Abstract
Pediatric obesity, a major risk factor for cardiovascular diseases and diabetes, has steadily increased in the last decades. Although excessive inflammation and oxidation are possible biochemical links between obesity and cardiovascular events in adults, little information is available in children. Furthermore, effects of gender and fitness on the interaction between dyslipidemia and oxidative or inflammatory stress in children are mostly unknown. Therefore, we measured systemic markers of oxidation (F2-isoprostanes, F2-IsoP, and antioxidants) and inflammation (interleukin-6, IL-6, and leukocyte counts) and metabolic variables in 113 peripubertal children (55 obese, Ob, age and gender-adjusted BMI%≥95th, 25F; 15 overweight, OW, BMI% 85th–95th, 8F; 43 normoweight, CL, 25F). Overall, when compared to CL, Ob displayed elevated F2-IsoP (99±7 vs. 75±4 pg/mL, p<0.005), IL-6 (2.2±0.2 vs. 1.5±0.3 pg/mL, p<0.005), elevated total WBC and neutrophils, and altered levels of total cholesterol, LDL-C, HDL-C, triglycerides, free fatty acids, glucose, and insulin (all p<0.005). This pattern was independent of gender and not caused by reduced fitness in Ob. Our data indicate that alterations in metabolic control and a concomitant increase in inflammation and oxidative stress occur early in life in obese children, likely exposing both genders to a similar degree of increased risk of future cardiovascular diseases.
Keywords: dyslipidemia, inflammation, oxidative stress, childhood obesity
INTRODUCTION
The widespread obesity epidemic affecting developed countries is a leading cause of type 2 diabetes, hypertension, stroke, myocardial infarction, and other cardiovascular diseases (1), accounting for more cumulative morbidity and mortality than any other cause (2). Alarmingly, obesity and its complications have especially increased in the last three decades among pediatric populations (1). While some underlying molecular mechanisms remain undefined, the association of cardiovascular disease with overt dyslipidemia and the progression of atherosclerotic lesions are well characterized in both adult and animal models (3). The combination of atherogenic diet, sedentary lifestyle, and genetic makeup in some ethnic groups results in excessive weight gain, dyslipidemia (low HDL cholesterol, high LDL cholesterol and triglycerides), and insulin resistance (4). When elevated, LDL cholesterol (LDL-C) infiltrates the arterial endothelium, producing a fatty streak; if dyslipidemia persists, various white blood cell (WBC) subtypes also infiltrate the lipid-laden vascular wall and secrete oxidative molecules and inflammatory cytokines that cause endothelial erosion and damage. In turn, this activates the clotting cascade and platelet aggregation attempting to repair the atheromatous lesion, producing occlusive thrombi and ultimately downstream cellular infarctions and generalized micro- and macro-vascular diseases (4).
These processes are modulated by complex interactions by cytokines and chemokines, such as IL-6, IL-1β, TNF-α, IL-8, MCP-1 (5), and many others. Increased secretion of these molecules by activated leukocytes, macrophages, and other tissues has been demonstrated in obese, diabetic, and cardiovascular patients (5). Signaling pathways regulating cytokine expression, such as the NF-κB pathway, are also activated by oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS), such as superoxide ion (toxic by-products of oxidative processes), and antioxidant defenses, (superoxide dismutase, SOD, catalase, glutathione, glutathione peroxidase, etc.). ROS are also released by activated neutrophils and other leukocytes; indeed, WBC secretion of oxidative and inflammatory mediators surrounding the lipid droplets of the atheromatic core is a crucial biochemical step linking atherosclerotic plaques to thrombotic luminal occlusion (3). Multiple biomarkers of ROS interaction with specific substrates (i.e., DNA, lipids) have been identified; whole-body oxidative stress is best reflected by systemic levels of lipid peroxidation (for which F2-isoprostanes, F2-IsoP, are considered the most reliable biomarker) (6).
Despite the rapid global increase and potential devastating impact of pediatric obesity on western societies in coming decades, very little is known about the progression of obesity complications in children, which may significantly differ from what occurs in adults (7). The overarching hypothesis of this study is therefore that pediatric obesity induces early, comprehensive alterations of substrate metabolism (dyslipidemia, impaired glucose tolerance), paralleled by elevated systemic oxidative/inflammatory status (increased inflammatory cytokines, activated leukocytes, markers of lipid peroxidation, reduced antioxidants), prolonging exposure of patients to the pathogenetic mechanisms associated with long-term development of cardiovascular disease. To elucidate these mechanisms, we performed a comprehensive assessment of inflammatory and oxidative status, leukocyte counts, and components of lipid and carbohydrate metabolism in 113 peripubertal healthy and obese children.
METHODS
Participants and Preliminary Visit
This study was approved by the University of California, Irvine Institutional Review Board (UCI IRB). All 113 children (12.9±0.3 yr, 58F) and their guardians provided informed consents/assent; none had any history of acute or chronic disease (diabetes, hypertension, hyperlipidemia, other familial dysmetabolic conditions, asthma, other immunologic diseases, recent infection or injury, or use of medication).
Percent body mass index adjusted for age and gender (BMI%) was determined according to current criteria by the Center for Disease Control and Prevention (8). Skinfold thickness measurements were also performed, and total fat and lean body mass calculated using Slaughter et al equations for age<16 yr and Siri equations for ≥16 yr (9), confirming that elevated BMI% was due to excess adiposity. All subjects also completed a standard questionnaire regarding their developmental status to assess pubertal stage (10).
Participants were divided into a control group (CL, n=43, 25F, BMI%<85th) and an obese group (Ob, n=55, 25F, BMI% ≥95th). To maintain a clear separation between groups, children with BMI% ≥85th but <95th BMI% (overweight but not obese) were not included in data analysis; however, as a small group of enrolled children were within this category (OW, n=15, 8F), their data will also be shown for completeness. Since physical fitness may independently affect oxidative and inflammatory status, groups were normalized by VO2max, measured via a standardized incremental cycle ergometer exercise protocol (Ergoline 800S, Sensors Medic, Yorba Linda, CA) until the limit of exercise tolerance (11). Gas exchange was measured breath-by-breath (Sensors medic, Yorba Linda, CA) and VO2max was calculated and expressed as mL O2/min/kg lean body mass.
Collection of Blood Samples
After an overnight fast, participants presented to the laboratory at ~7:30 am; vital signs were verified, and an intravenous catheter was inserted into a median cubital vein. To minimize the effect of venipuncture stress on measured variables, subjects rested for 90 min prior to blood draw. Peripheral whole blood was then collected in sterile EDTA tubes, and plasma was isolated and frozen at −80° C until day of assay.
Laboratory Measurements
The F2-IsoP assay was performed by the Eicosanoid Core Laboratory in the Division of Clinical Pharmacology at Vanderbilt University, using a gas chromatographic/negative ion chemical ionization mass spectrometric (GC/NICI-MS) method with stable isotope dilution (the Laboratory originally discovered and pioneered precise F2-IsoP measurement techniques beginning in the early 1990’s) (12). The remaining assays were performed by the Pediatric Growth Factor and Cytokine Laboratory of the UCI Institute for Clinical Translational Science (ICTS). SOD and GSH-420 were assayed using ELISA and a kinetic, rate based high-sensitivity colorimetric method (Northwest Life Science Specialties, Vancouver, WA), respectively. High sensitivity ELISA was used for quantification of IL-6 (R&D Systems, Minneapolis, MN) and free insulin (LINCO Research, St. Charles, MO). Plasma glucose was measured with a Beckman Coulter Glucose II Analyzer (Beckman Coulter, Fullerton, CA, USA). Standard ELISA was used for cortisol (Diagnostic Systems Laboratories, Webster, TX) and colorimetric techniques for glycerol (Sigma-Aldrich, St. Louis, MO), free fatty acid (FFA) (Zen-Bio, Research Triangle Park, NC), and triglyceride, total cholesterol, LDL-C, and HDL-C (BioAssay Systems, Hayward, CA).
Statistical Analysis
Data are shown as group means±SE. IL-6 required log transformation to normalize the distribution of data prior to statistical analyses. Significant differences between experimental groups were detected using unpaired, two-tailed Student’s t-test with a level of significance set at p<0.05. All statistical procedures were performed using JMP software (SAS Institute, Cary, NC).
RESULTS
In Ob, body weight, BMI%, and % body fat were significantly greater than in CL (Table 1), with no difference in mean age, gender distribution, Tanner stage, or VO2max. Mean weight, BMI%, and % body fat for OW were in between Ob and CL values.
TABLE 1.
Demographic characteristics of the 2 groups and gender subgroups.
| Age (yr) | Height (cm) | Weight (kg) | BMI% | % Body Fat | VO2max (mL/min/lean kg) | Tanner | n | |
|---|---|---|---|---|---|---|---|---|
| Ob | 12.7 ± 0.3 | 157.1 ± 1.9 | †74.2 ± 3.0 | †97.7 ± 0.2 | †37.3 ± 1.2 | 41.8 ± 1.1 | 3.0 ± 0.2 | 55 |
| CL | 13.4 ± 0.5 | 156.2 ± 2.4 | 49.2 ± 2.2 | 55.8 ± 3.5 | 20.1 ± 1.3 | 43.2 ± 1.5 | 3.1 ± 0.2 | 43 |
|
| ||||||||
| OW | 12.4 ± 0.9 | 156.4 ± 4.8 | 60.2 ± 5.7 | 89.9 ± 0.8 | 28.2 ± 1.9 | 46.3 ± 2.0 | 2.9 ± 0.4 | 15 |
|
| ||||||||
| Ob-F | 12.8 ± 0.6 | 154.2 ± 2.3 | †74.9 ± 4.9 | †98.0 ± 0.3 | †37.4 ± 0.9 | *38.6 ± 1.5 | 3.3 ± 0.2 | 25 |
| CL-F | 13.0 ± 0.6 | 154.8 ± 2.9 | 48.6 ± 2.6 | 60.0 ± 4.8 | 24.8 ± 1.3 | 43.4 ± 1.4 | 3.2 ± 0.3 | 25 |
|
| ||||||||
| Ob-M | 12.6 ± 0.4 | 159.5 ± 2.9 | †73.6 ± 3.7 | †97.5 ± 0.3 | †37.2 ± 2.0 | 44.2 ± 1.5 | 2.7 ± 0.2 | 30 |
| CL-M | 14.0 ± 0.8 | 158.2 ± 4.1 | 50.0 ± 3.8 | 50.0 ± 5.0 | 13.6 ± 1.7 | 42.8 ± 3.1 | 2.9 ± 0.4 | 18 |
Ob, obese; CL, healthy control; OW, overweight; F, female; M, male; BMI%, BMI percentile adjusted by age (in months) and gender;
p <0.05 and
p <0.005 Ob vs. CL in respective groups. Data are means±SE.
Fasting systemic concentrations of F2-IsoP, SOD, GSH-420, IL-6
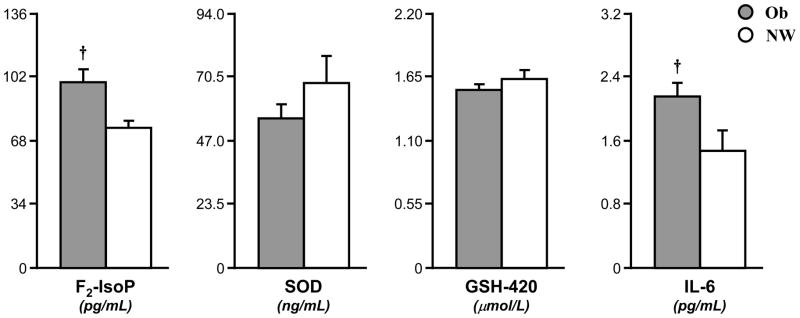
Compared to CL, the Ob group had increased concentrations of F2-IsoP (99±7 vs. 75±4 pg/mL, p<0.005) and IL-6 (2.2±0.2 vs. 1.5±0.3 pg/mL, p<0.005) (Fig. 1). The antioxidants SOD (58±6 vs. 72±11 ng/mL) and GSH-420 (1.45±0.04 vs 1.54±0.08 μM) were moderately reduced, albeit not significantly, in Ob children (Fig. 1). While OW was not included in the comparative analysis, their F2-IsoP (87±11 pg/mL) and IL-6 (2.0±0.4 pg/mL) concentrations were in between Ob and CL, while SOD (47±8 ng/mL) and GSH-420 (1.3±0.1 μM) were somewhat lower than either group.
Fig. 1. Elevated F2-isoprostane (F2-IsoP) and interleukin-6 (IL-6) but not superoxide dismutase (SOD) or reduced glutathione (GSH-420) in Ob.
Fasting plasma F2-IsoP and IL-6 were significantly higher in Ob (grey bar) than CL (white bar); SOD and GSH-420 were greater in CL, although not significantly. Data are mean±SE. * (p<0.05) and † (p<0.005) are significant differences between Ob and CL.
Lipid profiles, glucose, insulin, cortisol, and leukocytes
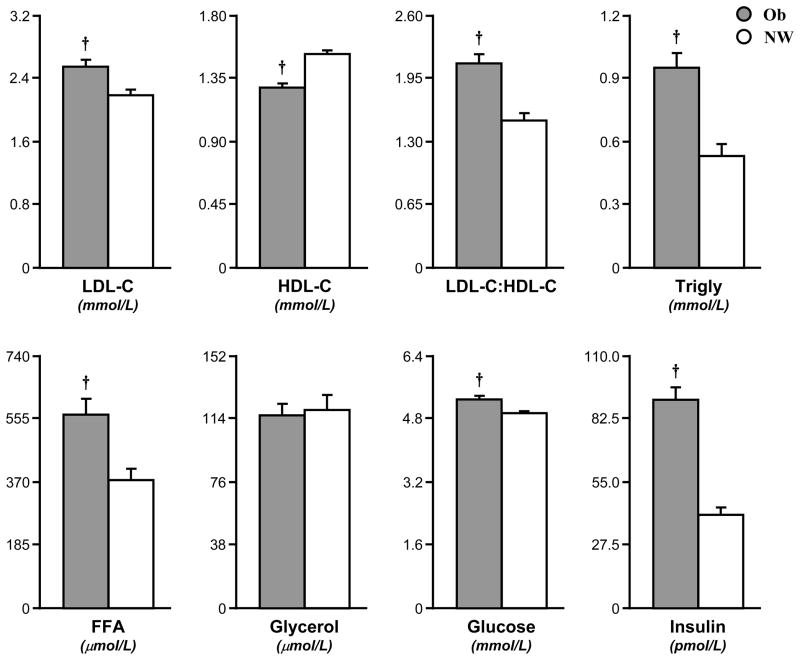
When compared to the CL group, Ob children displayed widespread alterations in their lipid profile, with significantly elevated total cholesterol (5.1±0.2 vs. 3.9±0.2 mM, p<0.005), LDL-C (2.5±0.1 vs. 2.2±0.1 mM, p<0.005), LDL:HDL ratio (2.1±0.1 vs. 1.5±0.1, p<0.005), triglycerides (1.0±0.1 vs. 0.5±0.1 mM, p<0.005), FFA (564±46 vs. 372±34 μM, p<0.005), and reduced HDL-C (1.3±0.1 vs. 1.5±0.1 mM, p<0.005) (Fig. 2). Ob also had elevated plasma glucose (5.3±0.1 vs. 4.9±0.1 mM, p<0.005) and insulin (90±6 vs. 40±3 pM, p<0.005) compared to CL, (Fig. 2). Plasma glycerol (Ob 116±7, CL 119±9 μM) and cortisol (Ob, 747±58 vs. 706±48 nM) were similar across groups. Again, values from the OW group mostly fell in between Ob and CL (total cholesterol, 4.5±0.3 mM; LDL-C, 2.5±0.1 mM; LDL-C:HDL-C, 1.4±0.2; triglyceride 0.7±0.1 mM; FFA 512±68 μM; glucose 5.2±0.1 mM; insulin 60±17 pM).
Fig. 2. Elevated fasting lipid profile, glycemia, and insulinemia in Ob.
Fasting blood LDL-C, LDL to HDL ratio, triglyceride (Trigly), free fatty acid (FFA), glucose, and insulin were significantly higher in Ob (grey bar) than CL (white bar); HDL-C was lower in Ob than CL. Data are mean±SE. * (p<0.05) and † (p<0.005) are significant differences between Ob and CL.
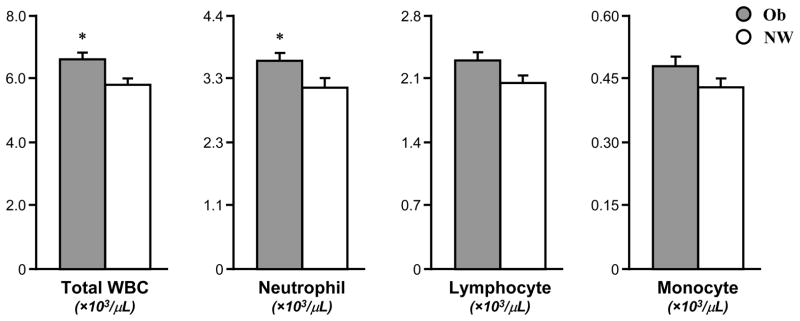
Total WBC (6.6±0.2 vs. 5.8±0.2, ×103/μL for all units of leukocytes, p<0.05) and neutrophil (3.6±0.2 vs. 3.1±0.2, p<0.05) counts were elevated in Ob compared to CL (Fig. 3), while lymphocytes (2.3±0.1 vs. 2.0±0.1) and monocytes (0.48±0.02 vs. 0.43±0.02) were not different between groups (Fig. 3).
Fig. 3. Elevated total white blood cells (WBCs) and neutrophils but not lymphocytes or monocytes in Ob.
Circulating total WBC and neutrophils were significantly higher in Ob (grey bar) than CL (white bar); lymphocytes and monocytes were also somewhat elevated in OB but not significantly. Data are mean±SE. * (p<0.05) significant differences between Ob and CL.
Gender effects
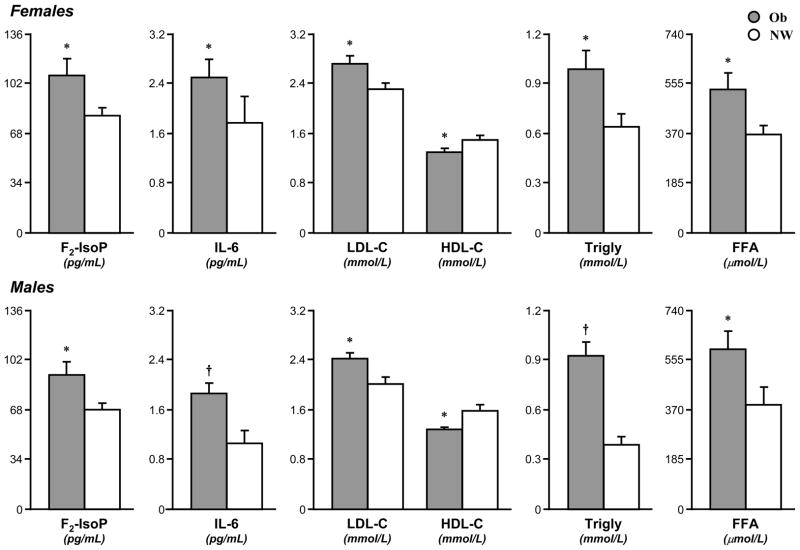
When the Ob and CL groups were subdivided by gender, the differences observed in the groups as a whole proved to be substantially gender-independent (Fig. 4). Among female participants, Ob subjects, compared to CL, displayed elevated F2-IsoP (107±12 vs. 80±6 pg/mL, p<0.05), IL-6 (2.5±0.3 vs. 1.8±0.4 pg/mL, p<0.05), total cholesterol (5.4±0.3 vs. 4.2±0.2, p<0.005), LDL-C (2.7±0.1 vs. 2.3±0.1 mM, p<0.05), LDL:HDL ratio (2.3±0.2 vs. 1.6±0.1, p<0.005), triglycerides (1.0±0.1 vs. 0.6±0.1 mM, p<0.05), FFA (531±62 vs. 363±34 μM, p<0.05), and lower HDL-C (1.3±0.1 vs. 1.5±0.1 mM, p<0.05). Insulin (88±9 vs. 45±5 pM, p<0.005), total WBC (6.9±0.3 vs. 6.0±0.3, p<0.05) and neutrophils (3.9±0.2 vs. 3.2±0.2, p<0.05) were also significantly higher.
Fig. 4. Elevated F2-isoprostane (F2-IsoP), interleukin-6 (IL-6), and fasting lipid profile between OB and CL within genders.
Ob (grey bar) and CL (white bar) were compared within genders. F2-IsoP, IL-6, LDL-C, triglyceride (Trigly), and free fatty acid (FFA) were significantly higher in Ob females (-F) and Ob males (-M) than CL-F and CL-M, respectively; HDL-C was lower in Ob-F and Ob-M than CL-F and CL-M, respectively. Data are mean±SE. * (p<0.05) and † (p<0.005) are significant differences between Ob and CL.
Among males, Ob subjects displayed a similarly altered pattern when compared to CL (Fig. 4): elevated F2-IsoP (91±9 vs. 68±5 pg/mL, p<0.05), IL-6 (1.9±0.2 vs. 1.1±0.2 pg/mL, p<0.005), total cholesterol (4.8±0.2 vs. 3.5±0.2, p<0.005), LDL-C (2.4±0.1 vs. 2.4±0.1 mM, p<0.05), LDL:HDL ratio (1.9±0.1 vs. 1.4±0.1, p<0.005), triglycerides (0.9±0.1 vs. 0.4±0.1 mM, p<0.005), FFA (592±67 vs. 385±67 μM, p<0.05), reduced HDL-C (1.3±0.1 vs. 1.6±0.1 mM, p<0.05). Glucose (5.4±0.1 vs. 5.0±0.1 mM, p<0.005), insulin (92±7 vs. 34±3 pM, p<0.005) and total WBC (6.3±0.3 vs. 5.5±0.3, p<0.05) were also elevated.
SOD and GSH-420 were generally lower in the Ob than respective CL gender subgroups, without however reaching statistical significance: SOD (ng/mL), females, 61±9 vs. 69±15; males, 56±8 vs. 76±16; GSH-420 (μM), females, 1.3±0.1 vs. 1.4±0.1; males, 1.6±0.1 vs. 1.7±0.1; (Ob vs. CL, respectively).
No difference in oxidative and inflammatory variables between females versus males within Ob and CL
When females and males were compared within Ob and CL, no difference was observed for F2-IsoP (107±12 vs. 91.9 and 80±6 vs. 68±5 pg/mL), IL-6 (2.5±0.3 vs. 1.9±0.2 and 1.8±0.4 vs. 1.1±0.2 pg/mL), SOD (61±9 vs. 56±8 and 69±15 vs. 76±16 ng/mL), or GSH-420 (1.3±0.1 vs. 1.6±0.1 μM and 1.7±0.1 vs. 1.4±0.1) (Ob-F vs. Ob-M and CL-F vs. CL-M, respectively).
DISCUSSION
In this study, a cohort of 70 overweight and obese children showed simultaneous elevations in inflammatory and oxidative status, paralleled by marked alterations in carbohydrate and lipid metabolism, when compared to healthy, age-, gender-, and fitness-matched controls. Obese children displayed significantly increased markers of systemic inflammation (IL-6, total WBC, neutrophils), systemic lipid peroxidation (F2-IsoP), and moderately decreased antioxidant defenses. Further, while the female gender commonly displays greater resistance than males against inflammatory and oxidative insults (13), in our study population, obesity-related changes were gender-independent. Taken together, these observations indicate that at an early age, obesity activates key pathogenetic mechanisms known to induce long-term cardiovascular complications. If left untreated, these complications are likely to occur earlier in life, displaying a more aggressive progression, and disrupting multiple additional aspects of physiological growth and development during childhood and adolescence.
The evolution of the cardiovascular complications of obesity has been clearly associated with exaggerated inflammation in animal and adult human models (3),(4). In coronary artery disease, elevated cytokines, chemokines, adhesion molecules and leukocytes have been consistently reported both within the vascular endothelium and in systemic circulation (3),(14). Among these biomarkers, one of the best characterized and most consistently defined as proinflammatory is IL-6; secreted primarily by macrophages, T-lymphocytes, adipocytes, skeletal muscle, and osteoblasts, IL-6 is known for its role as an acute phase reactant, inducing fever, modulating innate and adaptive immunity, and activating mobilization of energy substrates (15). Long-term systemic IL-6 elevations have been implicated in atherosclerosis, cardiac events, and all-cause mortality (16). In our study, IL-6 was significantly elevated in obese children, confirming prior observations from our and other laboratories (17),(18) and corresponding to results from studies in children with other important pediatric dysmetabolic conditions, such as type 1 diabetes (19),(20). An exaggerated inflammatory status in our obese group was also demonstrated by higher leukocyte counts, which also confirm prior observations from our laboratory (21). Importantly, among WBC subtypes, the greatest quantitative increase was in polymorphonuclear cells (PMNs), now identified as an independent risk factor and prognostic indicator for future cardiovascular outcomes regardless of disease status (22).
In the context of the interactions between inflammation and dyslipidemia resulting in vascular atherosclerosis, increasing importance is being attributed to the contributory role of oxidative stress, which independently enhances inflammatory processes via common biochemical pathways (i.e., NF-κB) and accelerates atherosclerotic plaque formation via oxidized LDL molecules (23). Elevations of biomarkers of oxidative stress can be induced by acute hyperlipidemia and hyperglycemia, while chronic elevations have been associated with dyslipidemic states, obesity, poorly-controlled diabetes, and coronary artery disease (24). While considerable analytical challenges rendered biomarkers of systemic oxidation traditionally difficult to measure, a recent major multi-laboratory study concluded that the most accurate biomarker of systemic oxidative injury in vivo is F2-IsoP (25), the most stably quantifiable of several forms of isoprostanes (IsoPs) formed through non-enzymatic free radical-mediated lipid peroxidation of arachidonic acid (12). F2-IsoP levels parallel systemic lipid peroxidation, and reflect the biological activity of the highly reactive cyclopentenone IsoPs (Cyc-IsoPs, products of an alternate lipid oxidation pathway) (6). Cyc-IsoPs have been shown in vitro and in vivo to induce apoptosis of neural cells and leukocytes and to modulate inflammation by interfering with the NF-κB pathway and serving as ligands for prostaglandin and PPAR-γ receptors (6). Elevated F2-IsoPs have consistently been detected in plasma of cardiovascular patients as well as in atherosclerotic plaques (26),(27), and have been identified as an independent prognostic indicator for the development of cardiovascular diseases and diabetes (28). The observed 32% F2-IsoP elevation in our group of 55 obese children seems to indicate that excessive lipid peroxidation, with the associated future risk of cardiovascular events, is already present when obesity is established in the pediatric age. Our results are in agreement with recent studies in smaller groups of overweight and metabolic syndrome children, displaying elevated plasma 8-isoprostaglandin F2α when compared to healthy controls (18). Interestingly, a study on 295 15-year old normoweight children demonstrated that F2-IsoP levels increase with BMI even within the range of healthy body mass (29). Oxidative stress has also often been associated with reduced concentrations of antioxidant molecules. In this study, concentrations of the two measured antioxidants (SOD and GSH-420) were 10–20% lower in obese children than in controls. While differences did not reach statistical significance, the possibility that these modest changes may reflect an initial weakening of antioxidant defense in our obese group is concordant with prior reports on obese animal models (30), obese adult populations (31), and a previous pediatric Hungarian study in which α-tocopherol, β-carotene, and total antioxidant capacity were significantly reduced in obese versus healthy children (32).
Numerous, often marked gender differences exist in many aspects of lipid and carbohydrate metabolism (33). In animal models, females have been shown to display higher concentrations of antioxidant (34) and greater resistance to oxidative damage (35). Similarly, in human studies, higher glutathione peroxidase (36) and lower lipid and DNA oxidation (37),(38) were reported in young women compared to men, while higher glutathione were also reported in newborn baby girls versus boys (39), suggesting that some level of protection against oxidant insults in females may already be established at birth. In our study, however, obesity-related alterations in lipid profiles and carbohydrate metabolism, as well as increases in inflammatory and oxidative stress markers, were largely gender independent. This suggests that the onset of the pathogenetic pathways triggered by obesity may override gender-related protective mechanisms, equally increasing future risk of cardiovascular events in both genders.
Physical fitness is known to exert independent positive effects on inflammatory and oxidative homeostasis regardless of adiposity status (40). As overweight and obese subjects tend to be generally less fit than their healthy-weight counterparts, expressing across-group differences without normalizing for fitness levels renders it almost impossible to separate effects of obesity per se from those of differing fitness. In our study, we took great efforts to accurately measure fitness via standardized, incremental exercise tests to exhaustion, and to normalize experimental groups by levels of maximal oxygen uptake per lean body mass. To our knowledge, this is the first study in children in which effects of fitness-independent obesity on inflammation and oxidative stress have been reported.
The small group of children in our study population in the 85th–95th BMI% range was not included in our statistical analysis; interestingly, however, mean plasma concentrations of key variables measured in the study (F2-IsoP, IL-6, leukocytes, components of the lipid panel) were, in general, intermediate between levels of the control and obese groups. This supports the notion of a dose-dependent relation between BMI and oxidative/inflammatory stress, with alterations beginning with relatively minor BMI increases. In fact, by further fractioning our experimental population in smaller groups based on increasing BMI, most key variables appear to increase step-wise; mean plasma F2-IsoP, for instance, was 75 pg/mL in CL, 87 pg/mL in overweight children, 90 pg/mL in children with BMI% 95th–97.5th, 102 pg/ml with BMI% 97.5th–99th, and 105 pg/ml with BMI%>99th. These data indicate the necessity of additional, large scale trials to fully identify optimal BMI% cut-offs for diagnostic, disease management and therapeutic purposes.
In conclusion, the obese children in our study displayed dyslipidemia, altered glucose metabolism, and elevated biomarkers of inflammation and oxidative stress, independent of gender and fitness levels. Our data support the concept that already at an early age, obesity activates biochemical mechanisms responsible for long-term cardiovascular complications, thereby considerably increasing, if left untreated, the number of years of expected severe morbidity in patients. Our data underscore the urgent necessity of the immediate implementation of widespread preventive strategies to limit the projected, devastating consequences of pediatric obesity in the upcoming decades.
Acknowledgments
The authors thankfully acknowledge all efforts and contribution by the entire UCI ICTS staff. Supported by NIH grants M01-RR00827-28 and K-23 RR018661-01.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Nabel EG. Cardiovascular disease. N Engl J Med. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis -- an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283:15533–7. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DA, Xu H, Cancelas JA. Children are not little adults: just ask their hematopoietic stem cells. J Clin Invest. 2006;116:2593–6. doi: 10.1172/JCI30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow SE the Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 9.Hansen JR, Wu YT, Cook JS, Cassady SL, Nielsen DH, Janz KF. Cross-validation of the Slaughter skinfold equations for children and adolescents. Med Sci Sports Exerc. 1993;25:1070–6. [PubMed] [Google Scholar]
- 10.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–34. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 12.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 13.Kerksick C, Taylor LI, Harvey A, Willoughby D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med Sci Sports Exerc. 2008;40:1772–80. doi: 10.1249/MSS.0b013e31817d1cce. [DOI] [PubMed] [Google Scholar]
- 14.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 15.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54:S114–24. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 16.Stefan, Richard AF, Annewieke WvdB, et al. Prediction of mortality risk in the elderly. Am J Med. 2006;119:519–25. doi: 10.1016/j.amjmed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 17.McMurray RG, Zaldivar F, Galassetti P, et al. Cellular immunity and inflammatory mediator responses to intense exercise in overweight children and adolescents. J Investig Med. 2007;55:120–9. doi: 10.2310/6650.2007.06031. [DOI] [PubMed] [Google Scholar]
- 18.Kelly AS, Steinberger J, Kaiser DR, Olson TP, Bank AJ, Dengel DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. 2006;1:248–52. doi: 10.1111/j.1559-4564.2006.05758.x. [DOI] [PubMed] [Google Scholar]
- 19.Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr Diabetes. 2006;7:16–24. doi: 10.1111/j.1399-543X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 20.Galassetti P, Tate D, Neill RA, Richardson A, Leu SY, Davis SN. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E1109–17. doi: 10.1152/ajpendo.00244.2005. [DOI] [PubMed] [Google Scholar]
- 21.Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM. Body fat and circulating leukocytes in children. Int J Obes. 2006;30:906–11. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- 22.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: Implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Hervas S, Fandos M, Real JT, et al. Insulin resistance and oxidative stress in familial combined hyperlipidemia. Atherosclerosis. 2008;199:384–9. doi: 10.1016/j.atherosclerosis.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Musiek E, Yin H, Milne G, Morrow J. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40:987–94. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 27.Praticò D, Iuliano L, Mauriello A, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–34. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–30. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 30.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–34. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46:785–90. doi: 10.1515/CCLM.2008.166. [DOI] [PubMed] [Google Scholar]
- 32.Molnar D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes. 2004;28:1197–202. doi: 10.1038/sj.ijo.0802719. [DOI] [PubMed] [Google Scholar]
- 33.Galassetti P, Tate D, Neill RA, Morrey S, Davis SN. Effect of gender on counterregulatory responses to euglycemic exercise in type 1 diabetes. Journal of Clinical Endocrinology Metabolism. 2002;87:5144–50. doi: 10.1210/jc.2002-020757. [DOI] [PubMed] [Google Scholar]
- 34.Coto-Montes A, Boga JA, Tomas-Zapico C, et al. Physiological oxidative stress model: Syrian hamster Harderian gland--sex differences in antioxidant enzymes. Free Radic Biol Med. 2001;30:785–92. doi: 10.1016/s0891-5849(01)00468-3. [DOI] [PubMed] [Google Scholar]
- 35.Bureau I, Gueux E, Mazur A, Rock E, Roussel A-M, Rayssiguier Y. Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr. 2003;22:239–46. doi: 10.1080/07315724.2003.10719299. [DOI] [PubMed] [Google Scholar]
- 36.Rush JWE, Sandiford SD. Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem. 2003;36:345–51. doi: 10.1016/s0009-9120(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 37.Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: Role of reactive oxygen species. Free Radic Res. 2000;33:287–93. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- 38.Proteggente AR, England TG, Rehman A, Rice-Evans CA, Halliwell B. Gender differences in steady-state levels of oxidative damage to DNA in healthy individuals. Free Radic Res. 2002;36:157–62. doi: 10.1080/10715760290006475. [DOI] [PubMed] [Google Scholar]
- 39.Lavoie J-C, Chessex P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic Biol Med. 1997;23:648–57. doi: 10.1016/s0891-5849(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 40.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]