Abstract
Background
Antibiotics are a mainstay of treatment for chronic rhinosinusitis (CRS) and recurrent acute rhinosinusitis (RARS). Although quality-of-life outcomes following endoscopic sinus surgery (ESS) have been previously studied, the change in antibiotic utilization following ESS is less well known.
Objective
We aimed to determine the effect of ESS on antibiotic utilization in CRS and RARS.
Methods
A multi-institutional, prospective cohort of patients with CRS and RARS was enrolled between January, 2001 and January, 2009. Patients completed the medication subscale of the Chronic Sinusitis Survey, and the Wilcoxon signed-rank test was used to compare differences in the overall reported time of antibiotic between preoperative and postoperative time points.
Results
503 patients were followed for an average 17.3 months. Overall, patients reported a 57.2% reduction in time on antibiotics following ESS. The majority of patients (60.4%) reported significantly less antibiotic utilization after ESS (p<0.001) consisting of an 83.7% reduction in the time on antibiotics. Subgroup analysis also revealed a significant reduction in antibiotic utilization for patients with and without nasal polyposis (59.0% and 58.2%; both p<0.001) as well as RARS (61.2%; p=0.001).
Conclusion
ESS significantly reduces antibiotic utilization for CRS and RARS. This finding demonstrates potential for lower health care expenditures related to antibiotics, as well as reduced risk of both antibiotic related morbidity and development of bacterial resistance.
Keywords: Chronic rhinosinusitis, sinusitis, endoscopic, surgery, antibiotics, sinus, reduction, quality-of-life, outcomes, recurrent acute
INTRODUCTION
Antibiotics are a mainstay of treatment for adult rhinosinusitis, which ranks 5th among all diseases requiring antibiotic prescriptions.1 Rhinosinusitis is characterized into acute, sub-acute, and chronic states depending on the duration of symptoms. Patients who develop more than 4 acute bacterial infections each year are further classified as recurrent acute rhinosinusitis (RARS). Chronic rhinosinusitis (CRS) involves symptoms lasting beyond 12 weeks, and patients may frequently develop acute exacerbations that require antibiotics.2
Antibiotic therapy is associated with several well documented disadvantages. First, adverse effects are more common with antibiotics versus placebo, with gastrointestinal symptoms occurring most frequently.3 Second, induction of bacterial resistance can occur from selective pressure of antibiotic therapy on existing microflora.4,5 Finally, the economic impact of antibiotic use in patients with rhinosinusitis who meet criteria for endoscopic sinus surgery (ESS) has been estimated at an average of $772 per patient per year.6 Considering these disadvantages and given that rhinosinusitis affects approximately 31 million people each year,7 it is clear that a reduction in antibiotic utilization would be beneficial from multiple perspectives.
Endoscopic sinus surgery is indicated for surgical treatment of both CRS and RARS recalcitrant to medical therapy. Favorable quality-of-life and symptom outcomes following ESS are well documented in previous studies. 8-11 However, postoperative trends in antibiotic utilization following ESS are less well known. We aimed to determine the specific effect of ESS on antibiotic utilization for patients with both CRS and RARS.
METHODS
Enrollment Procedures
Adult (≥ 18 years) patients with CRS or RARS were prospectively enrolled from three academic, tertiary care centers between January, 2001 and January, 2009. Comprehensive outcomes of this cohort have been previously reported.10,11 Inclusion criteria included a confirmed diagnosis of CRS or RARS in concurrence with guidelines established by the Rhinosinusitis Task Force.2 Enrolled patients with CRS were required to fail medical management for resolution of sinus symptoms, including, at a minimum, three or more weeks of broad-spectrum or culture directed antibiotics in addition to one or more trials of systemic corticosteroid therapy. Enrollment occurred at the visit when the patient opted for endoscopic sinus surgery, which was not necessarily the first clinic visit nor with any specific temporal relationship to failed medical therapy. Patients with RARS were required to have four documented episodes of acute bacterial rhinosinusitis in the prior year requiring antibiotics, in accordance with the above mentioned guidelines.2 Demographic and comorbidity data were collected at enrollment by the Principal Investigator (PI) at each site via patient history or medical record review and included: age, gender, sinonasal polyposis, prior sinus surgery, asthma, acetylsalicylic acid (ASA) intolerance, allergy (confirmed by modified radioallergic sorbent testing or skin prick), depression, and current smoking. Objective disease severity was evaluated through computed tomography (CT) in the coronal plane and rigid sinonasal endoscopy. Scoring of these baseline measures utilized the Lund-Mackay and Lund-Kennedy scoring systems.12,13 An institutional review board at each performance site provided annual approval of research protocol and the informed consent process.
Chronic Sinusitis Survey
Study patients completed the Chronic Sinusitis Survey (CSS) during preoperative enrollment meetings and at least 6 months postoperatively at routine follow-up appointments. A trained research coordinator assisted in the completion of the CSS, a 6-question, duration based, sinus-specific outcome instrument developed to measure both medication and symptom aspects of rhinosinusitis.8 Patients were prompted to report the number of weeks they have taken antibiotics for sinusitis during the previous eight weeks. Total and subscale scores were transformed to a standardized scoring scheme [range: 0 – 100] with lower scores representing a greater impact of disease. The PI was blinded to all CSS responses for the duration of the study.
Evaluation of Antibiotic Utilization
The ordinal CSS medication subscale was used as a self-reported, proxy measure of antibiotic utilization prior to and following ESS. While completing the CSS both pre- and postoperatively, subjects were asked the duration of time, in two week increments, they had taken sinusitis-related antibiotics in the preceding 8 week period: “0 weeks”, “1-2 weeks”, “3-4 weeks”, “5-6 weeks”, or “7-8 weeks”. The higher value of the response range was used to represent weeks of antibiotic use.
Statistical Analysis
Data was collected on standardized clinical research forms and recorded in a database system (Visual FoxPro, Microsoft Corp., Redmond, WA) before being transferred into statistical software (SPSS v.17.0, SPSS Inc., Chicago, IL) for analysis. Descriptive analyses were calculated for all cohort measures including: means (standard deviations), ranges, and frequency counts. The percentage of improvement in the reported CSS antibiotic use was assessed using the following formula: [(mean preoperative weeks –mean postoperative weeks) / mean preoperative weeks] × 100. The Wilcoxon signed-rank test and paired t-test were used to evaluate improvement in CSS total scores and antibiotic utilization over time. A p-value less than 0.05 was considered statistically significant.
RESULTS
Demographics and Comorbid Characteristics
A total of 718 patients electing ESS completed site enrollment procedures at three academic, tertiary care centers between January, 2001 and January, 2009. Final patient cohort characteristics and comorbid factors are described in Table 1. A total of 503 patients completed the CSS before and after surgery with a mean follow-up time of 17.3(6.6) months and were included in the analyses. No clinically meaningful differences in baseline characteristics were found between patients with and without follow-up (all p >0.05).
Table 1.
Demographic and comorbid factors for subjects undergoing endoscopic sinus surgery (n=503)
| Characteristics: | [range] | mean (SD) | n (%) |
|---|---|---|---|
| Age | [18 - 82] | 47.4 (13.7) | |
| Follow-up (months) | [6 - 50] | 17.3 (6.6) | |
| Gender | |||
| Males | 238 (47.3) | ||
| Females | 265 (52.7) | ||
| Nasal polyposis | 193 (38.4) | ||
| Prior sinus surgery | 297 (59.0) | ||
| Recurrent acute sinusitis | 21 (4.2) | ||
| Asthma | 206 (41.0) | ||
| ASA intolerance | 61 (12.1) | ||
| Allergy | 160 (31.8) | ||
| Depression | 80 (15.9) | ||
| Current smoking | 37 (7.4) | ||
| Lund-Mackay CT score | [0 - 24] | 12.5 (6.7) | |
| Lund-Kennedy endoscopy score | [0 - 20] | 7.2 (4.8) |
SD, standard deviation; ASA, acetylsalicylic acid; CT, computed tomography
Mean Preoperative and Postoperative CSS Scores
Overall, significant improvement in mean CSS medication and symptom subscale scores, as well as average CSS total score, were reported over time. Preoperative CSS medication subscale scores improved from 44.0(25.5) to 56.9 (24.5; p<0.001),. Mean preoperative CSS symptom subscale scores improved from 28.5(26.0) to 59.4(29.2; p<0.001), while CSS total scores significantly improved (p<0.001) from 36.3(19.5) to 58.2(21.0).
Decrease in Reported Antibiotic Use
The CSS medication subscale measures patient-reported antibiotic use in 2-week increments during the 8 weeks immediately preceding survey completion. This information served as a proxy for antibiotic utilization. At the time of enrollment, the majority of patients (79.5%; n=400) reported at least 2 weeks of antibiotics use in the prior 8 weeks (Figure 1) with an average of 3.4(2.5) weeks of antibiotic use in that same time period.
Figure 1.

Overall cohort antibiotic utilization before and after endoscopic sinus surgery.
Postoperatively, 40.0% of patients reported at least 2 weeks of antibiotic use in the preceding eight weeks with an average of 1.5(2.2) weeks in the prior eight weeks. Overall, patients reported a 57.2% reduction in time on antibiotics following ESS. The majority (60.4%) of all patients reported significantly less antibiotic utilization after ESS (p<0.001). When considering only the subgroup of patients who improved, there was an 83.7% reduction in average time on antibiotics while the number of patients reporting no antibiotic use nearly tripled from 103 preoperatively to 300 postoperatively (p<0.001; Figure 1).
Improvement in Nasal Polyp and RARS Subgroups
Subgroup analyses revealed a consistent, significant reduction in reported antibiotic use for two unique subsets of patients with rhinosinusitis. Patients presenting with CRS and nasal polyposis had a 58.7% reduction in average time on antibiotics from 3.1(2.6) weeks preoperatively to 1.3(2.1) weeks following ESS (p<0.001). The number of patients with sinonasal polyps who reported no antibiotic use increased greater than 200%, from 56 preoperatively to 125 patients postoperatively. Likewise, patients with RARS reported a 61.2% reduction in the average time on antibiotics from 3.9(2.6) weeks preoperatively to 1.5(2.3) weeks postoperatively following ESS (p=0.001). Trends in antibiotic frequency for these subsets are denoted in Figures 2-3. Regardless of comorbidity or clinical characteristic, significant postoperative reduction in the time on antibiotics was observed with mean improvements ranging between 54.9% and 63.9% (all p ≤ 0.005).
Figure 2.
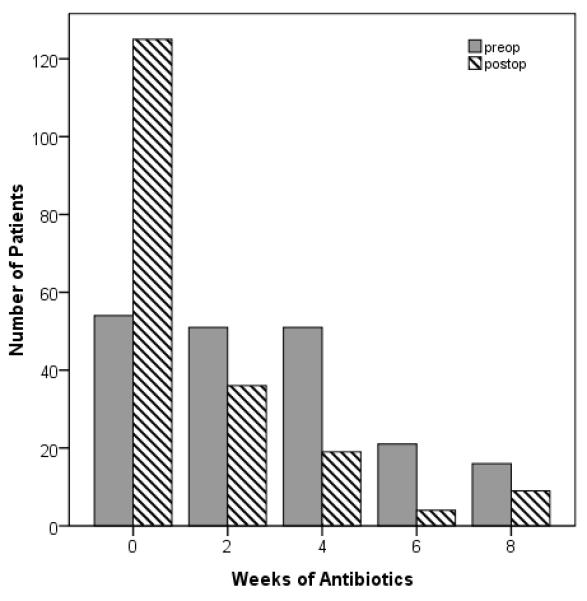
Antibiotic utilization for subjects with nasal polyposis before and after endoscopic sinus surgery.
Figure 3.
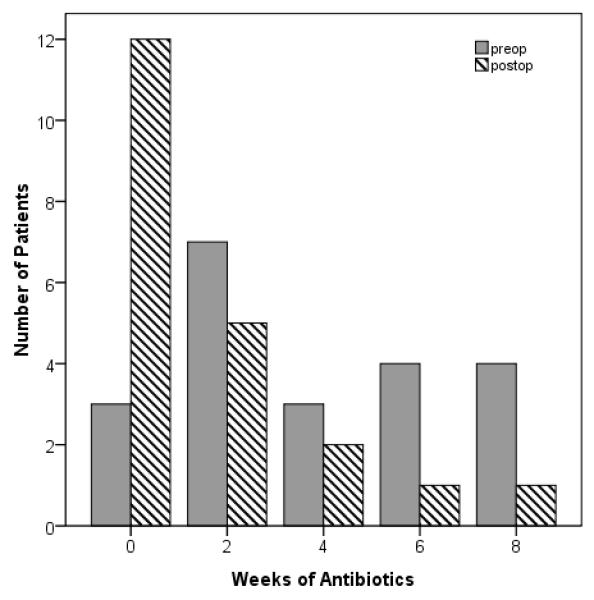
Antibiotic utilization for subjects with recurrent acute rhinosinusitis before and after endoscopic sinus surgery
DISCUSSION
We have presented a large prospective cohort who experienced a significant reduction in antibiotic utilization following ESS. Overall, patients reported a 57.2% reduction in time on antibiotics following ESS. Similar reduction in antibiotic utilization was observed for patient subgroups with polyps (58.7%) or RARS (61.2%). Although not reported extensively in the results, we also observed similar improvement in patients with comorbid conditions such as asthma, aspirin triad disease, cystic fibrosis, and immune dysfunction, with a reduction in mean antibiotic utilization in these groups ranging from 55.0% to 65.9%.
Comparable trends of reduced antibiotic utilization within smaller cohorts are found within the current available literature. Gliklich and Metson performed a 1 year, single institution analysis of 100 patients using the CSS medication subscale and reported a 58.0% reduction (weeks/year) in postoperative antibiotic use.6 Our investigation provides a strong degree of external validity for these previous findings through the use of a significantly larger cohort in a multi-institutional setting, and for a longer follow-up period. Similarly, Bhattacharyya utilized the Rhinosinusitis Symptom Inventory (RSI) to prospectively evaluate trends in antibiotic use in 100 patients undergoing ESS for a mean follow-up of 19.0 months.9 Review of data in that study reveals only a 34.8% reduction in time on antibiotics (p=0.020). One potential explanation for this slight disparity in results may be recall bias, where patients misestimate antibiotic use in the previous 12 months, as prompted by the RSI survey, compared to the previous 8 weeks in the CSS survey. In addition, the number of patients completing RSI survey between 6.4 and 36.6 months postoperatively is unreported. Respondents with less than 12 months follow-up may have resulted in the inclusion of preoperative antibiotic usage for a proportion of subjects with shorter term follow-up and an underestimate of the reduction of antibiotic use after surgery. Our investigation did not incorporate an overlap of postoperative data collection into the preoperative time frame, helping to minimize the potential for misclassification bias.
The results regarding patients with RARS in this study deserve mention. This RARS patient population comprises a small proportion of patients with rhinosinusitis and is consequently more challenging to study. A larger cohort of patients with RARS would be ideal however the magnitude of reduction in antibiotic utilization for our RARS cohort was still highly statistically significant. Previous investigations have reported a greater number of antibiotic courses in RARS versus CRS, but similar overall time on antibiotics between the two groups which is consistent with our data.14 Through direct comparison of these groups, we found that patients with RARS had a reduction in antibiotic utilization that was not significantly different from that seen in patients with CRS.
Our findings of reduced antibiotic utilization are important from both economic and public health perspectives. The annual aggregate health care expenditure for rhinosinusitis has been estimated at $5.8 billion,15 but there is little data that evaluates expenditures specifically related to antibiotic prescriptions or antibiotic related adverse effects. For patients who undergo ESS, Gliklich estimated an annual per patient cost reduction of $442 for antibiotic prescriptions.6 That estimate did not include the cost reductions related to fewer office visits, emergency room visits, alternative prescriptions, or lost productivity (workdays) for antibiotic related adverse effects or allergic reactions. We did not perform a cost analysis in this study, but our data implies significant reduction in antibiotic related health care expenditures and fewer medication related adverse events.
There are several mechanisms of antibiotic treatment failures, one of which relates to bacterial resistance as a result of selective pressure from frequent or incomplete antibiotic administration.4,5 Antibiotic compliance was not evaluated in this study, however, we would surmise that a direct benefit in the reduction of bacterial resistance would be realized through significant decreased antibiotic utilization in this patient population.
There are study limitations that must be considered. First, responses on the CSS survey reflect the immediately preceding 8 week time period. Seasonal variations in disease exacerbations may exist and result in increased or decreased antibiotic utilization at that specific point in time. Even though this data may not be completely reflective of all time on antibiotics during the investigational period, the CSS medication subscale is a validated instrument and proxy for antibiotic utilization and the degree of improvement seen was highly significant. Second, findings may not be applicable beyond the average 17.3 month follow-up period given that the natural history of the disease process, along with antibiotic utilization, could significantly change. Third, in this observational study, we could not specifically control for differences in physician prescribing patterns between institutions or among referring clinicians or timing of such therapy with respect to completion of the survey. The duration of antibiotics prescribed for disease exacerbation was variable both prior to and following surgical intervention and therefore any bias introduced by this variation affected both pre and post-operative data. With regards to timing, medical therapy for disease exacerbation was given as frequently as necessary for symptomatic improvement, and patients underwent such therapy regardless of timing of initial consultation or timing of study enrollment. Finally, our study involved a 29.9% loss to follow-up. Although this may introduce some selection bias, we believe this attrition rate to be representative of ambulatory patients with sinusitis who may be lost due to potential changes in insurance status, preferred providers, or patient travel requirements over time. No significant differences in baseline characteristics or comorbid status were found between patients with follow-up and those without.
CONCLUSION
Endoscopic sinus surgery significantly reduces antibiotic utilization in patients with CRS and RARS. These findings demonstrate the potential for lower health care expenditures related to antibiotic use, as well as reduced risk of both antibiotic related morbidity and development of bacterial resistance.
ACKNOWLEDGMENT
This investigation was made possible by a grant funding from the National Institute on Deafness and Other Communication Disorders (R01 DC005805), one of the National Institutes of Health, Bethesda, MD, as well as administrative support by the Department of Otolaryngology – Head and Neck Surgery, Oregon Health & Science University, Portland, Oregon.
Grant funded by the National Institutes of Health (NIDCD), Bethesda, MD. R01 DC005805 (PI: TL Smith)
There is no conflict of interest or financial disclosure for Naveen D. Bhandarkar, MD. Timothy L. Smith, MD, MPH, and Jess C. Mace, MPH were funded by a grant from the NIH/NIDCD. Timothy L. Smith is also a consultant for Sinexus, Inc. (Palo Alto, CA.) which provided no financial support for this investigation.
Footnotes
Oral presentation and clinical research award at the American Rhinologic Society Meeting at the 113th Annual Combined Otolaryngology Spring Meetings (COSM) in Las Vegas, NV., April 28 – May 2, 2010.
Public clinical trial registration (http://www.clinicaltrials.gov) ID: NCT00799097
The Institutional Review Board at OHSU provided approval and oversight for all research activities.
REFERENCES
- 1.Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 Suppl):1–45. doi: 10.1016/j.otohns.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2 Suppl):S1–7. doi: 10.1016/S0194-59989770001-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 4.Levy SB. How the misuse of antibiotics destroys their curative powers. Perseus Publishing; Cambridge (MA): 2002. The antibiotic paradox. [Google Scholar]
- 5.Schrag SJ, McGee L, Whitney CG, et al. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob Agents Chemother. 2004;48(8):3016–23. doi: 10.1128/AAC.48.8.3016-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gliklich RE, Metson R. Economic implications of chronic sinusitis. Otolaryngol Head Neck Surg. 1998;118(3):344–49. doi: 10.1016/S0194-59989870313-4. [DOI] [PubMed] [Google Scholar]
- 7.Lethbridge-Cejku M, Rose D, Vickerie J. Summary health statistics for U.S. adults: National Health Interview Survey, 2004. National Center for Health Statistics. Vital Health Stat. 2006;10(228):19–22. [PubMed] [Google Scholar]
- 8.Gliklich RE, Metson R. Effect of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117(1):12–7. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya N. Symptom outcomes after endoscopic sinus surgery for chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130(3):329–33. doi: 10.1001/archotol.130.3.329. [DOI] [PubMed] [Google Scholar]
- 10.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: A multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142(1):55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith TL, Mendolia-Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115(12):2199–205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 12.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–84. [PubMed] [Google Scholar]
- 13.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2 Suppl):S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya N, Lee KH. Chronic recurrent rhinosinusitis: disease severity and clinical characterization. Laryngoscope. 2005;115(2):306–10. doi: 10.1097/01.mlg.0000154738.40690.dd. [DOI] [PubMed] [Google Scholar]
- 15.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;193(Suppl):3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]


