Abstract
The study of emotions elicited by human-computer interactions is a promising field that could lead to the identification of specific patterns of affective states. We present a heart rate variability (HRV) assessment of the autonomic nervous system (ANS) response and respiratory sinus arrhythmia during PC-mediated stimuli by means of standard and multivariate autoregressive spectral methods. 35 healthy volunteers were exposed to computer-mediated tasks during data collection. The stimuli were designed to elicit: relaxation (R), engagement (E) and stress (S); half of the subjects were exposed to E before S (RES) while the other to S before E (RSE). HRV measures clearly separate the ANS response among R, S and E. Less significant differences are found between E and S in RSE, suggesting that S stimuli may cause a lasting response affecting the E period. Results from the bivariate analysis indicate a disruption of the cardio-respiratory coupling during non-relax conditions.
1. Introduction
The impact of computational devices in modern everyday life has raised an increasing interest on the study of emotions elicited by human-computer interactions, with particular focus on better understanding the link between emotional responses and learning processes mediated by computers. Many studies have shown interesting results that support the feasibility to detect affective states by means of psycho-physiological data acquisitions and analysis, with the critical purpose to transform the correlation between biological signals and emotional reactions into additional inputs for innovative human-computer interactions [1-5].
In particular, heart rate variability (HRV) measured as the variations of the time interval between two consecutive cardiac beats registered by means of electrocardiogram (ECG), is influenced by multiple neural and hormonal inputs that generate specific observable rhythms in the series. These rhythms provide a quantitative noninvasive measure of the autonomic nervous system regulatory action. Moreover the quantification of respiratory sinus arrhythmia (RSA), the influence of respiration on heart rate, provides information about the mechanisms involved in respiratory coupling [6].
Transfer function analysis [7,8] has been used in cardiovascular modeling in order to describe the relationship between different cardiac variables in different frequency bands, thus focusing on various underlying physiological mechanisms. In particular, the coherence function can be considered as a measure of the strength of the linear coupling between the two time series. As nonzero coherence values are likely to occur at some frequency even in the case of complete uncoupling between two time series, a threshold level has to be defined in order to assess whether the two series are significantly coupled or not. Previous studies [9] showed that the use of surrogate series, preserving the power spectrum of the original series but being structurally uncoupled, are recommended to avoid false coupling detections in the presence of oscillations occurring at nearby frequencies but produced by different mechanisms.
Accordingly, the aim of our study is to evaluate the autonomic nervous system response and the mechanical and autonomic effect of respiration on heart rate [10] during different digital stimuli efficient in simulating good elicitors of the targeted affective states [11-12].
2. Methods
2.1. Experimental Protocol
A group of 35 healthy volunteers (20 to 25 years old) is considered in this study. Each subject was monitored during the following experimental conditions:
Baseline (B): fixation of a white screen (3 mins)
Relaxation (R): fixation of relaxing panoramas slides (10 mins)
Engagement (E): detective story reading on the PC screen (10-15 mins)
Stress (S): frustrating task performance (i.e. stroop task) (4 mins)
In order to account for possible effects due to the sequential order of the emotion-induced conditions, the sample was split into two groups: half of the subjects (RES group) were exposed to E before S, whereas the other half (RSE group) to S before E. Such setting allows for evaluation of both effects of Engagement on Stress and vice versa, as well as the effects of Engagement and Stress on Relaxation. The entire session was repeated for each student after one month.
Every subject was briefed in the Lab in order to become more familiar with the Lab environment and equipment. When the subject reached a comfortable status, he/she was asked to start the experiment. Since during the stress task subjects have to speak, they were instructed to loudly read statements written on some relaxation and engagement slides, in order to fairly compare stress to the other conditions.
2.2. Signal Acquisition and Preprocessing
ECG and respiration signals were continuously acquired with subjects sitting and interacting in front of a personal computer. The ECG electrodes and respiration belts were connected to a multi-modality device for real-time computerized biofeedback and data acquisition (Flexcomp Infinity™ encoder, Thought Technology Ltd., Montreal, Canada). Every channel was acquired at 256 Hz sampling rate. The ECG signal was analyzed with custom software developed using Matlab (The Mathworks, Inc.; Natick, MA) in order to detect the R peaks and calculate the RR series as the time interval between two consecutive R peaks, detected by a traditional derivative threshold procedure. The respiratory signal was low-pass filtered and sampled in correspondence to the R waves, thus deriving the respiratory series. All data analysis was performed at the Neuroscience Statistic Research Laboratory, Department of Anesthesia (Massachussetts general hospital).
2.3. Autoregressive Spectral Analysis
Spectral analysis was performed by means of autoregressive (AR) spectral methods with custom software. The Levinson–Durbin recursion was used to identify the coefficients of the autoregressive model [13] and the order was chosen (between 4 and 12) according to the Akaike figure of merit. The AR spectral decomposition procedure was applied to calculate the power of the oscillations embedded in the series [14]. The rhythms were classified [15] as very low frequency (VLF, <0.04 Hz), low-frequency (LF, from 0.04 to 0.15 Hz) and high frequency (HF, from 0.15 to 0.5 Hz) oscillations. The power was expressed in absolute (LFRR, LFresp and HFRR, HFresp) and in normalized units [e.g. RR series: LFnu and HFnu as 100*LFRR/(σ2 RR-VLFRR) and 100*HFRR/(σ2 RR-VLFRR), where σ2 RR was the RR variance and VLFRR was the VLF power expressed in absolute units].
2.4. Bivariate AR Yule-Walker Algorithm
A bivariate autoregressive model was employed to study the interaction between RR and respiration [8]. The model is defined as
| (1) |
where M is the order and is set at 14, and
x1(n) and x2(n) are the RR and respiration respectively, w(n) represents the white noise and aij (k) represents the autoregressive coefficients. We used a recursive algorithm to determine the coefficients of the autoregressive model; spectral components are determined from these coefficients.
The coherence γ2 as a function of frequency f is evaluated as
| (2) |
where Px1 (f) and Px2 (f) are the auto-spectral density functions of RR and respiration respectively. Px1x2 (f) is the cross spectral density between RR and respiration.
The transfer function as a function of frequency for the model in Eqn (1) is obtained as
| (3) |
with i = 1,2; j = 1,2; .
2.5. Surrogate Data Analysis
For each pair of respiratory and RR series, we generated an ensemble of 100 pairs of AR surrogate time series preserving the power spectrum of the original ones but being completely uncoupled, as generations of two linearly independent stochastic processes [9].
These surrogates were obtained by fitting an AR model to each of the two original series, using pairs of independent white noises as model inputs to produce completely uncoupled surrogate series. The order of the model used to fit the data was optimized by means of the Akaike criterion.
The coherence was then estimated between each pair of surrogate series and its empirical sampling distribution computed accordingly at each frequency of the signal bandwidth. The threshold for zero coherence was set at the 95th percentile of the coherence sampling distribution.
2.6. Statistical Analysis
Median, 5th, 25th, 75th and 95th percentiles were computed for the group, for each index considered, for each epoch. Statistical analysis was performed to compare the R, S, E emotional states and B, using a paired t- test with Bonferroni multiple comparison correction (p<.05).
3. Results
Table I reports the median, 25th and 75th percentile values of mean, variance and spectral analysis indexes obtained from the RR series and respiration separately, during all the experimental conditions for both the RES and RSE groups. Mean RR significantly decreased during S compared to B and E in both groups. Moreover a significant decrease in HFnu and a parallel increase in LFRR and LFnu indexes was also observed during both E and S compared to B. Interestingly, HFRR and LFnu indexes showed significant differences between E and S phases only in the group which performed E before S (RES group).
TABLE 1.
SPECTRAL ANALYSIS RESULTS OBTAINED IN 35 SUBJECTS FROM THE RR SERIES DURING THE 4 EXPERIMENTAL CONDITIONS
| RES | RSE | |||||||
|---|---|---|---|---|---|---|---|---|
| B | R | E | S | B | R | E | S | |
| mean [ms] | 837(763-906) | 822(791-913) | 839(762-913) | 764(691-858)*# | 827(729-940) | 824(720-910) | 846(740-879) | 757(711-834)*# |
| var [ms*10−3] | 2.37(1.5-4.4) | 3.14(1.8-5.0) | 3.17(1.8-5.3)* | 3.40(2.3-4.4) | 2.08(1.2-2. 9) | 2.42(1.7-3.7) | 3.12(1.5-4.1)* | 2.43(1.8-3.8) |
| LFRR [ms2] | 896(489-1519) | 777(457-1594) | 1271(531-2029)* | 1597(937-2473)* | 656(369-1221) | 614(380-1017) | 1062(617-2140)* | 1316(760-2106)* |
| HFRR [ms2] | 437(193-1494) | 470(191-1319) | 398(139-1159) | 269(161-533)*# | 236(144-731) | 307(133-865) | 248(96-504) | 202(110-437) |
| LFnu [%] | 55.0(38.7-80.2) | 60.4 (45.5-77.6) | 70.8(56.4-80.2)* | 78.7(69-92.3)*# | 65.6(41.8-84.4) | 63.2(42.1-76.7) | 81.9(63.8-89.0)* | 82.6(72.6-92.4)* |
| HFnu [%] | 43.4(24.4-64.4) | 39.2(22.8-56.0) | 28.8(18.9-42.9)* | 22.1(12.9-37)* | 31.6(14.8-58) | 36.0(21.5-56.0) | 18.0(10.8-33.8)* | 15.8(8.0-23.4)* |
| LF/HF [−] | 1.40(.64-4.16) | 1.63(.87-3.96) | 2.56(1.47-4.56) | 4.15(2.79-18.5) | 2.07(.73-5.69) | 1.76(0.75-3.63) | 4.99(1.93-8.86) | 5.37(3.16-14.3) |
| LFresp[*103] | 79.3(15.1-179) | 35.4(8.6-62.0) | 38.6 (11.0-105) | 66.1 (37.9-112.5) | 11.9(2.2-53.3) | 20.9(5.1-158.7) | 34.8(7.0-94.3) | 57.9 (39.0-145.0) |
| HFresp [*103] | 86.9 (56.6-20.3) | 101.1(51.2-193) | 95.6 (46.9-183.8) | 128.6(66.7-264) | 73.7(25.2-211) | 62.6(22.2-180) | 52.2(24.7-134.4) | 92.5(45.7-238.4) |
Median (25th-75th percentile) are shown;
:p<0.05 paired t-test with Bonferroni correction vs B ;
:p<0.05 paired t-test with Bonferroni correction vs E
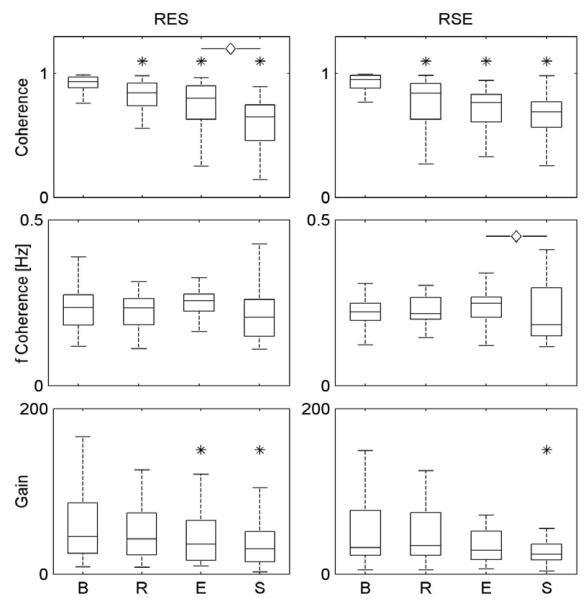
Fig.1 shows the values of coherence and gain of the transfer functions from respiration to RR interval, sampled at HF for the two groups of subjects (RES and RSE). We presented the results relevant to E before that to S even for RSE group for better visual comparison of the two groups. We found that even if the coherence between the respiratory and RR series significantly decreased during S compared with the other protocol phases, its value was upon the threshold level set by means of AR surrogates to reject the hypothesis of uncoupling in 93% of the series. Interestingly, in the RES group the gain of the transfer function from respiration to RR interval (the RSA gain) significantly decreased during E and S compared to B, while in the RSE group a significant decrease was found only with S. Moreover, significant differences were found between S and E phases in both groups.
Fig. 1.
The box-and-whisker plots summarize the results relevant to the bivariate AR analysis for the coherence and gain estimation performed on the RES and RSE groups at B, R, E and S phases. The symbol * indicates p<0.05 vs B; ◇: p<0.05 vs E.
4. Discussion
We have reported a preliminary quantitative analysis where standard autoregressive spectral and bivariate algorithms have been applied to R-R interval and respiratory time series with the goal to identify changes in the autonomic nervous system response and respiratory sinus arrhythmia as associated to three target affective states elicited by specific computer-mediated stimuli. Surrogate data analysis was further performed in order to accept or reject the hypothesis of uncoupling between the two time series.
Our results demonstrated that both standard and bivariate AR spectral analysis were able to detect significant differences in the autonomic response between the target phases of the protocol; moreover, the observed trends generally reflect the ones assessed through psychological self-rated tests [16]. In this sense the results give reasonable perspectives regarding an effective use of HRV and RSA measures in order to characterize the target states in question.
Significant differences were found between the two groups of subjects. In particular, standard spectral analysis was able to detect significant differences in the autonomic nervous system response between E and S in RES group only; accordingly we hypothesized that the stress-related stimuli cause a more lasting response influencing the E phase of the RSE group. The bivariate AR analysis was also able to identify differences between RES and RSE groups: while in RSE the gain of the transfer function significantly decreased during both E and S compared to B, in the RSE group a significant decrease was found only during S. Because there is a sequence effect, RES gradually improves the arousal reactions from the first to the last epoch, while RSE shows how engagement after stress is affected by the lingering stress arousal. This has important implications in setting up sequential stimuli, and/or using a randomized experimental design.
In conclusion, this preliminary study showed that the influence of stress and engagement states on heart rate and respiration could be identified by means of spectral and bivariate AR methods; accordingly these signal processing methods could be able to play a key role in the identification of specific affective states elicited by human-computer interactions.
Acknowledgements
This work was supported by NIH Grants R01-HL084502, and DP1-OD003646. We thank the Brown Lab at MIT, 77 Mass Ave Cambridge MA, for funding the experimental recordings, and the Clinical Research Center at MIT for all the support and help in carrying out all the experiments. We also thank Luca Citi for helping with signal preprocessing and analysis.
References
- [1].Wastell DG, Newman M. Stress, control and computer system design: a psychophysiological field study. Behaviour and Information Technology. 1996;15:183–192. [Google Scholar]
- [2].Wilson G, Sasse MA. Do users always know what’s good for them utilising physiological responses to assess media quality. In: McDonald S, Waern Y, Cockton G, editors. People and Computers XIV-Usability or Else! Proceedings of HCI 2000; Berlin: Springer; 2000. pp. 327–339. [Google Scholar]
- [3].Picard R. Affective Computing. 1997.
- [4].Scheirer J, Fernandez R, Klein J, Picard R. Frustrating the user on purpose: a step toward building an affective computer. Interacting with Computers. 2002;14:93–118. [Google Scholar]
- [5].Picard RW, Vyzas E, Healey J. Toward Machine Emotional Intelligence: Analysis of Affective Physiological State IEEE Transactions on pattern analysis and machine intelligence. 2001. pp. 1175–1191.
- [6].Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol. 1985;54:961–66. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- [7].Bendat JS, Piersol AG. Random Data. Wiley; New York: 1986. [Google Scholar]
- [8].Barbieri R, Triedman JK, Saul JP. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. AJP-Regu. 2002;283:R1210–20. doi: 10.1152/ajpregu.00127.2002. [DOI] [PubMed] [Google Scholar]
- [9].Faes L, Pinna GD, Porta A, Maestri R, Nollo G. Surrogate data analysis for assessing the significance of the coherence function. IEEE Trans Biomed Eng. 2004;51:1156–66. doi: 10.1109/TBME.2004.827271. [DOI] [PubMed] [Google Scholar]
- [10].Saul JP, Berger RD, Chen MH, Cohen RJ. Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am J Physiol. 1989:H153–H161. doi: 10.1152/ajpheart.1989.256.1.H153. [DOI] [PubMed] [Google Scholar]
- [11].Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- [12].Scotti S, Mauri M, Barbieri R, Jawad B, Cerutti S, Mainardi L, Brown EN, Villamira MA. Automatic Quantitative Evaluation of Emotions in E-Learning Applications. Conf Proc IEEE Eng Med Biol Soc. 2006:1359–62. doi: 10.1109/IEMBS.2006.260601. [DOI] [PubMed] [Google Scholar]
- [13].Kay SM. Modern Spectral Analysis: Theory and Applications. Prentice Hall; 1988. [Google Scholar]
- [14].Zetterberg LH. Estimation of parameters for a linear difference equation with application to EEG analysis. Math Biosci. 1979:227–75. [Google Scholar]
- [15].Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Standard of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- [16].Mauri M, Magagnin V, Cipresso P, Mainardi L, Brown EN, Cerutti S, Villamira MA, Barbieri R. Psychophysiological signals associated with affective states. Conf Proc IEEE Eng Med Biol Soc. 2010:3563–66. doi: 10.1109/IEMBS.2010.5627465. [DOI] [PMC free article] [PubMed] [Google Scholar]