Abstract
The superacid-promoted Houben-Hoesch reactions of amino-nitriles and related compounds have been studied. The nitriles form dicationic electrophiles and react with benzene in fair to good yields (12-95%). The intermediate iminium ions may also be reduced to the benzylic amines by NaBH4 or H2.
Keywords: Houben-Hoesch Reaction, Superelectrophile, Superacid, Nitrilium ion, Freidel-Crafts
1. Introduction
The Houben-Hoesch reaction is an acid-catalyzed reaction of nitriles with aromatic compounds leading to aryl ketones.1 It has been known for many years that the conversion works best with activated arenes (i.e., good nuclephiles) and a mechanism is proposed which involves protonation at the nitrile nitrogen to form the nitrilium ion. Typically, nitrilium ions are only moderately electrophilic, as they are incapable of reacting with benzene or deactivated arenes (i.e., weak nucleophiles). However, Shudo and Ohwada found evidence for diprotonated, superelectrophilic species in the reactions of nitriles in Brønsted superacids (eq 1). Superacidic media was shown to enhance the reactivities of the nitriles towards weak nucleophiles such as benzene. Upon hydrolysis of the iminium ion intermediates, the aryl ketones were obtained. In work with superelectrophiles, it has been shown that electrophiles may exhibit greatly enhanced reactivities when adjacent to a stable cationic center (i.e., an ammonium or pyridinium group).3 In the following manuscript, we describe our studies of Houben-Hoesch-type reactions involving ammonium-nitrilium dications and other related superelectrophiles. We also describe our efforts to reduce the iminium ion intermediates as a direct route to benzylic amines.
 |
2. Results and discussions
Our initial experiments examined the reactions of aminonitriles and related substrates. For example, 3-aminopropionyl nitriles were reacted in superacidic CF3SO3H with benzene (Table 1, entries 1,2). These reactions provided the expected aryl ketones in excellent yields, following aqueous hydrolysis. In the case of the secondary amine, product isolation was facilitated by conversion to its amide (11). This represents a new route the Mannich base 10, a useful intermediate in the synthesis of biologically active compounds and an analogue of kynuramine.4,5 The 2-aminoethanoyl nitriles also gave the desired products, however the yields were somewhat lower (entries 3-5). When product 14 was isolated, a minor biproduct was also obtained. Benzil was formed in 10% yield along with product 14. With cyanamide (5), benzamide (13) is formed as a product, although it is isolated in just 12% yield (entry 6). Aryl nitriles were also found to give the expected diaryl ketones (entries 8-9).
Table 1.
Products and isolated yields for the reaction of compounds 1-9 with CF3SO3H and C6H6.
| entry | nitrile | product | yield |
|---|---|---|---|
| (1) |

|

|
95% |
| (2) |

|

|
98% |
| (3) |

|

|
59% |
| (4) |

|

|
44% |
| (5) |

|

|
64% |
| (6) |

|

|
12% |
| (7) |

|

|
70% |
| (8) |

|

|
88% |
| (9) |

|

|
25% |
When H2SO4 or CF3CO2H were used as acid catalysts, little or no product 10 was formed in the reaction of compound 1 with benzene. The stronger acid system CF3SO3H-SbF5 was also used in a reaction 1 with benzene. Although product 10 was formed, it was also contaminated with significant amounts of antimony(III)triphenyl.
The final step of the Houben-Hoesch reactions involves hydrolysis of the iminium ion intermediate to form the aryl ketone (eq 1). It is known that imines and iminium ions can be reduced to give amine-type products. For example, amines have been prepared from the nitriles and Grignard reagents via imine reduction.6 There are no similar reports however of the reduction the Houben-Hoesch intermediates, and so we examined this as a possible route to benzylic amines. In this regard, 2-aminobenzonitrile (8) reacts with benzene in CF3SO3H to produce the iminium dication (19, eq 2). When this intermediate product is reacted with NaBH4 in methanol followed by acetic anhydride, the amide product (20) is isolated. The same reaction sequence provides the pyridine derivative 22 in 20% yield. We have also found that the Houben-Hoesch products may be reduced to the amine-type products using H2 with Pd-C. A common impurity in these reactions is the diaryl ketone, suggesting that the dicationic iminium ions (i.e., 19) may not be stable. Formation of the respective aryl ketones probably limits the yields for these conversions, nevertheless this Houben-Hoesch chemistry represents a direct route to the benzylic amines.
 |
 |
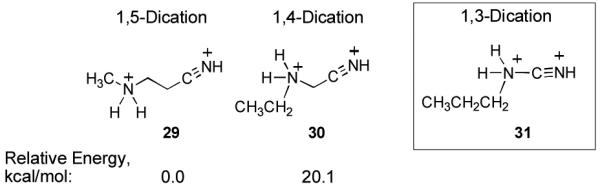
In considering the mechanisms for these conversions, it is proposed that diprotonated superelectrophilic species are involved in the conversions. Initial protonation is expected to occur at the strong base site (amine or pyridine nitrogen). A Freidel-Crafts-type reaction with benzene requires further protonation at the nitrile group. This suggests superelectrophilic intermediates 23-28. Although a second protonation at the nitrile group is conceivable, especially in light of Shudo and Ohwada’s previous work (vide supra),2 this seems unlikely due to the very high charge density on these ions (23-28). There does appear to be a correlation between reaction yields and the relative distance between charge centers. As the charge centers are in closer proximity on the diprotonated superelectrophile, the reaction yields drop off. For example, superelectrophile 23 is a 1,5-dication and it gives product 10 in 95% yield, superelectrophile 24 is a 1,4-dication and it gives product 13 in 44% yield, while superelectrophile 25 is a 1,3-dication and it gives benzamide (15) in just 12% yield. This trend probably reflects the relative ease by which the corresponding dications are formed in the superacid, since it is expected that the 1,4-dication would be a more powerful electrophile than the 1,5-dication. Electron-deficient nitriles are well known for their tendencies to form the triazine ring system7 and this may also be a competing side reaction. If this is the case for cyanamide, the competing trimerization to melamine limits the yield of benzamide (15).

Isomeric dications 29-31 were also studied by DFT calculations at the B3LYP 6-311G (d,p) level of theory and charge proximity dramatically influenced the relative stability of the ions (Figure 1).8 As such, the 1,5-dication (29) is found be 20 kcal/mol more stable than the 1,4-dication (30). Attempts to locate a stable minimum for dication 31 were not successful.
Fig. 1.
Calculated relative energies for superelectrophiles 29 and 30.
NMR experiments were also done to characterize the intermediates formed from the amino-nitriles in acidic media. Compounds 1, 4, 9, and dimethylcyanamide (32) were studied in solutions of increasing acidity (Table 2). Both compounds 1 and 4 show similar trends in the 13C NMR spectra. In CF3CO2H solution (Ho −2.7), compounds 1 and 4 exhibit significant shifts for the methyl groups and the nitrile carbons. This is a consequence of the amine group being protonated by the CF3CO2H. When the compounds are dissolved in superacidic CF3SO3H (Ho −14.1), the nitrile carbons are shifted further upfield. This shift of the 13C resonances is likely due to protonation of the nitrile and formation of the nitrilium ion. Interestingly, compound 1 shows a change of chemical shift for the nitrile carbon of 3.0 ppm in CF3SO3H versus CF3CO2H, while compound 4 shows a change of just 1.2 ppm for the nitrile carbon. This may suggest a greater degree of protonation for compound 1 in the superacid compared to compound 4. Dimethylcyanamide (32) was also studied in these acidic solutions, however the data suggests rapid conversion to the triazine. When compound 32 is dissolved in CF3SO3H, two sets of peaks are observed. Although the peaks cannot be definitively assigned, it is likely that they are from protonated triazine species. Compound 9 exhibits a fairly significant shift of the nitrile 13C resonance in CF3CO2H solution (δ 108.8) compared to CDCl3 solution (δ 117.3). When compound 9 was dissolved in CF3SO3H, the 13C spectrum suggested an equilibrium between two major components (see Supporting Information). With the complexity of this spectrum, it was difficult to make peak assignments. Another sample was prepared with FSO3H, a slightly stronger superacid (Ho −15.1).9 The resulting 13C spectrum had six major resonances (Table 2). Five small peaks (from a minor componant of the equilibrium) were also visible in the spectrum: δ, 127.7, 132.7, 134.0, 144.2, 148.8, 163.7.
Table 2.
13C NMR data for compounds 1, 4, 30, and 9 in solutions of varying acidity (analyses done at 25°C, except CF3SO3H samples which were done at −30°C).
| Substrate | Solvent | 13C NMR Siignals |
|---|---|---|

|
CDCl3 | 16.2, 45.0, 54.5, 118.7 |
| CF3CO2H | 12.5, 42.8, 52.4, 113.8 | |
| CF3SO3H | 12.6, 43.0, 51.4, 110.8 | |

|
CDCl3 | 44.0, 47.3, 114.5 |
| CF3CO2H | 43.1, 44.3, 108.4 | |
| CF3SO3H | 43.7, 44.5, 107.2 | |

|
CDCl3 | 40.3, 119.2 |
| CF3CO2H | 35.1, 158.5 | |
| CF3SO3H | a. 39.3, 133.1 b. 41.2, 144.0 |
|

|
CDCl3 | 117.3, 127.2, 128.6, 133.9, 137.3, 151.2 |
| CF3CO2H | 108.8, 123.2, 130.3, 131.7, 144.3, 147.3 |
|
| FSO3H | 109.2, 122.3, 130.8, 132.3, 144.2, 148.3 |
There is very little published 13C NMR data for nitrilium ions.10 Shudo and Ohwada have reported that N-protonated benzonitrile (33) exhibits a 13C NMR resonance at 103 ppm for the nitrilium carbon, shifted upfield from benzonitrile itself (119 ppm).2 This is consistent with the results from compounds 1 and 4, where increasing levels of acidity shift the nitrile resonance upfield. This again suggests formation of the superelectrophilic, dicationic species 23 and 24 in CF3SO3H. Interestingly, the earlier studies also found evidence for the formation of a triflate adduct (34) from benzonitrile.2b In our study of compound 9, we observed a complex 13C spectrum from CF3SO3H, including two triflate quartets. These observations are consistent with the formation of the triflate adduct 36. In FSO3H solution, compound 9 appears to form mainly the monocationic species 37. There is very little change in the 13C spectra from CF3CO2H and FSO3H solutions, suggesting that the superelectrophile 28 is formed in low concentration in superacid. The minor component of the equilibrium is assumed to a fluorosulfonate adduct. With the CF3SO3H solutions of compounds 1, 4, and 32, the 13C spectra showed no evidence for the triflate adducts.
As described in the Shudo and Ohwada’s study, there is evidence for the involvement of diprotonated, superelectrophilic species (i.e., 35) in the superacid-promoted Houben-Hoesch reaction.2 Superelectrophile 35 is formed by double protonation at the nitrile group of benzonitrile and it is capable of reacting with the weak nucleophile benzene. In our results, the dicationic elelctrophiles are generated by protonation at a strong base site followed by protonation at the nitrile group. These superelectrophiles exhibit similar, or in some cases enhanced, reactivities compared to dications such as 35. For example, it was reported that benzonitrile reacts with benzene in CF3SO3H to give benzophenone in 14% yield.2b Similar conversions involving superelectrophiles 26-28 give diaryl ketones in yields ranging from 25-70% (Table 1).
3. Conclusion
In summary, we have found that amino-nitriles and related substrates may be effective reagents in the superacid-promoted Houben-Hoesch reaction. With 3-aminopropionyl nitriles, this represents a new method for preparing Mannich base-type products. Reactivity trends and NMR data indicate that dicationic superelectrophiles are involved in these transformations. Moreover, we have found that the iminium ion intermediates may be directly reduced to amine-type products in a one-pot conversion.
4. Experimental Section
4.1 General Methods
Trifluoromethanesulfonic acid was freshly distilled prior to use. Reagents and solvents were obtained from commercial suppliers and used as received. Reactions were performed using oven-dried glassware with an argon atmosphere. Low resolution mass spectra were obtained from a commercial GCMS equipped with a mass selective detector. High resolution mass spectra were obtained from external an analytical service organization. All products were characterized by 1H and 13C NMR and mass spectroscopic methods. Known compounds exhibited spectral data consistent with those reported in the literature.
4.2 General preparation of the ketones
The amino-nitrile (1 mmol) is dissolved in dry CH2Cl2 (5 mL) to which is added benzene (2 mL). With stirring, triflic acid (2 mL) is then slowly added and the reaction is stirred overnight at 60– 80°C. The reaction is then quenched by pouring the solution over ice/water. The aqueous solution is then made basic by slow addition of 10 M NaOH and the solution is extracted twice with chloroform. The organic layer is washed with water, brine (2 × 20 ml) and dried over anhydrous sodium sulfate. In the event that imine/ketone mixtures are obtained, it may be necessary to stir the triflic acid/ice/water mixture for few hours in order to ensure complete hydrolysis. The products are purified via column chromatography (silica gel; hexanes:ether).
4.3 General preparation of the amides
Using the same procedure, the triflic acid-promoted reaction is conducted and then the mixture is cooled in an ice bath and a methanolic solution (10 mL) of NaBH4 (5 mmol) is added (argon atmosphere). The reaction is left to stir overnight at room temperature. The mixture is then pouring over ice/water and made basic by slow addition of 10 M NaOH. The solution is extracted twice with chloroform. The organic layer is washed with water, brine (2 × 20 ml) and dried over anhydrous sodium sulfate. The amine is further acylated by direct addition of acetic anhydride (1 mmol). The products are purified via column chromatography (silica gel; hexanes:ether).
4.3.1 N-ethyl-N-(3-oxo-3-phenylpropyl)acetamide 11.
98% as a viscous, yellow oil. 1H NMR (CDCl3, 300 MHz; mixture of cis-trans isomers) minor component: δ 1.12 (t, 3H, J=4.2 Hz), 2.12 (s, 3H), 3.25 (t, 3H, J=4.2 Hz), 3.40 (q, 2H, J=4.2 Hz), 3.73 (t, 3H, J=4.2 Hz), 7.45-7.49 (m, 2H), 7.56-7.60 (m, 1H), 7.92-7.94 (m, 2H); major component: δ 1.18 (t, 3H, J=4.2 Hz), 2.07 (s, 3H), 3.30 (t, 3H, J=4.2 Hz), 3.37 (q, 2H, J=4.2 Hz), 3.68 (t, 3H, J=4.2 Hz), 7.42-7.45 (m, 2H), 7.53-7.56 (m, 1H), 7.95-7.97 (m, 2H). 13C NMR (CDCl3, 300 MHz; mixture of cis-trans isomers) minor component: δ 12.9, 21.5, 37.4, 40.4, 43.3, 127.9, 128.8, 133.4, 136.5, 170.4, 197.8; major component: δ 14,1, 21.4, 37.3, 42.0, 44.5, 128.1, 128.6, 133.3, 136.7, 170.6, 199.2. Low resolution mass spectrum (EI) m/z: 219 (M+), 176, 105, 77, 58. High resolution mass spectrum, calcd for C13H17O2N, 219.12593, found 219.12710.
4.3.2 N-(2-(acetamido(phenyl)methyl)phenyl)acetamide 20.
22% as a light yellow solid, mp: 178-180°C. 1H NMR (CDCl3, 500 MHz) δ 2.16 (s, 3H), 2.24 (s, 3H), 2.54 (s, 1H), 6.61 (d, 1H, J = 9.5), 6.98-7.00 (m, 2H), 7.08 (t, 1H, J = 7.5 Hz), 7.30-7.45 (m, 4H), 7.95 (d, 1H, J = 8 Hz), 9.16 (s, 1H). 13C NMR (CDCl3, 500 MHz) δ 23.2, 24.2, 51.5, 124.9, 125.2, 126.3, 126.9, 127.6, 128.5, 128.7, 128.8, 133.8, 134.8, 136.3, 139.4, 169.7, 170.9. Low resolution mass spectrum (EI) m/z: 283 (M+), 264, 239, 222, 197, 180, 164, 145, 119, 104, 77, 62, 43. High resolution mass spectrum, calcd for C17H18O2N2, 282.13683, found 282.13759
4.3.3 N-((3-methylpyridin-2-yl)(phenyl)methyl)acetamide 22.
20% as a viscous, colorless oil. 1H NMR (CDCl3, 500 MHz) δ 2.07 (s, 3H), 2.26 (s, 3H), 6.34 (d, 1H, J= 8 Hz), 7.19-7.26 (m, 2H), 7.29-7.34 (m, 2H), 7.48 (dd, 1H, J= 0.5, 7.5 Hz), 7.91 (d, 1H, J= 7 Hz), 8.48 (dd, 1H, J= 1.0, 5.0 Hz). 13C NMR (CDCl3, 500 MHz) δ18.2, 23.5, 53.8, 122.7, 127.5, 128.2, 128.5, 131.3, 138.9, 140.6, 145.7, 156.6, 169.1. Low Resolution Mass Spectra (EI): m/z: 240 (M+), 197, 181, 147, 106, 43. Anal. calcd for C15H16N2O: C, 74.97; H, 6.71; N, 11.66. Found: C, 72.72; H, 6.82; N, 10.79.
Supplementary Material
Acknowledgments.
We gratefully acknowledge the support of the National Science Foundation (CHE-0749907) and the NIH-National Institute of General Medical Sciences (GM085736-01A1).
Footnotes
Supplementary data
Computational methods and results, characterization data and spectra of compounds are associated with this article as supplementary data. Supplementary data associated with this article can be found in the online version, at doi:.
References and notes
- 1.a Hoesch K. Ber. Dtsch. Chem. Ges. 1915;48:1122. [Google Scholar]; b Houben J. Ber. Dtsch. Chem. Ges. 1926;59:2878. [Google Scholar]; c Smith MB, March J. March’s Advanced Organic Chemistry. 6th Ed. Wiley; NY: 2007. pp. 732–733. [Google Scholar]
- 2.a Sato Y, Yato M, Ohwada T, Saito S, Shudo K. J. Am. Chem. Soc. 1995;117:3037. [Google Scholar]; b Yato M, Ohwada T, Shudo K. J. Am. Chem. Soc. 1991;113:691. [Google Scholar]
- 3.Klumpp DA. In: Recent Developments in Carbocation and Onium Ion Chemistry. Laali K, editor. American Chemical Society; Washington, DC: 2007. pp. 144–159. ACS Symposium Series 395. [Google Scholar]
- 4.a Gracias V, Ji Z, Akritopoulou-Zanze I, Abad-Zapatero C, Huth JR, Song D, Hajduk PJ, Johnson EF, Glaser KB, Marcotte PA, Pease L, Soni NB, Stewart KD, Davidsen SK, Michaelides MR, Djuric SW. Bioorg. Med. Chem. Lett. 2008;18:2691. doi: 10.1016/j.bmcl.2008.03.021. [DOI] [PubMed] [Google Scholar]; b Raja AS, Pandeya SN, Panda SS, Stables JP. Pharm. Chem. J. 2007;41:302. [Google Scholar]
- 5.Chung F, Tisne C, Lecourt T, Dardel F, Micouin L. Angew. Chem. Int. Ed. 2007;46:4489. doi: 10.1002/anie.200605201. [DOI] [PubMed] [Google Scholar]
- 6.a Leclerc E, Vrancken E, Mangeney P. J. Org. Chem. 2002;67:8928. doi: 10.1021/jo025872t. [DOI] [PubMed] [Google Scholar]; b Weiberth FJ, Hall SS. J. Org. Chem. 1986;51:5338. [Google Scholar]
- 7.Pankratov VA, Chesnokova AE. Russ. Chem. Rev. 1989;58:879. [Google Scholar]
- 8.Calculations done using Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Gaussian, Inc.; Wallingford CT: 2004.
- 9.Olah GA, Prakash GKS, Molnar A, Sommer JM. Superacids. 2nd Ed Wiley and Sons; NY: 2009. [Google Scholar]
- 10.Olah GA, Kiovsky TE. J. Am. Chem. Soc. 1968;90:4666. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



