Abstract
Mycobacterium tuberculosis dosRS two-component regulatory system controls transcription of approximately 50 genes including hspX, acg and Rv2030c, in response to hypoxia and nitric oxide conditions and within macrophages and mice. The hspX lies between acg and Rv2030c. However, the functions of the dosR regulated genes in vitro and in vivo are largely unknown. Previously, we demonstrated that deletion of hspX gene produced a mutant which grew faster in macrophages and in mice. In this study, we attempted to determine the functions of acg and Rv2030c by gene inactivation. We demonstrate that Rv2030c is dispensable for virulence and growth. However, deletion of acg produced a mutant which is attenuated in both resting and activated macrophages and in acute and persistent murine infection models. Surprisingly, deletion of acg did not compromise the viability of the mutant to nitrosative and oxidative stresses in vitro and in vivo. In addition, when the WT and the acg mutants were treated with antibiotics such as the prodrugs nitrofurantoin and nitrofuran, the acg mutant became more sensitive than the WT strain to these drugs. This suggests that Acg may not function as a nitroreductase. These data indicate that acg encodes an essential virulence factor for M. tuberculosis and enables it to grow and survive in macrophages and in mouse organs.
Introduction
Tuberculosis (TB) remains one of the major infectious diseases, causing 8% of all deaths worldwide [1]. Currently, over two billion people are infected with the causative agent, Mycobacterium tuberculosis. Once infected, an individual becomes a life-long carrier, with a 5–10% lifetime risk of contracting acute disease [2], [3]. A major biological question is the mechanisms by which M. tuberculosis controls its virulence. Clearly, a latent state plays an important role in which it needs to be non-virulent and non-transmissible.
In experimental models, the latent state is thought to be regulated by hypoxia [4] whose response in the bacterium is controlled by the dosRS two-component regulatory system (2CR) [5], [6]. The dosRS system controls the transcription of about 50 genes under hypoxic conditions and in response to nitric oxide [5], [7]. Recent work demonstrated that the dosR regulon is regulated by carbon monoxide which is produced by M. tuberculosis infected macrophages and other in vitro stress conditions [8], [9], [10]. Also, many genes in the dosR regulon show increased expression in murine macrophages and in murine lung tissues [9], [11], [12] and these genes may be involved in survival and persistence of the bacterium in vivo.
Most of the genes controlled by dosR have unknown or predicted functions. For example, narX [13], is predicted to encode a nitrate reductase which may help the bacterium to adapt to hypoxia. Protection against nitrogen stress is predicted [13] to be mediated by the nitroreductase genes acg (Rv2032), Rv3127 and Rv3131. It is thought that hspX [14], [15] may be involved in latency. A recent study using bioinformatic analysis showed that the dosR regulated genes may be involved in carbohydrate and fatty acid metabolism [16]. It is important to investigate if the genes within the dosRS regulon exhibit similar or diverse functions in vivo as this will help us to understand the biological relevance of the class of the genes and their roles in survival and persistence of the organism in human infection. Previously, studies using high density mutagenesis showed that most of the dosR regulated genes were not essential for growth [17]. In the past, with the aim of dissecting the potential mechanism which underlies the dosR regulatory system, dosR has been inactivated in M. tuberculosis. Counter intuitively, this produced a mutant that was hypervirulent in activated macrophages and in murine tuberculosis [18]. We made an unmarked hspX deletion mutant of M. tuberculosis [19] which showed faster growth in macrophages and in mice. This was also confirmed by another study of hspX gene knock out [20]. However, later studies produced dosR deletion mutants which either showed an attenuated phenotype in guinea pigs, mice and rabbits [21] or had no growth deficit in mice [22]. This raised the question as to the in vivo functions of the genes adjacent to the hspX gene. It has been shown previously that a hspX mutant in which hspX was replaced by a hygromycin-resistance gene [14] was attenuated in a macrophage model, suggesting that it is required for virulence. We hypothesised the reason for the contradictory findings between the hygromycin-resistance hspX gene deletion [14] and our unmarked deletion [19] was that the hygromycin-resistance gene deletion mutant had alterations in the genes which are immediately adjacent to hspX, namely acg which lies upstream, and Rv2030c which is downstream (see Fig. 1). The hspX and acg promoters which express divergently share the intergenic region [13], [23]. Interruption of this intergenic region may affect expression of both genes [14]. It is particularly interesting to investigate the function of the acg gene which has been suggested to encode a putative classical nitroreductase [13], [24]. acg is one of the most upregulated genes in the dosR regulon. The expression of acg was found to be coregulated with that of the hspX gene under low O2 conditions, within macrophages, especially activated macrophages and in mice [11], [13]. It has been suggested that acg might play an important role in M. tuberculosis detoxification of nitrogen intermediates [13]. The downstream gene Rv2030c of unknown function is co-transcribed with hspX [15].
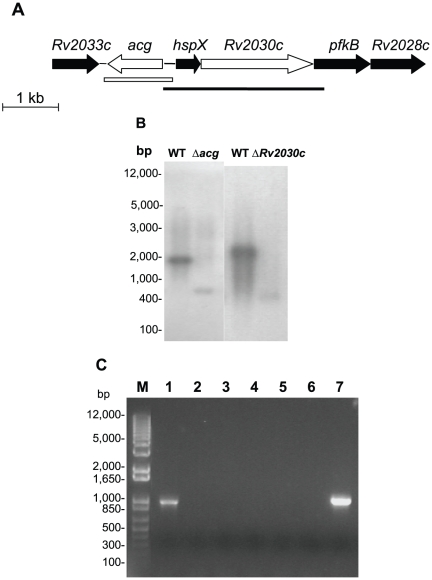
Figure 1. Construction of the M. tuberculosis acg and Rv2030c mutants.
A. Genomic context of the genes upstream and downstream of hspX gene. The open arrows indicate the acg or Rv2030c gene which was deleted in the mutant. The open box and the line show the location of the fragments used for complementation of YHΔacg or YHRv2030c, respectively. These were also used as probes for southern blotting analysis. B. Southern blotting analysis to confirm the deletion of the genes. DNA from WT and YHΔacg or YHRv2030c was digested with Pvu II and Btr I, respectively and hybridised with the probes which used to make the complemented constructs. bp, molecular weight marker (Invitrogen). The experiments were repeated twice, with identical results. C. Detection of acg mRNA in WT and YHΔacg by RT-PCR. RNA was extracted from 7 day-old cultures. M. molecular weight marker (Invitrogen). 1. RNA extracted from the WT strain, 2. DNase I-treated RNA isolated from the WT culture but no RT enzyme. 3. PCR negative control with each primer and water instead of template. 4. RNA extracted from YHΔacg. 5. DNase I-treated RNA isolated from the acg mutant culture but no RT enzyme. 6. PCR negative control with each primer and water instead of template. 7. PCR positive control with each primer and M. tuberculosis WT DNA.
In this paper, we deleted acg and Rv2030c. Deletion of acg led to an attenuated mutant in macrophage infection and in vivo acute and persistent murine models. The M. tuberculosis mutant lacking the Rv2030c gene was identical to the parent strain. This suggests that the hspX and its adjacent genes although regulated by DosR are functionally distinct.
Results
Construction of acg and Rv2030c mutants of M. tuberculosis
Previously, we showed that deletion of hspX results in rapid growth of the organism in vivo. As seen in Fig. 1A, hspX is the first gene of a possible operon with several downstream genes RV2030c, pfkB and Rv2028c. The intergenic region between each gene only contains a few base pairs of nucleotides. Upstream of hspX is acg which translates in the opposite direction. In order to verify the essentiality of the genes adjacent to hspX in vivo, we made two M. tuberculosis mutants which deleted acg or Rv2030c using a two-step mutagenesis strategy [25]. The generation of the mutant strain was confirmed by PCR (data not shown) and Southern blotting analysis (Fig. 1B). The expression of acg gene (Fig. 1C) and Rv2030c (data not shown) was also confirmed by RT-PCR. The M. tuberculosis strains lacking these genes were defined as YHΔacg and YHΔRv2030c, respectively. Both sequences of the PCR products amplified from WT and the mutants were analysed by DNA sequencing to confirm the deletion of these two genes.
The deletion of acg or Rv2030c was complemented by the introduction of the integrating plasmid pUC-Gm-Int containing the native gene and promoter into the mutant. This suicide plasmid contains a gene which encodes the integrase of phage L5, the attP site and a gentamicin resistant marker cassette. The gene integration into the chromosome of the mutant was selected for gentamicin resistance. The complemented strains were called YHacgComp and YHRv2030cComp.
Growth and survival of YHΔacg and YHΔRv2030c in macrophages
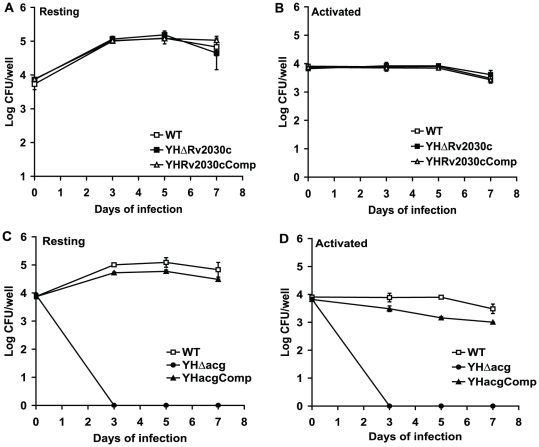
We examined the ability of both YHΔacg and YHΔRv2030c to infect and proliferate within bone marrow derived macrophages from BALB/c mice. As shown in Fig. 2A and 2B, the growth of WT and YHΔRv2030c in both resting and activated macrophages was similar over the 7 days of infection. These data indicate that the loss of Rv2030c has no detectable effect on intracellular survival and proliferation. Although YHΔacg was able to invade macrophages as seen in Fig. 2C and 2D showing similar CFU counts to the WT strain recovered from the macrophages at time 0, the mutant was unable to grow and survive in both resting and activated macrophages. After three days of infection, the entire macrophage lysates (1 ml/well) were plated on 7H11 agar. No CFU counts of YHΔacg were recovered. Similar profiles of YHΔacg were seen in the macrophage-like cell line J774A.1 (data not shown). These results indicated that acg is essential for intracellular survival.
Figure 2. Growth and survival of M. tuberculosis YHΔacg and YHΔRv2030c in macrophages.
Infection of YHΔRv2030c in resting bone marrow derived macrophages (A) and in IFNγ activated bone marrow derived macrophages (B). Infection of YHΔacg in resting bone marrow derived macrophages (C) and in IFNγ activated bone marrow derived macrophages (D). These results are the means and standard deviation derived from one representative of three independent experiments.
As the acg gene has been predicted to encode a nitroreductase in M. tuberculosis [13], deletion of the gene might render the bacilli sensitive to macrophage nitrogen intermediates. In order to confirm this, we infected macrophages derived from iNOS knock out mice with M. tuberculosis WT, YHΔacg and the complemented strains. These macrophages produce reduced levels of nitric oxide [26]. If acg was a nitroreductase, the growth of the mutant would be restored to its parental level or to a certain extent to the parental level. However, the YHΔacg mutant failed to grow in macrophages derived from the iNOS KO mice (Fig. S1) showing the similar survival patterns in the macrophages derived from WT mice (Fig. 2C and 2D).
Growth and survival of YHΔacg and YHΔRv2030c in mice
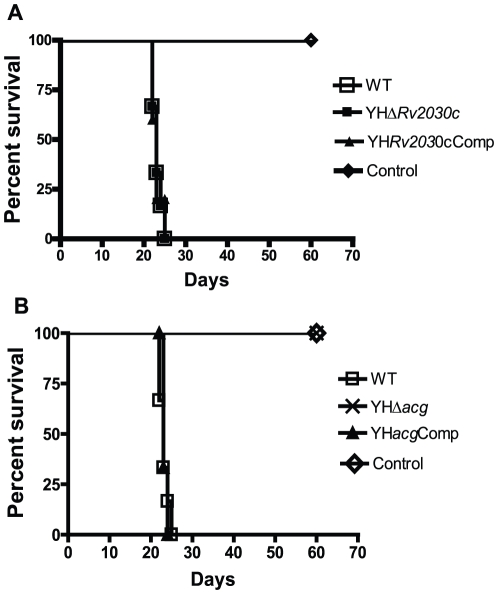
In order to verify if the mutants changed their virulence in acute infection in the absence of host acquired immunity, virulence assays of the M. tuberculosis strains in immunocompromised SCID mice were carried out as described previously [27]. The mice were infected intravenously with 106 CFU counts of each bacterial strain, and the survival of the SCID mice was observed over 60 days. As shown in Fig. 3A, the mice infected with WT and YHΔRv2030c survived for 23 and 22.5 days, respectively. In contrast, all mice infected with YHΔacg, like the uninfected control mice, survived for 60 days at which point the experiments were terminated (Fig. 3B). The attenuation of YHΔacg strain was reversed in the acg complemented strain (Fig. 3B).
Figure 3. Virulence of M. tuberculosis strains in SCID mice.
A. WT, YHΔRv2030c and complemented strain. B. WT, YHΔacg, and complemented strain. Mice (n = 6) were infected intravenously with 106 CFU counts of M. tuberculosis H37Rv strains. Survival of SCID mice was observed for 60 days. The results were repeated once with reproducible results. There were significant differences between WT and YHΔacg infected mice (P<0.0001).
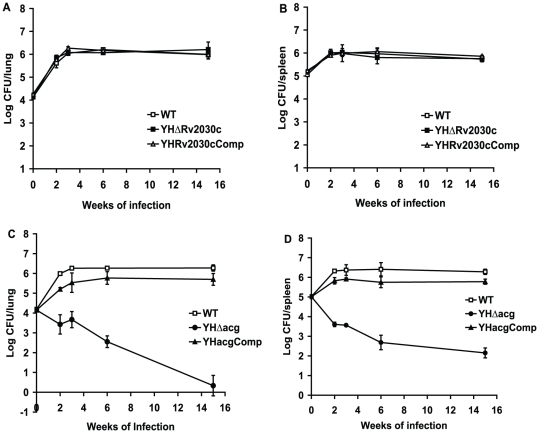
In order to examine the growth and survival of the mutants in the face of host acquired immunity, BALB/c mice were intravenously infected with 1.2×104 CFU counts of the WT and the mutant strains. As shown in Figure 4, typical growth of the WT M. tuberculosis H37Rv in the immunocompetent mice was seen. There was exponential growth for approximately three weeks, the CFU counts of the WT reached 106 CFU/lung, followed by a plateau as the acquired immune response inhibited the bacterial growth. The growth of YHΔRv2030c was similar to that of WT strain (Fig. 4A and 4B). However, after infection with YHΔacg mutant, no growth of the mutant was seen, the CFU counts of the mutant gradually decreased in both lungs and spleens (Fig. 4C and 4D) throughout the course of infection. Almost no acg mutant cells were recovered from the infected lungs at 15 weeks of infection which was confirmed by plating the entire lung homogenate on 7H11 agar. Growth characteristics which were similar to WT were seen in the acg complemented strain. These data indicate that YHΔacg is attenuated.
Figure 4. Growth and survival of YHΔacg and YHΔRv2030c in the lungs and spleen of BALBc mice.
Mice were infected with 104 CFU counts of each strain. At different time points the infected mice were sacrificed and the numbers of bacteria in the lungs and spleens were measured. CFU counts in lungs (A) and spleens (B) in mice infected with WT, YHΔRv2030c and complemented strain. CFU counts in lungs (C) and spleens (D) in mice infected with WT, YHΔacg and complemented strain. The reported values represent the average and the standard deviation obtained for each point from three mice. The experiments have been repeated twice with similar CFU counts in lungs and spleens.
The acg mutant stimulated less inflammatory response
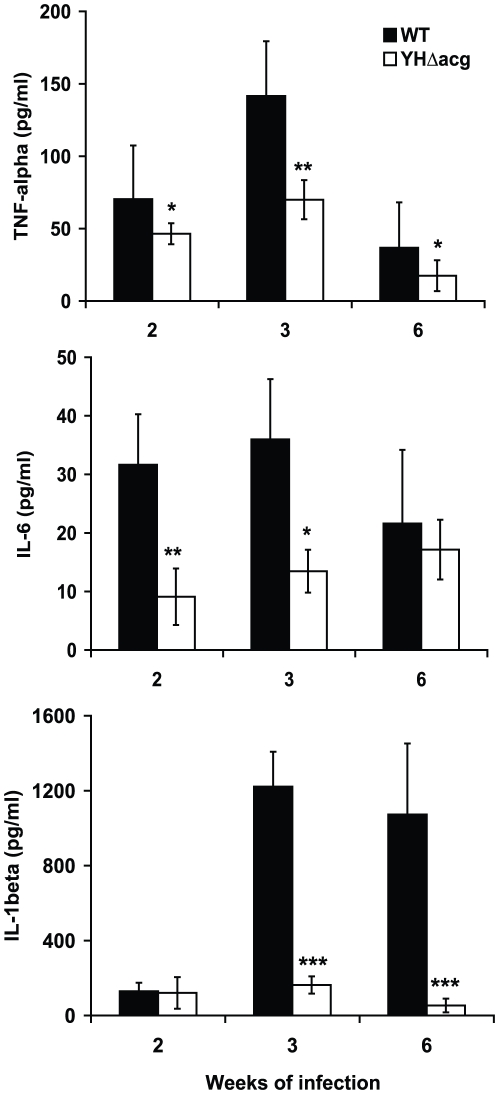
To examine if the mice infected with YHΔacg mutant produced stronger inflammatory response than those infected with the WT strain, which might inhibit the growth of the mutant in the mouse organs, we examined the production of the proinflammatory cytokines TNF-α, IL-6, and IL-1β in the lungs of mice infected with the WT and YHΔacg strains for 2, 3 and 6 weeks by ELISA. As shown in Fig. 5, the mutant stimulated less cytokine production in the host lungs, especially at 2 and 3 weeks of infection for both TNF-α and IL6 and at 3 and 6 weeks for IL-1β.
Figure 5. TNF-α, IL-6 and IL-1β levels in BALB/c mice infected with WT and YHΔacg measured by ELISA.
The data shown are the averages for the lungs from three mice ± standard errors. Statistical significance was determined by Student's t test (n = 3) (***, P<0.001).
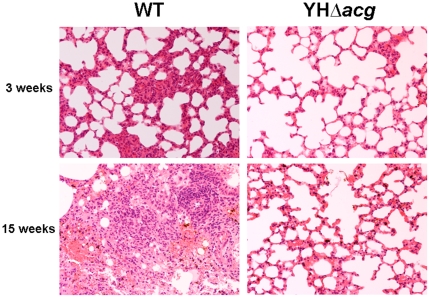
The histopathological changes of mice infected with WT and mutant strains were also examined. As shown in Fig. 6, the WT infected mice developed alveolar consolidation at 3 weeks and this was accompanied by the presence of granulomatous inflammation containing lymphoplasmacytic cells, alveolar macrophages, neutrophils, and multinucleated cells at 15 weeks. In contrast, the acg mutant infected lungs showed normal lung structure with the alveolar space preserved throughout the lung, indicating that the reduced host immune response and the lack of histopathological changes were due to insufficient bacilli present in the host organs.
Figure 6. Hematoxylin- and eosin-stained sections of lungs in mice infected with WT and YHΔacg for 3 and 15 weeks.
Histopathological examination was carried out using three mice in each group. Two sections from each mouse were examined. The data shown are representative of lung sections from three animals in each experimental group.
Response to in vitro stress
The WT, YHΔacg and YHΔRv2030c were grown in 7H9 broth without disturbance for 60 days as described previously [28]. Under these conditions, the bacilli grow in the top layer of the unagitated culture, where oxygen is available. Then they settle to the bottom of the container, where there is a low concentration of oxygen, and slowly adapt to microaerophilic and eventually to anaerobic conditions. Growth characteristics of the mutants were similar to those of the wild-type strain (data not shown), indicating that acg and Rv2030c genes are not required for the bacterium to grow under in vitro hypoxic conditions.
In order to investigate if stress conditions, especially those which M. tuberculosis encounters in macrophages, affect the mutants, WT, YHΔacg, YHΔRv2030c and complemented strains were exposed to hydrogen peroxide (H2O2), nitric oxide (NO) and acidic conditions. Survival of the strains was examined by CFU counting. No increased susceptibility to NO, H2O2 and acidic conditions was observed in these mutants in comparison to the WT strain (Fig. S2).
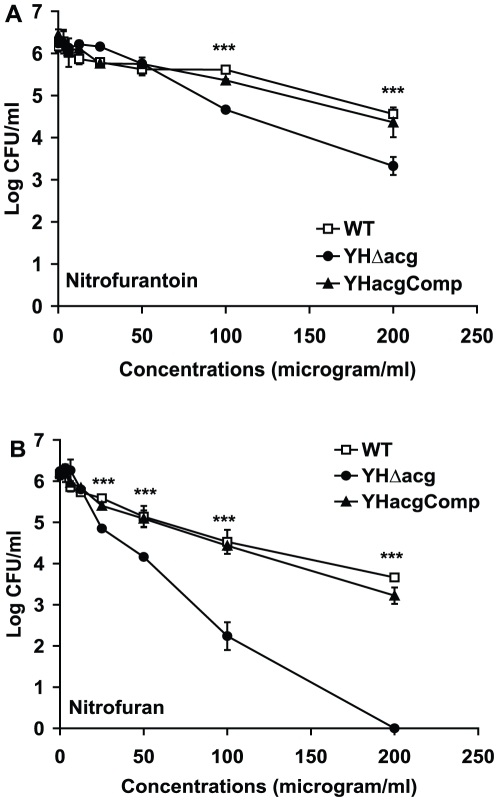
In order to investigate if the acg gene encoded a nitroreductase, the WT, YHΔacg and the complemented strains were incubated with the antibiotics such as prodrugs nitrofurantoin and nitrofuran, each containing a nitro group in its furan ring. The bactericidal activation of these drugs needs the NAD(P)H dependent nitroreductase. The bactericidal activities of these drugs were examined by CFU counting after two days of incubation with 7 days old cultures. As shown in Fig. 7A, the WT strain was relatively tolerant to nitrofurantoin. At 200 µg/ml, about 1.6 log kill was seen. However, YHΔacg became more sensitive to it than the WT strain; there was about 3 log kill at 200 µg/ml. The mutant was exceptionally sensitive to nitrofuran causing a 6 log kill at 200 µg/ml and a 4 log kill at 100 µg/m (Fig. 7B). The CFU counts of the WT strain were reduced to 2.5 logs and 1.6 logs at 200 and 100 µg/ml, respectively (Fig. 7B). This suggested that Acg protein might not have nitroreductase activity to reduce the nitro group of the drugs; otherwise deletion of the gene should render the bacteria more resistant to the drugs.
Figure 7. Survival of YHΔacg in response to nitrofuran drugs.
The WT, YHΔacg and the complemented strains were treated with nitrofurantoin (A) and nitrofuran (B) at different concentrations for 48 hours. These results are the means and standard deviation derived from one representative of three independent experiments. Statistical significance was determined by Student's t test (n = 3) (***, P<0.001).
Nitroreductase activity of YHΔacg
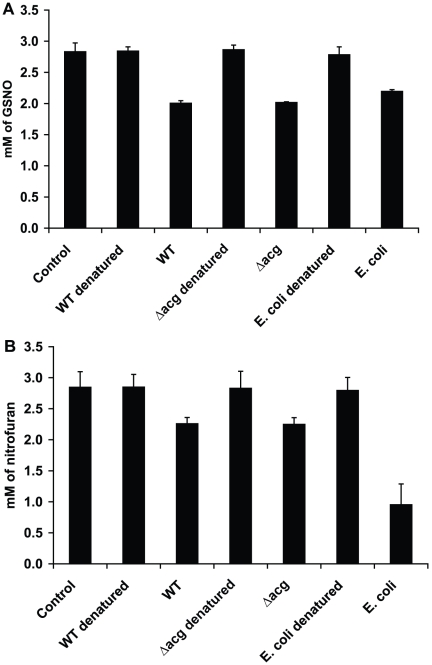
In order to investigate if the acg mutant contained a nitroreductase activity, the nitroreductase activities of the WT and YHΔacg lysates were measured using GSNO or nitrofuranoin or nitrofuran as a substrate. The Acg protein might be contained in the cell lysate as expression of recombinant M. tuberculosis Acg in E. coli produced a soluble protein (communication with Nicolas Keep). As seen in Fig. 8A, addition of WT and YHΔacg lysates significantly reduced the level of GSNO. However, there is no significant difference between WT and YHΔacg in term of nitroreductase activity (p>0.5). The reduction of GSNO was confirmed by using E. coli cell lysate. No nitroreductase activities were observed using denatured cell lysates from the WT, YHΔacg and E. coli. As seen in Fig. 8B, there was a reduction of the drug followed by addition of WT, YHΔacg and E. coli proteins. Again, there was no difference between the WT strain and the mutant in reduction of nitrofuran. The same results were observed using nitrofurantoin as a substrate (data not shown).
Figure 8. Measurements of nitroreductase activities in YHΔacg using GSNO (A) or nitrofuran (B) as a substrate.
Presence of nitroreductase activities was measured by the reduction of the substrate using the bacterial cell lysates at 24 hours for GSNO and 48 hours for nitrofuran. The cell lysate extracted from E. coli was added as a positive control. The denatured cell lystaes which were produced by boiling the cell lysates at 100°C for 10 minutes were included as the negative control. The experiments were repeated twice with the similar results.
Discussion
M. tuberculosis virulence is measured by the ability of the bacterium to invade, grow and persist in macrophages. This infection causes host tissue damage which is characterised by the formation of a compact cluster of cells, called a granuloma, around the foci of infection. Here we demonstrate that the deletion of acg yields an attenuated mutant which fails to grow and persist in macrophages and in immune deficient and immune competent mice. It also showed a deficit in causing tuberculosis granulomatous disease. These data demonstrate that acg is an essential factor for M. tuberculosis to establish growth in the host cells. Deletion of Rv2030c was dispensable to M. tuberculosis virulence and persistence in macrophages and in mice.
The hspX, acg and Rv2030c belong to the DosR regulated gene family. Although it has been suggested that DosR is a dormancy survival regulator [6], [23], [29], this supposition is only based upon gene expression under a variety of different stress conditions, in macrophages and in mice. Only a few reports [19] have demonstrated discrete gene functions, for example hspX, within the dosR regulon using allelic exchange mutants in animal infection models. The differential expression patterns of hspX under stress conditions and in vivo may be required to slow the growth of M. tuberculosis in order to retain a non-replicating state in latency. This is consistent with the putative function of the dosRS regulon. However, the phenotype of the acg mutant found in this study suggests that acg does not seem to fit into the dormancy associated role of the dosR regulon. It is associated with full virulence of M. tuberculosis and is required by the bacilli to sustain infection, but not to initiate the infection process because it was able to invade macrophages but unable to grow. Rv2030c is directly regulated by dosR and is co-transcribed with hspX. Surprisingly, deletion of Rv2030c has no effect on M. tuberculosis growth and persistence in macrophages and in acute and persistent animal models. Recent work suggested that M. tuberculosis persistence modulated by dosR was regulated by a complex multilayer regulatory network [29]. This was strongly evidenced by the fact that deletion of hspX and acg resulted in contradicting effects. There is significant discrepancy between dosR regulated persistence and the bacterial virulence in mice [22]. This was shown by the generation of a dosR mutant which survived normally in mice although the dosR regulon was highly upregulated in vivo [22]. Additional evidence which supports this hypothesis comes from the data that suggests dosR only controls the initial hypoxic response, whilst enduring hypoxic responses are independent of dosR regulation [22]. These results, together with our finding suggest that dosR may regulate functionally diverse genes; some are involved in M. tuberculosis persistence and others in M. tuberculosis virulence or in unknown physiological and pathological roles. In addition, these findings illustrate that information obtained by genome-wide analysis only gives clues as to the function of genes. Discrepancies between gene function and gene expression patterns can only be resolved by other techniques such as gene inactivation.
How does acg contribute in M. tuberculosis growth and survival in vivo? As described previously [13], [24], sequence homology analysis suggests that acg belongs to a nitroreductase family which consists of oxygen-insensitive flavin-dependent enzymes that reduce nitrosubstituted compounds such as nitroaromatics quinones, and riboflavin derivatives to either a hydroxylamino- or aminoaromatic endproduct in a FMN-, FAD-dependent or NAD(P)H dependent manner [30]. As acg is upregulated in response to NO, in macrophages [13] and in mice [11], it has been predicted that the product of acg may be responsible for M. tuberculosis detoxification of nitroaromatic compounds which may be present within macrophages or granulomas [13]. Our results indicated that the acg mutant was not able to grow and survive in macrophages which produce reactive oxygen and nitrogen intermediates, acidic conditions and lytic enzymes. However, unlike other M. tuberculosis mutants which lack the oxidative and nitrosative detoxification genes such as ahpC [31] or tpX [26], deletion of acg did not compromise the bacterium to grow and survive in acidic, nitric oxide and hydrogen peroxide environments in vitro. Furthermore, the growth and survival of the mutant was not restored in macrophages isolated from iNOS knockout mice. This was confirmed by the fact that acg mutant contained the same level of nitroreductase activity to reduce GSNO as the wild type (Fig. 8). It is not known if the acg gene product possesses a nitroreductase activity as we are not able to detect it using in vitro methods or using iNOS deficient mice. Further work is underway in our laboratory to study the enzymatic activity of the recombinant Acg protein to metabolise nitroaromatic compounds. Surprisingly, YHΔacg was more sensitive than the WT strains to nitrofurantoin and especially nitrofuran. Nitrofurans are prodrugs which exhibit antimicrobial activity against Escherichia coli and Staphylococcus saprophyticus as well as other facultative anaerobes, anaerobic bacterial and protozoan species [32], [33]. The antimicrobial activities of these prodrugs are largely dependent on the rapid reduction of the 5-nitro groups by a wide range of enzymes including the NAD(P)H nitroreductases produced by these bacteria, which leads to accumulation of redox active intermediates, including hydroxylamine adducts which cause lethality to the bacterial cells. Nitrofurantoin has a relatively high MIC against M. bovis BCG (12 µg/ml) [34] and M. tuberculosis H37Rv (49.5 µg/ml) [35]. M. tuberculosis is relatively tolerant to nitrofurantoin and nitrofuran showing 1.6 and 2.5 log killing at 200 µg/ml, respectively (Fig. 7). We showed that both the WT and YHΔacg exhibited low activities to reduce nitrofuran drugs (Fig. 8). However, YHΔacg became more sensitive to the drugs. This indicated that the nitrofuran drugs might function in unknown mechanisms in M. tuberculosis which differed from the activation of the drugs by reduction of the nitro-group. Further investigation is underway in our laboratory.
In summary, this work demonstrates that one of the M. tuberculosis dosR regulated genes, acg, is associated with M. tuberculosis virulence. The acg mutant is unable to grow and survive in macrophages and mice. This attenuation is not associated with a deficiency in response to oxidative and nitrosative stresses. Our data suggest that DosR may control an array of genes which shows functional diversity in vitro, in macrophages and in mouse infections. We have identified a key target against which new anti-TB drugs could be designed, or with which a live vaccine could be developed to prevent M. tuberculosis infection.
Materials and Methods
Ethics Statement
All animal experiments were conducted according to the Animals Scientific Procedures Act, 1986 (an Act of the Parliament of the United Kingdom 1986 c. 14) (Home Office Project licence Number 70/5933) with approval from St George's, University of London ethics committee. The animal husbandry guidelines and animal procedure for this study were followed according to the Animals Scientific Procedures Act, 1986. The sacrifice of the mice was performed in accordance with humane end point protocols under the Animals Scientific Procedures Act, 1986.
Bacterial strains and growth conditions
M. tuberculosis strain H37Rv was used as a parental strain to construct the mutants. M. tuberculosis strains were grown in 7H9 medium supplemented with 10% albumin dextrose complex (ADC, Becton Dickinson) and 0.05% Tween 80, or on 7H11 agar medium supplemented with oleic albumin dextrose complex (OADC, Becton Dickinson). E. coli XL2 was used as a host strain for cloning and plasmid propagation in or on liquid and solid Luria-Bertani medium. Antibiotics were used as follows: ampicillin (Sigma) 100 µg/ml, kanamycin (Sigma) 20 µg/ml, gentamicin (Sigma) 20 µg/ml and hygromycin (Invitrgen) 100 µg/ml.
Mutant construction
The mutant construction was carried out in a 2-step strategy using the pNIL/pGOAL plasmids as described previously [25]. DNA manipulations including DNA isolation, ethanol precipitation of DNA, electrophoresis of DNA in agarose and transformation were performed by standard procedures [36]. Enzyme reactions were performed according to the manufacturer's instructions (Invitrogene and New England Biolabs). To construct M. tuberculosis acg deletion, a 2949 bp PCR product containing the acg gene (996 bp) and about 1 kb flanking sequences adjacent to each end of the gene was amplified using M. tuberculosis H37Rv genomic DNA as template and primers acg1 (5′- AATAAGCTTAGCAGTTCCCGACCCTCAC-3′) and acg2 (5′- AATAAGCTTCGCTATGGAACCGACGTGC -3′). The PCR product was purified from agarose gel using a QIAquick Gel Extraction Kit (Qiagen) and was cloned into the Hind III site of pGEM3Z (Promega) to form pGEMacg. A Nco I fragment of 777 bp in the acg coding region was deleted. The disrupted acg gene with the flanking sequences was cloned into the Hind III site of p2NIL [25] to make p2NILacg1. A hyg-sacB marker cassette from the pGOAL 19 [25] was cloned into the Pac I site of p2NILacg1 to form the final mutant construct p2NILacg2. To construct M. tuberculosis Rv2030c deletion, a 4129 bp PCR product containing the Rv2030c gene and 1 kb flanking sequences adjacent to each end of the gene was amplified using M. tuberculosis genomic DNA as template and primers Rv2030c1 (5′- AATAAGCTTCGACTCCACCAAGTCCCAA -3′) and Rv2030c2 (5′- AATAAGCTTGGCTTCCGGGTTAACGATC -3′). The PCR product was cloned into the Hind III site of pGEM3Z (Promega) to form pGEMRv2030c. The Rv2030c gene was deleted by PCR with primers Rv2030c3 5′- ATTACGCGTAAACCTACCCGACCGGTCT-3′and Rv2030c4 (5′- ATTACGCGTACGGACCCAGTGGTCAG>TT-3′) which were designed outwardly starting from the start and stop codons of the Rv2030c gene using pGEMRv2030c as a template. The PCR product which contains only the flanking sequences of Rv2030c gene was cut with Mlu I and ligated to form pGEMRv2030cΔ. The Rv2030c flanking sequences containing no Rv2030c gene was cloned into the Hind III site of p2NIL [25] to form p2NILRv2030cΔ1. Finally, a hyg-sacB marker cassette from the pGOAL 19 [25] was cloned into the Pac I site of p2NILRv2030cΔ to form the final mutant construct p2NILRv2030cΔ2. Plasmid DNA was isolated with a QIAprep Spin Miniprep Kit (Qiagen). The sequences of purified PCR products were determined commercially (QIAGEN). Both DNA strands were sequenced by using the two primers that were used to generate each PCR product. The plasmids p2NILacg2 and p2NILRv2030cΔ2 were electroporated into M. tuberculosis H37Rv cells, respectively, and the selection of the mutants was performed as described previously [25], [37].
To complement the acg deletion, a 1395 bp DNA fragment containing the acg gene and 324 bp of upstream sequence was amplified by PCR using primers acgC1 5′- AATAAGCTTTCCAGCCGCATCAACCGGGT-3′) and acgC2 (5′- AATAAGCTTTCGCCGGATCCGCTCATCGA -3′). The PCR product was cloned into the Hind III sites of the integrating plasmid pUC-Gm-Int [38]. For complementation of Rv2030c mutant, a 2796 bp PCR product amplified using primer Rv2030c5 (5′- ATTTCTAGAGTCACCATGGTGTCCGGCAT-3′) and Rv2030c6 (5′-ATTTCTAGATCCAAGGCGGGGTTCATGGT-3′) containing 216 bp of the upstream sequence of hspX gene, hspX gene and Rv2030c was cloned into the Hind III sites of pUC-GM-Int. The constructs of complementation were electroporated into YHΔacg and YHΔRv2030c mutants, respectively, and GmR transformants were selected.
Estimation of viability under in vitro stress conditions
M. tuberculosis strains were grown in 7H9 medium containing 0.05% Tween 80 supplemented with 10% ADC for 10–15 days. A series of 10 ml standing cultures were used for the determination of CFU counts after exposure to stress. For oxidative stress, hydrogen peroxide (H2O2) (5 mM and 10 mM) was added to the cultures. For nitric oxide (NO) stress, diethylenetriamine/nitric oxide adduct (DETA/NO) and S-Nitrosoglutathione (GSNO) were added to the cultures at the final concentrations of 5 and 10 mM, and the cultures were incubated at 37°C. For treatment with drugs containing a nitro group, nitrofurantoin and 2-nitrofuran were incubated with cultures at the final concentrations of 200, 100, 50, 25, 12.5, 6.25, 3.125 and 0 µg/ml. For acidic stress, the culture medium was replaced with acidic 7H9 medium (pH 4). CFU counts of the treated cultures and the non-treated cultures were determined at 0 and 7 days (acidic conditions), at 0 and 24 hours (H2O2, DETA/NO and GSNO) or 0 and 48 hours (nitrofurantoin and nitrofuran). Each stress treatment was carried out in triplicate.
Nitroreductase assay
Nitroreductase activity was measured using cell lysates of WT, YHΔacg and E. coli K12 with GSNO, 2-nitrofuran or nitrofurantoin as a substrate. M. tuberculosis strains were grown in 7H9 medium containing 0.05% Tween 80 supplemented with 10% ADC without disturbance for 7 days. E. coli was grown in nutrient broth overnight at 37°C with constant shaking at 120 rpm. Bacterial numbers of the cultures were determined by optical density reading at 600 nm and CFU counts. The cultures were washed three times in chilled 50 mM Tris-HCI (pH 7.5) and were then resuspended in the same buffer. The cell suspension was transferred into 2 ml tubes each containing 75 to 150 µm glass beads and lysed by homogenisation using a FastPrep Instrument (Fisher Scientific) for 40 seconds at 6.5 speed. The cell debris was removed by centrifugation at 13,000 rpm for 20 minutes at 4°C followed by filtration. Total protein concentrations of the cell lysates were determined with the Bio-Rad protein assay using bovine serum albumin as a standard. The reduction of GSNO or nitrofuran drugs was determined by a decrease in the absorbance at 335 nm for GSNO (molar extinction coefficient 586 M−1cm−1) [39], at 373 nm for nitrofurantoin (molar extinction coefficient of 2.63×104 M−1cm−1) [40] and at 340 nm for 2-nitrofuran. The reaction mixture (1 ml) consisted of enzyme preparation, 50 mM Tris-HCl (pH 7.0), 5 mM GSNO or 0.5 mM nitrofurantoin and 2-nitrofuran, and 0.1 mM NADPH. Equal amounts of the WT and the mutant proteins were used in each preparation.
Mouse infection models
BALB/c mice (6 to 8 weeks old, female, body weight 18–20 g) were used (Harlan UK Ltd). M. tuberculosis cells were resusupended in phosphate-buffered saline (PBS) and were intravenously injected into the mouse with 1.2×104 CFU of the bacterial cells. At different time points, spleens and lungs from 4 mice were removed rapidly after sacrifice and a sterile autopsy was performed. The organs were transferred into 2 ml tubes each containing 1 ml sterile distilled water and 2 mm diameter glass beads. Lungs and spleens of the mice were homogenised using a reciprocal shaker (Thermo Hybaid Ltd) for 40 seconds at 6.5 speed. CFU counts from each lung and spleen were performed using serial dilutions of the homogenates.
Virulence assays of the M. tuberculosis strains with SCID mice (6 to 8 weeks old, female, Harlan UK Ltd) were carried out as described previously [27]. 9 mice in each group were intravenously infected with 106 CFU of the WT, mutant and the complemented strains. After 4 hours of infection, three mice in each group were sacrificed and CFU counts in lungs and spleens were estimated. The remaining 6 infected mice were observed for 60 days and the death time for each group was recorded. Median survival times were calculated for each group, and statistical analysis was performed using the log rank tests of survival.
Macrophage infection
Bone marrow-derived macrophages were isolated from BALB/c, C57BL/6 and the iNOS KO (Inducible Nitric Oxide Synthase Knockout) mice and cultured for 7 days as described previously [27]. Also macrophage J774A.1 cell line was used. Adherent macrophages were harvested and seeded at 2×105 cells per well of 24 well plates in Dulbecco's Modified Eagle Medium (Invitrogen) without L-cell conditioned medium, penicillin and streptomycin. Activation of macrophage cells was carried out by the addition of interferon-γ (IFN-γ, 100 U/ml. R&D Systems) for 24 h, followed by the addition of lipopolysaccharides (LPS, 200 ng/ml, Sigma) for 3 h. The cells were infected with the bacterial strains at a multiplicity of infection of 1∶1 for 4 hours, then washed 6 times with warm Hank's Buffered Salt Solution (HBSS). At 0, 3, 5 and 7 days after infection, the cells were washed and lysed with 0.1% Triton-X 100, and CFU counts were performed. At each time point, the infected macrophages in two wells were individually harvested using Trypsin-EDTA and stained for acid-fast bacilli (AFB) in order to check macrophage viability. The experiments were carried out three times in triplicate.
Supporting Information
Growth and survival of YHΔ acg in resting and IFN-γ-activated macrophages derived from iNOS knockout mice. These results are the means and standard deviation derived from one representative of three independent experiments.
(EPS)
Survival of YHΔ acg and YHΔ Rv2030c in response to DETA/NO at 5 and 10 mM (A), H2O2 at 5 and 10 mM (B), and under acidic condition (C). The data shown is a representative of three independent experiments. Data are represented as mean ± SD of triplicate tests.
(EPS)
Acknowledgments
We thank Neil Stoker, Department of Pathology and Infectious Diseases, The Royal Veterinary College, London, for providing the mutagenesis plasmids and helpful discussions, and Adrian Hobbs, Department of Pharmacology, University College London, for providing the iNOS KO mice. We thank Nicolas Keep and Francois-Xavier Chauviac, Institute for Structural and Molecular Biology, Department of Biological Sciences, Birkbeck, University of London, for helpful discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by both the European Community's Sixth Framework Programme to the project NEWTBDRUGS and the European Community's Seventh Framework Programme to the project StopLATENT-TB. The publication reflects only the authors' views. The European Community is not liable for any use that may be made of the information herein. The financial support of the Burton Medical Trust is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Geneva: World Health Organization.; 2008. Global tuberculosis control : surveillance, planning, financing. viii, 294 p. [Google Scholar]
- 2.Flynn JL, Chan J. What's good for the host is good for the bug. Trends Microbiol. 2005;13:98–102. doi: 10.1016/j.tim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 4.Wayne LG. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 5.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, et al. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha -crystallin. Proc Natl Acad Sci U S A. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voskuil MI, Visconti KC Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 2004;84:218–227. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall SL, Movahedzadeh F, Rison SC, Wernisch L, Parish T, et al. The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis (Edinb) 2004;84:247–255. doi: 10.1016/j.tube.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, et al. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci U S A. 2003;100:241–246. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purkayastha A, McCue LA, McDonough KA. Identification of a Mycobacterium tuberculosis putative classical nitroreductase gene whose expression is coregulated with that of the acr aene within macrophages, in standing versus shaking cultures, and under low oxygen conditions. Infect Immun. 2002;70:1518–1529. doi: 10.1128/IAI.70.3.1518-1529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, et al. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci U S A. 1998;95:9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Coates AR. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J Bacteriol. 1999;181:1380–1387. doi: 10.1128/jb.181.5.1380-1387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy DJ, Brown JR. Identification of gene targets against dormant phase Mycobacterium tuberculosis infections. BMC Infect Dis. 2007;7:84. doi: 10.1186/1471-2334-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 18.Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, et al. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun. 2003;71:1134–1140. doi: 10.1128/IAI.71.3.1134-1140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Movahedzadeh F, Stoker NG, Coates AR. Deletion of the Mycobacterium tuberculosis alpha-crystallin-like hspX gene causes increased bacterial growth in vivo. Infect Immun. 2006;74:861–868. doi: 10.1128/IAI.74.2.861-868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart JN, Rivera HN, Karls R, Quinn FD, Roman J, et al. Increased pathology in lungs of mice after infection with an alpha-crystallin mutant of Mycobacterium tuberculosis: changes in cathepsin proteases and certain cytokines. Microbiology. 2006;152:233–244. doi: 10.1099/mic.0.28275-0. [DOI] [PubMed] [Google Scholar]
- 21.Converse PJ, Karakousis PC, Klinkenberg LG, Kesavan AK, Ly LH, et al. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun. 2009;77:1230–1237. doi: 10.1128/IAI.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One. 2008;3:e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florczyk MA, McCue LA, Purkayastha A, Currenti E, Wolin MJ, et al. A family of acr-coregulated Mycobacterium tuberculosis genes shares a common DNA motif and requires Rv3133c (dosR or devR) for expression. Infect Immun. 2003;71:5332–5343. doi: 10.1128/IAI.71.9.5332-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 25.Parish T, Stoker NG. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146 ( Pt. 2000;8):1969–1975. doi: 10.1099/00221287-146-8-1969. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Coates AR. Acute and Persistent Mycobacterium tuberculosis Infections Depend on the Thiol Peroxidase TPX. PLoS ONE. 2009;4:e5150. doi: 10.1371/journal.pone.0005150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun. 2001;69:1142–1150. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Butcher PD, Mangan JA, Rajandream MA, Coates AR. Regulation of hmp gene transcription in Mycobacterium tuberculosis: effects of oxygen limitation and nitrosative and oxidative stress. J Bacteriol. 1999;181:3486–3493. doi: 10.1128/jb.181.11.3486-3493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasudeva-Rao HM, McDonough KA. Expression of the Mycobacterium tuberculosis acr-coregulated genes from the DevR (DosR) regulon is controlled by multiple levels of regulation. Infect Immun. 2008;76:2478–2489. doi: 10.1128/IAI.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Oliveira IM, Henriques JA, Bonatto D. In silico identification of a new group of specific bacterial and fungal nitroreductases-like proteins. Biochem Biophys Res Commun. 2007;355:919–925. doi: 10.1016/j.bbrc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Master SS, Springer B, Sander P, Boettger EC, Deretic V, et al. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology. 2002;148:3139–3144. doi: 10.1099/00221287-148-10-3139. [DOI] [PubMed] [Google Scholar]
- 32.Brondani DJ, Caetano N, Moreira DR, Soares RR, Lima VT, et al. Novel nitrofurazone derivatives endowed with antimicrobial activity. Arch Pharm (Weinheim) 2008;341:655–660. doi: 10.1002/ardp.200700243. [DOI] [PubMed] [Google Scholar]
- 33.Kashanian J, Hakimian P, Blute M, Wong J, Khanna H, et al. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 2008;102:1634–1637. doi: 10.1111/j.1464-410X.2008.07809.x. [DOI] [PubMed] [Google Scholar]
- 34.Murugasu-Oei B, Dick T. Bactericidal activity of nitrofurans against growing and dormant Mycobacterium bovis BCG. J Antimicrob Chemother. 2000;46:917–919. doi: 10.1093/jac/46.6.917. [DOI] [PubMed] [Google Scholar]
- 35.Taneja NK, Tyagi JS. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J Antimicrob Chemother. 2007;60:288–293. doi: 10.1093/jac/dkm207. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. In: Cold Spring Harbor NY, editor. Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Parish T, Stoker NG. Electroporation of mycobacteria; Parish TSNG, editor: Humana press: Totowa. 1998.
- 38.Lee MH, Pascopella L, Jacobs WR, Hatfull GF. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guerin. Proc Natl Acad Sci U S A. 1991;88:3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okado-Matsumoto A, Fridovich I. Putative denitrosylase activity of Cu,Zn-superoxide dismutase. Free Radic Biol Med. 2007;43:830–836. doi: 10.1016/j.freeradbiomed.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 40.McOsker CC, Fitzpatrick PM. Suppl A. Vol. 33. J Antimicrob Chemother; 1994. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. pp. 23–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth and survival of YHΔ acg in resting and IFN-γ-activated macrophages derived from iNOS knockout mice. These results are the means and standard deviation derived from one representative of three independent experiments.
(EPS)
Survival of YHΔ acg and YHΔ Rv2030c in response to DETA/NO at 5 and 10 mM (A), H2O2 at 5 and 10 mM (B), and under acidic condition (C). The data shown is a representative of three independent experiments. Data are represented as mean ± SD of triplicate tests.
(EPS)