Abstract
Tumorigenesis is a multi-step process due to an accumulation of genetic mutations in multiple genes in diverse pathways which ultimately lead to loss of control over cell growth. It is well known that inheritance of rare germline mutations in genes involved in tumorigenesis pathways confer high lifetime risk of neoplasia in affected individuals. Furthermore, a substantial number of multiple malformation syndromes include cancer susceptibility in their phenotype. Studies of the mechanisms underlying these inherited syndromes have added to the understanding of both normal development and the pathophysiology of carcinogenesis. Myotonic dystrophy (DM) represents a group of autosomal dominant, multisystemic diseases that share the clinical features of myotonia, muscle weakness, and early-onset cataracts. Myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2) result from unstable nucleotide repeat expansions in their respective genes. There have been multiple reports of tumors in individuals with DM, most commonly benign calcifying cutaneous tumors known as pilomatricomas. We provide a summary of the tumors reported in DM and a hypothesis for a possible mechanism of tumorigenesis. We hope to stimulate further study into the potential role of DM genes in tumorigenesis, and help define DM pathogenesis, and facilitate developing novel treatment modalities.
Keywords: Tumorigenesis, Myotonic dystrophy, Repeat expansion disorders, Pilomatricoma, β-Catenin
Introduction
Myotonic dystrophy (DM) is the most common adult muscular dystrophy, and is primarily associated with myotonia and muscle weakness. However, a broad range of clinical features are variably seen including early-onset cataracts, cardiac conduction defects, respiratory insufficiency, insulin-resistance, reduced immunoglobulin levels, frontal balding, testicular atrophy, and varying degrees of mental impairment. Both myotonic dystrophy type-1 (DM1) and myotonic dystrophy type-2 (DM2) result from unstable repeat expansions in untranslated regions of two unrelated genes, and yet both have similar clinical manifestations. In 1992, it was determined that myotonic dystrophy of Steinert, now classified as DM1, was due to an unstable trinucleotide (CTG) expansion in the 3′ untranslated region of the dystrophia myotonica-protein kinase (DMPK) gene, located on chromosome 19q13.3 [1]. The availability of DNA testing for this gene revealed that a subset of DM patients who had myotonia, weakness more proximal than distal, and cataracts, lacked CTG expansion in DMPK. Many of these families, which were subsequently classified as proximal myotonic myopathy, had a single disorder resulting from an unstable tetranucleotide (CCTG) repeat expansion in intron 1 of the zinc finger 9 (ZNF9) gene, on chromosome 3q21; this is now classified as DM2 [2]. The prevalence of DM1 is approximately 1:8,000. However, the prevalence of DM2 has yet to be determined, but it appears to be less common than DM1, with evidence that there may be a predominant northern European ancestry in families with DM2 [1]. Unfortunately, the molecular basis for the genetic instability and the exact mechanism of pathogenesis of DM remain unclear.
There are multiple case reports describing DM patients with pilomatricoma, a benign calcifying cutaneous neoplasm thought to be derived from hair matrix cells. In reflecting on our clinical experience caring for large numbers of DM patients, we have questioned whether there might be an increased incidence of other tumors in patients with DM. Furthermore, in the limited number of tumors that have been studied in DM patients, increased trinucleotide repeat expansion in the DM1 has been demonstrated, suggesting that somehow this underlying genetic instability may predispose these patients to developing tumors; no DM2 tumors have been studied [3–7].
There is some urgency to this question because several growth hormones and other anabolic factors have been proposed to treat the muscle weakness and wasting associated with DM. Using these anabolic agents shows promising results both in vivo and in vitro, but more research is needed regarding their potential therapeutic and adverse effects [8, 9]. The Neuromuscular Disease Center at the University of Rochester is currently conducting a treatment trial in DM patients, to determine the safety and feasibility of using insulin-like growth (IGF) factor 1 complexed to IGF binding protein 3 (IGFBP3) [IPLEX®, Insmed Incorporated, Richmond, VA, USA]. However, elevated serum levels of IGF-1 have been implicated in the etiology of several common adult neoplasms, most notably cancers of the breast, colon and prostate, leading to theoretical concerns that long-term treatment of DM patients with IGF-1 might promote tumor growth [10]. If IGF-1 or other growth factor therapies prove beneficial in DM, establishing whether myotonic dystrophy patients have an intrinsic susceptibility to specific neoplasms could influence how this treatment is used.
In this article, we provide a summary of published reports describing pilomatricomas and various other neoplasms observed in patients with DM. Any retrospective analysis of neoplasms in DM patients is constrained by the relatively recent advent of molecular diagnostic testing. Therefore, the existing literature primarily includes patients in whom the diagnosis was made on clinical grounds alone, who likely had the classical myotonic dystrophy of Steinert, DM1.
We also present an initial summary of tumors reported by DM1 and DM2 patients who enrolled in the NIH-funded National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy (FSHD) Patients and Family Members. Registry patients are classified by utilizing strict clinical definitions to distinguish between patients with DM1 and DM2 (www.dystrophyregistry.org), and a large proportion of the Registry patients have had DNA testing. In our comments, we offer a hypothesis for a molecular mechanism which might account both for the risk of pilomatricoma and, perhaps, other neoplasms in patients with DM.
Neoplasms in DM
Sporadic pilomatricomas are rare, benign epithelial tumors that usually present as solitary skin nodules measuring 0.5–6 cm. They are more common in females and typically occur in individuals under the age of 20. The prevalence of pilomatricomas in the general population is unclear; however, one study showed that they account for one of every 500 specimens submitted by dermatologists [11]. Multiple pilomatricomas in the same individual are even less common: in a study of 1,569 individuals with pilomatricomas, the incidence was 3.5% [12]. Common cutaneous sites of origin include the neck as well as frontal, temporal, periorbital, and periauricular skin of the head. Pilomatricomas may be clinically mistaken for epidermoid cysts or cutaneous malignancy [12, 13]. Several genetic disorders include multiple pilomatricomas as part of their phenotype, including Rubinstein–Taybi syndrome and Gardner syndrome both associated with a cancer predisposition [14, 15]. Multiple pilomatricomas have occurred in a few families in the absence of an identifiable underlying systemic disorder [16]. Pilomatrix carcinomas do occur, but are exceedingly rare [13].
Cantwell and Reed first reported an association between DM and pilomatricoma in 1965 [17]. In our review of the literature (Table 1), we have identified 35 published cases, with multiple pilomatricomas present in 31 (89%) individuals [17–42]. In four families, multiple family members had both pilomatricoma and DM. There was also one report of a DM patient with a follicular cyst that had pilomatricoma-like changes, resembling lesions observed in Gardner syndrome. In DM-associated pilomatricoma, the scalp was the most common site of origin, with the upper extremities, face, neck, trunk, and groin less frequently involved.
Table 1.
Myotonic dystrophy patients with pilomatricoma—literature summary
| Article | Age at tumor diagnosisb |
Sex | # Of tumors |
Tumor description | Other tumors | Family history of DM |
|---|---|---|---|---|---|---|
| Cantwell [17] | 52 (50) | M | 4 | Scalp; arms | Colloid goiter, thyroid; Angiolipoma (arm/trunk) | Y |
| Harper [30]a | 14 | F | 1 | Left forearm | Y | |
| 13 | F | 1 | Scalp | Y | ||
| Harper [29]a | 28 | M | >13 | Scalp; arms; back | Y | |
| 46 | F | >1 | Scalp | Y | ||
| 26 | F | >2 | Forehead; back; scalp | Y | ||
| 12 | M | >2 | Shoulder; back; scalp | Y | ||
| 52 | M | 3 | Scalp | Y | ||
| Chiaramonti [37]a | 24 (22) | M | 5 | Scalp | Y | |
| 37 (32) | F | 2 | Scalp | Y | ||
| Aso [41] | 46 (40) | F | 7 | Scalp; nuchal region | Apocrine cystadenoma (face) | Y |
| Runne [23] | 42 | F | 11 | Scalp; trunk | Multiple pigmented nevi | |
| Filla [33]a | 42 (15) | M | 1 | Gluteal region | Y | |
| (same case) | 41 (20) | M | >10 | Scalp; neck; extremities; pubis | Y | |
| Delfino [36] | 26 (14) | M | 8 | Scalp | Y | |
| 8 (3) | F | 1 | Neck | Y | ||
| Schwartz [21] | 49 (39) | M | 3 | Neck; forearms | Y | |
| Ribera [24] | 57 | M | 20 | Scalp; neck; shoulder; extremities | Medullary thyroid carcinoma (age 54) | Y |
| Kopeloff [28] | 32 (12) | F | >28 | Scalp; forehead | Y | |
| Salerni [22] | 43 (19) | F | 5 | Scalp; face; neck | Uterine fibroids | |
| Street [20] | 35 | M | >2 | Face; scalp | Y | |
| 1 | Face—epidermoid cyst | |||||
| White [18] | 41 | F | 24 | Scalp | ||
| Bouadjar [38] | 20 | M | >4 | Face; thigh; shoulder | ||
| Martinez-Albaladejo [26] | 39 (38) | F | >1 | Scalp; back | ||
| Farrell [34] | 43 | M | 3 | Scalp | ||
| Graells [31] | 25 | M | 8 | Scalp; arms; trunk | ||
| 51 | M | 5 | Scalp; arms | |||
| Urvoy [19] | 5 | M | >5 | Face; shoulder; back; neck | ||
| Berberian [42] | 26 (19) | M | >1 | Scalp; right arm | Maternal and paternal grandfathers—melanoma | Y |
| Father-multiple compound nevi | ||||||
| Laredo Ortiz [27] | 24 | M | 14 | Scalp; axilla; extremities | ||
| Betlloch [39] | 28 | M | >1 | Neck; face; extremities | Y | |
| Geh [32] | 3 | F | >30 | Scalp; trunk | Y | |
| Draper [35] | ? | F | >1 | Back; shoulder | Pleiomorphic parotid adenoma (age 29) | Y |
| Nishie [25] | 41 | F | 1 | Scalp—follicular cyst (pilomatricoma-like); axilla, inguinal—inflamed cystic lesions |
||
| 37 | >1 | |||||
| Barberio [40] | 18 (9) | M | 12 | Scalp; arm; neck |
Note: DM1 mutation status was only reported by Geh et al. However, the size of mutation was not reported
Multiple family members with pilomatricomas reported
In parentheses: age tumor first noted by patient
In addition to pilomatricomas, a variety of benign and malignant neoplasms have been described in 47 DM patients. Table 2 summarizes forty-one reported cases. Table 1 includes six individuals with pilomatricoma who were also reported to have other tumors [17, 22, 24, 41, 43–72]. Thymoma was the most commonly reported tumor (9 cases), followed by neoplasms of the parotid gland (6 pleomorphic adenomas), parathyroid (7 adenomas), and thyroid (1 adenoma, 2 colloid goiters, 5 thyroid cancers). Five patients developed multiple basal cell carcinomas. Two cases of gastric cancer, 2 insulinomas, and 2 pituitary tumors were reported. There were single cases of chronic myelogenous leukemia, B-cell gastric lymphoma, testicular cancer, multiple carcinoid tumors of the bowel, endometroid carcinoma of the ovary, spinal hemangioblastoma, sigmoid colon adenocarcinoma, maxillary jaw odontoma, laryngeal squamous cell carcinoma, and renal cell carcinoma. Thirteen individuals had multiple independent primary neoplasms (including those with pilomatricomas). The average age at the time of diagnosis of first tumor was 44 years.
Table 2.
Myotonic dystrophy patients with neoplasms (excluding pilomatricomas)—literature summary
| Article | Age at tumor diagnosis |
Sex | Neoplasm diagnosis | Family history of cancer | Family history of DM |
|---|---|---|---|---|---|
| Caughey [66] | 43 | M | Pituitary tumor, NOS (necropsy) | Y | |
| Fraumeni [65] | 47 | F | Chronic myelogenous leukemia | Father—prostate, 62 | Y |
| Mother—ovarian, 64 | |||||
| Goto [63] | 51 | M | Pleomorphic parotid tumor, thymoma | ||
| Banna [70] | 49 | M | Pituitary adenoma | Y | |
| Johannesson [59] | 51 | F | Pleomorphic parotid adenoma | Y | |
| 38 | F | Pleomorphic parotid adenoma | |||
| Mudge [51] | 32 | M | Thymoma, odontoma (maxillary tumor) | Y | |
| Kuroiwa [55] | 46 | F | Thyroid adenoma, Thymoma (necropsy) | Father, paternal grandmother—esophageal cancer | Y |
| Carlin [67] | 64 | F | Thymoma | ||
| Canovas [68] | 32 | F | Thymoma | ||
| Stieler [47] | 34 | M | Multiple basal cell carcinomas | ||
| Kerbrat [58] | 27 | M | Testicular cancer (immature teratoma) | Y | |
| Harada [62] | 55 | M | Parathyroid adenoma | ||
| 57 | M | Parathyroid adenoma | |||
| Garcia Delgado [64] | 40 | M | Parathyroid adenoma | Y | |
| Rosenberg [49] | 12 | F | Medullary thyroid carcinoma Parathyroid adenoma | Mother—medullary thyroid carcinoma, parathyroid tumor, NOS | Y |
| 38 | F | Thyroid carcinoma | Y | ||
| 40 | Parathyroid adenoma | ||||
| Hirai [61] | 42 | F | Thymoma | ||
| Reimund [50] | 48 | M | Multiple carcinoid tumors of the small bowel | Y | |
| Hirai [61] | 36 | M | Thymoma | ||
| Bell [69] | 56 | F | Parathyroid adenoma | ||
| Molina [53] | 40 | M | Parathyroid adenoma, Thyroid goiter | ||
| Kinoshita [5]a | 54 | F | Endometroid carcinoma of the ovary | ||
| Ogata [4]a | 58 | M | Pleomorphic parotid adenoma | Y | |
| Mascalchi [54] | 17 | M | Spinal hemangioblastoma | ||
| Sugio [46]a | 51 | M | Insulinoma | Y | |
| Jinnai [6]a | |||||
| Azurdia [71] | 42 | F | Multiple basal cell carcinomas | Y | |
| Jinnai [6] | 58 | M | Adenocarcinoma of sigmoid colon | ||
| 47 | M | Gastric adenocarcinoma | |||
| Draper [35] | 9 | F | Pleomorphic parotid adenoma | ||
| Osanai [3]a | 66 | M | Laryngeal squamous cell carcinoma | Y | |
| Kinoshita [57]a | 70 | ||||
| Renal cell carcinoma with liver metastases | |||||
| Hirai [61] | 42 | M | Multiple thymoma | Y | |
| Itin [60] | 50 | F | Multiple basal cell carcinomas | Y | |
| Kudva [56] | 46 | F | Thymoma (Stage III) | Y | |
| Unknown | Thyroid cancer | ||||
| Vandecaveye [44] | 60 | M | Insulinoma | ||
| Varras [43] | 37 | F | Uterine leiomyoma | Y | |
| Suzuki [45] | 50 | M | Gastric adenocarcinoma | ||
| Banuls [7]a | 41 | M | Multiple basal cell carcinomas | Y | |
| Ando [72] | 54 | M | Pheochromocytoma | ||
| Montella [52] | 53 | M | B-cell gastric lymphoma | Y | |
| Saponaro [48]a | 42 | F | Multiple basal cell carcinomas | Y |
DM1 mutation reported
Rosenberg et al. [49] reported a 44 year old woman with DM and hyperparathyroidism, with no apparent parathyroid adenoma, who also had neurofibromatosis. She had several family members with neurofibromatosis, most of whom reportedly also had DM. There have been several similar case reports of families with both DM and neurofibromatosis, the latter characterized by multiple café-aulait spots and multiple cutaneous fibromas [73, 74]. Since these reports occurred prior to the availability of clinical genetic testing for either disorder, it is difficult to determine whether these were coincidental associations of the two diseases. Interestingly, neurofibromatosis is a complex neurocristopathy, a group of hereditary cancer syndromes which also includes the multiple endocrine neoplasias; the latter share many of the tumor features reported anecdotally in DM patients, including neoplasms of the pituitary, thyroid, and parathyroid glands, as well as insulinomas, and carcinoid tumors.
In 2001, NIH established a National Registry of DM and FSHD patients and family members, to facilitate research in these diseases by bringing affected persons and their relatives together with DM researchers. Patients complete a baseline medical history questionnaire at the time they enroll, and provide annual health updates. A preliminary assessment of the National Registry reveals that 53 of the first 441 DM patients enrolled reported having benign and/or malignant tumors (Table 3). Unfortunately, these were all self-reported neoplasms, and neither age at diagnosis nor pathologic confirmation was available. However, it can be assumed that they were diagnosed at some point prior to the age at which they completed the baseline questionnaire. Therefore, twenty-five individuals were diagnosed at or before age 50. The mean age at the time of questionnaire was 52.6 years, with a range from 18 to 75 years. Of note, sixteen DM patients reported a diagnosis of a “skin tumor,” including 4 with basal cell carcinoma and 2 with melanoma. Compared with the previously-published cases from the literature summarized above, Registry members reported 2 parotid tumors and 4 thyroid tumors, but no thymoma or parathyroid adenoma. One patient reported familial polyposis, which is a clinical feature of Gardner syndrome (a disorder also associated with multiple pilomatricomas).
Table 3.
Patients with self-reported neoplasms in the DM NIH registry (n = 52 self-reported patients; n = 441 total patients enrolled in NIH Registry)
| Tumor site | Gender | Age at time of questionnaire |
DM type |
|---|---|---|---|
| Skin (NOS) | M | 18 | 1 |
| M | 38 | 1 | |
| F | 43 | 1 | |
| M | 44 | 1 | |
| M | 48 | 1 | |
| F* | 49 | 1 | |
| M | 55 | 1 | |
| F | 59 | 1 | |
| F** | 71 | 1 | |
| F | 72 | 2 | |
| Basal cell | F | 41 | 1 |
| F | 44 | 1 | |
| M | 51 | 1 | |
| F*** | 54 | 1 | |
| Melanoma | F | 44 | 1 |
| F | 48 | 1 | |
| Breast | F | 43 | 2 |
| F**** | 52 | 1 | |
| F | 54 | 1 | |
| F | 57, 59 | 1 | |
| F | 68 | 1 | |
| F** | 68 | 1 | |
| Benign breast | F | 46 | 2 |
| F | 62 | 1 | |
| Colon | F | 50 | 1 |
| F***** | 54 | 1 | |
| M | 54 | 1 | |
| M | 74 | 2 | |
| Cervix | F | 38 | 1 |
| F* | 49 | 1 | |
| F | 50 | 1 | |
| F | 57 | 1 | |
| Thyroid | F | 21 | 1 |
| F | 48 | 1 | |
| M | 54 | 1 | |
| Lung | M | 50 | 1 |
| F**** | 55 | 1 | |
| Wilms tumor | F | 40 | 1 |
| F | 43 | 1 | |
| Benign tumor (NOS) | F | 50 | 1 |
| F | 57 | 1 | |
| Benign parotid | M | 52 | 1 |
| F | 56 | 1 | |
| Fibroid (NOS) | F | 43 | 1 |
| F*** | 56 | 1 | |
| Malignant (NOS) | F | 68 | 2 |
| Tumor (NOS) | F | 74 | 2 |
| Lipoma | M | 50 | 1 |
| Uterine | F** | 68 | 1 |
| Ovarian | F | 70 | 2 |
| Brain (NOS) | F***** | 54 | 1 |
| Hairy cell leukemia | F | 75 | 1 |
| Familial polyposis | M | 45 | 1 |
| Multiple myeloma | F | 54 | 2 |
*, **, ***, ****, ***** denote that this is the same individual with multiple cancers
Genetic instability and DM
The myotonic dystrophies are multisystem, autosomal dominant disorders. In most individuals, the presence of distal versus proximal muscular weakness allows for a clinical diagnosis of DM1 or DM2, respectively. However, the definitive diagnosis depends upon DNA testing. DM1 is associated with variable degrees of clinical severity among affected individuals even within the same family, as well as an earlier age-at-onset and an increase in the severity of disease through successive generations, a genetic phenomenon known as “anticipation.” The severity of symptoms and age-at-onset correlate positively with the length of the repeat expansion inherited by an affected individual [1, 2]. Somatic instability is also seen, with repeat size increasing with age, as well as at different rates in various tissues [75, 76]. In DM2, there has been no significant correlation identified between the age at disease onset and the length of the repeat expansion. However, DM2 does display intergenerational and somatic instability [77].
The underlying mechanisms for the repeat instability are unclear. Both genome replication and repair mechanisms, such as replication slippage, the direction of replication progression, DNA methylation, gap filling, mismatch repair, double strand break repair, and recombination may be involved. It is likely that multiple pathways at various stages of development and in different tissues contribute to repeat instability [78–80].
Pathogenetics of DM
The mutation for DM1 lies within the non-coding region of DMPK, and several pathogenetic mechanisms have been proposed. Haploinsufficiency of the DMPK protein was initially suspected; the altered expression of neighboring genes due to DMPK-related CTG repeat expansion effects on chromatin restructuring has also been implicated in the pathogenesis of DM1. However, mouse models developed to test these hypotheses have failed to reproduce most of the symptoms that are seen in humans with DM1 [81, 82]. The identification of the gene responsible for DM2 has provided new insights into the molecular pathogenesis of the common features of these two disorders.
Neighboring genes near the DM2 locus bear no obvious functional relationship to those at the DM1 locus, making it difficult to explain how dysregulation of genes at these two loci could result in such a similar phenotype. Parallel studies in DM1 and DM2 have shown that the CTG expansions in DMPK (DM1) and the CCTG expansions in ZNF9 (DM2) are both transcribed into RNA, and actually do not alter the protein coding portion of their respective genes, but their mutant mRNA are sequestered in the nucleus and alter the function of RNA splicing factors, such as muscleblind (MBNL1) and the CUG-BP1 and ETR-3-like factors (CELF) family of RNA-binding proteins [83]. Altering the functional levels of these proteins in turn results in abnormal splicing and patterns for their various target mRNAs, including cardiac troponin T (cTNT), insulin receptor (IR), muscle chloride channel (ClC-1), and microtubule-associated tau with downstream effects producing characteristic clinical features of DM1 and DM2 [84–86].
Hypothesis: tumorigenesis in DM
We suggest that DM patients may have a previously-unrecognized susceptibility to an unusual spectrum of neoplasms as part of their disease-related phenotype. The tumor types implicated based on the descriptive data currently available include pilomatricomas; thymomas; adenomas of the parotid, pituitary, and parathyroid; insulinomas; thyroid tumors; and multiple basal cell carcinomas. Here, we hypothesize a potential pathway of tumorigenesis in DM based on these tumor types and other associated genetic disorders.
Pilomatricoma is the most common neoplasm thus far described in DM patients. Benign pilomatricomas and pilomatrix carcinomas have been shown to contain somatic mutations in the gene encoding β-catenin, CTNNB1 [87–89]. Immunohistochemical analysis has demonstrated that these mutations result in nuclear accumulation of β-catenin, and transgenic mice expressing activating β-catenin mutations develop similar hair follicle tumors [90]. Furthermore, somatic mutations in salivary gland adenomas result in over-expression of the pleomorphic adenoma gene PLAG1, leading to upregulation of β-catenin expression [91]. Thyroid carcinomas, pituitary adenomas, basal cell carcinomas, and melanomas have also been shown to have aberrant accumulation of β-catenin [92–94]. However, many tumors accumulate β-catenin in the absence of mutations in CTNNB1, suggesting additional sources for aberrant β-catenin accumulation [95].
As described above, Gardner syndrome (GS) and Rubinstein–Taybi syndrome (RTS) have been associated with an increased risk of pilomatricomas. GS is the association of familial adenomatous polyposis (FAP) with osteomas and soft tissue neoplasms including lipomas, epidermoid cysts, fibromas, desmoid tumors; it is caused by mutations in APC [96]. These individuals are also at risk of developing colorectal cancer, hepatoblastoma, duodenal carcinomas, stomach and pancreatic adenocarcinoma, and follicular and papillary thyroid carcinoma [97]. RTS is an autosomal dominant, multiple congenital anomaly syndrome in which various neoplasms have been reported, including medulloblastoma, neuroblastoma, oligodendroglioma, meningioma, rhabdomyosarcoma, pheochromocytoma, and leukemia [98]. Structural chromosomal abnormalities affecting 16p13.3 have been identified in some RTS patients [99]. Mutations in the CREB-binding protein gene located in this region are present in 56% of RTS patients, and a few patients have been shown to have mutations in the p300 gene, a transcriptional co-activator which binds to CREBBP [100–102].
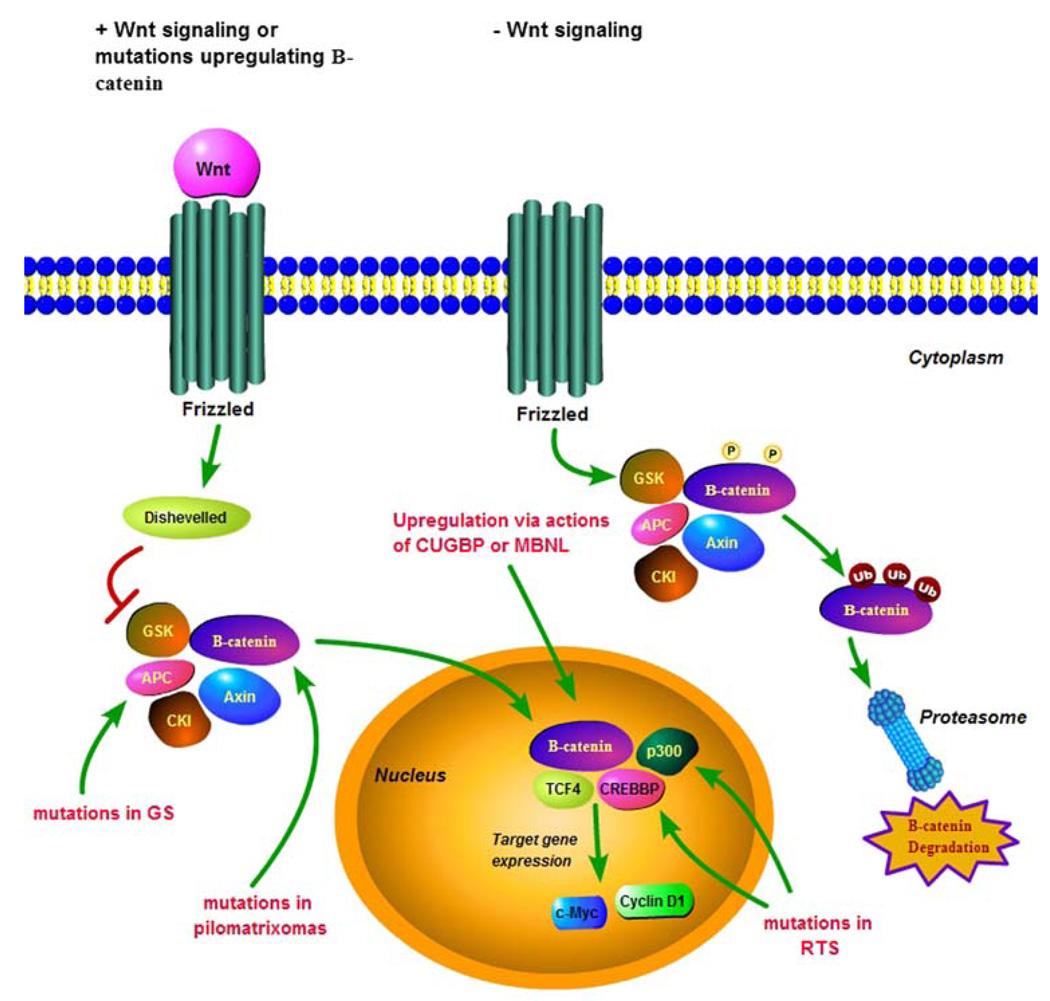
In GS, germline APC gene mutations result in a truncated APC protein. The native APC protein has multiple functional domains that interact with a variety of cytoplasmic proteins, including β-catenin. Inherited or somatic mutations in APC either remove or truncate its β-catenin regulatory domain. As shown in Fig. 1, the APC/AXIN/GSK3β/CKI/β-catenin complex is unable to phosphorylate β-catenin, leading to its accumulation in the nucleus. Accumulation of β-catenin allows it to complex with the transcription factor TCF-4, which activates downstream growth promoting genes including c-myc and cyclin D1 [95, 103, 104]. Mutations in CTNNB1 have also been seen in colorectal tumors expressing wild-type APC [105]. The CREBBP/p300 transcriptional co-activator complex associated with RTS has been shown to interact with β-catenin and activate transcription of downstream genes in mammalian cells [106–108]. Somatic mutations in both CREBBP and p300 have also been reported in various neoplasms [109].
Fig. 1.
Wnt signaling and regulation of β-catenin in tumorigenesis. In the presence of Wnt signaling, or after mutations of APC, axin, or β-catenin, the phosphorylation and degradation of β-catenin are blocked. This leads to the accumulation of β-catenin in the nucleus, where it binds to TCF4 and promotes transcriptional activation of target genes including c-Myc and cyclin D1. The transcriptional co-activator complex CREBBP/p300 interacts with β-catenin and synergistically activates β-catenin/TCF4 transcriptional activation. In the absence of Wnt signaling, the cytoplasmic degradation complex of APC, GSK3β, CKI, and β-catenin forms leading to the phosphorylation of β-catenin, ultimately leading to its ubiquitination and its degradation in the proteasome. Somatic β-catenin mutations have been seen in pilomatricomas as well as other tumors associated with DM. Germline APC mutations result in Gardner syndrome. Germline CREBBP and p300 mutations result in Rubinstein-Taybi syndrome. We believe that the association between DM and pilomatricomas, as well as the other tumors that have been reported in these patients involves the upregulation of β-catenin via the Wnt signaling pathway, possibly via the actions of CUGBP or MBNL
We hypothesize that tumor progression in DM also involves the upregulation of β-catenin via the Wnt signaling pathway, possibly via the actions of CUG-BP or MBNL. In human fibroblasts, CUG-BP1 has been shown to block calcireticulin-mediated repression of p21 translation [110]. Interestingly, increased expression of p21 is a somatic molecular characteristic of thymomas, one of the more commonly reported neoplasms in DM patients [111]. Furthermore, p21 plays a major role in oncogenesis, and has also been implicated in apoptosis, terminal differentiation, and replicative senescence via interactions with such well-known tumor suppressor genes as p53 and BRCA1, as well genes in the Wnt/β-catenin signaling pathway [112–114].
Since none of the genetic mechanisms proposed thus far explain the pathogenesis of DM completely, it is possible that multiple mechanisms may contribute to the differing clinical features seen in DM1 and DM2, which may depend on temporal variations in gene expression and/or tissue specificity. The majority of tumors reported thus far in the literature and in the NIH Registry were seen in individuals with a diagnosis of DM1, which could be due to the less common occurrence of DM1. However, to the extent that haploinsufficiency at the DM1 locus may account for some portion of the DM1 phenotype, it is conceivable that loss of heterozygosity mutations in DMPK might increase the risk of cancer. The catalytic domains of a subfamily of serine/threonine protein kinases in Drosophila melanogaster have shown homology to DMPK. The homozygous loss of one of these genes, the lats/warts gene, leads to excess growth and abnormalities of cell differentiation in Drosophila clones, suggesting that DMPK may function as a tumor suppressor gene within the Wnt/β-catenin signaling pathway [115].
Interestingly, in the limited number of instances in which DM1-related neoplasms have been studied, the tumors contained repeat expansions that were considerably longer than those assayed in non-neoplastic tissue from the same organ, or from the patient’s skeletal muscle and/or leukocytes [3–7]. It is uncertain whether this repeat expansion was secondary to tumorigenesis-related cell proliferation, or whether the somatic instability of repeat length seen commonly in DM, with repeat size increasing with age and at different rates in various tissues, leads to a critical threshold of sequestration of RNA binding proteins, possibly resulting in the upregulation of Wnt/β-catenin signaling in a tissue specific manner.
Conclusions and future directions
The study of familial clustering of cancer and multiple congenital anomalies has identified multiple cancer susceptibility genes and helped to define the pathogenesis of both hereditary and non-hereditary cancers. Despite the non-quantitative nature of the data available to date, the frequency, unusual types of tumors reported, and early age of tumor onset in DM patients are suggestive of a genetic mechanism. The apparent excess of pilomatricoma is the strongest candidate for a genuine relationship between DM and a risk of developing neoplasia. This observation led to our hypothesis that dysregulation of the Wnt/β-catenin signaling pathway may be the molecular basis for this relationship. This pathway has not only been implicated in the development of sporadic pilomatricomas and several other neoplasms reported in DM patients, but also in GS and RTS, both disorders characterized by an increased risk of pilomatricoma and multiple other tumors. A thorough, quantitative analysis of DM patients enrolled in the DM Registry, and population-based studies to quantify the risks of the various tumors found in DM1 and DM2 patients, are currently underway.
Various molecular mechanisms thought to be of importance in determining the classical DM phenotype have been shown to have links to previously-described oncogenic pathways. One or more of these may have a role in the pathogenesis of tumor development in DM patients, by dysregulation of a critical pathway in a tissue specific, time-dependent manner. Further defining these mechanisms may also help to explain any differences in tumor development that may occur between DM1 and DM2 patients. Additional systematic molecular and genetic studies of tumors arising in DM patients are needed to provide further insight into both tumorigenesis and the mechanism for genetic instability in DM, as well as influencing the medical management of these patients.
Acknowledgments
These observations are offered in memory of Dr. Robert W. Miller, Scientist Emeritus at the National Cancer Institute, who was a pioneer in leveraging alert clinical observations into paradigm-altering etiologic insights. He remains a role model and an inspiration to all those fortunate enough to have known him. Funding: This work was supported through funding provided by (1) Intramural Research Program of the National Cancer Institute (2) The National Registry of DM and FSHD Patients and Family Members is supported through the National Institute of Arthritis and Musculo-skeletal and Skin Diseases and the National Institute of Neurological Disorders and Stroke, #NO1-AR-5-2274 (3) University of Rochester Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center (NIH/NS48843).
Contributor Information
Christine M. Mueller, Email: muellerc@mail.nih.gov, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health/DHHS, 6120 Executive Boulevard, EPS 7101, Rockville, MD 20852-7231, USA
James E. Hilbert, Neuromuscular Disease Center, University of Rochester Medical Center, Rochester, NY, USA
William Martens, Neuromuscular Disease Center, University of Rochester Medical Center, Rochester, NY, USA.
Charles A. Thornton, Neuromuscular Disease Center, University of Rochester Medical Center, Rochester, NY, USA
Richard T. Moxley, III, Neuromuscular Disease Center, University of Rochester Medical Center, Rochester, NY, USA.
Mark H. Greene, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health/DHHS, 6120 Executive Boulevard, EPS 7101, Rockville, MD 20852-7231, USA
References
- 1.Harper PS. Myotonic dystrophy. London: WB Saunders; 2001. [Google Scholar]
- 2.The International Myotonic Dystrophy Consortium (IDMC) New nomenclature and DNA testing guidelines for myotonic dystrophy type 1 (DM1) Neurology. 2000;54:1218–1221. doi: 10.1212/wnl.54.6.1218. [DOI] [PubMed] [Google Scholar]
- 3.Osanai R, Kinoshita M, Hirose K, Homma T, Kawabata I. CTG triplet repeat expansion in a laryngeal carcinoma from a patient with myotonic dystrophy. Muscle Nerve. 2000;23:804–806. doi: 10.1002/(sici)1097-4598(200005)23:5<804::aid-mus19>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Ogata K, Takahashi A, Oguchi N, Ishitoya J, Fuse S, Shimpo T. Somatic mosaicism of p(CTG)n expansion in a case of myotonic dystrophy with parotid tumor. Rinsho Shinkeigaku. 1998;38:736–738. [PubMed] [Google Scholar]
- 5.Kinoshita M, Igarashi A, Komori T, et al. Differences in CTG triplet repeat expansions in an ovarian cancer and cyst from a patient with myotonic dystrophy. Muscle Nerve. 1997;20:622–624. doi: 10.1002/(sici)1097-4598(199705)20:5<622::aid-mus16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Jinnai K, Sugio T, Mitani M, Hashimoto K, Takahashi K. Elongation of (CTG)n repeats in myotonic dystrophy protein kinase gene in tumors associated with myotonic dystrophy patients. Muscle Nerve. 1999;22:1271–1274. doi: 10.1002/(sici)1097-4598(199909)22:9<1271::aid-mus16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Banuls J, Botella R, Palau F, et al. Tissue and tumor mosaicism of the myotonin protein kinase gene trinucleotide repeat in a patient with multiple basal cell carcinomas associated with myotonic dystrophy. J Am Acad Dermatol. 2004;50:S1–S3. doi: 10.1016/s0190-9622(03)00125-7. [DOI] [PubMed] [Google Scholar]
- 8.Moxley RTIII. Potential for growth factor treatment of muscle disease. Curr Opin Neurol. 1994;7:427–434. doi: 10.1097/00019052-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Vlachopapadopoulou E, Zachwieja JJ, Gertner JM, et al. Metabolic and clinical response to recombinant human insulin-like growth factor I in myotonic dystrophy—a clinical research center study. J Clin Endocrinol Metab. 1995;80:3715–3723. doi: 10.1210/jcem.80.12.8530624. [DOI] [PubMed] [Google Scholar]
- 10.Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found Symp. 2004;262:247–260. Discussion 260–268. [PubMed] [Google Scholar]
- 11.Marrogi AJ, Dehner LP, Coffin C, Wick MR. Pilomatrical neoplasms in children and young adults. Am J Dermatopath. 1992;14:87–94. doi: 10.1097/00000372-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Moehlenbeck FW. Pilomatricoma (calcifying epithelioma). A statistical study. Arch Dermatol. 1973;108:532–534. [PubMed] [Google Scholar]
- 13.Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39:191–195. doi: 10.1016/s0190-9622(98)70073-8. [DOI] [PubMed] [Google Scholar]
- 14.Masuno M, Imaizumi K, Ishii T, Kuroki Y, Baba N, Tanaka Y. Pilomatricomas in Rubinstein-Taybi syndrome. Am J Med Genet. 1998;77:81–82. doi: 10.1002/(sici)1096-8628(19980428)77:1<81::aid-ajmg19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Pujol RM, Casanova JM, Egido R, Pujol J, de Moragas JM. Multiple familial pilomatricomas: a cutaneous marker for Gardner syndrome? Pediatr Dermatol. 1995;12:331–335. doi: 10.1111/j.1525-1470.1995.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 16.Hills RJ, Ive FA. Familial multiple pilomatricomas. Br J Dermatol. 1992;127:194–195. doi: 10.1111/j.1365-2133.1992.tb08062.x. [DOI] [PubMed] [Google Scholar]
- 17.Cantwell AR, Jr, Reed WB. Myotonia atrophica and multiple calcifying epithelioma of Malherbe. Acta Derm Venereol. 1965;45:387–390. [PubMed] [Google Scholar]
- 18.White GM. Multiple pilomatricomas of the scalp. Int J Dermatol. 1992;31:348–350. doi: 10.1111/j.1365-4362.1992.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 19.Urvoy M, Legall F, Toulemont PJ, Chevrant-Breton J. Multiple pilomatricoma. Apropos of a case. J Fr Ophtalmol. 1996;19:464–466. [PubMed] [Google Scholar]
- 20.Street ML, Rogers RSIII. Multiple pilomatricomas and myotonic dystrophy. J Dermatol Surg Oncol. 1991;17:728–730. doi: 10.1111/j.1524-4725.1991.tb03426.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz BK, Peraza JE. Pilomatricomas associated with myotonic dystrophy. J Am Acad Dermatol. 1987;16:887–888. doi: 10.1016/s0190-9622(87)80236-0. [DOI] [PubMed] [Google Scholar]
- 22.Salerni E, Bonatti ML, D’Aurizio C, Baldassarre M, D’Alessandro E, Prencipe M. Multiple pilomatricomas and myotonic dystrophy: a case report. Riv Neurol. 1988;58:124–126. [PubMed] [Google Scholar]
- 23.Runne U, Chilf GN, Zentner J. Multiple pilomatricomas as symptoms of Curschmann-Steinert myotonia dystrophica. Hautarzt. 1982;33:271–275. [PubMed] [Google Scholar]
- 24.Ribera M, Calderon P, Barranco C, Ferrandiz C. Multiple pilomatricomas associated with myotonic dystrophy and medullary carcinoma of the thyroid. Med Cutan Ibero Lat Am. 1989;17:395–398. [PubMed] [Google Scholar]
- 25.Nishie W, Iitoyo M, Miyazawa H. Follicular cyst in a patient with myotonic dystrophy: a case of cyst with differentiation toward follicular infundibulum, isthmus, inner root sheath, and hair. Am J Dermatopathol. 2001;23:521–524. doi: 10.1097/00000372-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Albaladejo M, Alguacil-Garcia G, de Paco-Moya M, Moreno-Requena J. Dystrophia myotonica with multiple pilomatricomas. Rev Clin Esp. 1995;195:516–517. [PubMed] [Google Scholar]
- 27.Laredo Ortiz C, Munoz Romero F, Mallent Anon J, Domenech Miro E, Tafalla Pena M. Multiple pilomatricomas associated with Steinert disease. An Med Interna. 1997;14:409–411. [PubMed] [Google Scholar]
- 28.Kopeloff I, Orlow SJ, Sanchez MR. Multiple pilomatricomas: report of two cases and review of the association with myotonic dystrophy. Cutis. 1992;50:290–292. [PubMed] [Google Scholar]
- 29.Harper PS. Calcifying epithelioma of Malherbe. Association with myotonic muscular dystrophy. Arch Dermatol. 1972;106:41–44. [PubMed] [Google Scholar]
- 30.Harper PS. Calcifying epithelioma of Malherbe and myotonic dystrophy in sisters. Birth Defects Orig Artic Ser. 1971;7:343–345. [PubMed] [Google Scholar]
- 31.Graells J, Servitje O, Badell A, Notario J, Peyri J. Multiple familial pilomatricomas associated with myotonic dystrophy. Int J Dermatol. 1996;35:732–733. doi: 10.1111/j.1365-4362.1996.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 32.Geh JL, Moss AL. Multiple pilomatricomata and myotonic dystrophy: a familial association. Br J Plast Surg. 1999;52:143–145. doi: 10.1054/bjps.1998.3036. [DOI] [PubMed] [Google Scholar]
- 33.Filla A, Perretti A, Barbieri F, et al. Myotonic dystrophy and pilomatricomas: an unusual association. Acta Neurol (Napoli) 1982;4:79–91. [PubMed] [Google Scholar]
- 34.Farrell AM, Ross JS, Barton SE, Bunker CB. Multiple pilomatricomas and myotonic dystrophy in a patient with AIDS. Clin Exp Dermatol. 1995;20:423–424. doi: 10.1111/j.1365-2230.1995.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 35.Draper MR, Pickles JM. Pleomorphic adenoma and myotonic dystrophy: an association? J Laryngol Otol. 2000;114:985–987. doi: 10.1258/0022215001904563. [DOI] [PubMed] [Google Scholar]
- 36.Delfino M, Monfrecola G, Ayala F, Suppa F, Piccirillo A. Multiple familial pilomatricomas: a cutaneous marker for myotonic dystrophy. Dermatologica. 1985;170:128–132. doi: 10.1159/000249516. [DOI] [PubMed] [Google Scholar]
- 37.Chiaramonti A, Gilgor RS. Pilomatricomas associated with myotonic dystrophy. Arch Dermatol. 1978;114:1363–1365. [PubMed] [Google Scholar]
- 38.Bouadjar B, Masmoudi AN, Bouhadef A, Ysmail-Dahlouk M. Multiple pilomatricoma and myotonic dystrophy. Ann Dermatol Venereol. 1992;119:899–900. [PubMed] [Google Scholar]
- 39.Betlloch I, Morell A, Silvestre JF, Sevila A, Banuls J, Alfonso R. Pilomatricoma and myotonic dystrophy. Letter. Rev Neurol. 1997;25:306–307. [PubMed] [Google Scholar]
- 40.Barberio E, Nino M, Dente V, Delfino M. Guess what! Multiple pilomatricomas and Steiner disease. Eur J Dermatol. 2002;12:293–294. [PubMed] [Google Scholar]
- 41.Aso M, Shimao S, Takahashi K. Pilomatricomas: association with myotonic dystrophy. Dermatologica. 1981;162:197–202. doi: 10.1159/000250269. [DOI] [PubMed] [Google Scholar]
- 42.Berberian BJ, Colonna TM, Battaglia M, Sulica VI. Multiple pilomatricomas in association with myotonic dystrophy and a family history of melanoma. J Am Acad Dermatol. 1997;37:268–269. doi: 10.1016/s0190-9622(97)80138-7. [DOI] [PubMed] [Google Scholar]
- 43.Varras M, Polyzos D, Alexopoulos C, Pappa P, Akrivis C. Torsion of a non-gravid leiomyomatous uterus in a patient with myotonic dystrophy complaining of acute urinary retention: anaesthetic management for total abdominal hysterectomy. Clin Exp Obstet Gynecol. 2003;30:147–150. [PubMed] [Google Scholar]
- 44.Vandecaveye V, Verswijvel G, Colla P, Verhelst H, VanRobaeys J, Palmers Y. Cystic insulinoma of the pancreas in a patient with myotonic dystrophy: correlation of imaging and pathologic findings. Jbr-Btr. 2003;86:268–271. [PubMed] [Google Scholar]
- 45.Suzuki H, Aoyagi M. Anesthetic management with a laryngeal mask airway for gastrectomy in a patient with myotonic dystrophy. Masui. 2003;52:993–995. [PubMed] [Google Scholar]
- 46.Sugio T, Jinnai K, Ohara T, et al. Myotonic dystrophy associated with insulinoma. Intern Med. 1999;38:504–506. doi: 10.2169/internalmedicine.38.504. [DOI] [PubMed] [Google Scholar]
- 47.Stieler W, Plewig G. Multiple basaliomas in Curschmann-Steinert myotonia atrophica. Hautarzt. 1986;37:226–229. [PubMed] [Google Scholar]
- 48.Saponaro AE, Marini MA, Rossi GC, Casas JG. Multiple basal cell carcinomas in a patient with myotonic dystrophy type 1. Int J Dermatol. 2006;45:87–88. doi: 10.1111/j.1365-4632.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg NL, Diliberti JH, Andrews AM, Buist NR. Myotonic dystrophy and hyperparathyroidism: association with neurofibromatosis and multiple endocrine adenomatosis type 2A. J Neurol Neurosurg Psychiatry. 1988;51:1578–1580. doi: 10.1136/jnnp.51.12.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimund JM, Duclos B, Chamouard P, Warter JM, Weill JP, Baumann R. Intestinal carcinoid tumor and myotonic dystrophy. A new association? Dig Dis Sci. 1992;37:1922–1925. doi: 10.1007/BF01308090. [DOI] [PubMed] [Google Scholar]
- 51.Mudge BJ, Taylor PB, Vanderspek AF. Perioperative hazards in myotonic dystrophy. Anaesthesia. 1980;35:492–495. doi: 10.1111/j.1365-2044.1980.tb03827.x. [DOI] [PubMed] [Google Scholar]
- 52.Montella L, Caraglia M, Addeo R, et al. Atrial fibrillation following chemotherapy for stage IIIE diffuse large B-cell gastric lymphoma in a patient with myotonic dystrophy (Steinert’s disease) Ann Hematol. 2005;84:192–193. doi: 10.1007/s00277-004-0867-6. [DOI] [PubMed] [Google Scholar]
- 53.Molina MJ, Lara JI, Riobo P, et al. Primary hyperthyroidism and associated hyperparathyroidism in a patient with myotonic dystrophy: Steinert with hyperthyroidism and hyperparathyroidism. Am J Med Sci. 1996;311:296–298. doi: 10.1097/00000441-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Mascalchi M, Padovani R, Taiuti R, Quilici N. Syringomyelia in myotonic dystrophy due to spinal hemangioblastoma. Surg Neurol. 1998;50:446–448. doi: 10.1016/s0090-3019(97)00050-5. [DOI] [PubMed] [Google Scholar]
- 55.Kuroiwa Y, Yamada A, Ikebe K, Kosaka K, Sugita H, Murakami T. Myotonic dystrophy and thymoma: a necropsy case report. J Neurol Neurosurg Psychiatry. 1981;44:173–175. doi: 10.1136/jnnp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kudva GC, Maliekel K, Kim HJ, et al. Thymoma and myotonic dystrophy: successful treatment with chemotherapy and radiation: case report and review of the literature. Chest. 2002;121:2061–2063. doi: 10.1378/chest.121.6.2061. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita M, Osanai R, Kikkawa M, et al. A patient with myotonic dystrophy type 1 (DM 1) accompanied by laryngeal and renal cell carcinomas had a small CTG triplet repeat expansion but no somatic instability in normal tissues. Intern Med. 2002;41:312–318. doi: 10.2169/internalmedicine.41.312. [DOI] [PubMed] [Google Scholar]
- 58.Kerbrat P, Toussaint C, Ben Hassel M, Guerin D. Association of Steinert’s disease and testicular cancer. Presse Med. 1987;16:1434. [PubMed] [Google Scholar]
- 59.Johannesson G, Henriksson KG, Odkvist L. Coincidence of dystrophia myotonica and pleomorphic adenoma of the parotid gland. Acta Neurol Scand. 1978;57:275–278. doi: 10.1111/j.1600-0404.1978.tb05876.x. [DOI] [PubMed] [Google Scholar]
- 60.Itin PH, Laeng RH. Multiple pigmented basalioma of the scalp in a patient with Curschmann-Steinert myotonia dystrophica. Confirmation of a rare symptom constellation. Hautarzt. 2001;52:244–246. doi: 10.1007/s001050051298. [DOI] [PubMed] [Google Scholar]
- 61.Hirai T, Yamanaka A, Fujimoto T, Takahashi A, Takayama Y, Yamanaka K. Multiple thymoma with myotonic dystrophy. Jpn J Thorac Cardiovasc Surg. 2001;49:457–460. doi: 10.1007/BF02913913. [DOI] [PubMed] [Google Scholar]
- 62.Harada S, Matsumoto T, Ikeda K, Fukumoto S, Ihara Y, Ogata E. Association of primary hyperparathyroidism with myotonic dystrophy in two patients. Arch Intern Med. 1987;147:777–778. [PubMed] [Google Scholar]
- 63.Goto IK, Ichimaru K, Maruyama T, et al. An autopsy case of myotonic dystrophy with familial diabetes mellitus, and mixed tumor of the parotid gland and a thymoma. Fukuoka Acta Med. 1969;60:126–133. [Google Scholar]
- 64.Garcia Delgado E, Ruiz Galiana J. Association of primary hyperparathyroidism with myotonic dystrophy in two patients. Arch Intern Med. 1988;148:237–241. doi: 10.1001/archinte.148.1.237b. [DOI] [PubMed] [Google Scholar]
- 65.Fraumeni JF, Jr, Chabner BA, Li FP, Carbone PP. Myotonic dystrophy and leukemia. JAMA. 1969;208:696. [PubMed] [Google Scholar]
- 66.Caughey JE. Hypogonadism and pituitary tumours; report of a case of dystrophia myotonica with hypogonadism and acromegaly. N Z Med J. 1958;57:482–486. [PubMed] [Google Scholar]
- 67.Carlin L, Biller J. Myotonic dystrophy and thymoma. J Neurol Neurosurg Psychiatry. 1981;44:852–853. doi: 10.1136/jnnp.44.9.852-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Canovas A, Rodriguez Illera E, Arizcun A, Riva C, Diego J. Myotonic dystrophy and thymoma associated with myasthenic behavior on electromyography. Rev Clin Esp. 1981;160:405–407. [PubMed] [Google Scholar]
- 69.Bell E, Lorimer AR, Hinnie J. Association between myotonic dystrophy and primary hyperparathyroidism. J Int Med Res. 1994;22:296–298. doi: 10.1177/030006059402200508. [DOI] [PubMed] [Google Scholar]
- 70.Banna M, Bradley WG, Pearce GW. Massive pituitary adenoma in a patient with dystrophia myotonica. J Neurol Sci. 1973;20:1–6. doi: 10.1016/0022-510x(73)90113-5. [DOI] [PubMed] [Google Scholar]
- 71.Azurdia RM, Verbov JL. Myotonic dystrophy and basal cell carcinoma—a true association? Br J Dermatol. 1999;141:941–942. doi: 10.1046/j.1365-2133.1999.03184.x. [DOI] [PubMed] [Google Scholar]
- 72.Ando T, Goto R, Ohnishi H, Ishida M, Obara H. Anesthetic management for a patient of myotonic dystrophy with pheochromocytoma. Masui. 2004;53:1290–1292. [PubMed] [Google Scholar]
- 73.Ichikawa K, Crosley CJ, Culebras A, Weitkamp L. Coincidence of neurofibromatosis and myotonic dystrophy in a kindred. J Med Genet. 1981;18:134–138. doi: 10.1136/jmg.18.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pruzanski W, Adler H. Myotonic dystrophy, acute intermittent porphyria and neurofibromatosis in one patient. Acta Genet Stat Med. 1966;16:103–112. doi: 10.1159/000151955. [DOI] [PubMed] [Google Scholar]
- 75.Wong LJ, Ashizawa T, Monckton DG, Caskey CT, Richards CS. Somatic heterogeneity of the CTG repeat in myotonic dystrophy is age and size dependent. Am J Hum Genet. 1995;56:114–122. [PMC free article] [PubMed] [Google Scholar]
- 76.Ansved T, Edstrom L, Grandell U, Hedberg B, Anvret M. Variation of CTG-repeat number of the DMPK gene in muscle tissue. Neuromuscul Disord. 1997;7:152–155. doi: 10.1016/s0960-8966(97)00443-4. [DOI] [PubMed] [Google Scholar]
- 77.Day JW, Ricker K, Jacobsen JF, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 78.Nag DK. Trinucleotide repeat expansions: timing is everything. Trends Mol Med. 2003;9:455–457. doi: 10.1016/j.molmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Lenzmeier BA, Freudenreich CH. Trinucleotide repeat instability: a hairpin curve at the crossroads of replication, recombination, and repair. Cytogenet Genome Res. 2003;100:7–24. doi: 10.1159/000072836. [DOI] [PubMed] [Google Scholar]
- 80.Cleary JD, Pearson CE. The contribution of cis-elements to disease-associated repeat instability: clinical and experimental evidence. Cytogenet Genome Res. 2003;100:25–55. doi: 10.1159/000072837. [DOI] [PubMed] [Google Scholar]
- 81.Reddy S, Smith DB, Rich MM, et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat Genet. 1996;13:325–335. doi: 10.1038/ng0796-325. [DOI] [PubMed] [Google Scholar]
- 82.Jansen G, Groenen PJ, Bachner D, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13:316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- 83.Machuca-Tzili L, Brook D, Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve. 2005;32:1–18. doi: 10.1002/mus.20301. [DOI] [PubMed] [Google Scholar]
- 84.Kanadia RN, Johnstone KA, Mankodi A, et al. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–1980. doi: 10.1126/science.1088583. [DOI] [PubMed] [Google Scholar]
- 85.Ho TH, Charlet BN, Poulos MG, Singh G, Swanson MS, Cooper TA. Muscleblind proteins regulate alternative splicing. EMBO J. 2004;23:3103–3112. doi: 10.1038/sj.emboj.7600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho TH, Bundman D, Armstrong DL, Cooper TA. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum Mol Genet. 2005;14:1539–1547. doi: 10.1093/hmg/ddi162. [DOI] [PubMed] [Google Scholar]
- 87.Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–157. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 88.Kajino Y, Yamaguchi A, Hashimoto N, Matsuura A, Sato N, Kikuchi K. Beta-catenin gene mutation in human hair follicle-related tumors. Pathol Int. 2001;51:543–548. doi: 10.1046/j.1440-1827.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 89.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 90.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 91.Zhao X, Ren W, Yang W, et al. Wnt pathway is involved in pleomorphic adenomas induced by overexpression of PLAG1 in transgenic mice. Int J Cancer. 2006;118:643–648. doi: 10.1002/ijc.21400. [DOI] [PubMed] [Google Scholar]
- 92.El-Bahrawy M, El-Masry N, Alison M, Poulsom R, Fallowfield M. Expression of beta-catenin in basal cell carcinoma. Br J Dermatol. 2003;148:964–970. doi: 10.1046/j.1365-2133.2003.05240.x. [DOI] [PubMed] [Google Scholar]
- 93.Saldanha G, Ghura V, Potter L, Fletcher A. Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol. 2004;151:157–164. doi: 10.1111/j.1365-2133.2004.06048.x. [DOI] [PubMed] [Google Scholar]
- 94.Reifenberger J, Knobbe CB, Wolter M, et al. Molecular genetic analysis of malignant melanomas for aberrations of the WNT signaling pathway genes CTNNB1, APC, ICAT and BTRC. Int J Cancer. 2002;100:549–556. doi: 10.1002/ijc.10512. [DOI] [PubMed] [Google Scholar]
- 95.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 96.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 97.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 98.Miller RW, Rubinstein JH. Tumors in Rubinstein-Taybi syndrome. Am J Med Genet. 1995;56:112–115. doi: 10.1002/ajmg.1320560125. [DOI] [PubMed] [Google Scholar]
- 99.Taine L, Goizet C, Wen ZQ, et al. Submicroscopic deletion of chromosome 16p13.3 in patients with Rubinstein-Taybi syndrome. Am J Med Genet. 1998;78:267–270. [PubMed] [Google Scholar]
- 100.Roelfsema JH, White SJ, Ariyurek Y, et al. Genetic heterogeneity in Rubinstein-Taybi syndrome: mutations in both the CBP and EP300 genes cause disease. Am J Hum Genet. 2005;76:572–580. doi: 10.1086/429130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartsch O, Schmidt S, Richter M, et al. DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein-Taybi syndrome (RSTS) and in another patient with incomplete RSTS. Hum Genet. 2005;117:485–493. doi: 10.1007/s00439-005-1331-y. [DOI] [PubMed] [Google Scholar]
- 102.Bartsch O, Locher K, Meinecke P, et al. Molecular studies in 10 cases of Rubinstein-Taybi syndrome, including a mild variant showing a missense mutation in codon 1175 of CREBBP. J Med Genet. 2002;39:496–501. doi: 10.1136/jmg.39.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polakis P. Casein kinase 1: a Wnt’ er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 104.Behrens J. The role of the Wnt signalling pathway in colorectal tumorigenesis. Biochem Soc Trans. 2005;33:672–675. doi: 10.1042/BST0330672. [DOI] [PubMed] [Google Scholar]
- 105.Iwao K, Nakamori S, Kameyama M, et al. Activation of the beta-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res. 1998;58:1021–1026. [PubMed] [Google Scholar]
- 106.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun Y, Kolligs FT, Hottiger MO, Mosavin R, Fearon ER, Nabel GJ. Regulation of beta -catenin transformation by the p300 transcriptional coactivator. Proc Natl Acad Sci USA. 2000;97:12613–12618. doi: 10.1073/pnas.220158597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Labalette C, Renard CA, Neuveut C, Buendia MA, Wei Y. Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin. Mol Cell Biol. 2004;24:10689–10702. doi: 10.1128/MCB.24.24.10689-10702.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–4231. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- 110.Iakova P, Wang GL, Timchenko L, et al. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. EMBO J. 2004;23:406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mukai K, Sato Y, Hirohashi S, Shimosato Y. Expression of ras p21 protein by thymoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:11–16. doi: 10.1007/BF02899381. [DOI] [PubMed] [Google Scholar]
- 112.Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Beta-catenin simultaneously induces activation of the p53-p21WAF1 pathway and overexpression of cyclin D1 during squamous differentiation of endometrial carcinoma cells. Am J Pathol. 2004;164:1739–1749. doi: 10.1016/s0002-9440(10)63732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamei J, Toyofuku T, Hori M. Negative regulation of p21 by beta-catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem Biophys Res Commun. 2003;312:380–387. doi: 10.1016/j.bbrc.2003.10.129. [DOI] [PubMed] [Google Scholar]
- 114.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 115.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]