Abstract
Background
PDE5 inhibition has been shown to exert profound beneficial effects in the failing heart, suggesting a significant role for PDE5 in the development of congestive heart failure (CHF). The purpose of this study is to test the hypothesis that oxidative stress causes increased PDE5 expression in cardiac myocytes and the increased PDE5 contributes to the development of CHF.
Methods and Results
Myocardial PDE5 expression and cellular distribution were determined in left ventricular (LV) samples from patients with end stage CHF and normal donors, and from mice after transverse aortic constriction (TAC)-induced CHF. Compared to donor human hearts, myocardial PDE5 protein was increased ~4.5 fold in CHF samples, and the increase of myocardial PDE5 expression was significantly correlated with myocardial oxidative stress markers 3’-nitrotyrosine or 4-hydroxynonenal expression (p<0.05). Histological examination demonstrated that PDE5 was mainly expressed in vascular smooth muscle in normal donor hearts, but its expression was increased in both cardiac myocytes and vascular smooth muscle of CHF hearts. Myocardial PDE5 protein content and activity also increased in mice after TAC-induced CHF (p<0.05). When the SOD mimetic M40401 was administered to attenuate oxidative stress, the increased PDE5 protein and activity caused by TAC was blunted, and the hearts were protected against LV hypertrophy and CHF. Conversely, increased myocardial oxidative stress in SOD3 KO mice caused a greater increase of PDE5 expression and CHF after TAC. In addition, administration of sildenafil to inhibit PDE5 attenuated TAC-induced myocardial oxidative stress, PDE5 expression and CHF.
Conclusions
Myocardial oxidative stress increases PDE5 expression in the failing heart. Reducing oxidative stress by treatment with M40401 attenuated cardiomyocyte PDE5 expression. This and selective inhibition of PDE5 protected the heart against pressure overload induced LV hypertrophy and CHF.
Keywords: heart failure, oxidative stress, PDE5
Introduction
Congestive heart failure (CHF) is the leading cause of mortality in developed countries and continues to increase in prevalence. Phosphodiesterase type 5 (PDE 5) selectively hydrolyzes cGMP, and selective inhibition of PDE5 can increase cGMP bioavailability. It is generally believed that PDE5 is not present in normal cardiac myocytes, so that selective PDE5 inhibition has no direct inotropic effect in normal hearts1. However, recent work by Kass and associates demonstrated that selective inhibition of PDE5 with sildenafil markedly attenuated the LV hypertrophy and dysfunction produced by chronic pressure overload secondary to transverse aortic constriction (TAC) in mice 2. Thus, PDE5 inhibition has also been reported to attenuate myocardial infarct-induced LV remodeling3, and LV hypertrophy produced by chronic isoproterenol infusion in rats 4. In addition, PDE5 expression was increased in hypertrophied human right ventricles and left ventricles from humans with heart failure5,6, and over-expression of PDE5 in cardiac myocytes exacerbated myocardial infarction induced LV remodeling in mice6. However, the regulation of PDE5 expression in cardiac myocytes of the failing heart is not clear.
In this study, we provide evidence that PDE5 protein content is increased in cardiac myocytes in heart failure samples from human or mouse, and that expression of PDE5 in cardiac myocytes in the failing heart is regulated by myocardial oxidative stress. We find that the increase of myocardial PDE5 expression in human and mouse failing hearts was associated with myocardial 3’-nitrotyrosine and 4-hydroxynonenal (4-HNE) levels, (markers of oxidative stress). Importantly, attenuation of oxidative stress with the SOD mimetic M40401 decreased TAC induced PDE5 expression, ventricular hypertrophy, LV dysfunction and pulmonary congestion. Conversely, increasing myocardial oxidative stress by deletion of the SOD3 gene in mice increased myocardial PDE5 expression and exacerbated TAC-induced LV hypertrophy and heart failure. In addition, selective inhibition of PDE5 with sildenafil attenuated the TAC-induced LV oxidative stress, hypertrophy and dysfunction. Together, our study provides the first direct evidence that oxidative stress regulates PDE5 expression in cardiac myocytes.
Materials and methods
The experimental studies in mice and human tissues were approved by the Institutional Animal Care and Use Committee, and the Institute Review Board at the University of Minnesota, respectively.
Transverse Aortic Constriction (TAC) procedure and experimental treatments
Male C57BL/6 mice (Taconic, Germantown, NY) ~2 months old were used for the TAC procedure. TAC was performed using a 27G needle to calibrate the degree of aortic constriction as previously described 7,8. For the M40401 study, immediately after recovery from anesthesia following TAC, the mice were randomly assigned to two groups treated either with M40401 (10mg/kg/day, i.p.) or vehicle for 2 weeks. For the study in SOD3 KO mice, PDE5 expression and TAC-induced ventricular remodeling were determined in SOD3 KO mice and control wild type mice. For the sildenafil study, after TAC mice were randomly assigned to two groups, one group treated with sildenafil (50mg/kg twice a day for two weeks) by gavage and the other treated with vehicle.
Echocardiography
Mouse echocardiographic images were obtained with a Visualsonics high resolution Veve 660 or 770 system as previously described 8, 9.
Western blots, chemical and histological analysis
The detailed methods for sample collection, western blot, immunostaining, PDE5 and PKG activity measurement, cell culture and treatment are included in the online supplementary data, please see http://circ.ahajounals.org.
Data Analysis
Data in this study was first analyzed to determine if data was normally distributed using normality test (Kolmogorov-Smirnov) provided by Sigmastat. If data was normally distributed, the data was presented as mean ± standard error. If the data was not normally distributed, the data was presented as median (± standard error).
Data of two groups was compared with unpaired t-test
For the effect of M40401 or sildenafil on TAC-induced LV remodeling, one-way analysis of variance was used to test for differences among treatment groups followed by pairwise multiple comparisons with Fisher’s LSD method. If the data was not normally distributed, rank-based one-way analysis of variance was performed to test for differences among treatment groups followed by pairwise multiple comparisons with Dunn’s method. Two-way analysis of variance was used to test for differences between iNOS KO and wild type mice under control conditions and after TAC. In addition, two-way analysis of variance was also used to test for differences between SOD3 KO and wild type mice under control conditions and after TAC. For two-way analysis of variance, if analysis of variance demonstrated a significant effect, all pairwise multiple comparisons were made with Turkey’s method under the interaction term. Statistical significance was defined as p< 0.05.
Results
Increased PDE5 expression in human heart failure samples is correlated to myocardial oxidative stress
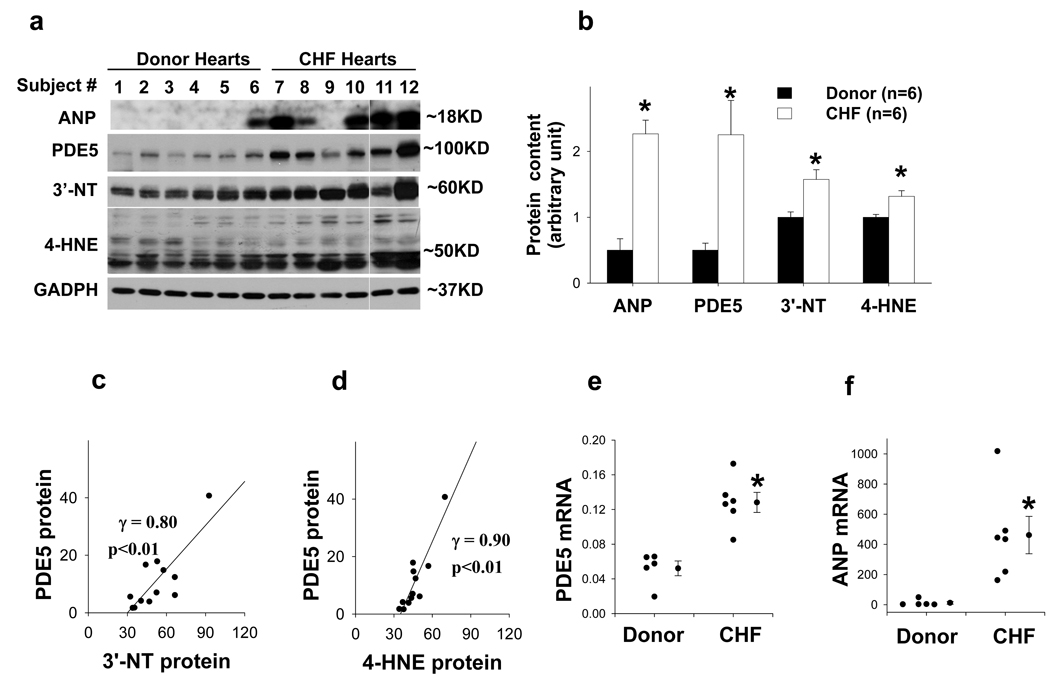
We studied LV PDE5 expression in 6 patients with CHF and 6 normal donor hearts (Table S1). PDE5 expression was increased in all CHF hearts, with the mean signal intensity increased over 4.5 fold as compared with the donor hearts (Figure 1a–b). Myocardial ANP protein, a marker associated with ventricular hypertrophy and heart failure, was also significantly increased in CHF hearts (Figure 1a–b). As previous studies indicate that oxidative stress can induce PDE5 expression in smooth muscle cells, levels of 3’-nitrotyrosine and 4-HNE were determined in the myocardial samples. Both 3’-nitrotyrosine and 4-HNE were significantly increased in the human CHF samples (Figure 1a–b). The increase of myocardial PDE5 was significantly correlated to the increase of 3’-nitrotyrosine and 4-HNE (Figure 1c–d).
Figure 1.
Increased PDE5 expression in human heart failure LV samples is correlated to the increases of ventricular oxidative stress markers 3’-nitrotyrosine (3’-NT) and 4-HNE. LV ANP, PDE5, 3’-nitrotyrosine and 4-HNE were increased in the congestive heart failure (CHF) samples (a, b), and the increase of ventricular PDE5 was significantly correlated to the increase of ventricular 3’-nitrotyrosine and 4-HNE (c, d). LV PDE5 and ANP mRNA contents were increased in CHF samples (e,f). *p<0.05 compared to normal donor hearts.
LV samples from 5 donor hearts and 5 failing hearts used for the western blots were also subjected to histological staining for PDE5 expression. Consistent with previous reports in normal human heart tissue, we found PDE5 predominantly expressed in vascular smooth muscle cells, with minimal expression in cardiac myocytes (Figure S1). Interestingly, in the samples from failing hearts, PDE5 was expressed in both cardiac myocytes and vascular smooth muscle cells.
Real time quantitative PCR demonstrated that PDE5 mRNA was significantly increased 2.4 fold in the human heart failure samples (Figure 1e) and ANP mRNA was increased 42 fold in the human heart failure samples(Figure 1f).
SOD mimetic M40401 attenuated myocardial oxidative stress and PDE5 expression in pressure overloaded hearts
Since PDE5 expression in the human heart samples was significantly correlated with 3’-nitrotyrosine and 4-HNE content, we speculated that pressure overload might increase both ventricular oxidative stress and PDE5 expression, and that decreasing the oxidative stress might attenuate PDE5 expression and LV dysfunction. To test this hypothesis, wild type mice were subjected to severe pressure overload using the TAC procedure and immediately randomized to treatment either with the SOD mimetic M40401 or vehicle. Ventricular structure and function were determined two weeks after TAC.
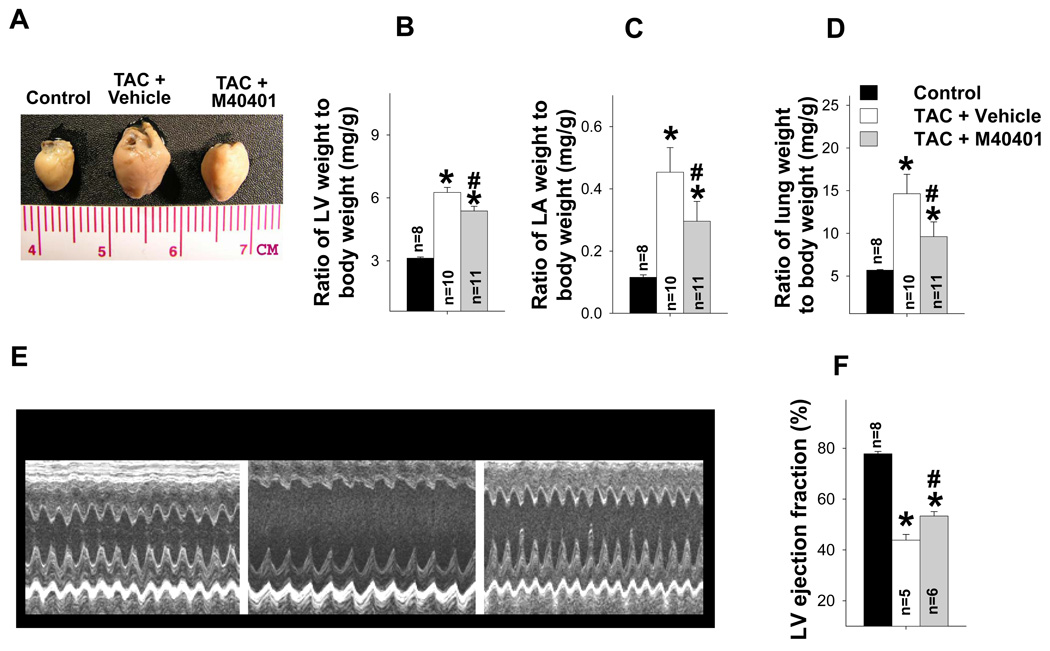
The increase in LV systolic pressure produced by TAC resulted in LV hypertrophy and systolic dysfunction. The LV hypertrophy and systolic dysfunction causes an increase in LV diastolic pressure that later results in left atrial (LA) hypertrophy, and ultimately pulmonary congestion. Therefore, TAC-induced cardiac remodeling can be evaluated by the progressive development of LV and LA hypertrophy, and increased lung weight.
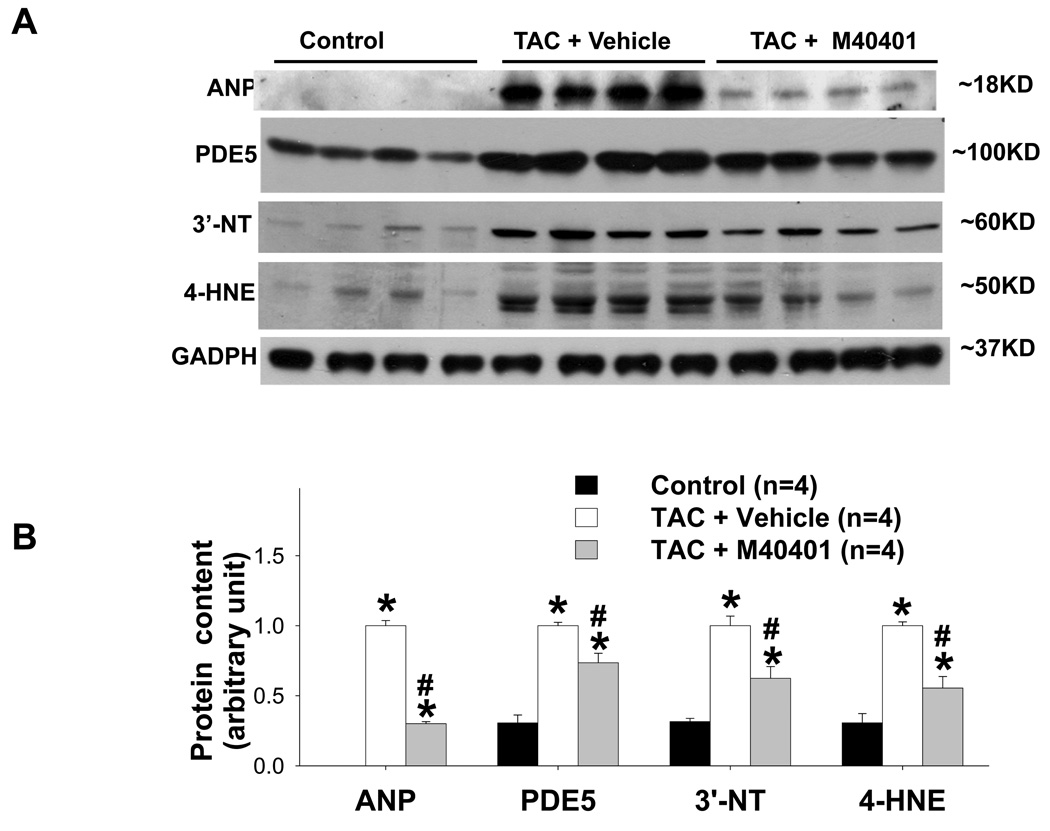
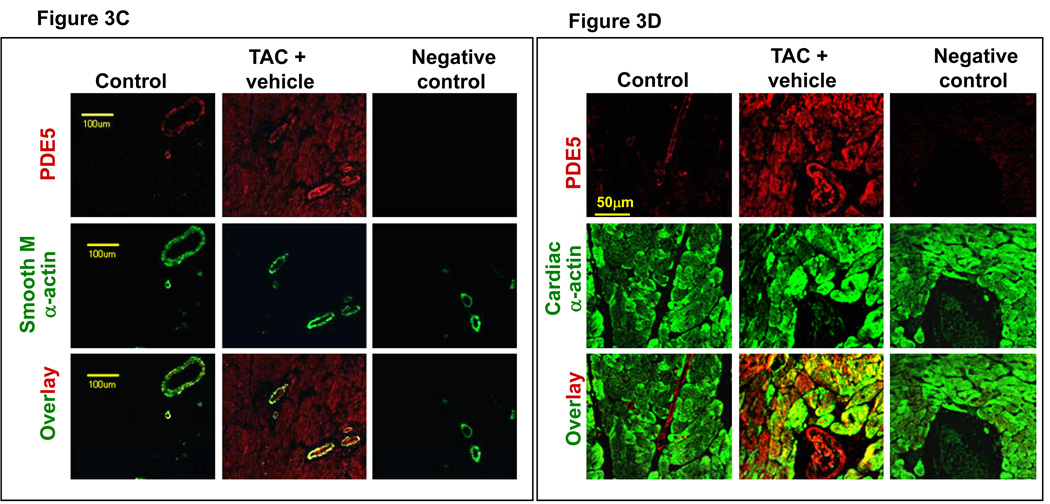
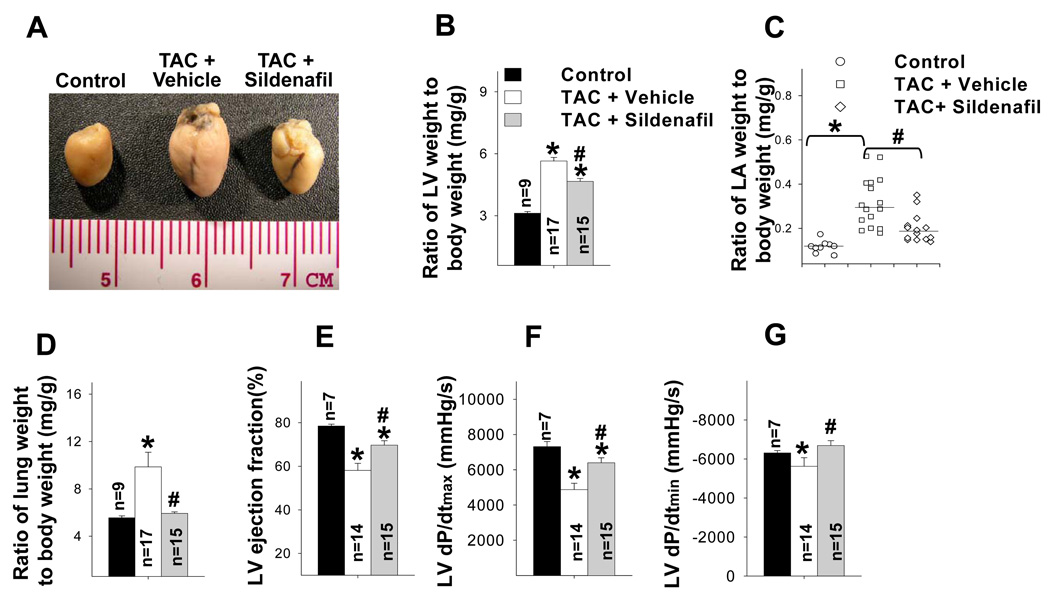
Two weeks after TAC the ratio of LV weight to body weight was increased by 81%, the ratio of LA weight to body weight increased 197%, and the ratio of lung weight to body weight increased 77% (Figure 2a–b). TAC also caused significant increases of tissue weight of LV, LA and lung, and their ratio to tibial length (Table S2). TAC also caused a significant decrease of LV ejection fraction and an increase of LV end systolic diameter (Figure 2c–d, Table S3). These changes were associated with significant increases of ventricular ANP, PDE5, and the oxidative stress markers, 3’-nitrotyrosine and 4HNE (Figure 3a–b). Histological staining demonstrated that TAC caused increased PDE5 protein expression in cardiac myocytes and vascular smooth muscle (Figure 3c). Scavenging superoxide with the SOD mimetic, M40401, significantly attenuated the TAC-induced increases of LV, LA and lung weight (Table S2), and their ratio to bodyweight or tibial length (Figure 2a–b, Table S2). M40401 also significantly attenuated the TAC-induced decrease of LV ejection fraction (Figure 2c–d) and the increase of the LV end systolic diameter (Table S3). M40401 significantly attenuated the TAC-induced increase of ventricular ANP, PDE5, and the oxidative stress markers 3’-nitrotyrosine and 4-HNE (Figure 3a,b).
Figure 2.
SOD mimetic M40401 significantly attenuated TAC-induced LV hypertrophy (a,b), left atrial hypertrophy (c), pulmonary congestion (d), and the decrease of LV ejection fraction (e,f). *p<0.05 compared to control group; #p<0.05 compared to TAC vehicle treatment.
Figure 3.
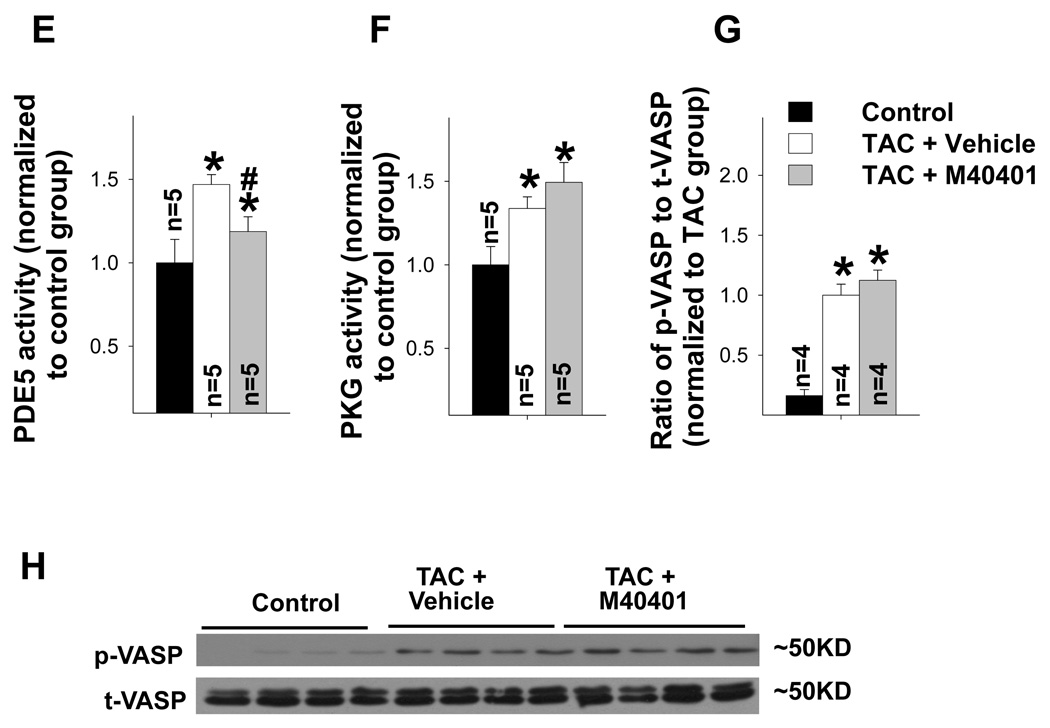
SOD mimetic M40401 significantly attenuated the TAC-induced increase in LV ANP, PDE5, 3’-nitrotyrosine (3’-NT) and 4-HNE (a, b). TAC caused increased PDE5 expression in cardiac myocytes (c,d), increased PDE5 activity (e), increased PKG activity (f) and expression of p-VASP (g,h). M40401 had no effect on TAC-induced increase of ventricular PKG activity (f) and the expression of p-VASP (g,h). *p<0.05 compared to control group; #p<0.05 compared to TAC group treated with vehicle.
Real time PCR showed that myocardial PDE5 mRNA was moderately increased as compared with the sham control group (p<0.05) in the animals exposed to TAC, and that TAC-induced increase of PDE5 was not statistically different after M40401 treatment (Figure S2). In addition, the overall increase of PDE5 mRNA in isolated cardiac myocytes from heart failure mice is similar to its increase in the myocardial tissues (Figure S2).
M40401 had no significant effect on PKG activity and phosphorylation of vasodilator-stimulated protein (VASP)
TAC caused a significant increase of myocardial PKG activity (Figure 3f), while SOD mimetic M40401 caused no significant further increase of myocardial PKG activity (Figure 3f).
cGMP causes PKG phosphorylation, and phosphorylated PKG induces phosphorylation of VASP at site of Ser239. Therefore p-VASPSer239 is often used as an indicator of PKG activity. We found that severe TAC caused a significant increase of p-VASPSer239 expression (Figure 3g–h), which is in agreement with previous report from Dr. Kass et al10 in the same model. M40401 also had no significant effect on TAC-induced increase of p-VASPSer239 expression (Figure 3g–h), which is consistent with myocardial PKG activity in these mice. These data suggest that PDE5 expression is not affected by PKG activity.
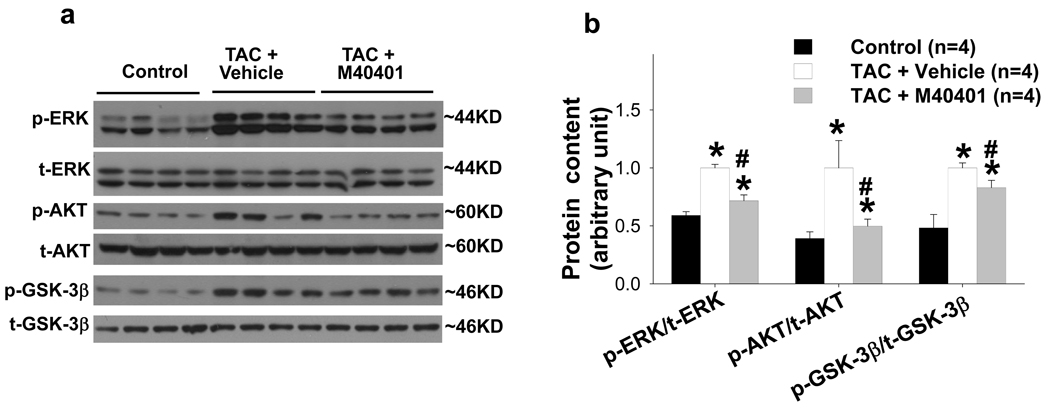
M40401 attenuated TAC-induced phosphorylation of ERK and Akt
TAC-induced LV oxidative stress, hypertrophy and heart failure are often associated with increased activation of ERK, Akt and GSK-3β. We found that chronic TAC caused significant increase of p-ERKThr202/204, p-AktSer473 and p-GSK-3βSer21/9, but not their total protein contents (Figure 4). M40401 significantly attenuated TAC-induced increase of p-ERKThr202/204, p-AktSer473 and p-GSK-3βSer21/9. These results are consistent with the decreased LV oxidative stress after M40401.
Figure 4.
SOD mimetic M40401 significantly attenuated the TAC-induced increases of p-ERK, p-AKT, p-GSK-3β (a), and their ratio to the total protein content (b). *p<0.05 compared to control group; #p<0.05 compared to TAC group treated with vehicle.
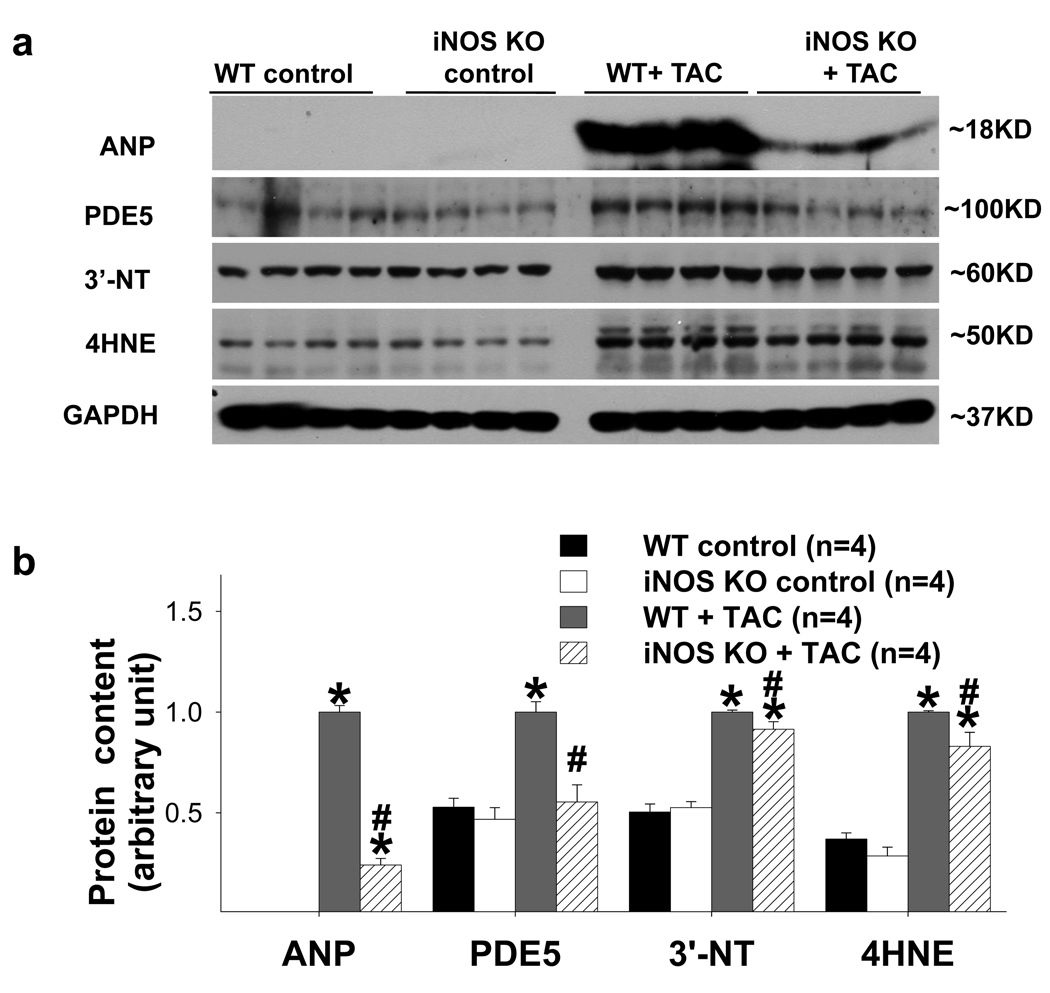
iNOS gene deletion (iNOS−/−) attenuated TAC-induced myocardial oxidative stress and PDE5 expression
Recently, we demonstrated that iNOS−/− significantly attenuated TAC-induced myocardial oxidative stress, LV hypertrophy and heart failure 11. We therefore speculated that the decrease of myocardial oxidative stress in iNOS−/− mice after TAC would consequently attenuate myocardial PDE5 expression in these mice. Consequently, LV ANP, PDE5, 3’-nitrotyrosine and 4-HNE expressions were determined in iNOS−/− hearts and wild type hearts under control conditions and after TAC for 2 weeks. As previously reported, LV ANP, 3’-nitrotyrosine and 4-HNE expressions were all significantly increased in both wild type and iNOS−/− hearts 11, while iNOS−/− significantly attenuated TAC-induced increases of LV ANP, 3’-nitrotyrosine and 4-HNE expression (Figure 5). As anticipated, TAC caused significant increases of LV PDE5 expression in wild type and iNOS−/− hearts, while iNOS−/− significantly attenuated TAC-induced increase of LV PDE5 expression (Figure 5). The relative small decreases of LV 3’-NT and 4-HNE in iNOS−/− mice after TAC suggest that iNOS or iNOS derived NO might play an important role for the PDE5 expression in the failing hearts.
Figure 5.
iNOS gene deletion (KO) significantly attenuated TAC-induced increase in LV ANP, PDE5, 3’-NT and 4-HNE (a, b). *p<0.05 compared to the control group; #p<0.05 compared to the TAC group treated with vehicle.
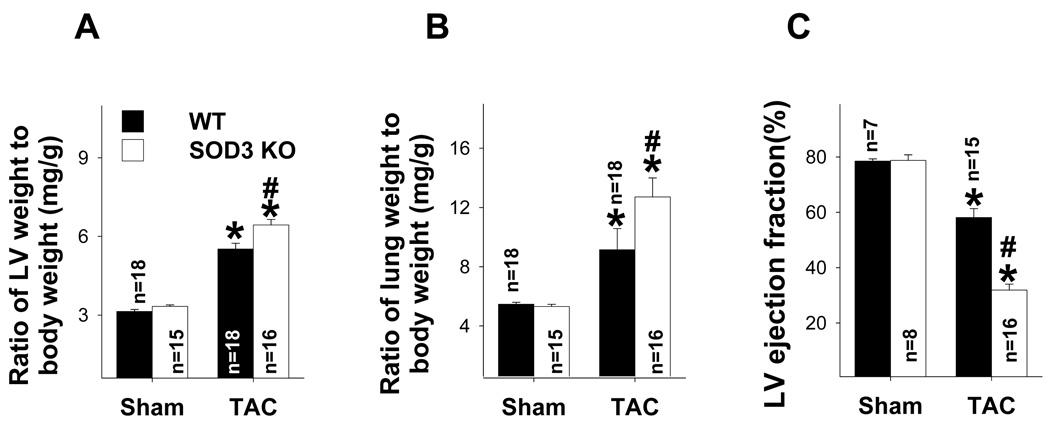
SOD3 gene deletion (SOD3−/−) increased myocardial PDE5 expression and exacerbated TAC-induced LV dysfunction
We previously demonstrated that SOD3−/− mice had mildly increased myocardial oxidative stress under basal conditions, but markedly enhanced TAC-induced LV oxidative stress, hypertrophy and dysfunction12. We subsequently hypothesized that the SOD3−/− heart would have exacerbated TAC-induced PDE5 expression. To test this hypothesis, wild type control mice and mice with SOD3−/− were subjected to TAC procedure for 2 weeks and ventricular structure and function were determined. Consistent with our previous report12, SOD3−/− has negligible effects on LV weight, lung weight and their ratio to bodyweight or tibial length (Figure 6a–b, Table S4) under control conditions. SOD3−/− had no effect on LV function under control conditions (Figure 6c, Table S5). However, the SOD3−/− had significantly exacerbated TAC-induced cardiac remodeling as demonstrated by significant increases of LV weight and lung weight ratios to bodyweight or tibial length (Figure 6a,b, Table S4). After TAC, the SOD3−/− heart had significantly decreased LV ejection fraction, a dramatic increase of LV end systolic diameter and an increase of LV end diastolic diameter (Figure 6c, Table S5).
Figure 6.
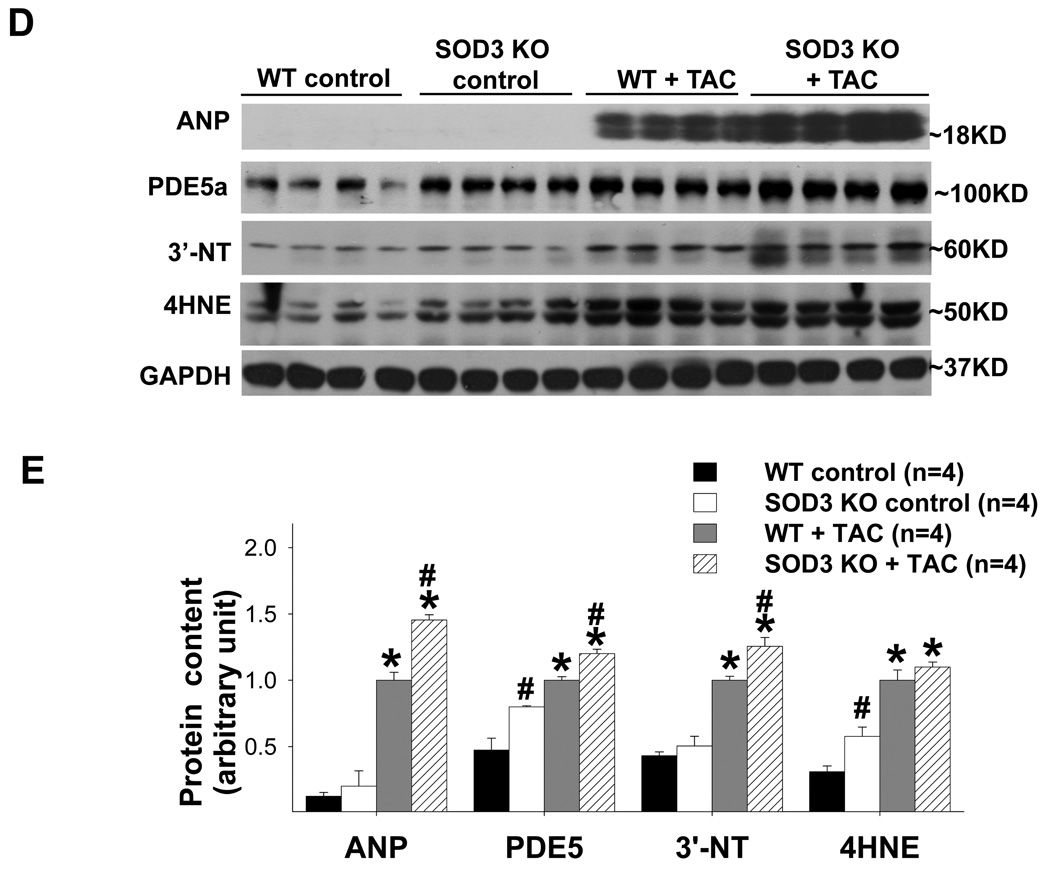
SOD3 gene deletion significantly exacerbated TAC-induced ventricular remodeling, myocardial oxidative stress and PDE5 expression. SOD3 KO exacerbated TAC-induced increase in the ratio of LV weight to body weigh (a), the ratio of lung weight to body weight (b), and the decrease of LV ejection fraction (c). SOD3 KO caused significant increase LV 4-HNE and PDE5 expression under control conditions (d,e), and greater increases LV ANP, PDE5, 3’-NT and 4-HNE after TAC (d,e). *p<0.05 compared to the control group; #p<0.05 compared to the TAC group treated with vehicle.
Interestingly, under control conditions, although the SOD3−/− heart had only moderately increased 4-HNE expression and did not significantly increase myocardial 3’-NT expression, myocardial PDE5 expression was significantly increased by 69% in these mice. As anticipated, the SOD3−/− heart had a significantly greater increase of ventricular ANP, 3’-nitrotyrosine, 4HNE and PDE5 expression after TAC (Figure 6d,e). The greater increase of LV PDE5 in SOD3−/− mice after TAC appears to be an additive effect.
Selective PDE5 inhibition attenuated TAC-induced LV dysfunction in wild type mice
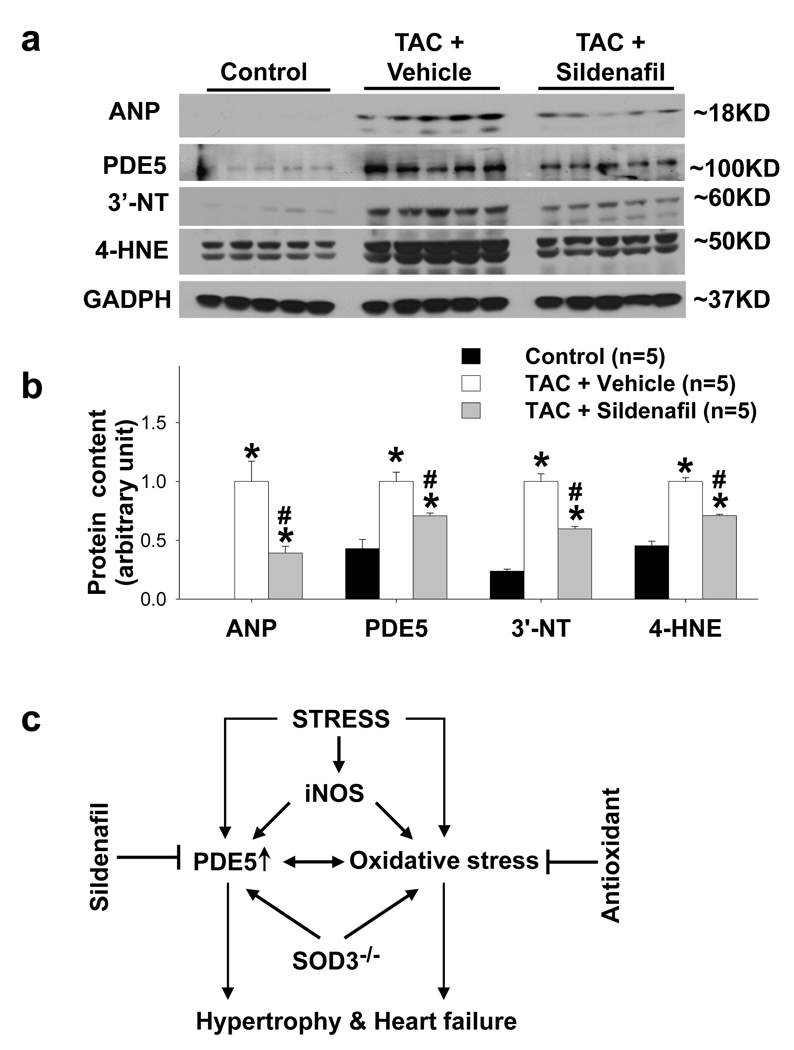
Since the TAC-induced LV dysfunction was associated with increased myocardial PDE5 RNA and protein expression, we determined the effect of PDE5 inhibition with sildenafil on TAC-induced ventricular hypertrophy and dysfunction. Briefly, mice after TAC were divided into two groups treated either with sildenafil or vehicle. Two weeks after TAC, mice in the vehicle treated group had significant increases in the LV, LA and lung weight (Table S6), ratio to body weight (Figure 7a–d) or tibial length (Table S6). TAC significantly decreased LV ejection fraction, increased LV end systolic diameter (Figure 7e, Table S7), and decreased LV dP/dtmax and LV dP/dtmin in the vehicle treated group (Table S7). Ventricular ANP, PDE5 and the oxidative markers 3’-nitrotyrosine and 4-HNE were also significantly increased after TAC (Figure 8a,b). Consistent with previous reports from Kass and associates 2, sildenafil significantly attenuated the TAC-induced LV hypertrophy and cardiac function as demonstrated in Figure 7, Supplementary Table 6 and 7. Interestingly, PDE5 inhibition with sildenafil also significantly attenuated the TAC-induced increases of ANP, 3’-nitrotyrosine, 4-HNE and PDE5 protein expression (Figure 8a,b).
Figure 7.
Selective PDE5 inhibitor sildenafil attenuated TAC-induced heart and lung remodeling and LV dysfunction. Sildenafil attenuated the TAC-induced increase in the ratio of LV weight to body weigh (a,b), the ratio of LA weight to body weight (c), and the ratio of lung weight to body weight (d). Sildenafil also attenuated the TAC-induced decrease of LV ejection fraction (e), and the decrease of LV dP/dtmax and dP/dtmin (c). *p<0.05 compared to the control group; #p<0.05 compared to the TAC group treated with vehicle.
Figure 8.
PDE5 inhibitor sildenafil significantly attenuated the TAC-induced increase of ventricular ANP, PDE5, 3’-nitrotyrosine (3’-NT) and 4-HNE (a, b). Diagram of the interaction of PDE5 and oxidative stress, and their effect on the development of ventricular hypertrophy and dysfunction (c). *p<0.05 compared to control group; #p<0.05 compared to TAC group treated with vehicle.
Selective PDE5 inhibition attenuated TAC-induced LV hypertrophy and dysfunction in SOD3−/− mice
Since LV PDE5 expression was increased in SOD3−/− mice, we studied the effect of PDE5 inhibition on TAC-induced LV hypertrophy and dysfunction. Result showed that sildenafil significantly attenuated TAC-induced LV hypertrophy, pulmonary congestion, decreased LV ejection fraction, and decreased LV contractility in SOD3−/− mice (Table S8, Table S9, Figure S3).
Global gene profiling indicates myocardial PDE5 mRNA was moderately increased in heart failure human LV samples
To understand the alteration of myocardial PDE5 mRNA in human heart failure samples, we downloaded an original microarray data set (GDS651; Reference Series:GSE1145) used for investigation of global gene profiles of left ventricular samples from normal donor hearts (n=11), and hearts with idiopathic dilated cardiomyopathy (n=15) or ischemic cardiomyopathy (n=11). These microarray data include three probe sets for PDE5 mRNA. The three probe sets revealed that LV PDE5 mRNA was significantly increased 1.3, 1.7, or 1.9 fold, respectively, in hearts with idiopathic dilated cardiomyopathy (Figure S4). Two probe sets revealed that LV PDE5 mRNA was significantly increased 1.6 and 2.1 fold in hearts with ischemic cardiomyopathy (Figure S4). These data suggest that increased PDE5 mRNA may be a common phenomenon in both ischemic and idiopathic dilated cardiomyopathy.
The effect of SOD mimetic on attenuation of PDE5 expression in cultured cardiomyocytes was unaffected by PKG inhibition
To further confirm that PKG activity had no effect on PDE5 expression, we subsequently determined the effect of SOD mimetic MnTMPyP on angiotensin-II induced PDE5 expression in cultured cardiac myocytes with or without PKG inhibitor. Angiotensin-II increased 3’-NT, PDE5 and ANP expression in cardiac myocytes. SOD mimetic MnTMPyP significantly attenuated Angiotensin-II induced increase of ANP expression in cultured cardiac myocytes, while the above effect was abolished by addition of PKG inhibitor KT5832 (Figure S5). SOD mimetic MnTMPyP also significantly attenuated Angiotensin-II induced expression of 3’-NT and PDE5 in cultured cardiac myocytes. However, addition of PKG inhibitor KT5832 after MnTMPyP had no effect on expression of 3’-NT and PDE5 in cardiac myocytes (Figure S5), indicating that the attenuation of PDE5 expression by reduction of oxidative stress was not PKG-dependent.
Discussion
To the best of our knowledge, this represents the first demonstration that oxidative stress regulates PDE5 protein expression in cardiac myocytes in the failing hearts. We demonstrated that increased myocardial PDE5 expression in the failing hearts is significantly correlated with increases of the ventricular oxidative stress markers 3’-nitrotyrosine and 4-HNE. Subsequently, we demonstrated that left ventricular oxidative stress and PDE5 expression were both increased in TAC-induced heart failure in mice, and decreasing the TAC-induced ventricular oxidative stress with the SOD mimetic M40401 resulted in lower PDE5 expression and improved LV dysfunction. Moreover, we demonstrated that iNOS gene deletion attenuated TAC-induced myocardial oxidative stress and PDE5 expression. Conversely, we found that increased ventricular oxidative stress in SOD3 KO mice was associated with increased ventricular PDE5 expression and significantly exacerbated TAC-induced LV hypertrophy and dysfunction. Finally, PDE5 inhibition with sildenafil diminished the TAC-induced ventricular oxidative stress, PDE5 expression, LV hypertrophy and LV dysfunction. Together, these data demonstrated that oxidative stress regulates the PDE5 expression in cardiac myocytes in the failing hearts, and the increased PDE5 expression in the failing heart contributes to the adverse ventricular remodeling in the failing hearts. The newly identified relationship between myocardial oxidative stress and PDE5 in the failing hearts suggests both antioxidant(s) and PDE5 inhibitor(s) may be promising therapeutic approaches for attenuating myocardial oxidative stress, PDE5 expression and PDE5 activity.
One of the interesting findings in the present study is the increased PDE5 expression in cardiac myocytes in both human and mouse failing heart. After the initial submission of this manuscript, another study also reported that PDE5 expression was increased in cardiac myocytes in failing human hearts6. This finding is important, as previous studies have demonstrated that PDE5 expression is very low in cardiac myocytes under basal conditions1, and on the basis of this finding, the prevailing hypothesis was that PDE5 inhibition would have minimal effects on cardiac function in diseased hearts 1. However, studies pioneered by Dr. Kass and his associates have demonstrated that selective PDE5 inhibition with sildenafil profoundly attenuates chronic pressure overload induced LV hypertrophy and dysfunction in mice subjected to TAC 2. Our results in the present study further demonstrate increased PDE5 expression in cardiac myocytes in the failing human and mouse heart. These results support and extend the previous work from Kass and his associates and support the notion that the use of PDE5 inhibitors as a novel approach to treat heart failure. Additionally, recent studies showed that PDE5 activity was increased in LV tissues obtained from heart failure mice 2, as well as in multiple tissues obtained from pacing-induced CHF in the dog model 13. This suggests that the increase of PDE5 protein and activity in heart failure subjects appears to be a common phenomenon across multiple species, and may even extend beyond the myocardial tissues.
The finding that M40401 reduced LV oxidative stress, hypertrophy and ventricular dysfunction in mice exposed to TAC is of considerable interest. M40401 is a potent highly diffusible SOD mimetic 14, 15 that has been shown to be effective in attenuating oxidative stress in many experimental models including shock, ischemia-reperfusion and inflammation16. M40403, a close analogue of M40401 is the only SOD mimetic advanced into phase-2 clinical trials. We found that M40401 moderately improved coronary blood flow in the CHF dog model and enhanced acetylcholine-induced coronary vasodilatation in dogs with pacing induced heart failure 17. Although no previous study has determined the effect of M40401 on the chronic systolic overload-induced heart failure, the finding that M40401 attenuated LV hypertrophy and dysfunction in the mice exposed to TAC is conceptually consistent with previous reports that decreasing oxidative stress attenuates LV hypertrophy and dysfunction produced by chronic systolic overload 18–20. Thus, SOD mimetic Mn-TBAP has been reported to attenuate both myocardial oxidative stress and LV dysfunction in PPARγ deficient mice 21, while administration of the antioxidant pyrrolidine dithiocarbamate significantly attenuated TAC-induced left ventricular oxidative stress and dysfunction in mice 22. The profound cardiac protective effect of M40401 in attenuating TAC-induced heart failure warrants additional studies in different or large animal models.
The findings that sildenafil attenuated the TAC-induced increases of LV mass, LA mass, lung weight and RV weight indicate that PDE5 inhibition attenuated TAC-induced ventricular dysfunction and cardiac remodeling. The findings of higher LV ejection fraction, LV systolic pressure, LV dP/dtmax and LV dP/dtmin in the sildenafil treated group indicate that PDE5 inhibition improved function of the chronically overloaded LV and are consistent with the previous report from Kass and associates 2. In addition to attenuating the TAC-induced LV hypertrophy and dysfunction in mice, sildenafil also lessened the TAC-induced increases of myocardial PDE5, 3’-nitrotyrosine and 4-HNE, suggesting that increased PDE5 activity in the failing heart might contribute to the increased oxidative stress and the up-regulation of PDE5. The decreased myocardial oxidative stress after sildenafil may be a result of decreased LV remodeling after PDE5 inhibition2, or may result from it’s capacity in scavenging superoxide anion or inhibition of H2O2 generation as recently reported by Fernandes et al23.
It is well established that oxidative stress is increased and contributes to cardiac hypertrophy24 . The finding that oxidative stress regulates LV PDE5 expression, and that this relationship may drive hypertrophy and heart failure is novel. The increased myocardial oxidative stress in response to pressure overload may arise from several sources, including mitochondrial electron transport leakage18, increases of nonphagocytic NADPH oxidase25, 26 and xanthine oxidase27, uncoupled NO synthase11, 19, or decreased antioxidant expression(such as SOD312, 28, 29 or SOD1). The content of 3’-nitrotyrosine and 4-HNE are often used as tissue oxidative stress markers, and the increase of myocardial oxidative stress as demonstrated by increased 3’-nitrotyrosine and 4-HNE in the LV of mice after TAC is consistent with previous reports from this laboratory 8, 11, 30 and others using the same experimental model 19. We recently demonstrated that iNOS KO significantly attenuated TAC-induced myocardial oxidative stress and heart failure 11. The greatly attenuated PDE5 expression and small reduction of 3’-NT in iNOS KO mice after TAC suggests that iNOS or iNOS-derived NO might play an important role for the increased PDE5 expression in the failing hearts. The finding that higher PDE5 expression in SOD3 KO under control conditions and after TAC is consistent with our previous reports that SOD3 plays an important role in regulating myocardial oxidative stress and protecting hearts against TAC or infarction-induced ventricular remodeling 12, 31. The finding that iNOS gene deletion, SOD3 KO and M40401 alter PDE5 expression in the failing hearts suggests that increase of PDE5 expression in the failing hearts is not limited to a particular source of increased oxidative stress.
M40401 attenuated myocardial PDE5 expression but had no effect on myocardial PKG activity and p-VASPSer239 expression. The unchanged myocardial PKG activity and p-VASP after M40401 in the failing hearts may reflect an overall balance of myocardial cGMP availability in cardiac myocytes. Specifically, decreased myocardial PDE5 expression after M40401 is anticipated to attenuate PDE5 dependent cGMP degradation, while decreased myocardial ANP (or BNP) after M40401 could simultaneously attenuate ANP or BNP-dependent cGMP production. Indeed, Kass and associates recently reported that addition of BH4 (a NOS cofactor and antioxidant) significantly attenuated TAC-induced myocardial oxidative stress and heart failure in mice, but also had no effect on myocardial PKG activity or p–VASPser239 content10. In the cultured myocytes, SOD mimetic attenuated angiotensin induced oxidative stress and PDE5 expression in cultured myocytes, while above effect was unaffected by PKG inhibitor KT5823. These data suggest that decreased PDE5 expression after antioxidant treatment may be through a PKG-independent pathway.
The significant increase of PDE5 expression in the human and mouse heart failure samples was associated with moderately increased PDE5 mRNA, suggesting that increased myocardial PDE5 protein in the failing heart is partially regulated at the transcriptional level. This mechanism is also supported by the finding of moderate increases of LV PDE5 mRNA in patients with idiopathic dilated cardiomyopathy or ischemic cardiomyopathy as revealed by the global microarray data.
The present data demonstrate that PDE5 protein expression is increased in the failing heart, and that this increase is related to the increased myocardial oxidative stress. However, since oxidative stress can broadly affect many signaling pathways and alter the expression and modification of many important molecules, the precise molecular mechanism by which oxidative stress can regulate PDE5 expression is yet to be fully defined.
In summary, our study demonstrated that myocardial oxidative stress causes increased PDE5 expression in the failing heart, and selective PDE5 inhibition or inhibition of oxidative stress induction of myocardial PDE5 expression using M40401 protected heart against pressure overload induced LV hypertrophy and CHF.
Clinical perspectives
Congestive heart failure (CHF) is a leading cause of mortality in developed countries that is increasing in prevalence. In the present study we found that myocardial PDE5 protein content is increased in patients with end stage CHF, and that this increase is correlated with markers of increased myocardial oxidative stress. Inhibition of PDE5 increases cGMP bioavailability and has been shown to attenuate systolic overload induced LV hypertrophy and CHF in animal models. Our finding that either decreasing oxidative stress with an SOD mimetic, or iNOS gene deletion, blunted the increase of myocardial PDE5 expression and LV dysfunction in mice subjected to systolic pressure overload implies that increased oxidative stress and iNOS expression at least partially contributed to the increased PDE5 expression and contractile dysfunction. The finding that inhibition of PDE5 with sildenafil reduced myocardial oxidative stress, PDE5 expression and LV dysfunction indicates a role for the increased PDE5 expression in contributing to the increased oxidative stress and LV dysfunction in the overloaded heart. These data provide the first direct evidence that oxidative stress and iNOS can regulate PDE5 expression in cardiac myocytes, and suggest that PDE5 inhibition, antioxidant treatment or iNOS inhibition can decrease PDE5 expression in the overloaded heart and might be effective approaches to attenuate systolic overload-induced LV hypertrophy and CHF. This work suggests that further exploration of the use PDE5 inhibitors or antioxidants in CHF is warranted.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge the expert technical assistance provided by Jennifer L. Fricton and the Histology & Microscopy Core of the Lillehei Heart Institute.
Sources of Funding: This study was supported by NHLBI Grants HL71790 (YC) and HL21872 (RJB) from the National Institutes of Health. Drs. Fassett, Hu and Zhang are recipients of Scientist Development Award from American Heart Association National Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts declared.
Reference List
- 1.Cheitlin MD, Hutter AM, Jr, Brindis RG, Ganz P, Kaul S, Russell RO, Jr, Zusman RM. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;33:273–282. doi: 10.1016/s0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- 2.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 3.Perez NG, Piaggio MR, Ennis IL, Garciarena CD, Morales C, Escudero EM, Cingolani OH, Chiappe de CG, Yang XP, Cingolani HE. Phosphodiesterase 5A inhibition induces Na+/H+ exchanger blockade and protection against myocardial infarction. Hypertension. 2007;49:1095–1103. doi: 10.1161/HYPERTENSIONAHA.107.087759. [DOI] [PubMed] [Google Scholar]
- 4.Hassan MA, Ketat AF. Sildenafil citrate increases myocardial cGMP content in rat heart, decreases its hypertrophic response to isoproterenol and decreases myocardial leak of creatine kinase and troponin T. BMC Pharmacol. 2005;5:10. doi: 10.1186/1471-2210-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St AC, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 6.Pokreisz P, Vandenwijngaert S, Bito V, Van den BA, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van LA, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 8.Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 Receptor Deficiency Exerts Unanticipated Protective Effects on the Pressure-Overloaded Left Ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of Sarcolemmal ATP-Sensitive Potassium Channel Activity Impairs the Cardiac Response to Systolic Overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 14.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Aston K, Riley DP, Salvemini D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br J Pharmacol. 2001;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Hou M, Li Y, Traverse JH, Zhang P, Salvemini D, Fukai T, Bache RJ. Increased superoxide production causes coronary endothelial dysfunction and depressed oxygen consumption in the failing heart. Am J Physiol Heart Circ Physiol. 2005;288:H133–H141. doi: 10.1152/ajpheart.00851.2003. [DOI] [PubMed] [Google Scholar]
- 18.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 19.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 21.Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med. 2005;39:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes MA, Marques RJ, Vicente JA, Santos MS, Monteiro P, Moreno AJ, Custodio JB. Sildenafil citrate concentrations not affecting oxidative phosphorylation depress H2O2 generation by rat heart mitochondria. Mol Cell Biochem. 2008;309:77–85. doi: 10.1007/s11010-007-9645-9. [DOI] [PubMed] [Google Scholar]
- 24.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 25.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Fassett J, Hu X, Zhu G, Lu Z, Li Y, Schnermann J, Bache RJ, Chen Y. Ecto-5'-nucleotidase deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:1557–1564. doi: 10.1161/HYPERTENSIONAHA.108.110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, Bache RJ, Duncker DJ, Chen Y. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44:1305–1313. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.