Abstract
BACKGROUND
Although the prevalence of oral clefts in China is among the highest in countries worldwide, little is known about its descriptive epidemiology.
METHODS
Data used in this study were collected from 1996 to 2005 using the nationwide hospital-based registry, the Chinese Birth Defects Monitoring Network. A total of 4,891,472 newborns (live or still births with 28 weeks of gestation or more) delivered in member hospitals were assessed for birth defects within 7 days following birth.
RESULTS
The prevalence of nonsyndromic, syndromic, and overall clefts was 14.23, 2.40, and 16.63 per 10,000, respectively. An upward time trend in the prevalence of nonsyndromic cleft palate and nonsyndromic cleft lip was detected. Cleft lip with or without cleft palate showed a different pattern by gender, urban-rural classification, and geographic location when compared to cleft palate, particularly for nonsyndromic cases. Maternal age was associated with prevalence of all oral clefts. Neonates with oral clefts had increased rates of mortality.
CONCLUSIONS
The observed complex patterns of prevalence of oral clefts from the Chinese national birth defects registry indicate that oral cleft subtypes by either cleft location or syndromic status should be considered in the development of intervention measures and in future analytical studies.
Keywords: birth defects, oral clefts, cleft palate, cleft lip, cleft lip and palate
INTRODUCTION
The term oral cleft (OC) refers to a group of craniofacial birth defects, usually including cleft lip (CL), cleft lip and palate (CLP), or cleft palate (CP), with a distinguishing feature of cleavage of the lip and/or palate resulting from abnormal embryological development of the primary palate and/or the secondary palate (Murray, 2002; Wantia and Rettinger, 2002). Oral clefts can appear as an isolated anomaly or as a part of a multiple congenital anomaly accompanied by other noncleft malformations. Both genetic and environmental factors are known to contribute to these congenital malformations (Murray, 2002; Wantia and Rettinger, 2002). The CL and CLP are collectively termed cleft lip with or without cleft palate (CL/P), which is distinct from the CP both in genetic and embryogenic aspects.
Oral clefts are some of the most common congenital anomalies around the world, affecting 1 in 500 to 2500 births (Murray, 2002). The epidemiology of these birth defects has been studied extensively in Caucasian populations, especially in Europe and North America. A small number of studies in Asian populations revealed that Chinese are among the ethnicities with the highest oral cleft occurrence, but little is known about the trends in China. There are several reports regarding OC prevalence, disparities, and long-term patterns in China; however, the findings have been inconsistent (Hu et al., 1982; Lian, 1989; Xiao, 1989; Wu et al., 1995; Wong and King, 1997; Chen et al., 1998; Cooper et al., 2000; Liang et al., 2000; Wang et al., 2001; Cheng et al., 2003; Dai et al., 2003, 2004a; Zhou et al., 2006). The inconsistency may be explained in part by unclear classification of clefts and syndromic status, differences in the underlying population, and geographic coverage and time span. These limitations made it previously impractical to achieve a comprehensive profile of OCs at the national level.
In China, OCs constitute one of the five leading causes of perinatal deaths (Xiao, 1989; Dai et al., 2004b). The survivors usually suffer from difficulties in feeding, swallowing, speaking, and hearing. In addition, children with OCs often face problems in cognition, communication, and education. To better understand the current burden of OCs in the Chinese population and to provide new insights into etiology, prevention, and management, we used the newly updated database of the Chinese Birth Defects Monitoring Network (CBDMN) to investigate the epidemiologic patterns and birth outcomes for infants with OCs from 1996 to 2005.
METHODS
Data Collection
The procedure for CBDMN data collection has been described elsewhere (Xiao, 1989; Xiao et al., 1990; Wu et al., 1995; Dai et al., 2002, 2003, 2004a, 2004b). In short, a three-level (county, province, and central) surveillance network and corresponding expert groups were established to undertake data collection. In member hospitals, every neonate is immediately examined after birth by trained health care professionals to screen for birth defects. For affected infants, individualized interviews of mothers and medical record reviews were used to gather information on routine obstetric items, family socioeconomic and demographic issues, clinical features, and exposures to harmful factors during the first trimester of pregnancy. The number of hospital delivery births was calculated monthly by mother’s age, residential area, infant gender, and pregnancy outcome. Data on births and birth defects were collected using standardized forms and were checked by senior professionals responsible for data quality in the hospital. When errors were identified, the form was returned and verified. Data were submitted quarterly at the provincial level to be checked and sorted. Finally, the data were sent to the National Center for Birth Defects Monitoring (central level), where a workgroup composed of clinicians, statisticians, epidemiologists and information technicians was responsible for diagnosis confirmation, data checking, encoding and inputting.
Data Quality Management
The expert group at each level verified disease diagnosis, data collection, data checking, and medical records according to the program manual to ensure high quality data. In addition, they organized an independent retrospective survey to find deficiencies and inaccuracies in data, and subsequently corrected the data prior to annual reporting. At the hospital level, the survey covered all data reported in the previous year. At the provincial and national level, cluster sampling covered approximately one third and 10% of member hospitals respectively.
Study Area and Coverage
From 1996 to 2005, a total of 517 hospitals at and above county level from 31 provinces, metropolitan and autonomous regions, which provide obstetric service, were included in the CBDMN. In 2001, 45 hospitals were replaced by 50 hospitals with similar socioeconomic and geographical circumstances, because of reorganization of their medical services. The covered areas were classified according to their geographical location and socioeconomic status as being in a coastal region, inner land, or remote area (Rao et al., 1989; Lin et al., 2002; Dai et al., 2004b). The live births monitored by CBDMN account for 3.67 to 5.59% of total live births in China with a mean proportion of 4.44%, based on the national number of live births (MOH of China, 2007).
Inclusion for Infants in the Registry
All neonates (live or stillbirths greater than or equal to 28 weeks’ gestation) delivered in member hospitals were enrolled, including spontaneous or legally induced fetal deaths that were grouped into the stillbirth category. The malformed infant must have been born in member hospitals with a birth defect diagnosed within 7 days after delivery.
Oral Clefts Definition and Classification
The CBDMN adopted the same definition of oral clefts by the International Clearinghouse for Birth Defects Surveillance and Research, a nongovernmental organization responsible for international information exchange on birth defects surveillance which had been known previously as International Clearinghouse for Birth Defects Monitoring System. Cleft palate includes submucous cleft palate and excludes CLP, cleft uvula, functional short palate, and high narrow palate. Cleft lip with or without cleft palate includes partial or complete clefting of the upper lip, with or without clefting of the alveolar ridge or the hard palate, excluding midline cleft of the upper or lower lip and oblique facial fissure. In the current study, nonsyndromic referred to an isolated oral cleft, and syndromic was defined as the OC case having multiple anomalies (i.e., a nonisolated OC case).
Statistical Methods
Birth prevalence was expressed as the number of oral cleft per 10,000 live and still births. Residential areas were categorized into urban (cities and urbanized area/town) and rural (villages and countryside) areas according to mother’s last residence address where she lived for at least 1 year. The 95% confidence interval for prevalence was calculated based on a Poisson distribution. The male-to-female prevalence ratio (PR) was calculated for OCs and each subtype by using the male prevalence as the numerator and the female prevalence as the denominator. Pearson chi-square test was used to compare the proportions and prevalence rates between different groups. Linear chi-square tests were used to detect trends in annual prevalence and maternal age-specific prevalence (Agresti, 2002). Statistical significance level for α was set at 0.05.
RESULTS
During 1996 to 2005, a total of 8133 infants with OCs were identified among 4,891,472 births, which yielded a prevalence of 16.63 per 10,000 births (Table 1). The majority of these cases were nonsyndromic OCs (NsOCs; 85.59%; prevalence = 14.23 per 10,000). The prevalence of nonsyndromic CP (NsCP), nonsyndromic CL (NsCL) and nonsyndromic CLP (NsCLP) was 2.00, 4.62, and 7.62 per 10,000 births, respectively. The prevalence of syndromic CP (SynCP), syndromic CL (SynCL) and syndromic CLP (SynCLP) was 0.57, 0.60, and 1.22 per 10,000 births.
Table 1.
The Prevalence (per 10,000 Births) and Relative Proportions (%) of Oral Clefts in Chinese Newbornsa in the Years 1996–2005
| Cleft type | Nonsyndromic
|
Syndromic
|
Total
|
|||
|---|---|---|---|---|---|---|
| N (%) | Rate (95% CI) | N (%) | Rate (95% CI) | N (%) | Rate (95% CI) | |
| CP | 976 (14.02) | 2.00 (1.87–2.12) | 279 (23.81) | 0.57 (0.50–0.64) | 1255 (15.43) | 2.57 (2.42–2.71) |
| CL/P | 5985 (85.98) | 12.24 (11.93–12.55) | 893 (76.19) | 1.83 (1.71–1.95) | 6878 (84.57) | 14.06 (13.73–14.39) |
| CL | 2258 (32.44) | 4.62 (4.43–4.81) | 295 (25.17) | 0.60 (0.53–0.67) | 2553 (31.39) | 5.22 (5.02–5.42) |
| CLP | 3727 (53.54) | 7.62 (7.37–7.86) | 598 (51.02) | 1.22 (1.12–1.32) | 4325 (53.18) | 8.84 (8.58–9.11) |
| Total | 6961 (100) | 14.23 (13.90–14.57) | 1172 (100) | 2.40 (2.26–2.53) | 8133 (100) | 16.63 (16.27–16.99) |
Total newborns in the years 1996–2005 were 4,891,472.
CI, confidence interval; CP, cleft palate; CL/P, cleft lip with or without cleft palate; CL, cleft lip; CLP, cleft lip with cleft palate.
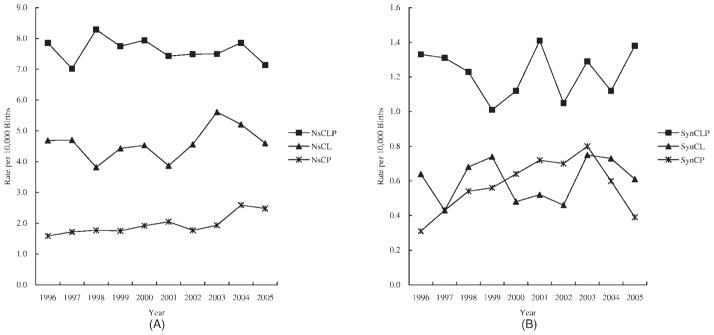
There was statistically significant variation in the annual prevalence of NsCLP, but not in the prevalence of NsCP and NsCL. Furthermore, we detected an upward trend in both NsCP and NsCL prevalence using linear chi-square test (p = 1.06 × 10−5 and 0.04, respectively; Fig. 1A). For syndromic OCs (SynOCs), significant variation was observed only in SynCP. There was no trend in any type of syndromic cleft (Fig. 1B).
Figure 1.
The time trends in prevalence of different oral clefts in China (per 10,000 births), 1996–2005. (A) The prevalence of nonsyndromic oral clefts in China,1996–2005. (B) The prevalence of syndromic oral clefts in China, 1996–2005. NsCLP, nonsyndromic cleft lip with cleft palate; NsCL, nonsyndromic cleft lip; NsCP, nonsyndromic cleft palate; SynCLP, syndromic cleft lip with cleft palate; SynCL, syncromic cleft lip; SynCP, syndromic cleft palate.
As shown in Table 2, a female excess was observed in NsCP, whereas a male excess was observed in NsCL and NsCLP. Consequently, the male-to-female PR was 0.56 for NsCP, 1.40 for NsCL, and 1.33 for NsCLP. There was no significant difference between gender in prevalence for SynCP and SynCL, but a male excess was observed for SynCLP with the male-to-female PR of 1.32.
Table 2.
The Prevalence of Nonsyndromic and Syndromic Oral Clefts (per 10,000 Births) in Chinese Newborns, 1996–2005
| Group | Births | Nonsyndromic oral clefts
|
Syndromic oral clefts
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NsCP
|
NsCL
|
NsCLP
|
NsOCs
|
SynCP
|
SynCL
|
SynCLP
|
SynOCs
|
||||||||||
| N | Prevalence | N | Prevalence | N | Prevalence | N | Prevalence | N | Prevalence | N | Prevalence | N | Prevalence | N | Prevalence | ||
| Gender | |||||||||||||||||
| Male | 2,596,731 | 379 | 1.46 (1.31, 1.61) | 1378 | 5.31 (5.03, 5.59) | 2304 | 8.87 (8.51, 9.23) | 4061 | 15.64 (15.16, 16.12) | 136 | 0.52 (0.44, 0.61) | 148 | 0.57 (0.48, 0.66) | 339 | 1.31 (1.17, 1.44) | 623 | 2.40 (2.21, 2.59) |
| Female | 2,293,540 | 595 | 2.59 (2.39, 2.80) | 872 | 3.80 (3.55, 4.05) | 1406 | 6.13 (5.81, 6.45) | 2873 | 12.53 (12.07, 12.98) | 134 | 0.58 (0.49, 0.68) | 131 | 0.57 (0.47, 0.67) | 227 | 0.99 (0.86, 1.12) | 492 | 2.15 (1.96, 2.33) |
| Residential area | |||||||||||||||||
| Urban | 3,370,829 | 707 | 2.10 (1.94, 2.25) | 1467 | 4.35 (4.13, 4.57) | 2400 | 7.12 (6.84, 7.40) | 4574 | 13.57 (13.18, 13.96) | 208 | 0.62 (0.53, 0.70) | 182 | 0.54 (0.46, 0.62) | 359 | 1.07 (0.95, 1.18) | 749 | 2.22 (2.06, 2.38) |
| Rural | 1,520,643 | 267 | 1.76 (1.55, 1.97) | 788 | 5.18 (4.82, 5.54) | 1319 | 8.67 (8.21, 9.14) | 2374 | 15.61 (14.98, 16.24) | 71 | 0.47 (0.36, 0.58) | 112 | 0.74 (0.60, 0.87) | 234 | 1.54 (1.34, 1.74) | 417 | 2.74 (2.48, 3.01) |
| Geographic locations | |||||||||||||||||
| Coast area | 1,915,288 | 478 | 2.50 (2.27, 2.72) | 749 | 3.91 (3.63, 4.19) | 1308 | 6.83 (6.46, 7.20) | 2535 | 13.24 (12.72, 13.75) | 117 | 0.61 (0.50, 0.72) | 82 | 0.43 (0.34, 0.52) | 173 | 0.90 (0.77, 1.04) | 372 | 1.94 (1.74, 2.14) |
| Inner land | 2,143,601 | 342 | 1.60 (1.43, 1.76) | 1035 | 4.83 (4.53, 5.12) | 1664 | 7.76 (7.39, 8.14) | 3041 | 14.19 (13.68, 14.69) | 93 | 0.43 (0.35, 0.52) | 77 | 0.36 (0.28, 0.44) | 187 | 0.87 (0.75, 1.00) | 357 | 1.67 (1.49, 1.84) |
| Remote area | 832,583 | 156 | 1.87 (1.58, 2.17) | 474 | 5.69 (5.18, 6.21) | 755 | 9.07 (8.42, 9.72) | 1385 | 16.63 (15.76,17.51) | 39 | 0.47 (0.33, 0.64) | 33 | 0.40 (0.27, 0.56) | 67 | 0.80 (0.61, 1.00) | 139 | 1.67 (1.39, 1.65) |
NsCP, nonsyndromic cleft palate; NsCL, nonsyndromic cleft lip; NsCLP, nonsyndromic cleft lip with cleft palate; NsOCs, total nonsyndromic clefts; SynCP, syndromic cleft palate; SynCL, syncromic cleft lip; SynCLP, syndromic cleft lip with cleft palate; SynOCs, total syndromic clefts.
The prevalence of NsCP was higher in urban areas (2.10 per 10,000 births) than in rural areas (1.76 per 10,000 births) (Table 2). In contrast, rates of NsCL and NsCLP were significantly higher in rural areas than in urban areas (p = 8.99 × 10−5 and 1.34 × 10−8, respectively). A similar pattern was also observed for syndromic OCs. The prevalence of SynCP was higher in urban areas than rural areas, whereas a higher prevalence of SynCL and SynCLP was observed in rural areas.
There was significant variation in the prevalence of NsOCs by geographic locations (Table 2). The NsCP prevalence was higher in coastal areas (2.50 per 10,000 births) than in remote areas (1.87 per 10,000 births), and the lowest prevalence was observed in inner land areas (1.60 per 10,000 births). The prevalence of NsCL and NsCLP was the highest in remote areas, followed by inner areas and coastal areas. For SynOCs, a higher prevalence of SynCP was observed in coastal areas than in inner or remote areas. No statistically significant differences of SynCL and SynCLP were identified by geographic location.
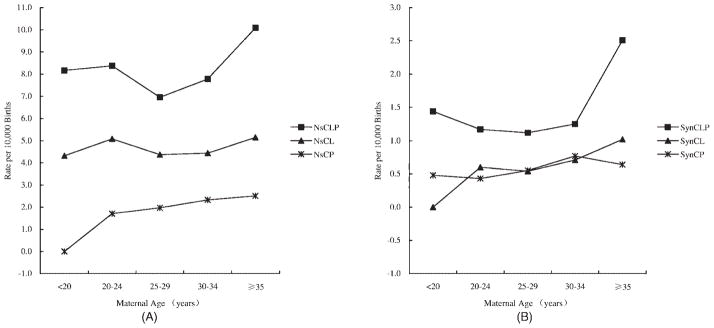
A significant difference in prevalence was also observed based on maternal age groups (Fig. 2A, B). The maternal age–specific NsCP prevalence increased with age (Fig. 2A). The highest prevalence of NsCL and NsCLP was observed in the ≥35-year age group, followed by the 20- to 24-year age group, and the lowest one was in 25- to 29-year age group. The maternal age–specific rates of all types of SynOCs increased with mother’s age (Fig. 2B).
Figure 2.
The maternal age–specific prevalence of different oral clefts in China (per 10,000 births), 1996–2005. (A) The maternal age–spefic prevalence of nonsyndromic oral clefts in China,1996–2005. (B) The maternal age–specific prevalence of syndromic oral clefts in China, 1996–2005. NsCLP, nonsyndromic cleft lip with cleft palate; NsCL, nonsyndromic cleft lip; NsCP, nonsyndromic cleft palate; SynCLP, syndromic cleft lip with cleft palate; SynCL, syncromic cleft lip; SynCP, syndromic cleft palate.
The perinatal mortality for all OCs subtypes was significantly higher than the rate in total births in our sample, and the proportion of low birth weight and preterm births was also higher than the rate of the normal birth population reported previously (Lin et al., 2002; Table 3). Moreover, rates of perinatal mortality, low birth weight, and preterm birth were much higher for all OC subtypes in syndromics than in nonsyndromics.
Table 3.
General Birth Characteristics of Infants Affected by Oral Clefts, China, 1996–2005
| Group | Nonsyndromic oral clefts
|
Syndromic oral clefts
|
||||||
|---|---|---|---|---|---|---|---|---|
| NsCP | NsCL | NsCLP | NsOCs | SynCP | SynCL | SynCLP | SynOCs | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Pregnancy outcomea | ||||||||
| Alive within 7 days | 916 (94.34) | 1959 (87.89) | 2930 (79.68) | 5805 (84.41) | 173 (62.68) | 97 (34.28) | 226 (39.30) | 496 (43.74) |
| Early neonate death (0.37%)b | 32 (3.30) | 38 (1.70) | 331 (9.00) | 401 (5.83) | 70 (25.36) | 40 (14.13) | 125 (21.74) | 235 (20.72) |
| Still birth (0.99%)b | 23 (2.37) | 232 (10.41) | 416 (11.31) | 671 (9.76) | 33 (11.96) | 146 (51.59) | 224 (38.96) | 403 (35.54) |
| Total mortality (1.36%)b | 55 (5.66) | 270 (12.11) | 747 (20.32) | 1072 (15.59) | 103 (37.32) | 186 (65.72) | 349 (60.70) | 638 (56.26) |
| Birth weight(g)c | ||||||||
| <2500 (5.87%)d | 113 (11.63) | 336 (15.03) | 657 (17.79) | 1106 (16.03) | 99 (35.61) | 157 (54.90) | 297 (51.30) | 553 (48.38) |
| 2500–3999 | 803 (82.61) | 1773 (79.29) | 2840 (76.90) | 5416 (78.48) | 174 (62.59) | 121 (42.31) | 270 (46.63) | 565 (49.43) |
| ≥4000 | 56 (5.76) | 127 (5.68) | 196 (5.31) | 379 (5.49) | 5 (1.80) | 8 (2.80) | 12 (2.07) | 25 (2.19) |
| Fetal age (weeks)e | ||||||||
| 28–36 (3.5%)f | 94 (9.64) | 333 (14.79) | 637 (17.14) | 1064 (15.32) | 64 (22.94) | 157 (53.22) | 280 (47.06) | 501 (42.86) |
| 37–41 | 847 (86.87) | 1844 (81.88) | 2942 (79.15) | 5633 (81.12) | 205 (73.48) | 129 (43.73) | 285 (47.90) | 619 (52.95) |
| ≥42 | 34 (3.49) | 75 (3.33) | 138 (3.71) | 247 (3.56) | 10 (3.58) | 9 (3.05) | 30 (5.04) | 49 (4.19) |
5 NsCP, 29 NsCL, 50 NsCLP, 3 SynCP, 12 SynCL and 23 SynCLP cases with unknown pregnancy outcome were excluded from this analysis.
The proportions of stillbirth, early neonate death and total perinatal death from the current sample were 0.99% (48,392/4,891,472), 0.37% (17,990/4,891,472) and 1.36%.
4 NsCP, 22 NsCL, 34 NsCLP, 1 SynCP, 9 SynCL and 19 SynCLP cases with unknown birth weight were excluded from this analysis.
The national estimate rate of low birth weight among livebirths was 5.87%.
1 NsCP, 6 NsCL, 10 NsCLP and 3 SynCLP cases with unknown fetal age were excluded.
The national estimate rate of preterm birth among livebirths was 3.5%.
NsCP, nonsyndromic cleft palate; NsCL, nonsyndromic cleft lip; NsCLP, nonsyndromic cleft lip with cleft palate; NsOCs, total nonsyndromic clefts; SynCP, syndromic cleft palate; SynCL, syncromic cleft lip; SynCLP, syndromic cleft lip with cleft palate; SynOCs, total syndromic clefts.
Among nonsyndromic cases (Table 3), perinatal mortality was higher among infants with NsCLP and NsCL than in those with NsCP. The proportions of low birth weight and preterm among infants with NsCLP and NsCL were also greater than those with NsCP. Similar patterns were also observed for syndromic cases.
DISCUSSION
This study examined the epidemiologic features of OCs in the Chinese population, using data from the largest birth defects registry in China. Our results illustrate that CLP is the most common type of OC in China in both syndromic and nonsyndromic patients. There were variations in OC subtypes by gender, residential area, and geographical locations. The relationships between perinatal outcomes and OCs were also found to vary by OC subtypes.
It has been reported that the prevalence of OCs ranged between 6.9 and 23.5 per 10,000 births in Caucasians (Vanderas, 1987; Mossey and Little, 2002; Gundlach and Maus, 2006), between 1.8 and 8.2 per 10,000 in U.S. blacks (Gundlach and Maus, 2006), and between 16.5 and 27.1 per 10,000 births in Japanese (Gundlach and Maus, 2006). The prevalence of 16.6 per 10,000 births for all OCs observed in our study was comparable to the report on Caucasians or Japanese, but was much higher than those observed in U.S. blacks. Earlier studies showed that Chinese, Japanese, and other Asian populations had the highest prevalence of NsCL/P, followed by Caucasians and then blacks (Lowry and Trimble, 1977; Croen et al., 1998; Mossey and Little, 2002; Hashmi et al., 2005; Cooper et al., 2006; Mossey, 2007). For NsCP, the prevalence in Chinese was lower than in Japanese and Caucasians, but was similar to the prevalence in other Asians (Korean, Thais, and Filipino) and blacks (Lowry and Trimble, 1977; Ogle, 1993; Croen et al., 1998; Mossey and Little, 2002; Hashmi et al., 2005; Cooper et al., 2006; Mossey, 2007). Cooper et al. (2006) conducted a pooled analysis and showed that the rate of NsCL/P and NsCP in Japanese was 11.8 and 2.8 per 10,000 live births, whereas these rates for other Asians were 12.2 and 2.1 per 10,000 live births, respectively. These findings are consistent with our results (12.2 and 2.0 per 10,000 for NsCL/P and NsCP, respectively).
In the current study, we observed a male excess in NsCL, NsCLP, and SynCLP, as well as a female excess in NsCP. A significant difference of gender distribution between NsCP and SynCP was consistent with previous reports (Wang et al., 2001; Dai et al., 2004a), highlighting the importance of distinguishing syndromic status when studying the role of gender in CP. Explanations for such gender differences in the prevalence of OC subtypes are currently unclear. One potential explanation is that craniofacial development varies by gender, as the palatal shelves in females are separated and vertical for a relatively longer time than in males (Burdi and Silvey, 1969a, 1969b).
A number of studies have investigated the relationship between maternal age and OCs. Some studies found that older maternal age increased the risk of NsCL/P (Shaw et al., 1991; Cooper et al., 2000; Bille et al., 2005) or NsCP (Shaw et al., 1991), whereas some did not (Baird et al., 1994; Vieira et al., 2002; DeRoo et al., 2003). Other researchers reported that maternal age increased the risk of syndromic clefts but not NsOCs (Chung et al., 1987; Baird et al., 1994; Vallino-Napoli et al., 2006). DeRoo et al. (2003) found that mothers younger than 20 years were twice as likely to have an infant with NsCL/P (relative risk, 2.0; 95% confidence interval, 1.3–2.9) than those aged 25 to 29 years. Robert et al. (1996) reported a U-shaped maternal-age relationship with CL/P in a large study with data from three congenital anomaly registries. In the current study, we also found a similar U-Shape in the NsCL/P maternal age–specific rate, an increasing trend of prevalence with increased maternal age in NsCP as well as for all types of SynOCs. When we examined these associations by gender, we obtained similar results. Our results suggest that older maternal age increases the risk of OC, and younger maternal age increases the risk of NsCL/P.
We observed an increasing time trend in NsCP and total CP with a p value <0.001, and in NsCL with a p value of 0.04, but not for overall OCs. There have been some studies suggesting that older maternal age increases the risk of OCs (Shaw et al., 1991; Vallino-Napoli et al., 2006), particularly the risk of isolated CP (Vallino-Napoli et al., 2006). The proportion of infants born to women greater than 35 years rose from 2.96% in 1996 to 7.70% in 2005 in our registry. It is possible that increasing maternal age may at least in part explain the observed trend of CP in our study. Interestingly, we did not observe any time trend for SynOCs, although their prevalence increased with maternal age. One possible explanation is that some syndromic cases diagnosed before 28 weeks’ gestation were aborted. The Ministry of Health of China issued a regulation that requests all pregnant women age 35 years or older to have an examination for birth defect screening in the second trimester of pregnancy starting from May 2003 (MOH of China, 2002). In fact, we detected a significant increasing time trend in prevalence of SynCP during 1996 to 2003, with a dramatic decrease afterward.
In general, there are large differences in economic level, education, occupational exposure, lifestyle, and health care between people who live in urban and rural areas in China (Rao et al., 1989; Shi, 1993; Tang et al., 2008). In the CBDMN system, the urban-rural classification depends on the place where the woman has lived for at least 1 year before her labor, which mainly reflects the combined exposure during her pregnancy (Xiao, 1989; Wu et al., 1995; Dai et al., 2003, 2004a). We found a higher CP prevalence but a lower CL and CLP prevalence in urban areas compared with rural areas, regardless of syndromic status. The findings from our study suggest that the etiology of CP may differ from that of CL and CLP.
Our study also demonstrated that the prevalences of NsCP and SynCP were similar according to the mother’s geographic location, whereas the prevalences of NsCL/P and SynCL/P showed different patterns according to mother’s geographic location. These findings further support the possibility that CP and CL/P have distinct etiologies. It is also suggested that genetic heritage might play an important role in CP, whereas environmental exposures play a more important role in CL/P. An earlier study (Croen et al., 1998) found a prevalence gradient for CL/P among Filipinos, with the highest rate in the Philippines, a lower rate in Hawaii, and the lowest in California. The authors also observed variation in OC prevalence among Chinese according to maternal country of birth. They suggested these variations could be related to environmental factors, like maternal diet changes following migration.
Our study also found that infants affected by OCs had poor pregnancy outcomes, particularly those affected by syndromic clefts. The high perinatal mortality of our study may partly result from those induced abortions included in our analysis, but grouped as stillbirths. Nevertheless, the much higher rate of early neonate death (5.83% for NsOC, 20.72% for SynOC) than in developed countries (Menegotto and Salzano, 1991; Hujoel et al., 1992; Vallino-Napoli et al., 2006), suggests a need for urgent improvement in perinatal intervention, such as nursing, breathing, and family support.
It is possible that hospital-based samples may introduce referral bias. However, our study used reliable CBDMN data that includes wide geographic coverage, consistent ascertainment methods, and large sample size, and our results provide valuable insights to our understanding of Chinese oral clefts time trends. The relative short monitoring period (28 weeks’ gestation to 6 days after delivery) may result in a lower detection rate of syndromic OCs.
In conclusion, high prevalence and high perinatal or early neonatal death of oral clefts indicate that OCs become one of the major public health concerns in China. The observed complex and distinctive prevalence patterns in different OC subtypes suggest that different OC subtypes by cleft location and by syndromic status might have different etiologies. The variations of OC prevalence by urban-rural classification and geographic location suggest that environmental exposures might play a critical role in the development of OCs, in addition to genetic factors. In China, future epidemiologic studies should be encouraged to explore environmental determinants of and hereditary susceptibilities to OCs, or the gene-environment interaction underlying urban-rural variations and geographic discrepancies. These studies would shed light on human embryology and its disturbance, aiding the prevention of OCs.
Acknowledgments
We thank the obstetricians, pediatricians, pathologists and other participants involved in the birth defects monitoring network. The opinions expressed are the views of the authors and do not necessarily reflect the official position of the National Center for Birth Defects Monitoring, China.
Supported in part by the National Key Technology R&D Program of China (Grant ID: 2006BAI05A01) and by the US Fogarty Training Grant 1D43TW007864-01 from the National Institutes of Health (NIH). This publication was made possible by CTSA Grant number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH, and NHL roadmap for medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR.
References
- Agresti A. Inference for contingency tables. In: Agresti A, editor. Categorical data analysis. 2. New York: John Wiley & Sons; 2002. pp. 70–114. [Google Scholar]
- Baird PA, Sadovnick AD, Yee IM. Maternal age and oral cleft malformations: data from a population-based series of 576,815 consecutive livebirths. Teratology. 1994;49:448–451. doi: 10.1002/tera.1420490604. [DOI] [PubMed] [Google Scholar]
- Bille C, Skytthe A, Vach W, et al. Parent’s age and the risk of oral clefts. Epidemiology. 2005;16:311–316. doi: 10.1097/01.ede.0000158745.84019.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi AR, Silvey RG. The relation of sex-associated facial profile reversal and stages of human palatal closure. Teratology. 1969a;2:297–303. doi: 10.1002/tera.1420020404. [DOI] [PubMed] [Google Scholar]
- Burdi AR, Silvey RG. Sexual differences in closure of the human palatal shelves. Cleft Palate J. 1969b;6:1–7. [PubMed] [Google Scholar]
- Chen S, Chen J, He L. An epidemiological survey of cleft lip and cleft palate in Fujian province. Zhonghua Kou Qiang Yi Xue Za Zhi. 1998;33:33–35. [PubMed] [Google Scholar]
- Cheng N, Bai Y, Hu X, et al. A base-line survey on birth defects in Gansu province, West China. Ann Trop Paediatr. 2003;23:25–29. doi: 10.1179/000349803125002823. [DOI] [PubMed] [Google Scholar]
- Chung CS, Mi MP, Beechert AM. Genetic epidemiology of cleft lip with or without cleft palate in the population of Hawaii. Genet Epidemiol. 1987;4:415–423. doi: 10.1002/gepi.1370040603. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Ratay JS, Marazita ML. Asian oral-facial cleft birth prevalence. Cleft Palate Craniofac J. 2006;43:580–589. doi: 10.1597/05-167. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Stone RA, Liu Y, et al. Descriptive epidemiology of nonsyndromic cleft lip with or without cleft palate in Shanghai, China, from 1980 to 1989. Cleft Palate Craniofac J. 2000;37:274–280. doi: 10.1597/1545-1569_2000_037_0274_deoncl_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Wasserman CR, Tolarova MM. Racial and ethnic variations in the prevalence of orofacial clefts in California, 1983–1992. Am J Med Genet. 1998;79:42–47. doi: 10.1002/(sici)1096-8628(19980827)79:1<42::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Dai L, Miao L, Zhou GX, et al. The prevalence analysis of cleft palate in Chinese perinatals: 1996–2000. Hua Xi Kou Qiang Yi Xue Za Zhi. 2004a;22:35–37. [PubMed] [Google Scholar]
- Dai L, Zhou GX, Zhu J, et al. Impacts of birth defects on perinatal deaths in Chinese population. Zhonghua Liu Xing Bing Xue Za Zhi. 2004b;25:138–141. [PubMed] [Google Scholar]
- Dai L, Zhu J, Zhou G, et al. Dynamic monitoring of neural tube defects in China during 1996 to 2000. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36(6):402–405. [PubMed] [Google Scholar]
- Dai L, Zhu J, Zhou GX, et al. The monitoring of cleft lip with or without cleft palate in China: 1996–2000. Zhonghua Kou Qiang Yi Xue Za Zhi. 2003;38:438–440. [PubMed] [Google Scholar]
- DeRoo LA, Gaudino JA, Edmonds LD. Orofacial cleft malformations: associations with maternal and infant characteristics in Washington State. Birth Defects Res A Clin Mol Teratol. 2003;67:637–642. doi: 10.1002/bdra.10114. [DOI] [PubMed] [Google Scholar]
- Gundlach KK, Maus C. Epidemiological studies on the frequency of clefts in Europe and world-wide. J Craniomaxillofac Surg. 2006;34(Suppl 2):1–2. doi: 10.1016/S1010-5182(06)60001-2. [DOI] [PubMed] [Google Scholar]
- Hashmi SS, Waller DK, Langlois P, et al. Prevalence of nonsyndromic oral clefts in Texas: 1995–1999. Am J Med Genet A. 2005;134:368–372. doi: 10.1002/ajmg.a.30618. [DOI] [PubMed] [Google Scholar]
- Hu DN, Li JH, Chen HY, et al. Genetics of cleft lip and cleft palate in China. Am J Hum Genet. 1982;34:999–1002. [PMC free article] [PubMed] [Google Scholar]
- Hujoel PP, Bollen AM, Mueller BA. First-year mortality among infants with facial clefts. Cleft Palate Craniofac J. 1992;29:451–455. doi: 10.1597/1545-1569_1992_029_0451_fymaiw_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Lian ZH. Birth defects surveillance in eight provinces, China. Zhonghua Yu Fang Yi Xue Za Zhi. 1989;23:222–225. [PubMed] [Google Scholar]
- Liang J, Wang Y, Miao L, et al. Nonsyndromic cleft lip with or without cleft palate in Chinese population: analysis of 3766 cases. Hua Xi Yi Ke Da Xue Xue Bao. 2000;31:408–410. [PubMed] [Google Scholar]
- Lin L, Liu Y, Zhang X, et al. Sampling survey on low-birth weight in China in 1998. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:149–153. [PubMed] [Google Scholar]
- Lowry RB, Trimble BK. Incidence rates for cleft lip and palate in British Columbia 1952–71 for North American Indian, Japanese, Chinese and total populations: secular trends over twenty years. Teratology. 1977;16:277–283. doi: 10.1002/tera.1420160306. [DOI] [PubMed] [Google Scholar]
- Menegotto BG, Salzano FM. Epidemiology of oral clefts in a large South American sample. Cleft Palate Craniofac J. 1991;28:373–376. doi: 10.1597/1545-1569_1991_028_0373_eoocia_2.3.co_2. discussion 376–377. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of China. Chan Qian Zhen Duan Ji Shu Guan Li Ban Fa (The regulation of prenatal diagnosis) 2002 http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohfybjysqwss/s6745/200804/217612.htm.
- Ministry of Health of China. Fu You Bao Jian Qing Kuang (The status of women and children health care) 2007 http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/ptjnj/year2007/p2191.htm.
- Mossey P. Epidemiology underpinning research in the aetiology of orofacial clefts. Orthod Craniofac Res. 2007;10:114–120. doi: 10.1111/j.1601-6343.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Mossey P, Little J. Epidemiology of oral clefts: an international perspective. In: Wyszynski DF, editor. Cleft lip and cleft palate: from the origin to treatment. New York: Oxford University Press; 2002. pp. 127–158. [Google Scholar]
- Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61:248–256. doi: 10.1034/j.1399-0004.2002.610402.x. [DOI] [PubMed] [Google Scholar]
- Ogle OE. Incidence of cleft lip and palate in a newborn Zairian sample. Cleft Palate Craniofac J. 1993;30:250–251. [PubMed] [Google Scholar]
- Rao K, Chen Y, Tian M. Research on the classification of health status in China. Zhong Guo Wei Sheng Tong Ji Za Zhi (Chinese Journal of Health Statistics) 1989;6:12–17. [Google Scholar]
- Robert E, Kallen B, Harris J. The epidemiology of orofacial clefts. 1. Some general epidemiological characteristics. J Craniofac Genet Dev Biol. 1996;16:234–241. [PubMed] [Google Scholar]
- Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43:225–228. doi: 10.1002/tera.1420430306. [DOI] [PubMed] [Google Scholar]
- Shi L. Health care in China: a rural-urban comparison after the socioeconomic reforms. Bull World Health Organ. 1993;71:723–736. [PMC free article] [PubMed] [Google Scholar]
- Tang S, Meng Q, Chen L, et al. Tackling the challenges to health equity in China. Lancet. 2008;372:1493–1501. doi: 10.1016/S0140-6736(08)61364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallino-Napoli LD, Riley MM, Halliday JL. An epidemiologic study of orofacial clefts with other birth defects in Victoria, Australia. Cleft Palate Craniofac J. 2006;43:571–576. doi: 10.1597/05-123. [DOI] [PubMed] [Google Scholar]
- Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987;24:216–225. [PubMed] [Google Scholar]
- Vieira AR, Orioli IM, Murray JC. Maternal age and oral clefts: a reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:530–535. doi: 10.1067/moe.2002.128875. [DOI] [PubMed] [Google Scholar]
- Wang YP, Liang J, Wu YQ, et al. Variation of incidence of nonsyndromic cleft lip and cleft palate from 1988 to 1992 in China. Xian Dai Yu Fang Yi Xue Za Zhi. 2001;28:40–42. [Google Scholar]
- Wantia N, Rettinger G. The current understanding of cleft lip malformations. Facial Plast Surg. 2002;18:147–153. doi: 10.1055/s-2002-33061. [DOI] [PubMed] [Google Scholar]
- Wong FW, King NM. A review of the rate of occurrence of cleft lip and palate in Chinese people. Hong Kong Med J. 1997;3:96–100. [PubMed] [Google Scholar]
- Wu Y, Zeng M, Xu C, et al. Analyses of the prevalences for neural tube defects and cleft lip and palate in China from 1988 to 1991. Hua Xi Yi Ke Da Xue Xue Bao. 1995;26:215–219. [PubMed] [Google Scholar]
- Xiao KZ. Epidemiology of cleft lip and cleft palate in China. Zhonghua Yi Xue Za Zhi. 1989;69:192–194. 114. [PubMed] [Google Scholar]
- Xiao KZ, Zhang ZY, Su YM, et al. Central nervous system congenital malformations, especially neural tube defects in 29 provinces, metropolitan cities and autonomous regions of China: Chinese Birth Defects Monitoring Program. Int J Epidemiol. 1990;19:978–982. doi: 10.1093/ije/19.4.978. [DOI] [PubMed] [Google Scholar]
- Zhou QJ, Shi B, Shi ZD, et al. Survey of the patients with cleft lip and palate in China who were funded for surgery by the Smile Train Program from 2000 to 2002. Chin Med J (Engl) 2006;119:1695–1700. [PubMed] [Google Scholar]