Abstract
A growing body of evidence has demonstrated a role for group II metabotropic glutamate receptors (mGluRs) in the reinforcing effects of cocaine. These receptors are important given their location in limbic-related areas, and their ability to control the release of glutamate and other neurotransmitters. They are also potential targets for novel pharmacotherapies for cocaine addiction. The present study investigated the impact of chronic cocaine self-administration (9.0 mg/kg/session for 100 sessions, 900 mg/kg total intake) on the densities of group II mGluRs, as assessed with in vitro receptor autoradiography, in the striatum of adult male rhesus monkeys. Binding of [3H]LY341495 to group II mGluRs in control animals was heterogeneous, with a medial to lateral gradient in binding density. Significant elevations in the density of group II mGluRs following chronic cocaine self-administration were measured in the dorsal, central and ventral portions of the caudate nucleus (P<0.05), compared to controls. No differences in receptor density were observed between the groups in either the putamen or nucleus accumbens. These data demonstrate that group II mGluRs in the dorsal striatum are more sensitive to the effects of chronic cocaine exposure than those in the ventral striatum. Cocaine-induced dysregulation of the glutamate system, and its consequent impact on plasticity and synaptic remodeling, will likely be an important consideration in the development of novel pharmacotherapies for cocaine addiction.
Keywords: cocaine self-administration, non-human primate, striatum, group II metabotropic glutamate receptors
Introduction
Cocaine abuse remains a significant problem worldwide with close to 40 million Americans age 12 and older reported to have tried cocaine or crack at least once, according to the National Survey on Drug Use and Health [1]. Of these individuals, nearly 4 million are considered regular users. Despite significant efforts effective treatment strategies for cocaine addiction have remained elusive. Thus, the identification of new therapeutic targets is of great importance. While research investigating the neurochemical effects of cocaine has traditionally focused on monoamine systems, recent studies have shown that there are also significant adaptations in glutamate systems [2], particularly following chronic cocaine exposure [3].
Group II metabotropic glutamate receptors (mGluRs), comprised of mGluR2 and mGluR3, are inhibitory Gi/Go-coupled G-protein receptors that are located both pre- and post-synaptically [4, 5]. When located pre-synaptically they function as autoreceptors, reducing glutamatergic transmission via negative feedback [6, 7]. Acting as heteroceptors, they can also influence the release of other neurotransmitters [8–10]. Group II mGluRs are heterogeneously distributed throughout the brain, and are particularly dense in areas such as the hippocampus, thalamus, striatum, amygdala and cortex [5, 11–14]. Given their location in limbic-related regions and their ability to modulate neurotransmission, it is not surprising that these receptors have been implicated in a variety of psychiatric disorders, such as schizophrenia and depression [15–17].
Recent data suggests that group II mGluRs are also involved in the reinforcing effects of cocaine [18–20]. For example, selective agonists of group II mGluRs, such as LY379268, block cocaine- and cue-induced reinstatement of cocaine-seeking in rats [21] when administered either systemically or directly into the nucleus accumbens [22]. In a similar fashion, LY379268 reduces cocaine self-administration and attenuates both cue- and cocaine-induced reinstatement of cocaine-seeking in non-human primates [23]. Few studies to date, however, have assessed how chronic cocaine exposure affects the regulation of group II mGluRs, an important consideration given the current interest in these receptors as targets for cocaine medications [24]. Thus, the present study investigated the impact of chronic cocaine self-administration on the densities of group II mGluRs, as assessed by in vitro receptor autoradiography, in the striatum of non-human primates. The cocaine self-administration paradigm involved 100 sessions of high-dose (0.3 mg/kg/injection) cocaine injections that resulted in a cumulative intake of 900 mg/kg; this regimen and total cocaine intake has been shown to significantly impact monoamine neurotransmission [25–28]. A non-human primate model was chosen because of the close homology of non-human primates to humans both in terms of striatal neuroanatomy and the glutamate system.
Materials and Methods
Subjects
Ten experimentally-naïve adult male rhesus monkeys (Macaca mulatta) weighing between 8.1–12.0 kg (mean ± SD; 10.1 ± 0.4 kg) at the start of the study served as subjects. All monkeys were individually housed with water available ad libitum in the home cage. Monkeys were weighed weekly and fed enough food daily (Lab Diet Monkey Chow and fruit supplementation) to maintain body weights at approximately 95% of free-feeding weights. Experimental procedures were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Self-administration
Details of surgical and self-administration procedures for this group of monkeys have been previously described [28, 29]. Briefly, monkeys were initially trained in operant chambers to respond on one of two levers under a fixed-interval (FI) 3-min schedule for 1g banana-flavored pellets. Daily training sessions terminated after 30 reinforcers were obtained and a minimum of 20 sessions with stable performance (± 20% of the mean for 3 consecutive sessions) satisfied training requirements. Upon achieving response stability, the feeder was unplugged and the effects of extinction on responding were examined for 5 consecutive sessions. Following extinction, food-maintained responding was reinstated for all monkeys. These extinction rates were used later to determine whether cocaine acted as a reinforcer in each monkey. Indwelling intravenous catheters were implanted into the femoral vein and subcutaneous ports were positioned in the lower back in all monkeys. After surgery, food-reinforced responding was re-established after which monkeys were divided into two groups. Control monkeys (n = 6) continued responding under the food-reinforced FI 3-min schedule for the duration of the study. Experimental monkeys (n = 4) began self-administration of cocaine (FI 3-min; 0.3 mg/kg per injection) for a period of 100 sessions. Daily sessions ended after 30 reinforcers were obtained. Animals obtained all of the reinforcers available each session. Detailed analyses of the behavioral data have been reported previously [28].
Tissue Processing
Monkeys utilized in this study were also used for 2-deoxy-d-[14C] glucose (2-DG) analysis. This technique enables us to visualize how patterns of functional brain activity shift as a consequence of chronic cocaine self-administration. The 2DG data from these animals has been reported previously [29–32]. The 2-DG experiment was initiated within 2 min of the final reinforcer on the last self-administration session (either food or cocaine reinforcement) and involved timed sampling of arterial blood for ~ 45 min. Immediately after the 2-DG procedure, animals were euthanized with sodium pentobarbital (100 mg/kg, i.v.). Brains were immediately removed, blocked, frozen in isopentane (−35 ºC to −50 ºC) and then stored at −80 ºC. Coronal sections (20 μm) were cut on a cryostat, thaw-mounted onto electrostatically charged slides, desiccated and stored at −80 ºC until autoradiography processing.
[3H]LY341495 Autoradiography
Autoradiography procedures for labeling group II mGluRs with the selective antagonist [3H]LY341495 were adapted for use in nonhuman primate tissue from those of Wright et al. [33]. Tissue sections were pre-incubated at room temperature in buffer (10 mM potassium phosphate buffer with 100 mM potassium bromide, pH 7.6) for 30 min. Pre-incubation removed any endogenous ligand, residual cocaine, and 2-DG. Sections were then incubated for 2 hours in buffer containing 4 nM [3H]LY341495 (40 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA) in the presence (non-specific binding) or absence (total binding) of 1 mM glutamate. Sections were rinsed 2 times (30 sec each) in ice-cold buffer, with a final 10 sec rinse in ice-cold water. Sections were immediately dried under a stream of cold air, dessicated overnight and apposed, along with calibrated [3H] autoradiographic standards (Amersham, Piscataway, NJ), to Kodak Biomax MR film (Perkin Elmer, Waltham, MA) for 9 weeks.
Densitometry and Data Analysis
Binding of [3H]LY341495 to group II mGluRs in the striatum was measured via densitometric quantification of autoradiograms. The level of striatum chosen for analysis was that at which the nucleus accumbens could most clearly be differentiated into core and shell divisions (Bregma 0.45 to – 0.45 mm; [34]). The striatum was then further divided into dorsal, central and ventral caudate nucleus and dorsal, central and ventral putamen, according to the atlas of Paxinos and colleagues (Panel A, Figure 1; [34]). Analyses of autoradiograms were conducted by quantitative densitometry with a computerized image processing system (MCID, InterFocus Imaging Ltd., Linton, United Kingdom). Tissue / mg wet weight tissue) were determined from optical densities using a calibration curve obtained by densitometric analysis of [3H] standards on each autoradiogram. Specific binding was determined by subtracting non-specific binding values from the total binding values, measured in adjacent sections. Statistical analysis was performed for each brain structure by means of a two-way independent Student’s t-test.
Results
[3H]LY341495 binding in control animals
Binding of [3H]LY341495 to group II mGluRs was seen throughout the striatum of non-human primates (Table 1). Specific binding of [3H]LY341495 accounted for greater than 85% of total binding (see Figure 1 supplementary data). In agreement with previous reports, group II mGluRs were observed throughout the caudate, putamen and nucleus accumbens [14, 35–38]. The topography of group II mGluR binding sites in non-drug exposed control animals was heterogeneous with a gradual medial to lateral gradient (highest to lowest) in binding density. Higher densities of binding were observed in the medial aspects of the central and ventral caudate, while more moderate levels of binding were observed in the medial aspects of the putamen. The lowest levels of binding were measured in the lateral portions of the caudate nucleus, putamen and nucleus accumbens core and shell (Panel A Figure 2).
Table 1.
Effect of chronic cocaine self-administration on the distribution of [3H]LY341495 binding to group II mGluRs in non-human primate striatum.
| Brain Region | Food Controls (N=6) | Chronic cocaine self-administration (N=4) |
|---|---|---|
| Dorsal caudate (1) | 440 ± 19.8 | 524 ± 29.8* |
| Central caudate (2) | 534 ± 16.8 | 606 ± 24.4* |
| Ventral caudate (3) | 508 ± 16.2 | 581 ± 22.7* |
| Dorsal putamen (4) | 406 ± 14.8 | 470 ± 39.4 |
| Central putamen (5) | 423 ± 16.5 | 481 ± 30.7 |
| Ventral putamen (6) | 457 ± 18.9 | 522 ± 29.2 |
| Nucleus accumbens core (7) | 387 ± 13.5 | 388 ± 39.2 |
| Nucleus accumbens shell (8) | 402 ± 14.7 | 422 ± 16.2 |
Mean ± S.E.M. data are presented as specific binding in fmols/mg of wet weight tissue.
Numbers in parentheses next to brain regions indicate their location in Figure 1, supplementary data.
P<0.05 compared to food controls, two-way unpaired Student’s t-test.
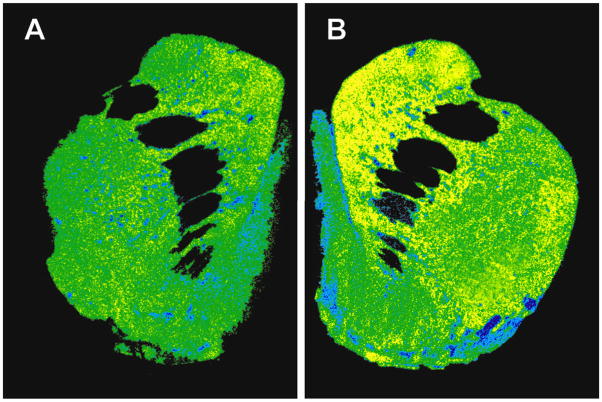
Figure 2.
Pseudocolor-enhanced autoradiogram of [3H]LY341495 binding in the striatum of a control (Panel A) and cocaine self-administering (Panel B) monkey.
Effect of chronic cocaine self-administration on [3H]LY341495 binding sites
In those animals that chronically self-administered cocaine, densities of [3H]LY341495 binding sites were significantly higher in all portions of the caudate nucleus (dorsal, +19 %, central, +13 % and ventral, +14 %; Table 1, Panel B Figure 2), compared to control animals. In contrast, there were no differences between control and cocaine-exposed animals in [3H]LY341495 binding sites in either the putamen or the nucleus accumbens core or shell (Table 1).
Discussion
The present study demonstrates that there are significant adaptations in the striatal glutamatergic system in response to chronic cocaine self-administration, though these appear to be restricted to the more dorsal territories of the striatum. We chose to focus on the striatum in this study based on three facts: 1) the striatum plays an essential role in the reinforcing effects of cocaine [39, 40], 2) group II mGluRs attenuate the reinforcing effects of cocaine [18–20] and 3) group II mGluRs are localized in the striatum [14, 35]. The finding of significantly higher densities of group II mGluRs in the caudate nucleus as a result of chronic cocaine self-administration indicates the presence of considerable plasticity within the dorsal striatum. In contrast, no differences in receptor density were observed between the groups in either the putamen or nucleus accumbens. These data suggest, therefore, that elements of the glutamate system within the dorsal striatum may be more sensitive, compared with the ventral striatum, to the effects of chronic cocaine exposure in the non-human primate.
The localization of [3H]LY341495 binding sites within the non-human primate striatum agrees with previous autoradiographic and immunohistochemical labeling studies [14, 35, 37]. A novel finding of the present study was the identification of a medial-lateral gradient (high to low) in the binding density of group II mGluRs. The highest levels were found in the medial caudate nucleus and lowest in the lateral portions of the caudate, putamen and nucleus accumbens core and shell. Given the topographical organization of glutamatergic projections to different regions of the striatum [41–43], the differential distribution of group II mGluRs within the medial and lateral portions of the striatum may have functional implications for how glutamate regulates the striatum.
Group II mGluRs in the striatum are thought to be localized primarily pre-synaptically [5, 11], where they can modulate the release of neurotransmitters [44–46]. As exposure to cocaine increases, there is hypothesized to be a gradual and escalating involvement of the glutamatergic system [2, 47]. Indeed, previous data in rodents have shown that acute administration of cocaine does not induce the release of glutamate [3, 48], whereas following chronic self-administration, levels of extracellular glutamate are progressively increased with each dose of cocaine [3]. One mechanism explaining our results, then, may be a compensatory increase in the levels of group II mGluRs autoreceptors in response to these sustained elevations in extracellular glutamate concentration. One of the predicted consequences of these up-regulated group II mGluR autoreceptors may be lower basal levels of glutamate, which have been reported to occur in cocaine-exposed rodents [3, 49, 50] and humans [51]. In agreement with our data, Weiss and colleagues reported functional increases in group II mGluRs in a number of brain areas such as the prefrontal cortex, central nucleus of the amygdala, and hippocampus following escalation of cocaine self-administration in rats [52]. Thus, future studies will be aimed at investigating how group II mGluRs are dysregulated in response to cocaine self-administration in these and other brain areas.
One of the interesting outcomes from the present study was the lack of effects in the ventral striatum (incorporating both ventral putamen and nucleus accumbens). A recent investigation in rodents also failed to observe any impact on the function of group II mGluRs in the nucleus accumbens following chronic cocaine self-administration [52]. Although the initial effects of cocaine have been shown to be primarily located in limbic-related areas, such as the ventral striatum [31], with longer exposure the effects of cocaine expand to incorporate more dorsal regions [53, 54]. Given the extended duration of cocaine self-administration in the present study, therefore, it is possible that group II mGluRs may have been transiently affected in the ventral striatum earlier in the course of exposure. Thus, the compartmentalization of effects and relative sensitivity of the glutamate system in the dorsal striatum following chronic exposure to cocaine self-administration is in accordance with these studies.
In contrast to the data reported here, data from rodent studies have reported decreased function, or lower levels, of group II mGluRs following repeated cocaine exposure [55, 56]. There are, however, important differences between these studies. First, there are considerable species differences in the glutamate system [57–59], as well as in the response of the brain to cocaine [60, 61]. Second, the monkeys in our study self-administered cocaine, while the rodents in the other studies received cocaine non-contingently. There is evidence of behavioral and neurochemical differences in the effects of cocaine delivered contingently versus non-contingently [62–65]. Third, the total intake of cocaine received by the animals between the studies was very different (180–200 mg/kg in the rodent studies, 900 mg/kg in the present study) and fourth, these previous studies involved a period of abstinence (up to 3 weeks) before measuring either the density or functionality of group II mGluRs. As it relates to the latter point, future studies are needed to investigate the role of cocaine abstinence following chronic cocaine self-administration to determine how malleable group II mGluR densities are in response to long-term cocaine exposure and abstinence in non-human primate brains.
Conclusions
Thus, our data suggests that group II mGluRs are altered as a direct consequence of chronic cocaine exposure, potentially leading to impaired regulation of glutamate transmission. As reviewed recently by Kalivas and O’Brien [47], the transition to addiction likely involves a shift in the pattern of neural circuits that control cocaine-related behavior. Correspondingly, there may also be a change to a more dominant role for glutamate versus dopamine in mediating the effects of chronic cocaine. Since there is a critical role for glutamate in the control of plasticity and synaptic remodeling, it is essential to understand how this system is impacted by long-term cocaine exposure.
Supplementary Material
Figure 1, supplementary data: Representative autoradiogram of [3H]LY341495 binding in a control monkey. Total (Panel A), and non-specific (Panel B) binding are shown. Numbers in Panel A represent areas chosen for densitometric analysis; 1, dorsal caudate; 2, central caudate; 3, ventral caudate; 4, dorsal putamen; 5, central putamen; 6, ventral putamen; 7, nucleus accumbens core; 8, nucleus accumbens shell.
Highlights.
Group II metabotropic glutamate (mGluRs) are involved in the effects of cocaine
We studied the density of group II mGluRs following chronic cocaine in monkeys
We found higher levels of binding in the dorsal striatum, not the ventral striatum
Therefore the dorsal striatum may be more sensitive to the effects of cocaine
Treatments for cocaine that target these receptors may be efficacious
Acknowledgments
This work was supported by NIDA DA26590 (TJRB), DA09085 (LJP) and DA06634 (P50).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
T.J.R. Beveridge, Email: tbeverid@wfubmc.edu.
H.R. Smith, Email: hsmith@wfubmc.edu.
M.A. Nader, Email: mnader@wfubmc.edu.
L.J. Porrino, Email: lporrino@wfubmc.edu.
References
- 1.SAMSHA. United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. National Survey on Drug Use and Health; Ann Arbor, MI: 2008. [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Miguens M, et al. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196(2):303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 4.Lujan R, et al. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13(4):219–41. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 5.Petralia RS, et al. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71(4):949–76. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich D, et al. Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 2002;42(3):297–305. doi: 10.1016/s0028-3908(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 7.Kew JN, et al. Activity-dependent presynaptic autoinhibition by group II metabotropic glutamate receptors at the perforant path inputs to the dentate gyrus and CA1. Neuropharmacology. 2001;40(1):20–7. doi: 10.1016/s0028-3908(00)00118-0. [DOI] [PubMed] [Google Scholar]
- 8.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29(1):83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 9.Marek GJ, et al. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292(1):76–87. [PubMed] [Google Scholar]
- 10.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299(1):12–20. [PubMed] [Google Scholar]
- 11.Gu G, et al. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 12.Ohishi H, et al. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335(2):252–66. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 13.Ohishi H, et al. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53(4):1009–18. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 14.Testa CM, et al. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390(1):5–19. [PubMed] [Google Scholar]
- 15.Bespalov AY, et al. Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. Eur J Pharmacol. 2008;592(1–3):96–102. doi: 10.1016/j.ejphar.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 16.Chaki S, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46(4):457–67. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Feyissa AM, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):279–83. doi: 10.1016/j.pnpbp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33(8):1818–26. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- 19.Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav. 2009;94(1):204–10. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morishima Y, et al. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102(11):4170–5. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24(20):4723–7. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186(2):143–9. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 23.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318(2):922–31. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 24.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25(5):265–72. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Beveridge TJ, et al. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34(5):1162–71. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letchworth SR, et al. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macey DJ, et al. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23(1):12–6. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nader MA, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 29.Beveridge TJ, et al. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobe of nonhuman primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- 30.Beveridge TJ, et al. Functional effects of cocaine self-administration in primate brain regions regulating cardiovascular function. Neurosci Lett. 2004;370(2–3):201–5. doi: 10.1016/j.neulet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Porrino LJ, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22(17):7687–94. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porrino LJ, et al. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24(14):3554–62. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright RA, et al. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther. 2001;298(2):453–60. [PubMed] [Google Scholar]
- 34.Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Vol. 1. San Diego, CA: Academic Press; 2000. (various pagings) [Google Scholar]
- 35.Phillips T, et al. Localization of metabotropic glutamate receptor type 2 in the human brain. Neuroscience. 2000;95(4):1139–56. doi: 10.1016/s0306-4522(99)00353-x. [DOI] [PubMed] [Google Scholar]
- 36.Richards G, et al. Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H]LY354740 binding in vitro and quantitative radioautography: correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPgammas binding. J Comp Neurol. 2005;487(1):15–27. doi: 10.1002/cne.20538. [DOI] [PubMed] [Google Scholar]
- 37.Samadi P, et al. Basal ganglia group II metabotropic glutamate receptors specific binding in non-human primate model of L-Dopa-induced dyskinesias. Neuropharmacology. 2008;54(2):258–68. doi: 10.1016/j.neuropharm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Smith Y, et al. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat. 2001;22(1–2):13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6(6):615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 40.Roberts DC, et al. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12(5):781–7. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 41.Fudge JL, et al. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110(2):257–75. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 42.Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350(3):337–56. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- 43.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20(10):3798–813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marti M, et al. Presynaptic group I and II metabotropic glutamate receptors oppositely modulate striatal acetylcholine release. Eur J Neurosci. 2001;14(7):1181–4. doi: 10.1046/j.0953-816x.2001.01750.x. [DOI] [PubMed] [Google Scholar]
- 45.Pisani A, et al. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22(14):6176–85. doi: 10.1523/JNEUROSCI.22-14-06176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xi ZX, et al. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300(1):162–71. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 47.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 48.Xi ZX, et al. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010;58(1):304–13. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker DA, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 50.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, et al. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174(3):171–6. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao Y, Martin-Fardon R, Weiss F. Behavioral and Functional Evidence of Metabotropic Glutamate Receptor 2/3 and Metabotropic Glutamate Receptor 5 Dysregulation in Cocaine-Escalated Rats: Factor in the Transition to Dependence. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porrino LJ, et al. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004;27(8):813–20. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159(1):414–26. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 56.Xi ZX, et al. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303(2):608–15. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 57.Hill MP, Brotchie JM. Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum. Br J Pharmacol. 1999;127(1):275–83. doi: 10.1038/sj.bjp.0702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21(6):1838–47. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500(4):788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- 60.Lyons D, et al. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16(3):1230–8. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zocchi A, Conti G, Orzi F. Differential effects of cocaine on local cerebral glucose utilization in the mouse and in the rat. Neurosci Lett. 2001;306(3):177–80. doi: 10.1016/s0304-3940(01)01898-5. [DOI] [PubMed] [Google Scholar]
- 62.Broadbear JH, et al. Effects of response contingent and noncontingent cocaine injection on hypothalamic-pituitary-adrenal activity in rhesus monkeys. J Pharmacol Exp Ther. 1999;290(1):393–402. [PubMed] [Google Scholar]
- 63.Hemby SE, et al. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133(1):7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- 64.Howell LL, et al. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208(2):191–9. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markou A, Arroyo M, Everitt BJ. Effects of contingent and non-contingent cocaine on drug-seeking behavior measured using a second-order schedule of cocaine reinforcement in rats. Neuropsychopharmacology. 1999;20(6):542–55. doi: 10.1016/S0893-133X(98)00080-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1, supplementary data: Representative autoradiogram of [3H]LY341495 binding in a control monkey. Total (Panel A), and non-specific (Panel B) binding are shown. Numbers in Panel A represent areas chosen for densitometric analysis; 1, dorsal caudate; 2, central caudate; 3, ventral caudate; 4, dorsal putamen; 5, central putamen; 6, ventral putamen; 7, nucleus accumbens core; 8, nucleus accumbens shell.