of Aβ assemblies is the metabolism of the dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) or a derivative. The reduction of the MTT tetrazole dye to the formazan form is believed to measure mitochondrial activity, which is taken as an indicator of cell viability. When exposed to low concentrations of Aβ(1-42) oligomers, many types of cells respond by vacuolation, inhibition of tetrazolium dye reduction activity, and increasing the rate of exocytosis [13]. Aβ oligomers activate P21-activated protein kinases (PAKs) which regulate actin cytoskeleton organization through cofilin phosphorylation and are translocated from the cytosol to membrane vesicles in cultured hippocampal neurons [10], and in the brains of AD cases and in aged Tg2576 mice [15]. Cultured mature oligodendroglia, but not oligodendroglial precursor cells, also increase exocytosis of formazan when incubated with oligomeric Aβ(1-42), and myelination inhibition is reminiscent of white matter degeneration in AD [7]. Hence, enhanced exocytosis is an early step in Aβ oligomer-mediated events, not restricted to neurons, and is disease-relevant.
Hence, while inhibition of formazan production is generally equated to cell death, this is an oversimplification, as a variety of conditions will interfere with the reduction process though the cells remain alive. Two discrete forms of formazan are visible in cells under the microscope: punctate granules (intracellular) and exocytosed membrane-associated elongated needles or plates (extracellular). The relative proportions of these forms depend on cell type and culture conditions. In 2007, Hong et al. [6] determined that these two forms were differentially soluble in Tween-20, and that soluble Aβ assemblies, but not the monomeric peptide, stimulated needle production shortly after exposure of cells to the assemblies. Hence, the initial cellular response to Aβ assemblies results in an increase in formazan production, not the decrease seen after lengthy incubations (24–48 hours) connected with cell death. We have taken advantage of this altered MTT reduction without direct cell death to monitor an early stage in the multistep cellular response to soluble Aβ oligomers.
Materials and Methods
Oligomer Generation
Oligomer preparation from synthetic Aβ(1-42) peptide diluted from DMSO solution was as described [8, 12] and Supplemental Material. Biological Aβ enriched in oligomers was collected by concentrating (10×) serum-free culture media supernatant of human H4 neuroglioma cells (H4 –APP) overexpressing human APP695 on a Microcon 10kDa cut-off centrifugal spin filter (Millipore) before chromatography on Sephadex G75 as for synthetic peptide. Synthetic Aβ(1-42) peptide was purchased from rPeptide (Bogart, GA). Oligomers were formed by dilution to 2μg/ml (443 nM) in 50mM sodium phosphate, 150mM NaCl, pH 7.5, and incubated at room temperature for two hours. After BSA addition (2 mg/ml), oligomers were separated from monomers on a Sephadex G-75 size-exclusion column equilibrated with an appropriate cell culture media containing 2 mg/ml BSA. Aβ(1-42) fibrils were produced by incubating the synthetic peptide at 1 mg/ml unstirred in 50mM sodium phosphate, 150mM NaCl, 0.02% sodium azide, pH 7.5 at 37°C for 5 days.
Quantifying Aβ
Aβ content was determined by sandwich ELISA based on [12] using an N-terminal Aβ specific capture antibody (6E10) and a mid-region Aβ-specific biotinylated detection antibody (bio4G8) and a Biotek Synergy HT plate reader. Sample Aβ content was quantified with reference to a concurrently run standard curve of monomeric Aβ(1–40).
Cell Culture
SH-SY5Y human neuroblastoma cells, C2C12 mouse myoblast cells, and human H4 neuroglioma cells were grown in standard culture media (MEM, DMEM and GlutaMAX -I, respectively; InVitrogen/Gibco) containing 10% FBS and 1% Penicillin/Streptomycin. Media for H4 cells stably transfected with human APP695 [17] was supplemented with 200 μg/ml Hygromycin B.
Partitioning of MTT
Cells were plated in a 96-well tissue culture plate (Greiner bio-one 655180) at 20,000 cells/well in 100 μl of media. The cells recovered at 37°C under 5% CO2 overnight. The next day, Aβ in cell type-specific growth media was pre-warmed to 37°C and added to each well (100 μl) after aspirating the old media. The plate was returned to the incubator for one hour, then 10 μl of 5 mg/ml MTT (Sigma-Aldrich) dissolved in growth media was added (final concentration of 0.5mg/ml) and incubated for an additional hour at 37°C.
Tween-soluble formazan was separated from Tween-insoluble MTT by the addition of 10 μl of 10% v/v Tween-20 in culture media (final concentration 1% v/v). The plate was shaken at 300 rpm at 37°C for 10 minutes to fully solubilize the detergent-soluble fraction of reduced MTT formazan. The media was collected as Tween-soluble MTT (sMTT). The remaining Tween-insoluble MTT (iMTT) was dissolved in 120μl of DMSO. The absorbance of both MTT pools was read with a Biotek plate reader at 594 nm, subtracting the background absorbance at 690 nm. Using the measured optical densities (OD 594 nm – OD 690 nm) of the iMTT and sMTT fractions, the % iMTT was calculated as [iMTT/(iMTT + sMTT)]. For comparison, the %iMTT of wells to which cell media lacking Aβ were added was set equal to 100.
Effects of Oligomeric Aβ in the presence of monomer
Purified synthetic Aβ(1-42) oligomers and monomers were diluted separately in MEM culture media to 30 nM. Concentrations of oligomers were determined by 2-site immunoassay (6E10/bio4G8) compared to a standard curve generated with Aβ(1–40) monomer. Additionally, they were combined to give 30 nM oligomers and 30 nM monomers. SH-SY5Y cells were treated with these preparations for one hour and processed for MTT partitioning.
Results
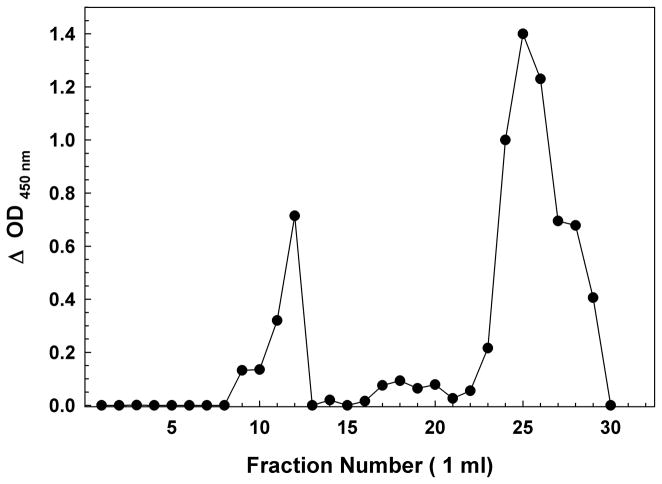
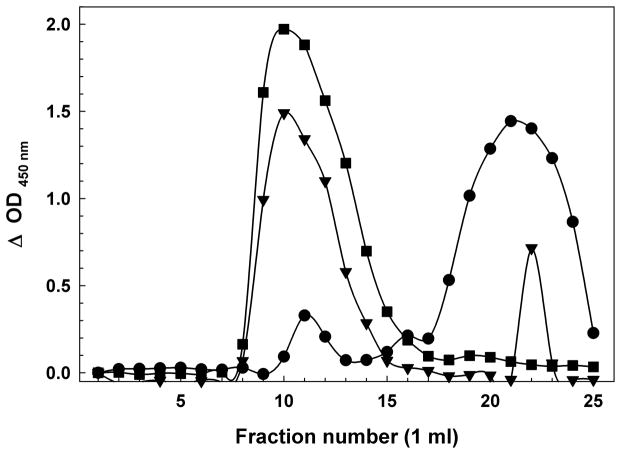
Oligomer preparations were fractionated on Sephadex G75 (exclusion limit ~70 kDa for globular proteins) in order to measure the amount of Aβ in oligomers and to separate them from monomeric peptide. The chromatography also permitted the exchange of the peptides into fresh culture media in the presence of BSA to prevent loss of oligomers on the chromatography resin. This choice of fractionation range combined the high molecular weight oligomers into a single pool. Figure 1A shows a gel filtration profile of synthetic Aβ(1-42) oligomers detected by two-site ELISA. Figure 1B shows profiles for biological Aβ oligomers concentrated from huAPP695-transfected H4 human neuroglioma cell media over three days in serum-free medium. Aβ concentrations are expressed as monomer equivalents. Only the void volume fractions and the included Aβ monomer fractions contained significant quantities of Aβ for both the synthetic peptide and the H4 media. Re-chromatography of purified G75 void volume synthetic or biologic oligomers returned only void volume material with little to no trace of monomer, even if stored at 4 °C for 24 hours, and their size distribution on Sepharose 12 was unaltered, demonstrating their stability in culture media (data not shown).
Figure 1.
(A) Sephadex G75 Size Exclusion Chromatography of a Synthetic Aβ(1-42) Oligomer preparation. The first peak of OD 450 nm (~fractions 9–12) represents the Aβ immunoreactivity of high molecular weight oligomeric Aβ eluting in the void volume, while the second peak (~fraction 23–30) represents primarily monomer and low-m.w. oligomers near the inclusion volume. BSA (66 kDa fr. 13); myoglobin (17 kDa fr. 20). (B) Sephadex G75 Size Exclusion Chromatography of H4 Neuroglioma-derived Aβ Peptides. Similar to the synthetic peptide, the first peak (~fractions 8–15) represents the Aβ immunoreactivity high molecular weight oligomeric Aβ eluting in the void volume, while the second peak (~fraction 18–25) represents primarily monomer and low-m.w. oligomers near the inclusion volume. BSA (66 kDa fr. 13); myoglobin (17 kDa fr. 20). As the H4 cells incubate in serum-free media, the population of Aβ in the media shifts from nearly all monomeric to largely high-m.w. oligomers. Day 1 (circles); Day 2 (inverted triangles); Day 3 (squares).
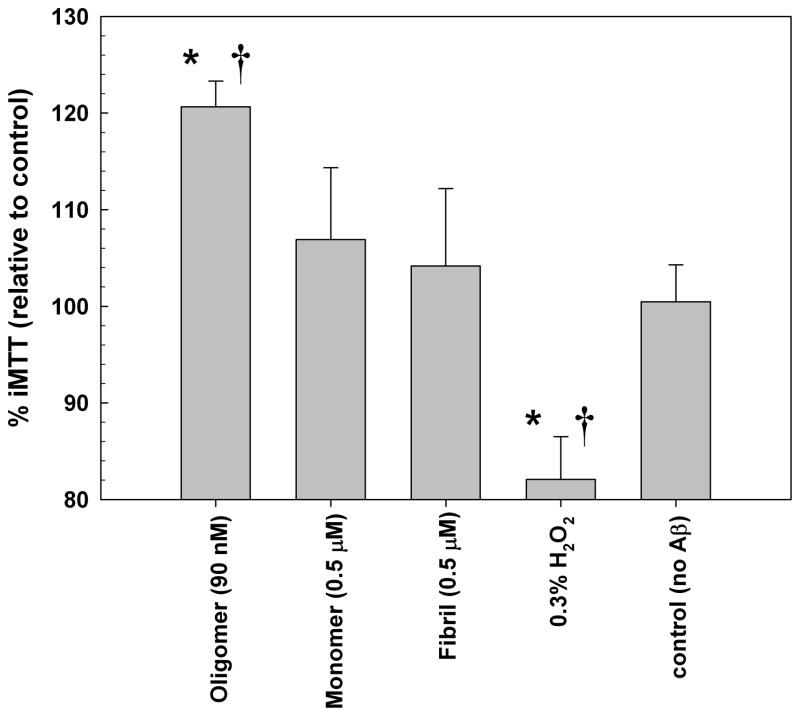
Figure 2A illustrates the distinction between Tween-insoluble needles and soluble granule structures in SH-SY5Y cells; after Tween-20 extraction only the needles remain. Figure 2B compares the effects of different assembly states of Aβ(1-42) on the processing of MTT by SH-SY5Y neuroblastoma cells using differential Tween-20 extraction. Neither 0.5 μM fibrils nor 0.5 μM monomers changed the proportion of iMTT produced compared to control media. In contrast, a significant increase (p<0.001) in iMTT was induced by the addition of a lower concentration (90 nM) of purified synthetic Aβ oligomers, and brief treatment with H2O2 (0.3%, 3 minutes) reduces iMTT in comparison to controls.
Figure 2.
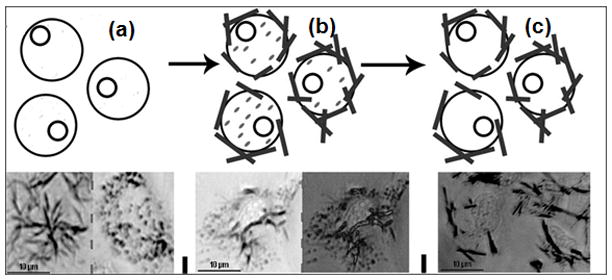
(A) Oligomeric Aβ Increases the Proportion of Tween 20-insoluble MTT Formazan in SH-SY5Y Neuroblastoma Cells. Two types of formazan are deposited: intracellular granules and extracellular needles. Cell surface (left) and intracellular (right) focal plane images are shown. Aβ oligomers induce increased formation of the Tween 20-insoluble needles; addition of Tween 20 solubilizes the granules, leaving behind the needle-like formazan. The supernatant is collected and the remaining formazan solubilized in DMSO. Thick lines = needles; dashes = granules. (a) untreated cells metabolizing MTT ; (b) cells treated with Aβ oligomers metabolizing MTT; (c) cells treated with Aβ oligomers metabolizing MTT after extraction with Tween 20. (B) Effect of Oligomeric, Fibrillar, and Monomeric Synthetic Aβ(1-42) Peptides on MTT Formazan Exocytosis from SH-SY5Y Neuroblastoma Cells. Oligomeric Aβ (90 nM) yields a distinct increase in the proportion of detergent insoluble MTT (iMTT) over normal baseline production (=100%). No detectable change from control proportions was seen with high concentrations (0.5 μM) of monomeric or fibrillar Aβ. Furthermore, the alteration by oligomeric Aβ is in a direction opposite of acute toxins such as hydrogen peroxide. Each bar represents ten replicates. Asterisk denotes significance from control (p<0.001) and dagger indicates significance from monomer/fibril (p<0.001).
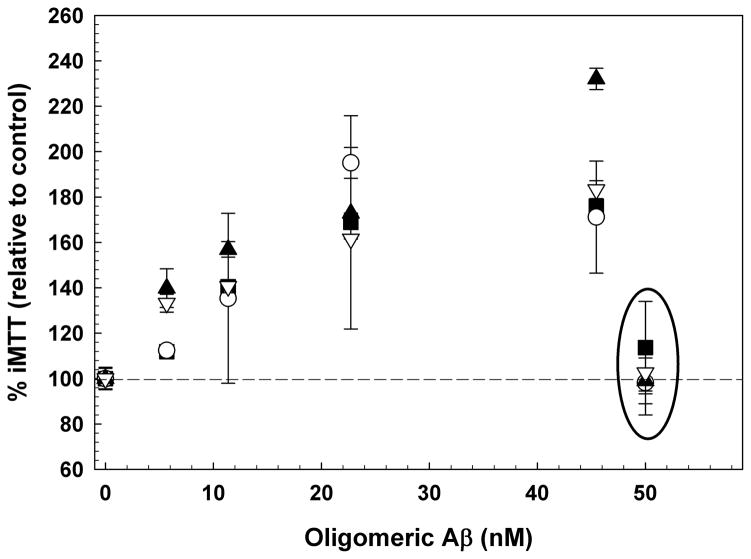
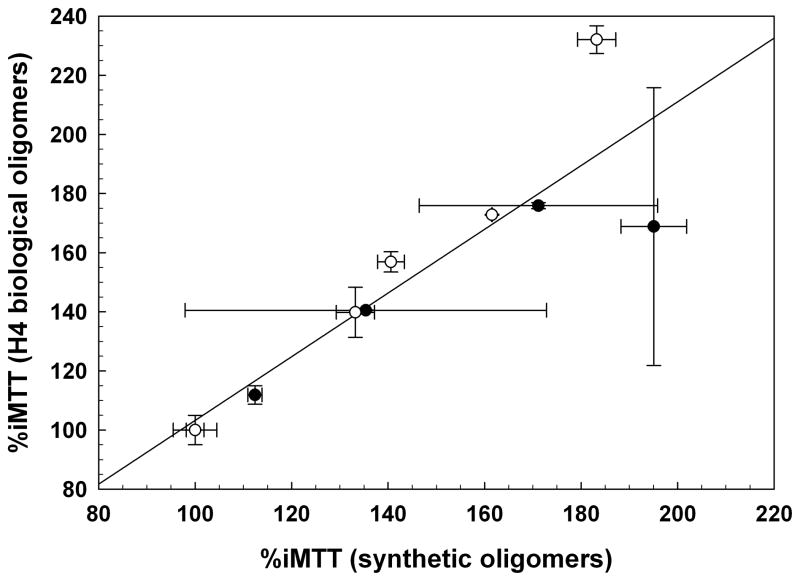
The effect of oligomers relative to monomer or fibrils is dose-dependent and comparable in both SH-SY5Y and C2C12 cells (Figure 3A). Fifty nM of either synthetic or biological monomer had no effect on iMTT for either cell type (circled). There was no statistically significant difference in potency between synthetic and H4-produced (biological) oligomers. Similarly fractionated non-transfected H4 cell-conditioned media had no effect on MTT metabolism. In SH-SY5Y cells, the EC50 for synthetic oligomers was 12.19 nM ± 0.969 nM, and 13.43 nM ± 0.725 nM for biologically derived oligomers. Similarly, in C2C12 cells the comparable EC50 values were 15.75 nM ± 0.838 nM (synthetic) and 19.22 nM ± 0.932 nM (biological). The dose-responses of synthetic and biological oligomers are positively correlated (Figure 3B). Therefore, biological and synthetic Aβ oligomers are indistinguishable as monitored by this method.
Figure 3.
(A) Dose-dependent increase of iMTT in response to oligomeric Aβ, but not monomer (50 nM; circled); N=3. SH-SY5Y cells with biological oligomers (closed squares); SH-SY5Y cells with synthetic oligomers (open circles); C2C12 cells with biological oligomers (closed triangle); C1C12 cells with synthetic oligomers (open inverted triangles). (B) Data from the dose-response experiment in (A) plotted as a correlation between the responses elicited from synthetic Aβ oligomers versus biological Aβ oligomers. The diagonal line indicates a linear fit to all of the data on the plot (slope = 1.07; r2 = 0.795). SH-SY5Y (closed circles); C2C12 (open circles). Biologically derived and synthetic oligomeric Aβ elicit essentially the same effects.
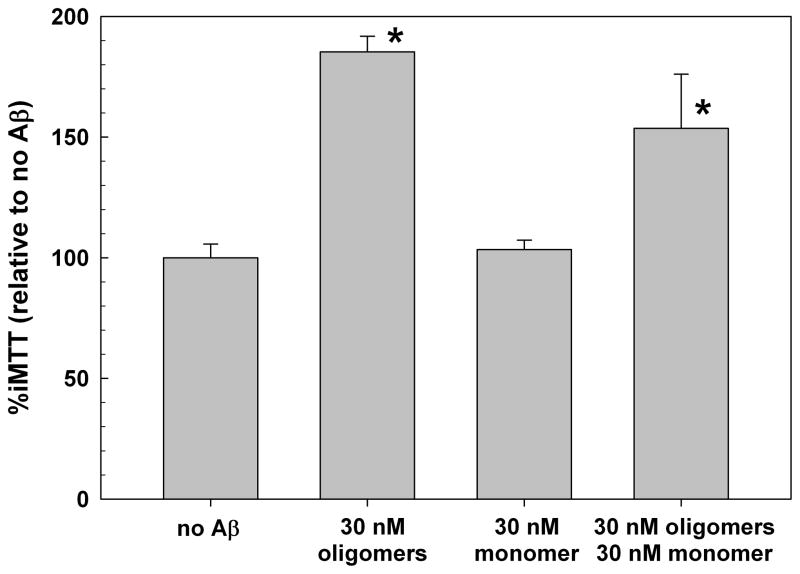
While oligomeric Aβ clearly has long term toxic effects on cells, the role of monomer in normal and pathobiological situations is less clear. There are reports of neuroprotective effects of the monomeric peptide in different situations [5]. Since in most studies with synthetic oligomers, as well as physiologically, Aβ is present as mixtures of oligomers and monomers, we took advantage of the availability of separate pools of purified oligomers and monomeric Aβ(1-42) to test whether monomer could exert protective effects against oligomer-induced exocytosis of MTT needles. The presence of an equal concentration of monomer partially mitigates the effect of a non-saturating dose of oligomers (Figure 4). While the difference between these two groups did not achieve statistical significance given the limited number of replicates (N=3), the trend suggests oligomer effects may be underestimated in the presence of monomeric peptide.
Figure 4.
Monomeric Aβ(1-42) Modulates Oligomer-induced iMTT Exocytosis in SH-SY5Y cells. The addition of 30nM monomeric Aβ to 30 nM oligomeric Aβ attenuates the ability of Aβ oligomers to increase iMTT. * = p<0.05 compared to no Aβ.
Discussion
We sought an assay that reflected oligomer effects at early times after exposure to low oligomer concentrations that also gave rise to a positive signal, rather than an inhibition which is a generic terminal response less informative of cellular events. The exocytosis of reduced formazan produced by reduction of the MTT tetrazolium dye by cellular enzymes [14] and the differential effects of Aβ oligomers on formazan deposits elaborated by Hong et al. [6] provide a rapid and sensitive marker of oligomer activity in a variety of cell types. The ratio of Tween-insoluble formazan to Tween-soluble formazan gives an indication of oligomer activity and a one hour treatment with nM concentrations of chromatographically purified oligomers is sufficient to elicit a maximal response.
The conditions for measurement of the biological activity of Aβ oligomers were chosen to reflect concentrations of the peptide approaching the nM and lower concentrations believed to be present in the AD brain [4]. At nM concentrations, Aβ oligomers bind to dendrites of some neurons in primary culture [9]. Binding is reduced by brief mild proteolysis of the cell surface [10], a hallmark of interaction with a receptor(s). In many studies, however, biological effects of Aβ oligomers are assessed on cultured cells at middle to high micromolar concentrations and following lengthy exposure times, often 24–48 hours, using cell death as an endpoint. Aβ oligomers are surface active materials and long incubations at high concentrations can lead to non-specific perturbation of the lipid bilayer.
Soluble synthetic or H4 neuroglioma cell culture-derived Aβ oligomers can be readily separated from monomer by chromatography on Sephadex G75. The lack of a consistent or significant concentration of intermediate size Aβ-containing structures (15–50 kDa) (also not observed on Sepharose 12 chromatography) suggests either that oligomer shape alters their apparent molecular weight on size exclusion chromatography, or that these structures are not stable on the time scale of the separation (20–30 minutes). The separated oligomers, by contrast, are stable for at least 24 hours at 4 °C.
Monomeric Aβ(1-42) peptide is believed to be nontoxic, and does not alter MTT metabolism [6, 10, 19], and fibrillar peptide is only weakly toxic. Neither of these forms of Aβ increased iMTT above controls. Synthetic and H4 neuroglioma-derived oligomers indistinguishably, potently, and dose dependently increase the proportion of iMTT on the surface of SH-SY5Y neuroblastoma cells and C2C12 myoblasts. This equivalence indicates that for this cellular measure of oligomer activity that the synthetic and biological oligomers are comparable. Recent studies with primary rat neuronal cultures report similar effects with synthetic and biological Aβ oligomers [20]. The potency of purified oligomers and the lack of effect of purified monomer on iMTT production suggested the possibility that the presence of monomeric Aβ could attenuate the effects of oligomers on cellular systems. Monomer-free Aβ oligomers are considerably more potent (EC50 10 – 20 nM, based on Aβ monomer content) on early stages of cellular activity measured by MTT exocytosis in two cell types than is generally reported with monomer-oligomer mixtures. Addition of monomeric Aβ(1-42) to fractionated Aβ oligomers reduced the efficacy of the oligomers by a mechanism that remains to be explored.
The observed similarities between the cellular effects of synthetic Aβ oligomers and H4-produced oligomers are a significant finding. Whether the similarity extends to oligomers produced in different ways or isolated from different sources should be validated. The mass and size distribution heterogeneity of synthetic Aβ oligomers depends on the conditions under which they are formed. Their resistance to dissociation by denaturants such as urea and SDS also reveal differences among the preparations in kinetic stability [16], which reflects underlying structural characteristics in protein structure. Increased stability is particularly evident in oligomers from biological sources such as those that accumulate in APP transgenic mouse models of Aβ pathology [11, 18] and SDS-stable dimeric species postulated to be unique to AD [19]. As the cellular responses elicited by soluble Aβ oligomers are probed in more detail it is likely that the more subtle structural details of both synthetic and biological oligomers will impact aspects of cellular biology differently. Not all forms of soluble oligomers, either synthetic or biological, may be equivalent physiologically, which would have consequences for biomarker and therapeutic strategies for Alzheimer’s disease.
Supplementary Material
Research Highlights
Purified soluble Aβ oligomers rapidly induce cellular MTT formazan exocytosis.
Synthetic Aβ(1-42) and cell-derived oligomers equipotently stimulate exocytosis .
Half-maximal stimulation of exocytosis induced by10-20 nM oligomers.
Monomeric Aβ attenuates the effect of soluble oligomers.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. Susan Catalano of Cognition Therapeutics (Pittsburgh, PA) for studies with primary rat neuronal cultures. We also acknowledge the support of NIH/NIA grants NS058382 (M. P. M., A. M. W.), AG005119 (M. P. M., H. L.) and the Alzheimer’s Association IIRG-06-27275 (A. M. W., H. L.). M. H. was supported by an NSF REU Site grant (DBI-0648233).
ABBREVIATIONS
- APP
amyloid precursor protein
- ELISA
enzyme-linked immunoassay
- FBS
fetal bovine serum
- HFIP
1,1,1,3,3,3-hexafluoroisopropanol
- HRP
horseradish peroxidase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam M. Weidner, Email: Weidner.Adam@gmail.com.
Molly Housley, Email: Molly.Housley@uky.edu.
M. Paul Murphy, Email: mpmurp3@email.uky.edu.
References
- 1.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 2.Cizas P, Budvytyte R, Morkuniene R, Moldovan R, Broccio M, Losche M, Niaura G, Valincius G, Borutaite V. Size-dependent neurotoxicity of beta-amyloid oligomers. Arch Biochem Biophys. 2010;496:84–92. doi: 10.1016/j.abb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georganopoulou DG. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc Natl Acad Sci U S A. 2005;102:1173–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong HS, Maezawa I, Yao N, Xu B, Diaz-Avalos R, Rana S, Hua DH, Cheng RH, Lam KS, Jin LW. Combining the rapid MTT formazan exocytosis assay and the MC65 protection assay led to the discovery of carbazole analogs as small molecule inhibitors of Abeta oligomer-induced cytotoxicity. Brain Res. 2007;1130:223–234. doi: 10.1016/j.brainres.2006.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiuchi M, Maezawa I, Itoh A, Wakayama K, Jin LW, Itoh T, Decarli C. Amyloid beta1-42 oligomer inhibits myelin sheet formation in vitro. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.05.007. e-published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein WL. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 12.LeVine H., 3rd Alzheimer's beta-peptide oligomer formation at physiologic concentrations. Anal Biochem. 2004;335:81–90. doi: 10.1016/j.ab.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Liu ML, Hong ST. Early phase of amyloid beta42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp Mol Med. 2005;37:559–566. doi: 10.1038/emm.2005.69. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Peterson DA, Schubert D. Amyloid beta peptide alters intracellular vesicle trafficking and cholesterol homeostasis. Proc Natl Acad Sci U S A. 1998;95:13266–13271. doi: 10.1073/pnas.95.22.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008;283:14132–14143. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning M, Colon W. Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward beta-sheet structure. Biochemistry. 2004;43:11248–11254. doi: 10.1021/bi0491898. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Shankar GM, Leissring MA, Adame A, Sun X, Spooner E, Masliah E, Selkoe DJ, Lemere CA, Walsh DM. Biochemical and immunohistochemical analysis of an Alzheimer's disease mouse model reveals the presence of multiple cerebral Abeta assembly forms throughout life. Neurobiol Dis. 2009;36:293–302. doi: 10.1016/j.nbd.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catalano SM, Ravenscroft J, Rehak C, Yurko R, Isso N, Hafferstein H, Rishton G, Staniszewski A, Arancio O. International Conference on Alzheimer's Disease, Abstract #; July 10 – July 15, 2010; Honolulu ,Hawaii. 2010. pp. 3–499. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.