Abstract
Adenocarcinomas of the lung commonly show an increase in the activity of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, yet many are resistant to apoptosis induced by the inhibition of PI3K. We hypothesized that Bcl-xL would have a synergistic effect on the apoptotic response induced by inhibition of the PI3K/Akt pathway in lung adenocarcinoma. To test this, we examined the effect of the PI3K inhibitor and LY294002 on lung adenocarcinoma cell lines expressing varying levels of Bcl-xL. We found that cells that overexpress Bcl-xL are resistant LY294002-induced apoptosis, while cells that express little Bcl-xL readily are not. Restoring Bcl-xL expression in cells that express low level of Bcl-xL conferred resistance to apoptosis in response to LY294002. The simultaneous inhibition of the PI3K/Akt pathway by LY294002 or Akt1 siRNA and Bcl-xL function by ABT-737 or Bcl-xL siRNA greatly enhanced the apoptotic response. Moreover, this response was associated with the induction of proapoptotic BH3-only BCL2 family member Bim. Our data suggest that PI3K/Akt and Bcl-xL pathways control cell death in lung adenocarcinoma cells in a synergistic manner. Modulation of Bcl-xL expression may represent one important strategy to optimize the efficacy of therapeutic agents targeting the PI3K/Akt pathway in adenocarcinoma of the lung.
Keywords: adenocarcinoma, PI3K/Akt pathway, Bcl-xL, apoptosis
Introduction
Lung cancer is the number one cause of cancer-related deaths worldwide with approximately 1.5 million cases each year (1). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancers, among which adenocarcinomas are the most common (40%). Adenocarcinomas of the lung have a high mortality rate, with a 5-year overall survival that is generally less than 15% (2). A major limitation to the curative potential of current therapy is resistance to chemotherapy (3). Anticancer drugs exert at least part of their cytotoxic effect by triggering apoptosis. Better understanding the molecular mechanisms controlling apoptosis is therefore crucial to defining new targets for therapeutic intervention in lung cancer.
Molecular genetic studies have led to the discovery of several potential targets for therapeutic design, such as PI3K and Akt. The PI3K signal transduction pathway was found to regulate cell proliferation and survival and to be closely associated with the development and progression of various tumors (4). We and others have suggested that the PI3K signaling pathway is involved in the early stage of lung cancer progression; increases in gene copy number of the PI3K catalytic subunit and increases in Akt activity, as detected by phosphorylation status, have been observed in premalignant and malignant human bronchial epithelial cells and in NSCLC cells (5–7). Downstream from PI3K, phosphorylated Akt is a powerful promoter of cell survival as it antagonizes and inactivates various components of the apoptotic cascade such as proapoptotic Bad, caspase-9, and forkhead transcription factor family members (8). Various drugs targeted against molecular changes in these pathways have been developed and some are being tested for clinical use in lung cancer (9, 10). The apoptotic response resulting from the inhibition of PI3K/Akt pathways have been observed to varying degrees in several types of cancer (11–14) including NSCLC cells (15–18). Therefore, it is important to identify mechanisms of sensitivity and resistance to these agents.
Proteins of the Bcl-2 family are key regulators of apoptosis. Overexpression of anti-apoptotic proteins like Bcl-2 and Bcl-xL can provide tumor cells with resistance to a variety of cellular insults including chemotherapeutic drugs in cell culture and in animal models (19, 20). There is evidence for a link between this survival mechanism and the PI3K pathway. The PI3K pathway targets members of the Bcl-2 family through phosphorylation and functional regulation (21). The PI3K pathway also regulates the expression of these proteins, as PI3K/Akt stimulates the expression of anti-apoptotic Bcl-2 proteins, such as Bcl-xL and Mcl-1, through the activation of NF-kB (22). However whether Bcl-2 or Bcl-xL contributes to the resistance of lung adenocarcinoma cells to apoptosis induced by the inhibition of the PI3K/Akt pathway is not established.
The current study was therefore designed to investigate the synergistic effect PI3K/Akt pathway and Bcl-xL in controlling apoptosis in adenocarcinoma cells of the lung. We show that Bcl-xL plays a critical role in mediating resistance of lung adenocarcinoma cells to cell death induced by the inhibition of the PI3K/Akt pathway. Combined inhibition of Bcl-xL and PI3K/Akt pathway may represent a useful strategy for the treatment of lung adenocarcinoma.
Materials and Methods
Cell lines and culture conditions
Five human lung adenocarcinoma cell lines A549, H23, H1793, H549 and H441 were purchased from the American Type Culture Collection (Manassas, VA). The PI3K/Akt inhibitor LY294002 was purchased from Cell Signaling ( Beverly, MA, USA); Bcl-2/Bcl-xL inhibitor ABT-737 or enantiomer of ABT-737 was obtained from Abbott Laboratories (Abbott Park, IL, USA). The concentrations of these inhibitors used are as follows: LY294002 (25–50 μM); ABT-737 or enantiomer of ABT-737 (1–8 μM). In some experiments, the inhibitors were titrated to determine the lowest concentration that resulted in specific kinase inhibition and induction of apoptosis. The cells were plated 24h prior to adding the inhibitor in the presence of 10% serum for 24, 48, or 72 h and were then subjected to the analysis of Akt activation, cell apoptosis and cell cycle progression. All inhibitors were resuspended in DMSO as a vehicle. Apoptotic and cell cycle assays were repeated at least three times.
Antibodies and Immunoblot Analysis
A mouse monoclonal antibody to phosphorylated Akt (Ser473), phosphorylated p70 S6K, rabbit polyclonal antibodies to Akt, rabbit polyclonal antibodies to Bcl-xL, Mcl-1, Bad, Bax, Bim and Bid, rabbit polyclonal antibodies to PARP, Caspase 3 and Cleaved Caspase 3 were obtained from Cell Signaling (Beverly, MA). Goat anti-β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Western blotting was performed using standard procedures as described in our previous study (23), with detection using the ECL chemiluminescent system (Pierce Biotechnology, Rockford, IL). Antibody dilutions for immunoblotting were 1:1000. The blots were reprobed with an anti-actin antibody to correct for protein loading differences. Anti-rabbit, anti-goat and anti-mouse secondary antibodies were purchased from Promega (Madison, WI).
Silencing of Bcl-xL or Akt1 gene expression
Oligofectamine, Opti-MEMI and Stealth™ RNAi Negative Control Med GC (12935-300) were purchased from Invitrogen (Carlsbad, CA, USA). Three double-stranded Bcl-xL siRNAs, HSS141361 sense sequence 5′-GAUACAGCUGGAGUCAGUUUAGUGA-3′, anti-sense sequence 5′-UCACUAAACUGACUCCAGCUGUAUC-3′, HSS141362 sense sequence 5′-CCCAGUGCCAUCAAUGGCAACCCAU-3′, anti-sense sequence 5′-AUGGGUUGCCAUUGAUGGCACUGGG-3′, HSS141363 sense sequence 5′-GCAGUUUGGAUGCCCGGGAGGUGAU-3′, anti-sense sequence 5′-AUCACCUCCCGGGCAUCCAAACUGC-3′ and negative control siRNA 5′-CGUACGCGGAAUACU-UCGA-3′; three double-stranded Akt1 siRNAs, HSS100346 sense sequence 5′-GAC GUG GCU AUU GUG AAG GAG GGU U-3′, anti-sense sequence 5′-AAC CCU CCU UCA CAA UAG CCA CGU C-3′, HSS100347 sense sequence 5′-AUU CUU GAG GAG GAA GUA GCG UGG C-3′, anti-sense sequence 5′-GCC ACG CUA CUU CCU CCU CAA GAA U-3′, HSS100345 sense sequence 5′-AUA CCG GCA AAG AAG CGA UC UGC A-3′, anti-sense sequence 5′-UGC AGC AUC GCU UCU UUG CCG GUA U-3′ were synthesized by Invitrogen (Dallas, TX, USA) and were suspended in water at a concentration of 20 μM. The transfections were done according to the manufacturer’s instructions. Briefly, 1 × 105 or 5 × 104 cells were seeded into 6-well plates with medium overnight. For each well, 5 or 10 μl of each siRNA duplex sequence were mixed together with 185 μl of Opti-MEMI and then combined with another mixture prepared using 3 μl of oligofectamine and 15 μl of Opti-MEMI. The final concentration of the siRNA was 100 or 200 nM. For the combination of LY294002 and Bcl-xL siRNA treatment, cells were incubated with 25 μM LY294002 in 10 % FBS serum for additional 24 or 48 h.
Flow cytometry
For analysis of DNA content and cell cycle by flow cytometry, cells were pelleted, washed once with PBS, fixed with ethanol. At the time for flow cytometry analysis, cells were washed once in PBS, and then stained for DNA content by use of 0.5 ml of 50 μg/ml propidium iodide (PI) (Sigma-Aldrich, MO, USA) and 100μg/ml RNAase A (Qiagen, Valencia, CA, USA) in PBS and 38 mM sodium citrate pH 7.4. A total of 10,000–20,000 stained nuclei were subjected to flow cytometry analysis. Data were collected on a Becton Dickinson FACSCalibur flow cytometer using Cellquestpro software (BD Biosciences, San Jose, CA). Cell cycle analysis was performed using the ModFit LT software (Verity Software House Inc., Topsham, ME, USA). The percentage of cells in sub-G1 was considered apoptotic.
Apoptosis was evaluated by assessment of Annexin V and PI double staining (Invitrogen, Carlsbad, CA). Briefly, 1 × 106 cells treated cells were pelleted, washed with PBS, resuspended in 100 μl of binding buffer and incubated at room temperature for 15 min in the presence of Alexa Fluor®-488-conjugated Annexin V (0.2 μM) and 1 μl of PI (10 μg/ml) solution. After staining, 400 μl of binding buffer was added and Annexin V staining was then quantified by FACS analysis. Cells of positive Annexin V and negative PI were considered apoptotic. Data acquisition and analysis were performed by the CellQuestpro program (BD Biosciences, San Jose, CA).
Stable transfection of Bcl-xL in H23 cells
Retroviral plasmid pBabe vector and pBabe-Bcl-xL are generous gifts of Elizabeth Yang at Vanderbilt University (24). 4 μg of plasmid DNA were transfected into Phoenix-eco packaging cells by using PolyFect Transfection kit (Qiagen, Valencia, CA) according to the instructions of the manufacturer. After 48 hr, virus-containing media was collected and used to immediately infect H23 cells in the presence of 4 μg/ml Polybrene (Millipore, Billerica, MA). After 24 h of incubation, media was changed. Puromycin was added 48 h post transfection at a final concentration of 4μg/ml to obtain stable clones overexpressing Bcl-xL.
Statistical analyses
All determinations were performed in duplicate or triplicate for each group and each experiment was repeated at least three times. Values are means ± SD. Representative results from western blot and flow cytometry analysis from a single experiment are presented. Statistical analyses were performed by paired t-test. Differences were considered to be statistically significant at P<0.05. Two-tailed P-values of <0.05 were regarded as significant.
Results
Lung adenocarcinoma cells are resistant to apoptosis induced by inhibition of the PI3K/Akt but undergo cell cycle arrest
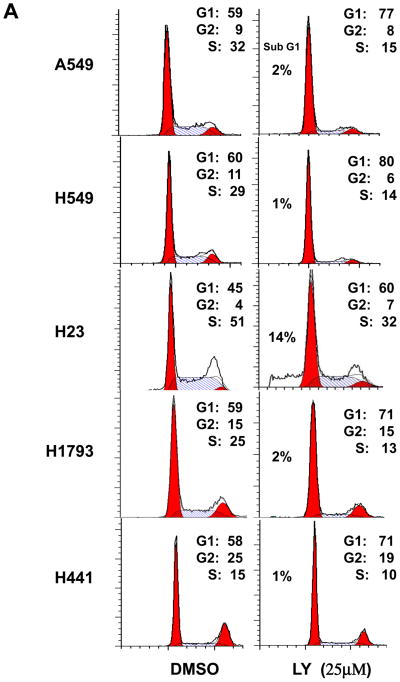
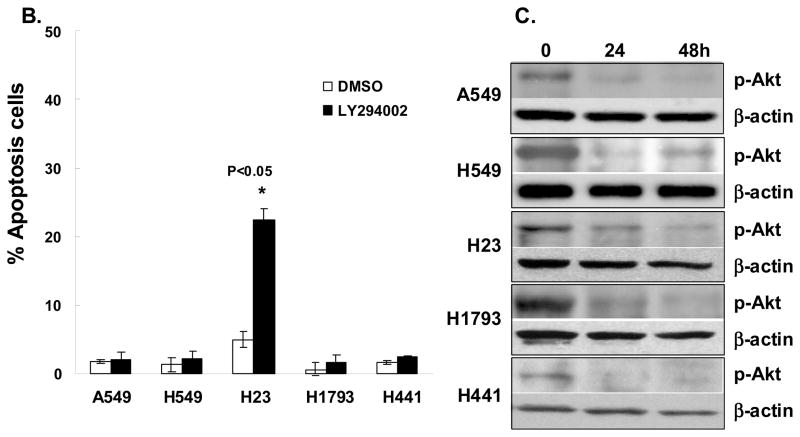
The apoptotic and cell cycle response to the PI3K/Akt inhibitor LY294002 were tested in a panel of five lung adenocarcinoma cell lines, A549, H549, H23, H1793 and H441 grown under normal growth conditions in the presence of 10% FBS. Akt activation was assessed by immunoblotting with phospho-specific antibodies to phosphorylated Akt at S473. Apoptosis was assessed by Annexin V binding assay and sub-G1 population by PI nuclear staining. Treatment of these cells with 25 μM LY294002 for 48 hours showed a negligible apoptotic response (<2%) in 4/5 cell lines tested (Figure 1A). Extending the treatment for up to 72 hours did not induce significant cell death in these cells (Figure 1B). In contrast, LY294002 induced apoptosis in more than 14–23 % in H23 cells (Figure 1A and B). While four out of five lung adenocarcinoma cell lines examined subjected to LY294002 failed to undergo apoptosis, this treatment was sufficient to inhibit cell growth and led to cell cycle arrest in G0/G1 in all five cell lines (Figure 1A). The ability of LY294002 to suppress the activation of Akt in these experiments was confirmed by western blotting with antibodies against phosphorylated Akt S473 as shown in Figure 1C. These data indicate that lung adenocarcninoma cells are commonly resistant to apoptosis induced by PI3K/Akt inhibition.
Figure 1.
Effects of PI3Kinase inhibition on lung adenocarcinoma cell survival. (A) LY294002 induced G1 cell cycle arrest in five human lung adenocarcinoma cells but only induced apoptosis in H23 cells. Cells were incubated for 48 hours with 25 μM LY294002. Apoptotic fraction was recognized as sub-G1 population of the cell cycle measured by flow cytometry with nuclear PI staining. One representative experiment of three with similar results is shown. The peak filled in red to the left denotes diploid cells in G1, the red peak to the right is G2 and the area marked by the hatched blue line denotes diploid cells in the S phase. (B) Cells were incubated for 72 hours with 25 mM LY294002 and followed by Annexin V and PI staining. Bars represent percentage of Annexin-V stained/PI negative cells for DMSO-treated control group (white bars), 25 μM LY294002-treated (black bars). Data are expressed as mean ± SD. Statistically significant differences between controls and inhibitor-treated group are indicated as: *, p < 0.05. (C) Immunoblot analysis of p-Akt on lung adenocarcinoma cell lines after 48h treatment with LY294002 (25 μM). Total cell lysates were extracted and immunoblotted with anti-p-Ser 473 Akt and then stripped and blotted with β-actin.
Bcl-xL is highly expressed in most lung adenocarcinoma cell lines examined and its expression is independent of PI3K/Akt signaling pathway
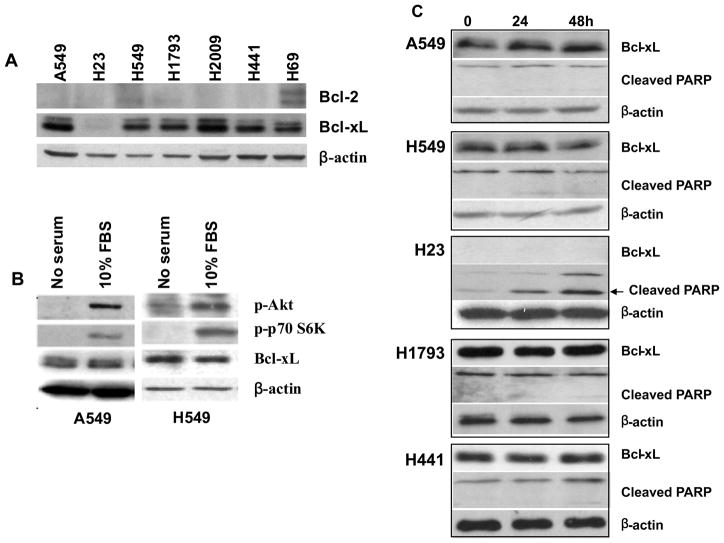
To explore the potential role of Bcl-2/Bcl-xL in the mechanism of the differential sensitivity to LY294002-induced apoptosis in lung adenocarcinoma cells, we first evaluated the expression level of both Bcl-2 and Bcl-xL in a subset of lung adenocarcinoma cell lines. Bcl-2 is barely detectable in all cell lines, which is consistent with the literature (25). This is not due to an inability of the antibody to detect Bcl-2 as the protein was readily detected in H69, a small cell lung cancer cell line included as a control (Figure 2A). In contrast, all cell lines, with the exception of H23, displayed high expression of Bcl-xL (Figure 2A). Interestingly, H23 is the cell line sensitive to LY294002-induced apoptosis (Figure 1). Recent publications implicate the role of Akt activation in Bcl-xL expression levels in some type of cells (26, 27). Therefore, we asked whether PI3K/Akt pathway activation regulates the expression of Bcl-xL in these lung adenocarcinoma cell lines. Tumor cell lines were treated with 25 μM LY294002, for up to 48 hours before analysis. As shown in Figures 2B and 2C Bcl-xL expression in A549 and H549 cells was independent of serum culture conditions (Figure 2B) or LY294002 treatment (Figure 2C) while phosphorylation of Akt was clearly modulated by these conditions.
Figure 2.
Effects of PI3K/Akt inhibition on expression of Bcl-xL in lung adenocarcinoma cell lines. (A) Immunoblot analysis of Bcl-2 and Bcl-xL proteins in lung adenocarcinoma cell lines. H69 is a small cell lung cancer cell line and was used as a positive control for Bcl-2 expression. Total cell lysates obtained from the cells maintained in normal 10% serum growth condition were immunoblotted with anti-Bcl-xL, anti-Bcl-2 and anti-β-actin antibodies. (B) Bcl-xL expression is independent of serum concentrations in A549 and H549 cells. Cells were cultured in serum-free media overnight followed by 10% serum for 24h. Immunoblot analysis was used to determine the expression level of Bcl-xL and phosphorylation status of Akt and p70S6K. (C) Bcl-xL expression is independent of PI3k inhibition in lung adenocarcinoma cells. Cells were incubated for 24–48 hours with 25 μM LY294002 and then cell lysate were immunoblotted with antibody against Bcl-xL and PARP.
Combined inhibition of Bcl-xL and PI3K/Akt works in synergy to promote apoptosis of lung adenocarcinoma cells
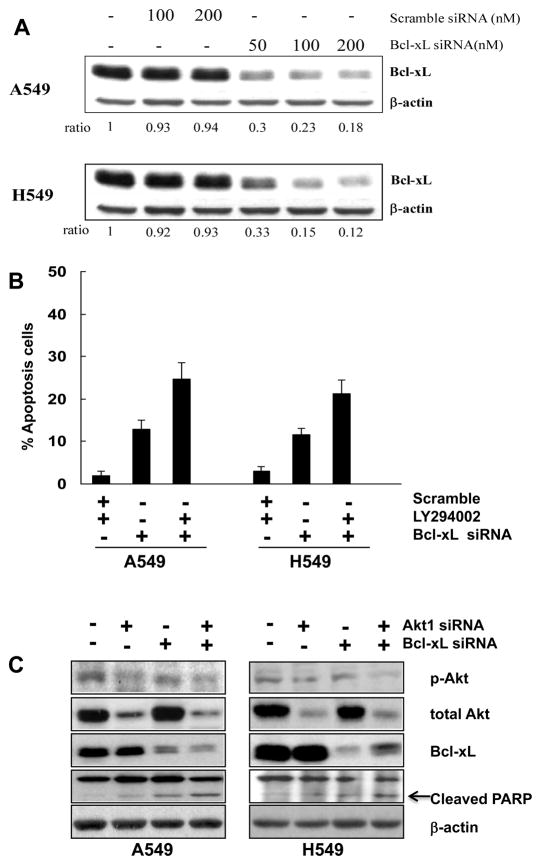
Based on the data presented in Figures 1 and 2, we hypothesized that Bcl-xL expression may provide an important mechanism for resistance to apoptosis induced by PI3K/Akt inhibition in lung adenocarcinoma cells. To test this hypothesis, we developed two strategies to inhibit the function of Bcl-xL. First, we silenced Bcl-xL expression using siRNA technology, and second we tested a potent novel small molecule Bcl-2/Bcl-xL inhibitor, ABT-737 (28). After Bcl-xL function was inhibited, we determined the effect this had on the ability of lung adenocarcinoma cell lines to undergo apoptosis in response to LY294002 treatment or Akt1 gene silencing. In these experiments we used A549 and H549 cells, as these cells are resistant to LY294002-induced apoptosis and express a high level of Bcl-xL. Treatment of these cells with various concentrations of Bcl-xL siRNA demonstrated a dose-dependent reduction in Bcl-xL protein level after 48 hours (Figure 3A). In contrast, scrambled siRNA had no significant effect on Bcl-xL expression. The addition of 25 μM LY294002 dramatically increased apoptosis of A549 and H549 cells subjected to Bcl-xL siRNA treatment up to 26% and 23% respectively after 48 hours of treatment (Figure 3B). Similar results were obtained with ABT-737. A549 and H549 cells were treated with DMSO, LY294002, ABT-737, and ABT-737 enantiomer as control or combined compounds for 48h. As shown in Figure 3E, combined LY294002 and ABT-737 treatments increased cell apoptosis significantly as compared to the effect induced by LY294002 or ABT-737 alone (p<0.05). Thus, Bcl-xL inhibition renders lung adenocarcinoma cells sensitive to apoptosis induced by the inhibition of the PI3K/AKT pathway.
Figure 3.
Apoptotic response induced by the inhibition of PI3K/Akt and Bcl-xL in A549 and H549 cells. (A) Dose-dependent Bcl-xL downregulation in response to Bcl-xL siRNA for 48 hours; expression levels were determined by immunoblot analysis. Results represent one of three experiments with similar results. (B) Bcl-xL gene silencing restores the sensitivity of A549 and H549 cells to PI3K inhibition-induced apoptosis. Percentage of apoptotic cells (sub-G1 population) detected by FACS analysis is represented after cells were transfected with 100 nM Bcl-xL siRNA or scramble siRNA control for 48 hours, followed by 25 μM LY294002 treatment for another 48 hours in the presence of 10% FBS. Knockdown of Bcl-xL alone induces a significant percentage of cells to undergo apoptosis. The combination of Bcl-xL knockdown and LY294002 treatment increased the apoptotic response in a synergistic manner. (C) Effects of combinatorial treatment of Akt1 and Bcl-xL siRNA on phosphorylated Akt (s473), total Akt, Bcl-xL and PARP in A549 and H549 cells. Cells were transfected with double-stranded Akt1 siRNA and/or Bcl-xL siRNA. After an initial transfection (5 h), cells were left untreated for an additional 48 h, and protein lysates were obtained for Western Blotting to measure the expression of Akt phosphorylation at Ser473, total Akt, Bcl-xL and cleaved PARP. (D) A representative histogram of cell cycle distributions and increase of cells in sub-G1 phase (percentage of subG1 fraction is shown in %) induced by combined Bcl-xL and Akt1 siRNA treatment for 48 h in H549 and A549 cells is shown. (E) Apoptosis induced by combined treatment with ABT-737 (8 μM) for 48h or with the enantiomer as negative control (ABT-ctl) and LY294002 (25 μM) in A549 and H549 cells.
Because LY294002 specificity for PI3Kinase inhibition is not perfect, we tested the effect of Akt1 gene silencing on the apoptotic response observed in these cells with Bcl-xL inhibition. Immunoblot analysis of A549 and H549 cells lysates after transfection with a control siRNA or with Akt1 siRNA for 48 h demonstrated a clear reduction in both phosphorylated and total Akt protein levels (Figure 3C). Consistent with the effect of LY294002 alone observed on apoptosis (Figure 3B), Akt down regulation by siRNA alone is not enough to induce significant apoptosis in A549 or H549 cells. In contrast, the combination of Akt1 and Bcl-xL gene silencing led to apoptosis in 22–34% of the cells (Figure 3D). The apoptotic effect induced by combined treatment of Bcl-xL and Akt1 siRNA for 48 hours was also confirmed by the cleavage of PARP (Figure 3B). Taken together, these results support the conclusion that PI3K/Akt and Bcl-xL closely cooperate to the survival of lung adenocarcinoma. There is true synergy between the two molecular pathways as combined effect is favored over the sum of individual component effect on apoptosis (Figure 3B, 3D and 3E).
Ectopic expression of Bcl-xL protects H23 cells from LY294002-induced apoptosis
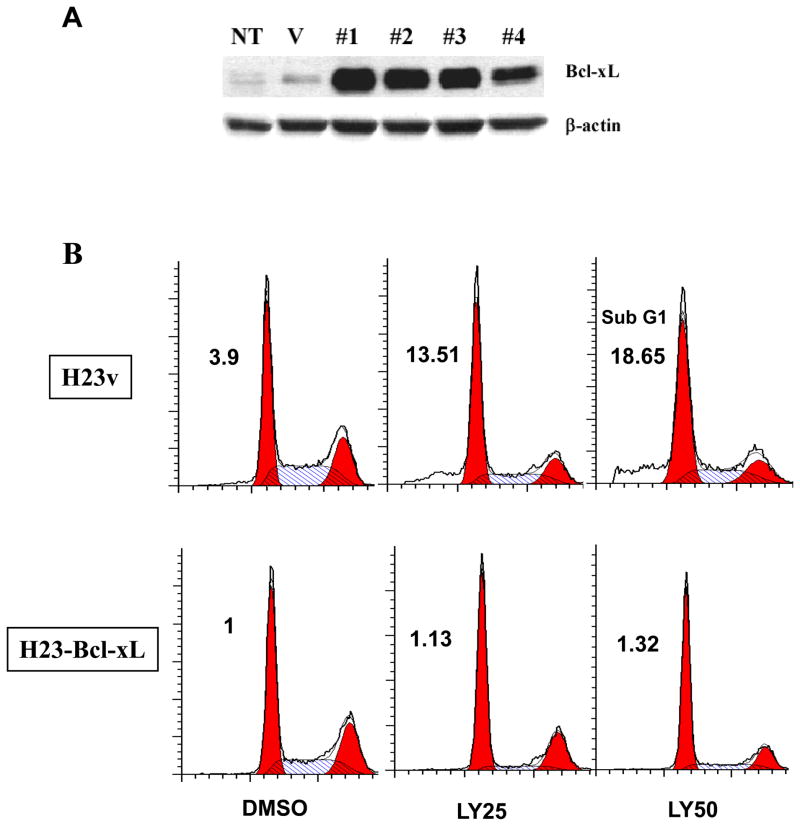
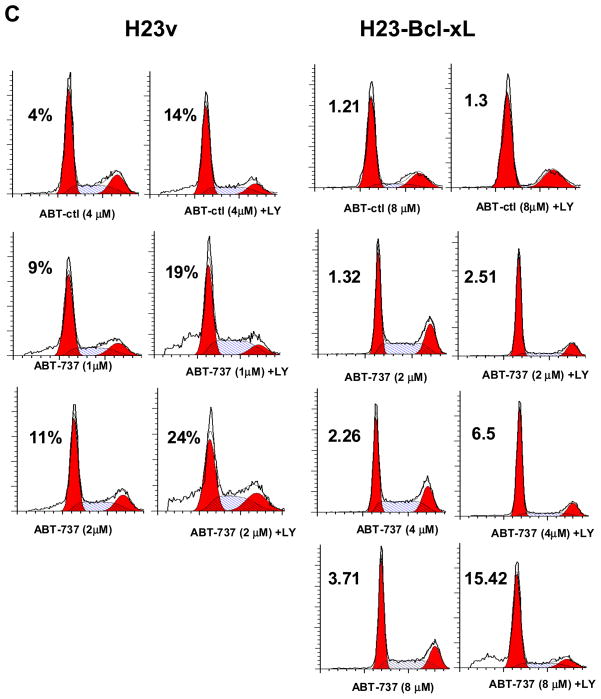
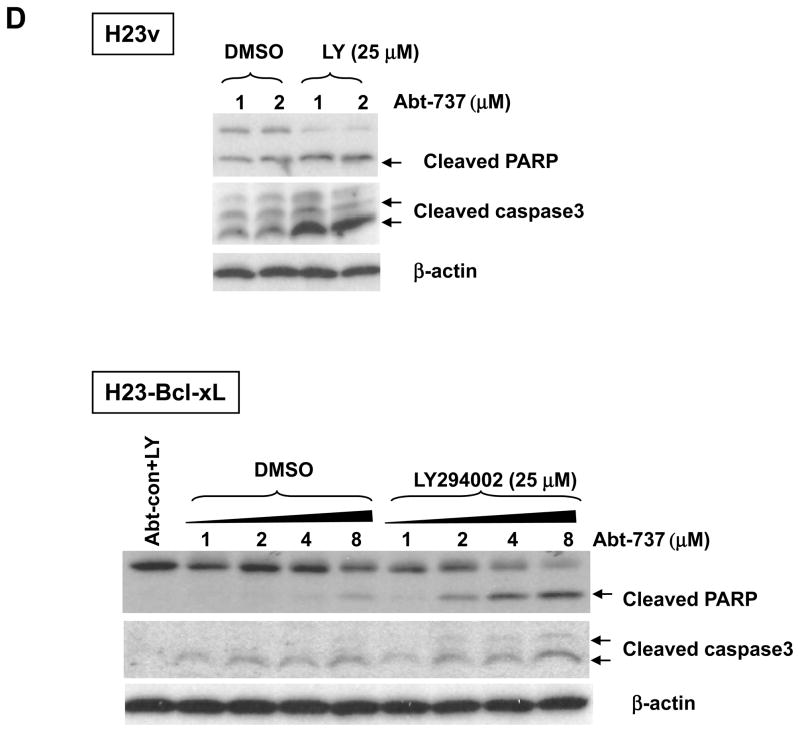
Because our results suggest a protective role for Bcl-xL in LY294002-induced apoptosis, we tested whether overexpression of Bcl-xL in H23 cells, which express a low level of Bcl-xL at baseline, may induce resistance to LY294002. To test this, we established H23 cell lines stably transfected with a Bcl-xL or control expression vector, and apoptosis was assessed following treatment with LY294002. Transfection with the Bcl-xL plasmid resulted in enhanced expression of Bcl-xL by more than 70% when compared to vector alone (Figure 4A). In H23 cells that had Bcl-xL expression restored, LY294002 induced cell death in less then 2% of cells, as compared to the 14% that was seen in the control cells after 48h treatment (Figure 4B). H23-Bcl-xL cells failed to undergo apoptosis even treated with high concentrations of LY294002 (50 and 75 μM) (Figure 4B and data not shown). These apoptosis rates are comparable to those of lung adenocarcinoma cancer cell lines resistant to LY294002-induced cell death (Figure 1A). This suggests that Bcl-xL is an important mediator of this resistance to apoptosis. Moreover, the overexpression of Bcl-xL increased the resistance of H23 cell to apoptotic effect induced by the combination of ABT-737 and LY294002. As shown in Figure 4C, combined 25 μM LY294002 and 1 μM ABT-737 is sufficient to induce apoptosis in 19% of H23, a response comparable to 18% induced by LY294002 at 50 μM alone (Figure 4B). Similarly, ABT-737 has to be increased up to 8 μM to induce comparable rate of apoptosis (15%) when combined with LY294002 in H23 cells transfected with Bcl-xL (Figure 4C). These results were confirmed by the cleaveage of PARP and Caspase 3 in H23 and H23-Bcl-xL cells treated combined ABT-737 and LY294002 in Figure 4D. Together, these results further demonstrate that Bcl-xL confers protection against PI3K inhibition-induced apoptosis in H23 cells.
Figure 4.
Overexpression of Bcl-xL increases H23 resistance to LY294002-induced apoptosis. (A) Immunoblot analysis of H23 cells stably over-expressing Bcl-xL. Four independent clones (labeled #1-4) are shown. “V” indicates cells that have been stably transfected with the vector alone (pBabe), and “NT” indicates the parental cell line that has not been transfected. (B) Representative FACS analysis of H23v ( pBabe vector contol) and H23-Bcl-xL (clone #1) cells cultured in 10% FBS media for 48 hours in the presence or absence of 25 and 50 μM LY294002. Percentages refer to the sub-G1 population, which corresponds to the apoptotic population. (C) Apoptosis induced by combined ABT-737 at indicated concentration ± LY294002 (25 μM) on H23v and H23-Bcl-xL cells. A representative FACS result post-48h treatment was shown. (D) Cleaved PARP and Caspase 3 induced by combined ABT-737 at indicated concentration and LY294002 (25 μM) on H23v and H23-Bcl-xL cells.
PI3K inhibition induced BIM expression in sensitive H23 cells
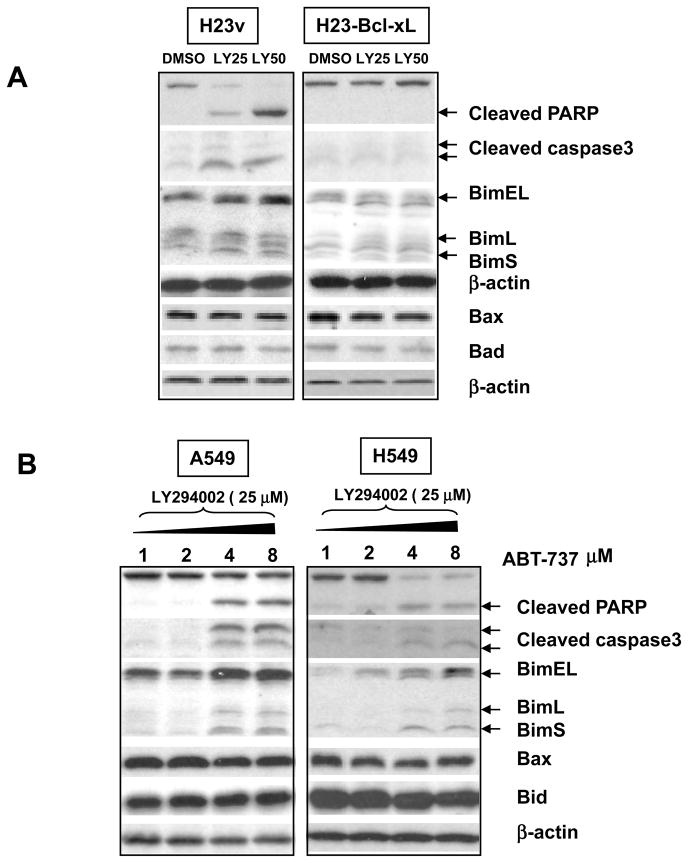
To provide further insights as to how other Bcl-2 family members may be involved in the PI3K inhibition-induced apoptosis in H23 cells, the expression of pro-apoptosis and antiapoptosis-related Bcl-2 family members including Bad, Bax, Bim, Bid was tested in H23 and H23-pBabe-Bcl-xL cells. Figure 5A illustrates a significant induction of the proapoptotic BH3-only protein BIM isoform long (BimEL) and the shortest form ( BimS) in H23 cells treated with LY294002 for 48 h. In contrast, Bim was not activated in resistant H23-pBabe-Bcl-xL cells. There were no significant differences in the protein level of Bad, Bax or Bid. In resistant A549 and H549 cells, only combined high concentration of ABT-737 (> 4 μM) and LY294002 (25 μM) induced Bim activation as well as apoptosis indicated by cleaved PARP and Caspase 3 (Figure 5B).
Figure 5.
Bim was activated in LY294002-induced apoptosis in lung adenocarcinoma cells. (A) LY294002 induced Bim activation in H23 cells but not in H23 overexpressing Bcl-xL cells. Both H23 and H23-pBabe-Bcl-xL cells were treated with 25 and 50 μM LY294002 for 48 hours in the presence of 10 % serum. (B) Bim activation was induced by combined ABT-737 and LY294002 in A549 and H549 cells at indicated concentration. After treatment, Cell lysates were examined by Western blot using anti-apoptotic and pro-apoptotic antibodies specific to the indicated proteins.
Discussion
Regulation of cell survival pathways is pivotal in not only cancer progression, but has also become increasingly important in understanding mechanisms that underlie resistance to therapy. Our study defined one potential mechanism by which lung adenocarcinoma cell lines could be resistant to apoptosis induced by the inhibition of such survival pathways. One pathway of particular clinical interest is the PI3K/Akt pathway. This pathway is disrupted in many cancer types, and resistance to inhibitors of PI3K has been reported in cancers, including lung cancer. Therefore, it is important understand the mechanisms by which these tumors develop resistance to these drugs to improve the therapeutic efficacy. Our results implicate another important survival protein, Bcl-xL, as one potential mechanism for resistance. First, our data demonstrate that by inhibiting the expression of Bcl-xL, the apoptotic response is restored in lung adenocarcinoma cells otherwise resistant to the cell death induced by the PI3K inhibitor LY294002. Furthermore, Bcl-xL and PI3K inhibition in combination had a synergistic effect on apoptosis. In a set of converse experiments, where Bcl-xL expression was restored in cells that lack Bcl-xL, cells did not undergo apoptosis in response to PI3K inhibition. These data taken together suggest that a combination therapy that inhibits two critical survival pathways may have a role in the treatment of adenocarcinomas of the lung and that Bcl-xL expression may be a predictor of a tumor’s resistance to chemotherapy involving inhibition of PI3K.
Molecular studies have led to the discovery of several potential targets for cancer therapeutic design, such as vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), PI3K/Akt/mTOR, MEK and Bcl-2/Bcl-xL (9, 28). Various drugs targeted against these molecular changes have been developed and some are being tested for clinical use in lung cancer therapy (9, 10). However, recent work suggests that mammalian cells have developed several different survival pathways that become activated in a cell type- and stimulus-dependent fashion, leaving the prospect of inhibiting these pathways alone may not be sufficient to induce cell death (29, 30). The inherited or acquired resistance to small molecular inhibitors such as PI3K/Akt inhibitor (LY294002 or wortmannin) (15, 16, 18), mTOR inhibitor (rapamycin) (17), EGFR inhibitor (gefitinib) and Bcl-2/Bcl-xL (ABT-737) inhibitor (28) is indeed observed frequently in various types of cancers including NSCLC. Our study shows that to overcome the cellular mechanisms of drug resistance to PI3K inhibition in adenocarcinoma of the lung, Bcl-xL expression needs to be down-regulated, and that process is associated with induction of proapoptotic BH3-only protein Bim. Proteins in the Bcl-2 family are central regulators of programmed cell death and contribute to chemotherapy resistance of cancer cells via growth factor-dependent or -independent mechanism. For example, high levels of the anti-apoptotic MCL-1 protein is the major factor that causes resistance to ABT-737 in small cell lung cancer and acute myeloid leukemia (31, 32). Pro-apoptotic BH3-only Bcl-2 family member Bim is essential for TKI-induced apoptosis in sensitive EGFR-mutant cells of lung cancer (33–35). Our results implicate Bcl-xL as another important survival protein in causing resistance to the PI3K inhibition in NSCLC cell lines that do not harbor EGFR mutations. Moreover, we show that Bim appears to be implicated in the apoptotic response to PI3K inhibition in lung adenocarcinoma cells expressing low levels of Bcl-xL though the exact mechanism by which Bcl-xL downregulation may promote Bim activation after PI3K inhibition remains to be determined. Our data warrant further investigation of the role of Bim induction in the apoptosis induced by LY294002 in lung adenocarcinoma cells.
Functional cooperation between PI3K/Akt and Bcl-2 family member proteins has emerged as an important mechanisms for preventing cells from apoptosis and promoting tumorigenesis (36–38). While Bcl-xL has been implicated in cell survival independent of the PI3K/Akt pathway in the prostate cancer cells (39), the data we report here suggests a cross talk between the mitochondrial and cytoplasmic cell survival machinery. Although our data indicate that Bcl-xL expression is independent of PI3K/Akt or mTOR pathway activation, we clearly demonstrate that Bcl-xL plays a role in the apoptotic response of lung cancer cell lines to LY294002. In fact, we report a synergistic effect when combining Bcl-xL inhibition, with PI3K inhibition, suggesting a coordination of function between these two pathways (Figure 3). In addition to the cooperation between Akt and Bcl-2 pathway, interactions between the PI3K/Akt and Raf/MEK/ERK pathways are also important for the regulation of cell cycle progression and apoptosis in several types of cancers including small cell lung cancer cells (40, 41). However, these interactions remain controversial. Future studies into these types of biomolecular interactions are therefore warranted.
In summary, we have shown that the resistance of adenocarcinoma of the lung to PI3K inhibitor-induced apoptosis can be overcome by downregulation of Bcl-xL. PI3K/Akt pathway and Bcl-xL expression cooperate to promote cell survival and the level of Bcl-xL expression is a key mechanism controlling the resistance to cell death induced by PI3K/Akt inhibition. These results may have important implications and suggest that an approach directed to both molecular targets PIK3K/AKT and Bcl-xL may offer greater therapeutic response to adenocarcinoma of the lung.
Acknowledgments
Acknowledgements of funding support: Dr. Massion is supported by RO1 CA102353
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Nadkar A, Pungaliya C, Drake K, Zajac E, Singhal SS, Awasthi S. Therapeutic resistance in lung cancer. Expert Opin Drug Metab Toxicol. 2006 Oct;2(5):753–77. doi: 10.1517/17425255.2.5.753. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003 Oct;4(4):257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 5.Massion P, Gray J. Molecular Cytogenetic Explorations of Human Genome. In: Galas SMaD., editor. Genomic Technologies: Present and Future Evolution of Genomic Technologies. 2002. [Google Scholar]
- 6.Shah A, Swain WA, Richardson D, et al. Phospho-akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res. 2005 Apr 15;11(8):2930–6. doi: 10.1158/1078-0432.CCR-04-1385. [DOI] [PubMed] [Google Scholar]
- 7.Massion PP, Taflan PM, Shyr Y, et al. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med. 2004 Nov 15;170(10):1088–94. doi: 10.1164/rccm.200404-487OC. [DOI] [PubMed] [Google Scholar]
- 8.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dy GK, Adjei AA. Novel targets for lung cancer therapy: part I. J Clin Oncol. 2002 Jun 15;20(12):2881–94. doi: 10.1200/JCO.2002.11.145. [DOI] [PubMed] [Google Scholar]
- 10.Reinmuth N, Mesters RM, Bieker R, Hoffknecht P, Berdel WE, Thomas M. Signal transduction pathways as novel therapy targets in lung cancer. Lung Cancer. 2004 Aug;45( Suppl 2):S177–86. doi: 10.1016/j.lungcan.2004.07.976. [DOI] [PubMed] [Google Scholar]
- 11.Pfeil K, Eder IE, Putz T, et al. Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. Prostate. 2004 Feb 15;58(3):259–68. doi: 10.1002/pros.10332. [DOI] [PubMed] [Google Scholar]
- 12.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002 Oct;1(12):989–97. [PubMed] [Google Scholar]
- 13.Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994 Feb 15;54(4):903–7. [PubMed] [Google Scholar]
- 14.Aguirre D, Boya P, Bellet D, et al. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis. 2004 Nov;9(6):797–805. doi: 10.1023/B:APPT.0000045781.46314.e2. [DOI] [PubMed] [Google Scholar]
- 15.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986–97. [PubMed] [Google Scholar]
- 16.Wei L, Yang Y, Yu Q. Tyrosine kinase-dependent, phosphatidylinositol 3′-kinase, and mitogen-activated protein kinase-independent signaling pathways prevent lung adenocarcinoma cells from anoikis. Cancer Res. 2001 Mar 15;61(6):2439–44. [PubMed] [Google Scholar]
- 17.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005 Aug 15;65(16):7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 18.Sithanandam G, Smith GT, Masuda A, Takahashi T, Anderson LM, Fornwald LW. Cell cycle activation in lung adenocarcinoma cells by the ErbB3/phosphatidylinositol 3-kinase/Akt pathway. Carcinogenesis. 2003 Oct;24(10):1581–92. doi: 10.1093/carcin/bgg125. [DOI] [PubMed] [Google Scholar]
- 19.Reed JC, Miyashita T, Takayama S, et al. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996 Jan;60(1):23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Page C, Beidler DR, Wicha MS, Nunez G. Overexpression of Bcl-x(L) promotes chemotherapy resistance of mammary tumors in a syngeneic mouse model. Am J Pathol. 1999 Dec;155(6):1861–7. doi: 10.1016/S0002-9440(10)65505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000 Nov;12(6):543–9. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Downes CP, Whetton AD, Owen-Lynch PJ. Role of phosphatidylinositol 3-kinase and specific protein kinase B isoforms in the suppression of apoptosis mediated by the Abelson protein-tyrosine kinase. J Biol Chem. 2000 Apr 28;275(17):13142–8. doi: 10.1074/jbc.275.17.13142. [DOI] [PubMed] [Google Scholar]
- 23.Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003 Nov 1;63(21):7113–21. [PubMed] [Google Scholar]
- 24.Greider C, Chattopadhyay A, Parkhurst C, Yang E. BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene. 2002 Nov 7;21(51):7765–75. doi: 10.1038/sj.onc.1205928. [DOI] [PubMed] [Google Scholar]
- 25.Reeve JG, Xiong J, Morgan J, Bleehen NM. Expression of apoptosis-regulatory genes in lung tumour cell lines: relationship to p53 expression and relevance to acquired drug resistance. Br J Cancer. 1996 May;73(10):1193–200. doi: 10.1038/bjc.1996.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrizas M, LeRoith D. Insulin-like growth factor-1 inhibition of apoptosis is associated with increased expression of the bcl-xL gene product. Endocrinology. 1997 Mar;138(3):1355–8. doi: 10.1210/endo.138.3.5103. [DOI] [PubMed] [Google Scholar]
- 27.Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax: Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005 May 5;24(20):3348–57. doi: 10.1038/sj.onc.1208471. [DOI] [PubMed] [Google Scholar]
- 28.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 29.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998 Feb;8(1):49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 30.Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem. 2000 Oct 27;275(43):33487–96. doi: 10.1074/jbc.M005824200. [DOI] [PubMed] [Google Scholar]
- 31.Wesarg E, Hoffarth S, Wiewrodt R, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007 Dec 1;121(11):2387–94. doi: 10.1002/ijc.22977. [DOI] [PubMed] [Google Scholar]
- 32.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006 Nov;10(5):375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007 Oct;4(10):1681–89. doi: 10.1371/journal.pmed.0040316. discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007 Oct 9;4(10):e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007 Oct;4(10):1669–79. doi: 10.1371/journal.pmed.0040315. discussion 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001 Apr 13;276(15):12041–8. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 37.Karnauskas R, Niu Q, Talapatra S, et al. Bcl-x(L) and Akt cooperate to promote leukemogenesis in vivo. Oncogene. 2003 Feb 6;22(5):688–98. doi: 10.1038/sj.onc.1206159. [DOI] [PubMed] [Google Scholar]
- 38.Umeda J, Sano S, Kogawa K, et al. In vivo cooperation between Bcl-xL and the phosphoinositide 3-kinase-Akt signaling pathway for the protection of epidermal keratinocytes from apoptosis. Faseb J. 2003 Apr;17(6):610–20. doi: 10.1096/fj.02-0597com. [DOI] [PubMed] [Google Scholar]
- 39.Yang CC, Lin HP, Chen CS, et al. Bcl-xL mediates a survival mechanism independent of the phosphoinositide 3-kinase/Akt pathway in prostate cancer cells. J Biol Chem. 2003 Jul 11;278(28):25872–8. doi: 10.1074/jbc.M301744200. [DOI] [PubMed] [Google Scholar]
- 40.Shelton JG, Steelman LS, Lee JT, et al. Effects of the RAF/MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003 Apr 24;22(16):2478–92. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- 41.Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 2005 Sep 15;65(18):8423–32. doi: 10.1158/0008-5472.CAN-05-0058. [DOI] [PubMed] [Google Scholar]