Abstract
Two types of endogenous cannabinoid-receptor agonists have been identified thus far. They are the ethanolamides of polyunsaturated fatty acids—arachidonoyl ethanolamide (anandamide) is the best known compound in the amide series—and 2-arachidonoyl glycerol, the only known endocannabinoid in the ester series. We report now an example of a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (noladin ether), isolated from porcine brain. The structure of noladin ether was determined by mass spectrometry and nuclear magnetic resonance spectroscopy and was confirmed by comparison with a synthetic sample. It binds to the CB1 cannabinoid receptor (Ki = 21.2 ± 0.5 nM) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds weakly to the CB2 receptor (Ki > 3 μM).
We have reported the isolation and identification of two types of endogenous cannabinoids that bind and activate the known cannabinoid receptors CB1 and CB2. Arachidonoyl ethanolamide (anandamide; ref. 1) and later two more polyunsaturated fatty acid ethanol amides (2) were found in porcine brain. An ester, 2-arachidonoyl glycerol (2-AG) was isolated by us from canine gut (3) and by Sugiura et al. from brain (4). Anandamide and 2-AG have been the objects of numerous investigations in various areas of biology and have been found to affect processes in the nervous, cardiovascular, immune, and reproductive systems (5–8). They interact with many neurotransmitters and affect hormone levels (9–10). This ubiquity of effects led us to look for additional endocannabinoids.
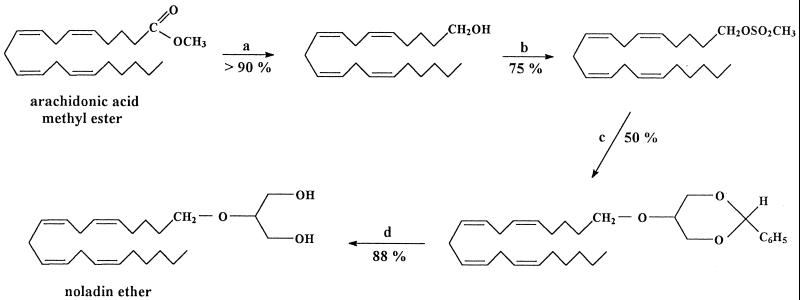
We report now that we have isolated from porcine brain a third endocannabinoid, 2-arachidonyl glyceryl ether, which we have named noladin ether (Fig. 1).
Figure 1.
Synthesis of noladin ether: step a, LiAlH4/ether, 25°C; step b, CH3SO2Cl/pyridine, 25°C; step c, benzylideneglycerol-KOH/benzene, 25°C; step d, HCl/CH3OH, 25°C. Percentages indicate yields of reactions.
Materials and Methods
Isolation of Noladin Ether.
Porcine brain (100 g, approximately a single brain) was added to a mixture of chloroform (200 ml) and methanol (200 ml) and mixed in a blender for 2 min. Water (100 ml) was added, and the mixing process was continued for another minute. The mixture was filtered. Two layers were formed. The water-methanol layer was separated and evaporated under reduced pressure. The residue obtained was extracted with methanol. The above isolation was repeated numerous times (from a total of 2.4 kg porcine brain). The extract obtained was chromatographed on a gravity column (i.d. 2.5 cm, height 28 cm, 82 g ICN Silica TSC, 60 A) with hexane/acetone initially in a ratio of 10:1 (vol/vol) (400 ml), then 9:1 (100 ml), and finally 4:1 (100 ml). The fractions eluted were monitored for binding to the CB1 receptor from rat brain synaptosomes (prepared as described below; ref. 11) on the basis of displacement of the potent labeled agonist [3H]HU-243 purchased from Tocris (Bristol, U.K.). The material eluted in fractions 45–54 (10-ml each) was found to bind to the receptor. These fractions were combined and purified further by HPLC (see below). A polar-active compound developed on a TLC plate (silica gel 60 F254, Merck) in a hexane/acetone (4:1) solvent system gave a single spot (Rf = 0.13). From 2 kg of porcine brain, we obtained 0.5 mg of noladin ether, approximately the amount of anandamide obtained from a comparable extraction (1).
Preparation of Synaptosomal Membranes.
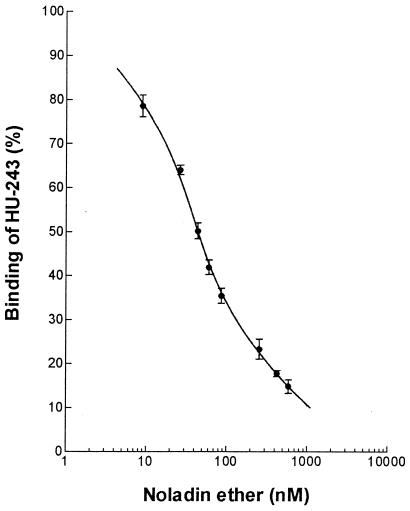
Synaptosomal membranes were prepared from the brains of Sabra male rats (250 g) after removal of the brainstem. The [3H]HU-243 probe, with a dissociation constant of 45 pM (11), was incubated with synaptosomal membranes (3–4 μg) for 90 min at 30°C with the indicated concentrations of noladine ether or the vehicle alone (fatty-acid-free BSA at a final concentration of 0.5 mg/ml). Bound and free radioligand were separated by centrifugation (see ref. 11 for full details of the assay). The data were normalized to 100% of specific binding, which was determined with 50 nM unlabeled HU-243. Data points represent the average of triplicate determinations from three independent experiments. The Ki value was determined with a GraphPad (San Diego) PRISM 2.01 program that follows the Cheng-Prusoff equation (12). A sigmoid dose-response (variable slope) built-in equation in PRISMwas used to fit the curve shown in Fig. 2.
Figure 2.
Competitive inhibition of [3H]HU-243 binding by natural noladin ether. For details see Materials and Methods.
HPLC.
The HPLC analysis was performed by using a Waters liquid chromatograph (Millipore) equipped with a Photodiode Array (model 996), 20- and 50-μl loops, a Waters 600E multisolvent delivery system, and a Millennium 2010 chromatography manager for the analysis of data. A reversed-phase Waters Symmetry C18 column (5 μm, 250 × 4.6 mm i.d.) was used. Reversed-phase solvents were HPLC grade. Acetonitrile was purchased from J. T. Baker. Water was doubly distilled and filtered by using a 0.2-μm cellulose mixed ester membrane (Schleicher & Schuell). Before injection into the HPLC, the sample was filtered by loading concentrated methanol extract onto a 0.2-μm Gelman nylon syringe filter.
The analyses were run under isocratic conditions at ambient temperature. The optimized mobile phase used for the isolation of the active noladin ether was acetonitrile/water [70:30 (vol/vol)]. The flow rate was 1.5 ml/min, and the monitoring wavelength was 210 nm. Under these conditions, noladin ether elutes at 22.88 min. Of 4.5 mg of column fraction, 0.5 mg of pure noladin ether was obtained.
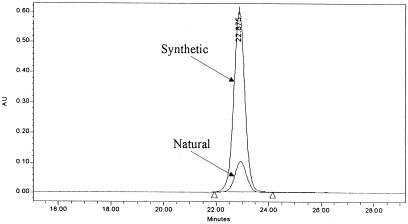
Synthetic standard (noladin ether) was injected by using the same working conditions. Similar elution time was noticed for both compounds. After comparing the UV/Vis spectrum of the synthetic compound with extracted noladin ether, excellent matching was obtained (Fig. 3). Moreover, when a synthetic sample was spiked with natural noladin ether and injected into the HPLC, one single peak was eluted at 22.88 min. Waters MILLENNIUM software purity calculations confirmed the existence of only one component under this peak.
Figure 3.
Overlay of HPLC chromatograms of natural and synthetic noladin ether. For details see Materials and Methods.
MS.
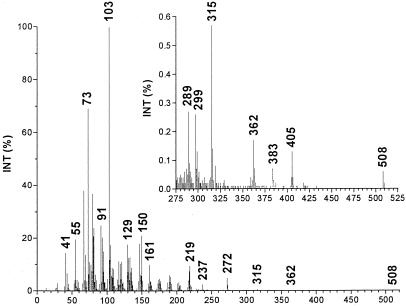
A Hewlett Packard G 1800B GCD system that has a HP-5971 GC with electron ionization detector was used. The software used was GCD PLUS CHEMSTATION. The column used was HP-5 (crosslinked 5% PH ME siloxane) with a film thickness of 28 m × 0.25 mm × 0.25 μm. Experimental conditions were: inlet, 250°C; detector, 280°C; splitless injection/purge time, 1.0 min; initial temperature, 70°C; initial time, 3.0 min; rate, 30°C/min; final temperature, 280°C. The MS spectrum is presented in Fig. 4.
Figure 4.
Electron impact/mass spectrum of di(trimethylsilyl) derivative of natural noladin ether. The relative intensity of the peaks above 275 m/z is given (Inset). For details see Materials and Methods.
NMR Spectroscopy.
NMR spectra were acquired on a Bruker (Billerica, MA) Advanced DMX 600-MHz spectrometer. Proton chemical shifts at 295.0 K were referenced to chloroform at 7.26 and 77.0 ppm for proton and carbon signals, respectively. One- and two-dimensional (2D) homo- and heteronuclear data were collected at natural abundance by using 2D-correlated spectroscopy, 2D double-quantum filtered correlated spectroscopy, 2D total-correlation spectroscopy, and 2D heteronuclear multiple-quantum correlation experiments.
All NMR data were processed by using XWINNMR 2.6 software (Bruker) running on Silicon Graphics (Mountain View, CA) INDY workstations. Zero-filling and suitable apodization functions were applied to enhance the spectral resolution. The spectra were analyzed by using Aurelia 2.7.5 software (Bruker).
The one-dimensional proton spectra and the 2D-correlated spectroscopy and total-correlation spectroscopy spectra of natural and synthetic noladin were compared and found to be identical under the acquisition conditions. The chemically identical alkene methine protons 6, 7, 9, 10, 12, 13, 15, and 16 are overlapped, as are the methylene protons 8, 11, and 14 that have double bonds at both sides. The alkane protons 2 and 3 are resolved only in the carbon dimension. The NMR data and assignments of noladin ether are given in Table 1.
Table 1.
NMR spectral parameters of noladin ether (600 MHz, in CDCI3)
| Carbon number | δC | δH (m, J in Hz) | Correlated spectroscopy | Total-correlation spectroscopy |
|---|---|---|---|---|
| 1 | 14.0 | 0.88 (t, 6.9) | 2/3* | 2/3*, 4, 5 |
| 2 | 22.5 | 1.30 (m) | 1 | 1, 4, 5, 6 |
| 3 | 31.4 | 1.30 (m) | 2, 4 | 1, 4, 5, 6 |
| 4 | 29.3 | 1.35 (m) | 3, 5 | 1, 2/3*, 5, 4, 8 |
| 5 | 27.1 | 2.05 (q, 7.0, 15.5) | 4, 6 | 1, 2/3, 4, 6 |
| *6, 7, 9, 10, 12, 13, 15, 16 | 128.0, 129.8 | 5.37 (m) | †5, 8, 11, 14, 17 | 2/3*, 4, 5, 8, 11, 14, 17, 18, 19† |
| *8, 11, 14 | 25.5 | 2.82 (m) | 5‡, 7, 9, 10, 12, 13, 15, 17‡ | 4, 5, 7, 9, 10, 12, 13, 15, 17, 18, 19 |
| 17 | 26.8 | 2.10 (m) | 16, 18 | 14, 16, 18, 20 |
| 18 | 26.0 | 1.44 (m) | 17, 19 | 14, 16, 17, 19, 20 |
| 19 | 29.6 | 1.62 (m) | 18, 20 | 14, 16, 18, 20 |
| 20 | 69.9 | 3.58 (t, 6.6) | 19 | 17, 18, 19 |
| 22 | 79.3 | 3.45 (p, 4.8, 9.4) | 23, 25 | 23, 25 |
| 23, 25* | 62.0 | 3.68, 3.76 | 22 | 22 |
| 24, 26* | — | 1.87 (b) | — | — |
Signals were unresolved in the spectra.
Presumably, the cross peaks are to adjacent protons.
Long-range correlations are common in alkene systems. For technical details see Materials and Methods.
Pharmacological Assays with Noladin Ether.
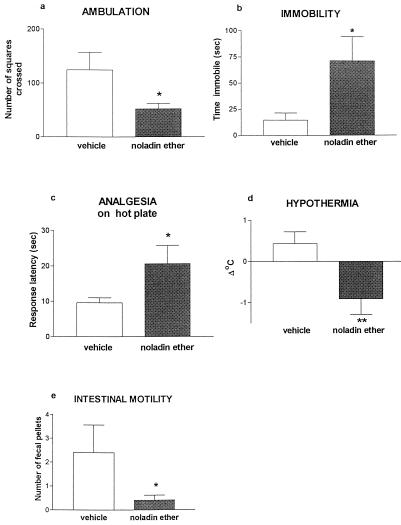
Female Sabra mice (Harlan, Jerusalem, Israel) were injected i.p. with vehicle (ethanol/emulphor/saline 1:1:18) or with 20 mg/kg synthetic noladin ether in the same vehicle. Each mouse was observed 10 min later in four consecutive assays as described (13): (i) ambulation in an open field (20 × 30 cm, divided into 12 squares) for 8 min; (ii) time spent immobile (over 4 min) after being placed on a metal ring (5.5-cm diameter) raised 15 cm above the surface; (iii) analgesia on a hot plate (55°C), on which latency was measured until the first hindpaw lick or jump from the plate (the latter was rarely observed); (iv) hypothermia (rectal temperature subtracted from that measured before injection); and (v) the number of fecal pellets produced over 8 min in the open field was recorded and taken as a measure of intestinal motility. Thus, the testing started: for ambulation, 10 min after injection; ring immobility, 18 min after injection; and rectal temperature and analgesia, 22–23 min after drug administration. The results are presented in Fig. 5.
Figure 5.
Performance of mice injected with synthetic noladin ether on pharmacological tests for cannabinoid effects. Noladin ether was injected 10 min before the start of testing. *, P < 0.05; **, P < 0.01 (difference from vehicle controls). For further details see Materials and Methods.
Each treatment group consisted of six mice. Control and synthetic noladin ether-treated mice were compared with t tests for independent samples.
Results and Discussion
The isolation procedure involves extracting porcine brain with chloroform/methanol followed by a series of chromatographic separations on a silica gel column, TLC, and reversed-phase HPLC. The purity of the isolated material (0.5 mg from 2.4 kg brain) was established by TLC and HPLC photodiodarray detection in which a single spot and a pure single peak (Fig. 3) were observed, respectively.
Initial indications for the structure of the endocannabinoids were noted from MS and NMR spectroscopy data. Thus, after silylation with bis(trimethylsilyl)- trifluoroacetamide, we observed a strong peak at m/z 219 typical for the glyceryl di(trimethylsilyl) moiety (m/z = 218 + 1) and a peak at m/z 508, which is 14 mass units lower than 522, the molecular weight of 2-AG (Fig. 4). The NMR spectrum indicated the presence of nonconjugated double bonds with an 8:6 ratio of double-bond protons/protons allylic to two double bonds, typical of the arachidonyl moiety (Table 1). These findings and other MS and NMR data suggested that the endocannabinoid noladin ether could be the ether of glycerol with arachidonyl alcohol.
Both anandamide, an amide, and 2-AG, an ester, are prone to facile hydrolytic and enzymatic hydrolysis. To prepare a more stable entity related to 2-AG, we had synthesized noladin ether already (see Fig. 1) and had initiated an evaluation of its biological properties before the completion of the work described now (14, 15). Independently, Sugiura et al. had prepared this compound also (16) and Suhara et al. had examined its effects on Ca2+ levels in cells (17). The availability of synthetic noladin ether made possible a direct comparison with the natural product. The GC/MS spectrum of the di(trimethylsilyl) ether of noladin ether and the NMR spectral parameters of the natural compound are presented in Fig. 4 and Table 1. These GC/MS and NMR data were identical for the natural and synthetic compounds. On TLC and HPLC (Fig. 3), again identity was noted (for full details see Materials and Methods).
Noladin ether inhibited the specific binding of [3H]HU-243 (11) to the CB1 receptor from rat brain synaptosomal membranes in a manner typical of competitive ligands (Fig. 2). The inhibition constant (Ki) value for noladin ether was 21.2 ± 0.5 nM (SEM, n = 3). Although anandamide and 2-AG bind to both CB1 and CB2, we observed low binding (above 3 μM) of noladin ether to CB2 prepared from COS cells transfected with a plasmid carrying the CB2 gene, following methods previously used by us (3).
Synthetic noladin ether was examined for behavioral effects by using tests frequently used for cannabinoid evaluation (13): the ring immobility (catalepsy) test, which measures the time over 4 min that mice spend motionless on a ring (5.5-cm diameter; ref. 18) and the open-field test, which measures locomotor activity (ambulation). Hypothermia, antinociception (hot-plate latency) and rate of defecation in the open field, a measure of intestinal motility, were assessed also. The results presented in Fig. 5 show that noladin ether has significant effects in all these assays.
1-Alkyl glyceryl ethers, with a large variety of alkyl chains, are well known natural products. They are present in mammals, birds, plants, protozoa, and bacteria. They are particularly wide spread in marine animals. They have been found in chicken egg yolk, human bone marrow and milk, pig spleen, and intestinal fat and liver in rat. For a recent review, see ref. 19; for a more detailed but older review see ref. 20. Some 1-alkyl glyceryl ether-based phospholipids such as platelet-activating factor, for example, have many physiological and pathological attributes (21), and related synthetic ether phospholipids have antineoplastic activity and are investigated widely in oncology (22–24) and against visceral leishmaniasis (25). Reduced levels of 1-alkyl glyceryl ethers have been noted in some peroxisomal diseases such as Zellweger syndrome (26). Surprisingly, very few 2-alkyl glycerol ethers are known as natural products and noladin ether seems to be, to our knowledge, the first such example of a long-chain fatty alcohol moiety forming an ether at the C-2 position of glycerol.
Anandamide and 2-AG do not have the same pharmacological profile (5–10). We expect that the spectrum of activities of noladin ether will differ also from that of the previously identified endocannabinoids. We assume that it will have a narrower profile of activity, because contrary to anandamide and 2-AG, which bind to both CB1 and CB2, noladin ether binds very weakly to CB2. Hence, noladin ether may be of importance in clarifying the CB1-based functions of the endocannabinoid system.
Ethers are generally stable in vivo. In view of the putative stability of noladin ether in comparison to anandamide, an amide, and 2-AG, an ester, which are hydrolysed rapidly in vivo (5), noladin ether may serve as a lead for drug development.
Acknowledgments
We thank the National Institute on Drug Abuse for Grant DA 9789 (to R.M.) and the Israel Science Foundation for a joint grant (to R.M. and Z.V.). R.M. is affiliated with the David R. Bloom Center for Pharmacy at the Hebrew University.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- 2D
two-dimensional
References
- 1.Devane W A, Hanuš L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 2.Hanuš L, Gopher A, Almog S, Mechoulam R. J Med Chem. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski N E, Schatz A R, Gopher A, Almog S, Martin B R, Compton D R, et al. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 4.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 5.Mechoulam R, Fride E, Di Marzo V. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod J, Felder C C. Neurochem Res. 1998;23:575–581. doi: 10.1023/a:1022418217479. [DOI] [PubMed] [Google Scholar]
- 7.Wagner J A, Varga K, Kunos G. J Mol Med. 1999;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- 8.Klein T W, Newton C, Friedman H. Immunol Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli D, Giuffrida A, Calignano A, de Fonseca F R. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 10.Pertwee R G. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- 11.Devane W A, Breuer A, Sheskin T, Järbe T U C, Eisen M S, Mechoulam R. J Med Chem. 1992;35:2065–2069. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Prusoff H. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 13.Fride E, Mechoulam R. Eur J Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- 14.Mechoulam R, Sheskin T, Ben-Shabat S, Fride E. Symposium on the Cannabinoids. Burlington, VT: International Cannabinoid Res. Soc.; 1998. p. 13. [Google Scholar]
- 15.Mechoulam R, Fride E, Ben-Shabat S, Meiri U, Horowitz M. Eur J Pharmacol. 1998;362:R1–R3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. J Biol Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 17.Suhara Y, Takayama H, Nakane S, Miyashita T, Waku K, Sugiura T. Chem Pharm Bull. 2000;48:903–907. doi: 10.1248/cpb.48.903. [DOI] [PubMed] [Google Scholar]
- 18.Pertwee R G. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taguchi H, Armarego W L F. Med Res Rev. 1998;18:43–89. doi: 10.1002/(sici)1098-1128(199801)18:1<43::aid-med3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Snyder F, editor. Ether Lipids. New York: Academic; 1972. [Google Scholar]
- 21.Barnes P J, Page C P, Henson P M, editors. Platelet Activating Factor and Human Disease. Oxford: Blackwell Scientific; 1989. [Google Scholar]
- 22.Terwogt J M M, Mandjes I A M, Sindermann H, Beijnen J H, Huinink WWTB. Br J Cancer. 1999;79:1158–1161. doi: 10.1038/sj.bjc.6690184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkovic D. Gen Pharmacol. 1998;31:511–517. doi: 10.1016/s0306-3623(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 24.Kostantinov S M, Berger M R. Cancer Lett (Shannon, Irel) 1999;144:153–160. doi: 10.1016/s0304-3835(99)00219-0. [DOI] [PubMed] [Google Scholar]
- 25.Herwaldt B L. N Engl J Med. 1999;341:1840–1842. doi: 10.1056/NEJM199912093412411. [DOI] [PubMed] [Google Scholar]
- 26.Poulos A, Bankier A, Beckman K, Johnson D, Robertson E F, Sharp P, Sheffield L, Singh H, Usher S, Wise G. Clin Genet. 1991;39:13–25. doi: 10.1111/j.1399-0004.1991.tb02980.x. [DOI] [PubMed] [Google Scholar]