Abstract
Context
The prevalence of obesity has risen sharply in the United States in the past few decades. Etiologic links between obesity and substance use disorders have been hypothesized.
Objective
To determine whether familial risk for alcohol dependence predicts obesity, and whether any such association became stronger between the early 1990s and early 2000s.
Design
Repeated cross-sectional surveys; analyses of the National Longitudinal Alcohol Epidemiologic Survey (1991–92) and the National Epidemiologic Survey on Alcohol and Related Conditions (2001–02) were conducted.
Setting
The non-institutionalized, adult population of the U.S. in 1991–92 and 2001–02.
Participants
Individuals drawn from population-based, multi-stage, random samples (N=39,312 and 39,625).
Main Outcome Measures
Obesity, defined as a body mass index >= 30 based on self-reported height and weight, and predicted from family history of alcoholism and/or problem drinking.
Results
In 2001–02, women with a family-history of alcoholism, operationalized as having biological parent or sibling with a history of alcoholism or alcohol problems, had 49% higher odds for obesity than those without a family history (OR=1.48, 95 % CI: 1.36, 1.61; p<0.0001), a highly significant increase (p<0.0001) from the odds ratio of 1.06 (95% CI: 0.97, 1.16) estimated for 1991–92. For men in 2001–02, the association was significant (OR=1.26; 95% CI: 1.14–1.38, p<0.0001), but not as strong as for women. Both the association and the secular trend for women were robust to adjustment for covariates, including sociodemographic variables smoking, alcohol use, alcohol/drug dependence, and major depression. Similar trends were observed for men, but did not meet statistical significance criteria after adjustment for covariates.
Conclusion
The results provide epidemiologic support for a link between familial alcoholism risk and obesity for women, and possibly for men. This link has emerged in recent years, and may result from an interaction between a changing food environment and predisposition to alcoholism and related disorders.
Introduction
Obesity, defined by the World Health Organization (WHO) as body mass indices (BMI) >=30 kg/m2, respectively1 is associated with increased incidence of hypertension, type 2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, respiratory problems, and certain cancers.2, 3 Moreoever, the prevalence of obesity in the United States has doubled in the past three decades, from 15% in 1976–1980 to 33% in 2003–2004.4 Correspondingly, there has been a marked increase in risk for premature death due to obesity-related disease, and the relative contribution of obesity-attributable mortality to total deaths in the U.S. rose substantially between 1990 and 2000.5, 6
Increases in obesity in the United States are not simply a result of an across-the-board population weight increase. Rather, distributions of BMI in American adults over time are characterized by a gradual shift toward higher mean BMI, but with a marked increase in the higher end of the distribution, indicating that the largest increases have occurred in the highest weight categories.4, 7 The increase in mean BMI may be due to changes in environmental factors that influence all individuals, such as greater availability of high-calorie foods.8, 9 However, the increased number of people in the rightward tail of the BMI distribution suggests that there are subgroups of individuals who are particularly vulnerable to such a changing environment.
Among the factors that might contribute to differential vulnerability to over-eating in an obesigenic environment are deficiencies in impulse control, possibly related to individual differences in sensitivity to neurochemical reward. These characteristics are hallmarks of substance use disorders, 10, 11 and behavioral and neurobiological commonalities between overeating-associated obesity and substance use disorders have been documented in recent years (e.g refs, 12–18). Both substance use disorders and overeating-associated obesity are complex and moderately heritable; both are influenced by availability and access to highly reinforcing substances (i.e., drugs or palatable foods), both are aggravated by stress, and both lead to dopamine-modulated neurobiological adaptations.15 Indeed, observational and laboratory studies have detected links between impulsive characteristics and overeating,12, 19, 20 as well as preference for highly palatable (e.g., sweet, salty, fatty) foods. 12 Therefore, it is plausible that individuals at risk for substance use disorders have been differentially impacted by the obesity epidemic in the United States.
The purpose of this report is to investigate whether the subset of the United States population at elevated risk for alcohol use disorders, as indicated by a family history of alcoholism, have experienced greater increases in obesity than the subset of the population with no family history of alcoholism. We accomplish this by examining data from repeated cross-sectional samples of the non-institutionalized, adult population of the United States in the National Longitudinal Alcohol Epidemiologic Survey (NLAES, 1991–92) and the National Epidemiologic Survey on Alcoholism and Related Conditions (NESARC, 2001–02). With over 40,000 participants in each survey, we were able to investigate whether increases in obesity have been more prominent in individuals with a family history of alcoholism, or in other words, whether the association between family history of alcoholism and obesity has increased over time. We also investigate whether any such changes can be attributed to confounding sociodemographic characteristics, smoking, alcohol use, or depression. A clearer understanding of the relationship between familial risk for addiction, obesity, and the changing environment, may help inform prevention and treatment efforts for the subpopulation of obese individuals who are prone to addictive behaviors.
Methods
Sample
The NLAES (1991–92) and NESARC (2001–02) surveys focused on alcohol and drug use, DSM-IV substance use disorders and associated impairment in samples representative of the adult, non-institutionalized, civilian population of the United States. There were many methodological similarities between the two surveys, including the sampling universe and instrumentation used to assess alcohol dependence and related risk factors, such as family history of alcohol problems, major depression, and other disorders. Blacks were oversampled in both surveys and Hispanics were oversampled in the NESARC. Face-to-face interviews were administered by experienced lay interviewers from the U.S. Census Bureau. Respondents were informed about measures taken to ensure the confidentiality of the information they provided, and informed consent was obtained from all subjects. Ethical review and approval of all procedures was conducted by the U.S. Census Bureau and U.S. Office of Management and Budget; all subjects provided informed consent. The final NESARC sample consisted of 43,093 persons; overall raw response rate was 81%. The final NLAES sample consisted of 42,862 persons with a response rate of 90%. The analytical samples excluded those with missing height or weight, or incomplete or indeterminate family history information, pregnant women, and underweight individuals (BMI <= 18.5 kg/m2). This resulted in N=39,312 for NLAES and N=39,625 for NESARC, or 91.7 and 92.0% of the total samples, respectively. Further details for both surveys, and comparative descriptions of methods are available elsewhere.21–24
Assessment
Psychiatric diagnoses, alcohol consumption, and smoking were assessed in both surveys with the Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV version (AUDADIS-IV),25 which covers DSM-IV substance use syndromes for past year and life time frames. The psychiatric assessments in both surveys included major depression, alcohol use disorders, and drug use disorders.
Measures of smoking history and past-year drinking were also administered in both the NLAES and NESARC versions of the AUDADIS. Questions about height and weight were included in both surveys. The AUDADIS queries family history of alcoholism by asking whether a relative has “been an alcoholic or problem drinker at ANY time in his/her life.” This probe is repeated for each relative type: mother, father, brother, sister, half-siblings, children, etc.
Variables and Covariates
Main Outcome and Predictors
BMI classification was chosen as the primary outcome variable; BMI was calculated from self-reported data as weight (in kg) divided by height (in meters) squared. BMI scores were used to classify participants as obese, defined as BMI >=30 kg /m2, and non-obese, defined as BMI <30 kg/m2..1 This dichotomous classification was used instead of BMI as a continuous variable because of the aforementioned change in the shape of the BMI distribution over time. Moreover, relationships between BMI and morbidity or mortality may be nonlinear,26–28 suggesting that changes in obesity are more relevant for public health considerations than the overall change in mean BMI. The primary predictor variable, family history of alcoholism, was defined as having either a biological parent or full biological sibling with a history of problem drinking or alcoholism based on the AUDADIS family history assessment.
Subjects who were not raised with biological relatives, or who reported “unknown” alcoholism/problem drinking status for all parents and siblings were excluded from the analysis. In addition, pregnant women and underweight individuals were excluded (underweight may be indicative of severe illness). The NLAES queried hospitalization due to pregnancy in the past year, whereas the NESARC asked whether women were currently pregnant. Hence, the NLAES exclusion was slightly broader.
Sociodemographic Covariates
Racial/ethnic categorization included non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, and “Other”. The “Other” category included groups that were too small for independent analysis, such as indigenous Americans, and non-Hispanic multi-racial individuals. Race/ethnicity was determined by self-report, and categories were collapsed from more detailed categories, defined by the survey administrators, that followed the 1990 and 2000 census conventions for the NLAES and NESARC, respectively. Race/ethnicity was assessed in these surveys as a demographic variable, and for statistical weighting purposes.
Age was categorized into six groups: 18–27, 28–37, 38–47, 48–57, 58–67 and 68+ years. With respect to educational achievement, individuals were categorized into one of four groups: no high-school diploma or GED, high-school diploma or GED only, some college or other post-secondary education but not a bachelor’s degree, bachelor’s degree or higher. Subjects from each survey were grouped into quartiles defined by total household income. Quartiles were defined separately for each survey, and cut-offs were determined by ranking of un-weighted data.
Behavioral Covariates
Based on responses to smoking history questions, individuals were categorized as current smokers, former smokers, or non-smokers. Former smokers were those who had smoked 100 or more cigarettes in their lives, but none in the past 12 months, while current smokers were defined as those who crossed the 100-cigarette threshold and had also smoked in the past 12 months. Non-smokers were those who never crossed the 100-cigarette threshold. The estimated number of drinks per month during the previous 12 months, was computed from retrospective self-report by multiplying the typical frequency of drinking a given beverage by the number of drinks of that beverage consumed on a typical occasion. This value was included as a continuous variable in several analyses. Subjects were also categorized by their DSM-IV alcohol dependence status, with “Current” alcohol dependent subjects meeting criteria for a past-year diagnosis, and “Former” alcohol dependent subjects meeting criteria for alcohol dependence prior to, but not during, the past 12 months. The same approach was taken for drug dependence, with all separate types of drug dependence assessed in both NLAES and NESARC categorized as a single diagnosis of current or former drug dependence. DSM-III-R diagnoses of major depression on a past-year and lifetime basis are available in both NLAES and NESARC and were used as covariates in multivariate analyses.
Statistical Procedures
All descriptive statistics and associated standard errors, as well as regression models, were calculated using the SUDAAN statistical software package.29 Variance estimation utilized a Taylor linearization method appropriate for the multistage design of the surveys. Significance of between-survey differences in odds ratios (ORs) were assessed using two-sample z-tests applied to the beta coefficients (log OR). This is the large-sample equivalent of a two-sample t-test; z is the between-sample difference in effect size, divided by the pooled standard error.
Results
Table 1 describes subjects with complete data on family history of alcoholism and BMI, after exclusions for current (or recent) pregnancy and underweight status (see Methods). Altogether, for the NLAES, 880 subjects were excluded due to pregnancy, 785 because of missing BMI, 630 because of missing family history, and 1,255 because of being underweight. In the NESARC, 453 were excluded due to pregnancy, 1,423 due to missing BMI, 762 because of missing family history, and 830 due to underweight status. This resulted in sample sizes of N=39,312 for the 1991–92 NLAES survey and N=39,625 for the NESARC, conducted ten years later, in 2001–02. In the NLAES, individuals with missing BMI were slightly more likely than subjects in the analysis samples to report a family history of alcoholism (OR=1.11 p=0.03), and this effect was more pronounced in the NESARC (OR=1.33 p<0.0001). There were no differences in obesity between the analysis sample and individuals with missing family history in either the NLAES or the NESARC.
Table 1.
Sample Descriptions
| NLAES (1991–92), N=39,312 a |
NESARC (2001–02), N=39,625 a |
|||||
|---|---|---|---|---|---|---|
| N | Col % (SE) [Weighted] | % FH+ (SE) | N | Col % (SE) [Weighted] | % FH+ (SE) | |
| Sex | ||||||
| Men | 17,130 | 50.1 (0.3) | 30.5 (0.4) | 17,650 | 49.7 (0.3) | 29.9 (0.6) |
| Women | 22,182 | 50.0 (0.3) | 34.0 (0.4) | 21,975 | 50.3 (0.3) | 34.8 (0.7) |
| Race | ||||||
| White | 29,852 | 76.8 (0.4) | 33.1 (0.3) | 22,572 | 71.1 (1.6) | 33.1 (0.5) |
| Black | 5,469 | 11.2 (0.3) | 30.8 (0.8) | 7,527 | 11.0 (0.7) | 31.9 (1.0) |
| Hispanic | 2,589 | 7.7 (0.3) | 30.3 (1.1) | 7,710 | 11.6 (1.3) | 32.4 (1.1) |
| Asian/Pacific | 865 | 2.8 (0.1) | 13.5 (1.4) | 1,055 | 3.8 (0.5) | 12.7 (1.3) |
| Other | 537 | 1.5 (0.1) | 43.3 (2.9) | 761 | 2.5 (0.2) | 42.5 (2.0) |
| Age | ||||||
| 18–27 | 6,731 | 19.2 (0.3) | 31.3 (0.7) | 6,403 | 17.8 (0.3) | 28.1 (0.9) |
| 28–37 | 9,233 | 23.4 (0.3) | 37.3 (0.6) | 7,774 | 19.1 (0.3) | 33.2 (0.8) |
| 38–47 | 7,387 | 20.0 (0.3) | 36.1 (0.7) | 8,236 | 21.5 (0.3) | 36.9 (0.8) |
| 48–57 | 4,818 | 13.4 (0.2) | 34.2 (0.8) | 6,384 | 17.1 (0.3) | 36.4 (1.0) |
| 58–67 | 4,643 | 11.1 (0.2) | 28.4 (0.8) | 4,498 | 10.9 (0.2) | 32.5 (1.0) |
| 68+ | 6,500 | 13.0 (0.2) | 20.0 (0.6) | 6,330 | 13.6 (0.3) | 24.4 (0.7) |
| Education | ||||||
| No HS | 8,057 | 19.5 (0.3) | 33.7 (0.7) | 7,146 | 15.5 (0.5) | 37.0 (1.0) |
| HS Only | 12,060 | 31.5 (0.3) | 33.3 (0.5) | 11,509 | 29.2 (0.6) | 33.5 (0.7) |
| Some College | 9,933 | 26.3 (0.3) | 33.8 (0.6) | 11,693 | 30.3 (0.4) | 34.4 (0.7) |
| College Degree | 8,823 | 22.7 (0.3) | 27.9 (0.6) | 9,277 | 25.0 (0.6) | 25.7 (0.6) |
| Household Inc. | ||||||
| Lowest Qrtl. | 8,945 | 17.9 (0.3) | 26.5 (0.6) | 9,850 | 19.0 (0.4) | 33.6 (0.8) |
| 2nd Qrtl. | 10,260 | 26.8 (0.3) | 32.4 (0.6) | 9,545 | 21.9 (0.4) | 34.0 (0.8) |
| 3rd Qrtl. | 9,878 | 26.2 (0.3) | 34.4 (0.6) | 9,806 | 26.0 (0.3) | 32.4 (0.8) |
| Top Qrtl. | 10,229 | 29.1 (0.3) | 33.7 (0.6) | 10,424 | 33.0 (0.8) | 30.5 (0.7) |
| Smoking Status | ||||||
| Never | 19,698 | 49.9 (0.3) | 27.1 (0.4) | 22,801 | 55.6 (0.7) | 26.7 (0.6) |
| Current | 11,514 | 29.6 (0.3) | 40.2 (0.6) | 9,169 | 24.5 (0.5) | 42.9 (0.8) |
| Former | 8,085 | 20.5 (0.3) | 33.3 (0.6) | 7,649 | 19.9 (0.4) | 35.3 (0.8) |
| Alcohol Dep. | ||||||
| Never | 34,255 | 86.5 (0.2) | 29.0 (0.3) | 35,098 | 87.1 (0.4) | 29.4 (0.5) |
| Current | 1,622 | 4.5 (0.1) | 52.5 (1.5) | 1,401 | 3.9 (0.1) | 49.1 (1.7) |
| Former | 3,435 | 9.0 (0.2) | 53.5 (1.0) | 3,126 | 9.0 (0.3) | 53.6 (1.0) |
| Drug Dep. | ||||||
| Never | 38,190 | 97.1 (0.1) | 31.3 (0.3) | 38,396 | 96.6 (0.2) | 31.2 (0.6) |
| Current | 189 | 0.5 (0.0) | 57.4 (4.2) | 229 | 0.6 (0.1) | 62.6 (3.9) |
| Former | 933 | 2.4 (0.1) | 66.5 (1.7) | 1,000 | 2.8 (0.1) | 64.5 (1.8) |
| Major Depression | ||||||
| No | 30,036 | 77.5 (0.3) | 27.8 (0.3) | 31,370 | 79.3 (0.4) | 28.5 (0.5) |
| Yes | 9,276 | 22.5 (0.3) | 47.5 (0.6) | 8,255 | 20.7 (0.4) | 47.3 (0.7) |
After exclusions for missing FH, height, or weight data, underweight status, or current pregnancy.
The prevalence of obesity was 14.9% (95% CI: 14.2%, 15.6%) in the 1991–92 NLAES sample and 23.0% (95% CI: 22.2%, 23.8%) in the 2001–02 NESARC sample. Mean BMI in the NLAES was 25.4 for women (SD=5.2) and 26.0 (SD=4.1) for men. In the NESARC, mean BMI was 27.0 (SD=6.1) for women and 27.3 (SD=4.8) for men.
In the NLAES, 32.2% of the sample reported a family history of alcohol-dependence (FH-Alc; 95% CI: 30.6%, 33.8%) with a very similar percentage reporting FH-Alc in the NESARC (32.4%, 95% CI: 31.3%, 33.5%). The likelihood of having a family history was similar across Whites, Blacks and Hispanics, but was much lower among Asians. Women were slightly more likely to report family history than men, a result that was consistent across types of relatives (i.e., mother, father, brother, sister) and surveys. Subjects between the ages of 28 and 57 reported higher rates of family history than either 18–27 year old or 58+ year old subjects.
The full BMI distribution in the combined gender NLAES and NESARC samples, stratified by FH-Alc, can be seen in Figure 1. The Figure shows remarkably little difference in BMI distribution between individuals with and without a family history of alcoholism in the NLAES. However, in the NESARC, the two sub-populations clearly diverge. At BMI values of 30 kg/m2 and above, the cut-point for obesity, individuals reporting a family history of alcoholism constitute a higher proportion of membership in any BMI range than those without a family history of alcoholism.
Figure 1.
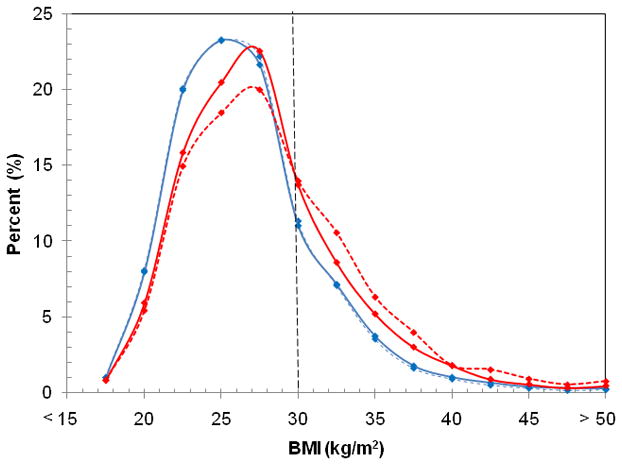
BMI Distributions of the 1991–92 NLAES (Blue) and 2001–02 NESARC (Red) weighted samples stratified by family history of alcoholism (solid lines = family history of alcoholism, dashed lines=no family history of alcoholism). Frequencies were calculated in 2.5 kg/m2 intervals, up to 50 kg/m2. Individuals below BMI of 18.5 kg/m2 were excluded. Data points were connected via a polynomial smoothed line.
The core result -- the prevalence of obesity stratified by family history of alcoholism in the 1991–92 NLAES and 2001–02 NESARC -- is quantified in Table 2. While there was only a modest association between FH-Alc and obesity in 1991–92, a highly significant association for both genders was observed in 2001–02. The bivariate odds ratio describing the association was significantly higher for women in 2001–02 compared with women ten years earlier (OR=1.48 vs. OR=1.06, cross survey difference test: z=5.77, p<0.0001). There was a similar secular trend for men, but lower in magnitude of marginal statistical significance (OR=1.26 vs. OR=1.08; z=1.92, p=0.055). Although the overall prevalence of obesity for both sexes increased from NLAES to NESARC, the increase was significantly stronger among those with a family history of alcoholism, and this effect was particularly pronounced for women.
Table 2.
Prevalence of Obesity by Alcoholism Family History: 1991–92 and 2001–02
| NLAES (1991–92) |
NESARC (2001–02) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Prevalence, % (SE) |
Odds Ratio (95% CI) |
Prevalence, % (SE) |
Odds Ratio (95% CI) |
||||
| FH- | FH+ | (FH+ vs. FH-) | N | FH- | FH+ | (FH+ vs. FH-) | ||
| Women | 22,182 | 15.4 (0.4) | 16.1 (0.5) | 1.06 (0.97–1.16) | 21,975 | 20.8 (0.5) | 28.0 (0.7) | 1.48 (1.36–1.61) b,c |
| Men | 17,130 | 13.9 (0.4) | 14.9 (0.6) | 1.08 (0.97–1.22) | 17,650 | 21.4 (0.6) | 25.5 (0.7) | 1.26 (1.14–1.38) b,d |
| Total | 39,312 | 14.6 (0.3) | 15.6 (0.4) | 1.08 (1.00–1.15) a | 39,625 | 21.1 (0.5) | 26.9 (0.5) | 1.37 (1.28–1.47) b,c |
p<0.05.
p<0.001.
NESARC OR differs from NLAES with p<0.0001.
NESARC OR differs from NLAES with p=0.05.
Similar comparisons between FH+ and FH- individuals stratified by major demographic and behavioral variables were conducted. Stratification variables included race/ethnicity, age, education and total household income, smoking status, alcohol dependence status, drug dependence status, and major depression. These results are listed in Supplemental Tables 1 and 2. Women (Supplemental Table 1) and men (Supplemental Table 2) are presented separately due to the differences exhibited in the primary comparisons. For almost all of the stratified comparisons, the association between FH-Alc and obesity in the NESARC was stronger than in the earlier NLAES, as evidenced by a trend toward higher odds ratios in the NESARC (though not all differences were statistically significant). The only exceptions to this trend were for Asian/Pacific Islander men and for “Other” race/ethnicity women. In both cases, these comprise relatively small and potentially heterogeneous strata. Still, there was a significant association (p<0.05) between FH-Alc and obesity in the NESARC for the majority of comparisons (36 out of 60, vs. 3 out of 60 for NLAES comparisons). These results suggest that the increased influence of FH-Alc over time is independent of a number of factors indicative of socioeconomic status and social disadvantage, and is not a result of confounding by sociodemographic or behavioral factors.
Table 3 lists adjusted odds ratios from a series of planned logistic regression analyses in which potentially explanatory covariates are entered sequentially into a model predicting obesity from FH-Alc. For both genders, the addition of sociodemographic covariates – race/ethnicity, age, educational attainment, and household income -- results in a small reduction in the difference in odds ratios between the NLAES and NESARC. For women, the odds ratios remain highly significant in NESARC, as does the difference in odds ratio between NLAES and NESARC. (Addition of state of residence as an additional demographic variable resulted in essentially no change in odds ratios, and therefore was not included in subsequent analyses). In the second model listed in Table 3, alcohol dependence, drug dependence, smoking status, and a quantitative measure of alcohol consumption were added to the model. These had minimal impact on the odds ratios in both surveys, for both genders.
Table 3.
Association between Family History of Alcoholism and Obesity Under Various Models: 1991–92 and 2001–02
| Additional Model Covariates | NLAES (1991–92) |
NESARC (2001–02) |
Comparison (NESARC vs. NLAES) |
|
|---|---|---|---|---|
| Odds Ratio (95% CI), FH+ vs. FH- | Odds Ratio (95% CI), FH+ vs. FH- | Z | p | |
| Women | ||||
| Race, Age, Education, Income | 1.02 (0.93–1.12) | 1.35 (1.23–1.48) b | 4.30 | <0.001 |
| Race, Age, Education, Income, Alcohol Consumption, Alcohol Dependence, Drug Dependence, Smoking | 1.05 (0.96–1.15) | 1.38 (1.25–1.51) b | 4.09 | <0.001 |
| Race, Age, Education, Income, Alcohol Use, Smoking, MDD | 1.00 (0.91–1.10) | 1.30 (1.19–1.43) b | 3.97 | <0.001 |
| Men | ||||
| Race, Age, Education, Income | 1.00 (0.89–1.13) | 1.13 (1.02–1.24) a | 1.49 | 0.13 |
| Race, Age, Education, Income, Alcohol Consumption, Alcohol | 1.06 (0.94–1.19) | 1.13 (1.03–1.25) a | 0.88 | 0.38 |
| Dependence, Drug Dependence, Smoking Race, Age, Education, Income, Alcohol Use, Smoking, MDD | 1.03 (0.91–1.16) | 1.11 (1.01–1.23) a | 0.52 | 0.60 |
p<0.05,
p<0.001.
Finally, because FH-Alc is likely to be associated with major depressive disorder, and obesity is also a correlate of major depression and/or other mood disorders in the United States,30, 31 life-history of major depression was added to the models. This resulted in modest, and non-significant changes in the association between FH-Alc and obesity for both genders in both surveys. In these final models, the adjusted odds ratios for women were 1.30 (95% CI: 1.19, 1.43) in the NESARC, and 1.00 (95% CI: 0.91, 1.10) in the NLAES; difference p<0.0001. Hence the association between FH-Alc and obesity among women remained highly significant in the NESARC (p<0.0001), and the difference in the association between the NLAES and NESARC remained significant for women (p<0.0001). A modest association between FH-Alc and obesity remained for men in the NESARC after adjustment (OR=1.11; 95% CI: 1.01, 1.23, p=0.02); this was a slight, but non-significant increase over the NLAES (OR=1.02; 95% CI: 0.94, 1.11)
Discussion
In this work, we use two large epidemiological samples representative of the non-institutionalized adult population of the United States to examine secular trends in the association between familial risk for alcoholism and obesity. We find a significant association between familial alcoholism and obesity among men and women surveyed in 2001–02. Furthermore, this association was substantially greater in 2001–02 than in 1991–92 among women, with a qualitatively similar, but less pronounced trend among men (p=0.05) in bivariate analyses. For men, the association between familial alcohol problems and obesity in 2001–02 is smaller than for women, but statistically significant. For women, both the association and the secular trend remain significant after adjustment for other sociodemographic variables, smoking, alcohol consumption, alcohol and drug dependence and major depression. For men, the secular trend is non-significant after adjustment for covariates.
Our findings suggest that a link between familial-alcoholism and obesity has emerged in recent years, particularly among women. In other words, the interaction between factors related to familial-alcoholism and the increasing obesigenicity of the environment may have resulted in a differential increase in the prevalence of obesity among individuals vulnerable to addiction. This may be specifically a result of a changing food-environment and the increased availability of highly palatable foods.18, 32–34 Hence, the present finding is consistent with a body of psychological and neurobiological literature describing over-eating as an addictive behavior.12, 14, 15, 17, 18, 35, 36 This point-of-view postulates that neurocircuitry activated by drugs of abuse overlaps regions involved in food-related rewards. There is also support for this idea from behavioral studies. For example, some studies have suggested an association between family history of alcoholism and a preference for sweet foods. 37, 38 However, to our knowledge, this is the first documentation of a link between alcoholism and obesity using epidemiological data, and more importantly, the first study to suggest that the epidemiological association between alcoholism-risk and obesity has grown over time, and perhaps emerged fairly recently.
There was a small, albeit non-significant reduction in effect size in the association between familial alcoholism and obesity among women after adjusting for major depression the only non-substance related psychiatric diagnosis assessed in both surveys and this reduction may have been larger if other psychiatric disorders has been assessed in both surveys. Familial alcoholism may lead to obesity, in part, through psychiatric comorbidity. Other causal mechanisms, including common etiology for obesity and other psychiatric disorders that correlate with alcoholism risk are also plausible.39–41 Twin models suggest some overlap between the genetic etiology of depression and alcoholism; interestingly, evidence for this overlap is stronger for women than for men.42–44 Regardless of the role of depression other psychiatric disorders in the causal pathway between familial alcohol risk and obesity, documentation of this association, and its change over time, is a significant step in understanding obesity-associated characteristics in a subset of the population.
It could be argued that the magnitude of the odds ratios describing the association between familial alcoholism and obesity, even among women, is not exceptionally large. However, our main finding is the change in the magnitude of this association between 1991–92 and 2001–02. It should be noted that the measurement uncertainty inherent in a brief, self-reported assessment of familial alcohol problems would likely bias effect size estimates downward. The fact that we observed a highly significant change in these odds ratios for women, and a suggestive trend for men, over the relatively short period of ten years, could have significant implications for understanding obesity in a sizeable subset of the population. If there is a portion of the population whose eating behaviors are more “addiction-like” than others, then characterizing such individuals could aid in the individualized treatment of obesity. Pharmacological and psychosocial interventions modeled on addiction treatment may be indicated in such individuals.
A better understanding of the emerging link between familial alcoholism and obesity requires further examination of this association in recently ascertained, high-quality, general population samples, or carefully designed case-control samples. A more complete psychiatric characterization of subjects in such samples could also help refine the pathways through which this association occurs. In addition, it would be desirable to identify specific eating behaviors and psychological characteristics that mediate the link between familial-alcoholism and obesity. These might include general dietary patterns; preferences for short-term rewards over delayed gratification; 45, 46 preferences for highly rewarding sweet, salty or fatty foods; 37, 38 18 and binge eating behaviors.47–49 To our knowledge, this is the first study to examine the association between family history of alcohol problems and BMI, and moreover, to study repeated cross-sections of the United States population to examine recent secular trends. Gearhardt and Corbin50 have noted an association between family history of alcohol problems and BMI in the NESARC, but in the context of understanding drinking behavior in relation to obesity. Barry and Petry51 noted an association between lifetime alcohol use disorder and obesity in men and an inverse association between past-year alcohol use disorders and obesity among women. The intent of their analysis, however, was not to specifically examine family history of alcoholism.
The large, population based samples, and analysis of repeated-cross sections of the population constitutes considerable strengths. The repeated cross-section approach is particularly well-suited for estimating overall change within a population.58 The use of self-reported height and weight to determine BMI are limitations, and could potentially bias the estimates of the association between FH-Alc and obesity. Self-reports are known to result in underestimated BMI, with effects that may differ by age, gender and measured BMI.52–55 On average, measured BMI is about 0.6 kg/m2 higher than self-report based BMI, and the discrepancy is larger for higher BMI individuals.55 Obesity prevalence estimates based on measurement are up to 50% higher than those based on self-report.56 On the other hand, prevalence estimates of obesity obtained here are quite close to the self-report based estimates produced by the Behavioral Risk Factor Surveillance System survey (BRFSS),57 and BRFSS estimates have exhibited similar secular trends and associations with health outcomes as those based on physical measurement.56, 58 The correlation between measured and self-reported BMI ranges from 0.89 to 0.97, 55,59 and correlation of reporting bias with sociodemographic variables is mitigated in these analyses by the inclusion of numerous socio-demographic covariates in multivariate models. As demonstrated in Supplemental Tables 1 and 2, the association between FH-Alc and self-report based obesity is consistent across socio-demographic categories. Considering all of these factors, the tendency for higher BMI individuals to underestimate their BMI to a larger degree, if independent of FH-Alc, could result in a slight underestimation of the true association between FH-Alc and obesity.
Missing data also may have an impact on effect size estimates; those with indeterminate family history statuses were no more or less likely to be obese than others in the NLAES or NESARC, but those with missing BMI data were more likely to report a family history of alcoholism, an effect that was larger in the NESARC. It is difficult to speculate on how this correlation might impact the results, but if social-desirability is a component of missing BMI data (i.e., passive refusal to report high weight), this may result in slight underestimates for both the association between FH-Alc and obesity, and the secular trend.
Although we tested for several potential confounding relationships, there may be other confounding variables that were not assessed in both the NLAES and the NESARC. In addition, potential explanatory variables, such as physical activity, caloric intake, binge-eating behaviors, and other psychiatric disorders were not measured. However, in order for the secular trend to be attributable to and unmeasured confounding variable, it would be necessary for the unmeasured variable to be correlated with both family history of alcoholism, and with obesity, and to have changed over time. This same principle applies in biases inherent in self-reports of height, weight, and family history of alcoholism. In other words, presuming such biases are stable over time, they are unlikely to account for the secular trend in the association between FH-Alc and obesity.
We emphasize that our findings apply only to trends in the United States. There are many environmental contributors to both alcoholism (and by extension, having a family member with alcoholism) and to obesity that vary from one cultural context to another. It is noteworthy that the United States has much higher rates of obesity than other developed countries,60 but has slightly lower rates of alcohol consumption than these countries.61 Cross-cultural replication of these analyses could provide further insights into the environmental factors that have contributed to these secular trends.
Conclusions
In the decade between the early 1990s and early 2000s, a clear link between familial alcoholism risk and obesity has become apparent in the United States. The link is more prominent among women, for whom it is not explained by potentially confounding sociodemographic variables, smoking, alcohol consumption, substance dependence, or major depression. These findings provide epidemiological support for the etiologic links between addiction and overeating or obesity documented in neurobiological studies.15 Moreover, our are consistent with the hypothesis that relatively recent environmental changes have contributed to this link. The fields of obesity research and addiction research have a mutual interest in working together to find treatments for obese individuals from high addiction-risk backgrounds, and developing a more detailed understanding of shared etiology between these conditions.
Supplementary Material
Acknowledgments
The authors are grateful to Professor Denise Wilfley, Washington University, for valuable comments on the results presented here. Data analysis, interpretation, and manuscript preparation were supported by R01 AA17444, R01 DA 019963, P01 CA 089392 (RAG), R21 DA026612 (RAG, KEN), K02 DA 021237 U10 AA 008401 (LJB), U01 AA018111 (RFK). Investigators from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) were involved in collection and management of data from the NLAES and NESARC, which were sponsored by the NIAAA with supplemental support from the National Institute on Drug Abuse. Sponsors had no further role in analysis or interpretation of the data; or preparation, review, or approval of the manuscript. NLAES and NESARC data were obtained from CSR, Incorporated and the NIAAA, (http://niaaa.census.gov), respectively. RAG, LJB, KEN, and PRH had full access to all of the data in the study (as obtained by CSR and NIAAA) and take responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analyses conducted by RAG. Dr. Bierut is an inventor on the patent “Markers for Addiction” (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. Bierut served as a consultant for Pfizer Inc. in 2008.
References
- 1.World Health Organization. The global epidemic of obesity. Geneva: WHO; 1997. [Google Scholar]
- 2.NIH. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States--no statistically significant change since 2003–2004. NCHS Data Brief. 2007;(1):1–8. [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery RW, Utter J. The changing environment and population obesity in the United States. Obes Res. 2003;11 (Suppl):12S–22S. doi: 10.1038/oby.2003.221. [DOI] [PubMed] [Google Scholar]
- 10.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 11.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28(3):343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48(1):12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, O’Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry. 2007;164(5):708–710. doi: 10.1176/ajp.2007.164.5.708. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 16.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53(1):1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Rapaka R, Schnur P, Shurtleff D. Obesity and addiction: common neurological mechanisms and drug development. Physiol Behav. 2008;95(1–2):2–9. doi: 10.1016/j.physbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Cocores JA, Gold MS. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med Hypotheses. 2009;73(6):892–899. doi: 10.1016/j.mehy.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007;48(1):119–122. doi: 10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri R, Nederkoorn C, Jansen A. The interaction between impulsivity and a varied food environment: its influence on food intake and overweight. Int J Obes (Lond) 2008;32(4):708–714. doi: 10.1038/sj.ijo.0803770. [DOI] [PubMed] [Google Scholar]
- 21.Grant BF, Moore TC, Shepard J, Kaplan K. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alchol and Related Conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- 22.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58(5):464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- 23.Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291(17):2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- 24.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Grant BF, Dawson DA, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV Version. Bethesda, Md: National Institute on Alcohol Abuse and Alcoholism; 2001. [Google Scholar]
- 26.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 27.Engeland A, Bjorge T, Selmer RM, Tverdal A. Height and body mass index in relation to total mortality. Epidemiology. 2003;14(3):293–299. [PubMed] [Google Scholar]
- 28.Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996;20(1):63–75. [PubMed] [Google Scholar]
- 29.SUDAAN Language Manual, Release 9.0 [computer program]. Version. Research Triangle Park, NC: Research Triangle Institute; 2004. [Google Scholar]
- 30.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickering RP, Grant BF, Chou SP, Compton WM. Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2007;68(7):998–1009. doi: 10.4088/jcp.v68n0704. [DOI] [PubMed] [Google Scholar]
- 32.Levin BE. Factors promoting and ameliorating the development of obesity. Physiol Behav. 2005;86(5):633–639. doi: 10.1016/j.physbeh.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 33.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Heber D. An integrative view of obesity. Am J Clin Nutr. 2010;91(1):280S–283S. doi: 10.3945/ajcn.2009.28473B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 36.Davis C. Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (Lond) 2009;33 (Suppl 2):S49–S53. doi: 10.1038/ijo.2009.72. [DOI] [PubMed] [Google Scholar]
- 37.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31(11):1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27(11):1743–1749. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- 39.Rivenes AC, Harvey SB, Mykletun A. The relationship between abdominal fat, obesity, and common mental disorders: results from the HUNT study. J Psychosom Res. 2009;66(4):269–275. doi: 10.1016/j.jpsychores.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 40.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–651. doi: 10.4088/jcp.v65n0507. quiz 730. [DOI] [PubMed] [Google Scholar]
- 41.Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, Ronnemaa T. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251(1):35–43. doi: 10.1046/j.1365-2796.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- 42.Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57(8):803–811. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. A twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50(9):690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- 44.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry. 1995;52(5):374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 45.Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Grucza RA, Przybeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry. 2007;48(2):124–131. doi: 10.1016/j.comppsych.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conason AH, Sher L. Alcohol use in adolescents with eating disorders. Int J Adolesc Med Health. 2006;18(1):31–36. doi: 10.1515/ijamh.2006.18.1.31. [DOI] [PubMed] [Google Scholar]
- 49.Piran N, Robinson SR. Associations between disordered eating behaviors and licit and illicit substance use and abuse in a university sample. Addict Behav. 2006;31(10):1761–1775. doi: 10.1016/j.addbeh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009;23(2):216–225. doi: 10.1037/a0015011. [DOI] [PubMed] [Google Scholar]
- 51.Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addict Behav. 2009;34(1):51–60. doi: 10.1016/j.addbeh.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc. 2001;101(1):28–34. doi: 10.1016/S0002-8223(01)00008-6. quiz 35–26. [DOI] [PubMed] [Google Scholar]
- 53.Roberts RJ. Can self-reported data accurately describe the prevalence of overweight? Public Health. 1995;109(4):275–284. doi: 10.1016/s0033-3506(95)80205-3. [DOI] [PubMed] [Google Scholar]
- 54.Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self-reported weight and height in the French GAZEL cohort. Int J Obes Relat Metab Disord. 2000;24(9):1111–1118. doi: 10.1038/sj.ijo.0801375. [DOI] [PubMed] [Google Scholar]
- 55.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001–2006. BMC Public Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 57.Paynter N, Denny CH, Greenlund KJ, Croft JBM, GA Declining prevalence of no known major risk factors for heart disease and stroke among adults - United States, 1991–2001. MMWR Morb Mortal Wkly Rep. 2004;53(1):4–7. [PubMed] [Google Scholar]
- 58.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 59.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 60.WHO Global InfoBase team. The SuRF Report 2. Surveillance of chronic disease Risk Factors: Country-level data and comparable estimates. Geneva: World Health Organization; 2005. [Google Scholar]
- 61.World Health Organization. [Accessed March 26, 2010];WHO Statistical Information System (WHOSIS) http://www.who.int/whosis/en/Updated February 9, 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


