Abstract
The link between brain structure and intelligence is a well-investigated topic, but existing analyses have mainly focused on adult samples. Studies in healthy children and adolescents are rare, and normative data specifically addressing the association between corpus callosum morphology and intellectual abilities is quite limited. To advance this field of research, we mapped the correlations between standardized intelligence measures and callosal thickness based on high-resolution magnetic resonance imaging (MRI) data. Our large and well-matched sample included 200 normally developing subjects (100 males, 100 females) ranging from 6 to 17 years of age. Although the strongest correlations were negative and confined to the splenium, the strength and the direction of intelligence-callosal thickness associations varied considerably with respect to age and sex. While significant correlations in females were mainly positive, significant correlations in males were exclusively negative. However, only the negative correlations in the overall sample (i.e., males and females combined) remained significant when controlling for multiple comparisons. The observed negative correlations between callosal thickness and intelligence in children and adolescents contrast with the positive correlations typically reported in adult samples. However, negative correlations are in line with reports from other pediatric studies relating cognitive measures to other brain attributes such as cortical thickness, gray matter volume, and gray matter density. Altogether, these findings suggest that relationships between callosal morphology and cognition are highly dynamic during brain maturation. Sex effects on links between callosal thickness and intelligence during childhood and adolescence are present but appear rather weak in general.
Keywords: brain, corpus callosum, development, gender, IQ, MRI
Introduction
The link between brain structure and intelligence is a well-investigated topic. However, the majority of existing studies have been conducted on adult samples. These prior studies mostly show modest positive correlations between intelligence and brain morphology in a network of brain areas, mainly comprising frontal and parietal regions, but also areas within the temporal and occipital lobes (Jung and Haier, 2007; Luders et al., 2009). Studies conducted in healthy children and adolescents are rare but seem to suggest that the relation of intelligence to brain structure changes over time, as children grow older. For example, Karama et al. (2009) reported no significant associations between cortical thickness and the general cognitive factor in children (6–11.9 years) when applying FDR corrections, while adolescents (12–18.3 years) showed numerous areas where correlations were significantly positive. Moreover, Shaw et al. (2006) reported a developmental shift from a primarily negative correlation between intelligence and cortical thickness in early childhood (3.8–8.4 years) to a positive correlation in late childhood (8.6–11.7), early adolescence (11.8–16.9 years), and early adulthood (17–29 years), where positive correlations were maximal in late childhood. Wilke et al. (2003) illustrated that positive and negative correlations were apparent in children and adolescents aged 5–19 years, but only positive correlations reached statistical significance starting after the age of 12 years. Similarly, the brain regions showing the strongest links to intelligence seemed to differ depending on the age group examined. For example, Wilke et al. (2003) reported more pronounced effects in younger children (<12 years) for deep gray matter structures, while correlations in older children (>12 years) were most significant in the cingulate. Frangou et al. (2004), who examined subjects aged 12–21 years (without splitting their sample into different age groups) detected significant positive correlations in the orbitofrontal cortex, cingulate, cerebellum, and thalamus as well as negative correlations in the caudate nucleus.
We hypothesized that such age-dependent associations between anatomical and cognitive measures are not only evident in cortical and subcortical regions, but also in the corpus callosum (i.e., the largest inter-hemispheric fiber structure connecting many of these regions). Midsagittal callosal area has been shown to be an indicator of the total number of small diameter fibers connecting both hemispheres (Aboitiz et al., 1992). Since small diameter fibers are particularly involved in transferring higher-order cognitive information (Aboitiz, 1992), callosal morphology may thus link with the capacity for inter-hemispheric transfer and/or with hemispheric specialization which may modulate intellectual abilities. Indeed, a number of studies suggest that the structural integrity of the corpus callosum is associated with intellectual abilities (Atkinson, Jr. et al., 1996; Aukema et al., 2009; Chiang et al., 2009; Davatzikos and Resnick, 1998; Fletcher et al., 1992; Kulak et al., 2007; Luders et al., 2007; Schatz and Buzan, 2006; Spencer et al., 2005; Strauss et al., 1994; Wozniak et al., 2009). However, there is a lack of such studies in healthy children and adolescents.
Thus, to advance this field of research and to characterize the link between callosal morphology and intelligence in younger populations, we selected a large sample of children and adolescents (n=200), aged 6–17 years, from a normative database of subjects (Evans, 2006). We applied advanced surface-based mesh-modeling methods and mapped correlations between callosal thickness and standardized intelligence measures for this overall sample but also within four equally-sized subgroups, each spanning three years of age. The overarching goal of our study was to establish the presence and direction of correlations between callosal thickness and intelligence in the developing brain (i.e., in individuals younger than 18 years old). Given that existing links between brain structure and cognitive measures may remain disguised or biased towards the effect of the sex that is more heavily represented in the sample when pooling males and females (Haier et al., 2005), our study also addressed possible sex effects. This was achieved by including equal numbers of males and females (both in the overall sample and within subgroups), by testing for significant interactions with sex, and by conducting analyses separately within males and females if significant sex interactions were detected.
Materials and Methods
Subjects
All subjects were selected from a database pertaining to the First Objective of the MRI Study of Normal Brain Development (Evans, 2006). Subjects were excluded if they met criteria “established or highly suspected to adversely impact healthy brain development” as detailed elsewhere (Evans, 2006). Informed consent was obtained from parents and adolescents, and assents were obtained from the children. All protocols and procedures were approved by the relevant Institutional Review Board at each pediatric study center and at each coordinating center (Evans, 2006). The final sample of the current study contained 200 subjects (100 males; 100 females) aged between 6 and 17 years. We divided this sample into four equally-sized age groups; each group contained 50 subjects and each spanned three consecutive years of age. That is, group 1 (G1) contained subjects aged 6–8 years, group 2 (G2) contained subjects aged 9–11 years, group 3 (G3) contained subjects aged 12–14 years, and group 4 (G4) contained subjects aged 15–17 years (see Table 1). Within each age group, we carefully matched the number of males (n=25) and females (n=25). To determine handedness, subjects were ask to perform eight different activities, modified from the Edinburgh Handedness Inventory (EHI; Oldfield, 1971). Right-handed activities were rated with 1; left-handed activities were rated with 0. Subjects with total scores of <7 were classified as non-right-handed. Importantly, each age group (G1-G4) contained the same number of non-right-handers (n=5) with the same sex ratio for these non-right handers (4 males, 1 female).
Table 1.
Sample and group-specific descriptive statistics.
| Age* Range [Ages*] | N of Subjects [Per Age*] | FS-IQ Mean ± SD Min – Max |
P-IQ Mean ± SD Min – Max |
V-IQ Mean ± SD Min – Max |
|
|---|---|---|---|---|---|
| Overall | 6–17 | 200 | 113.13 ± 11.61 86 – 158 |
111.52 ± 11.91 80 – 157 |
111.69 ± 12.82 82 – 151 |
| Group 1 | 6–8 [6-7-8] | 50 [12-21-17] | 115.60 ± 15.38 86 – 156 |
115.02 ± 14.82 89 – 157 |
112.66 ± 16.10 82 – 149 |
| Group 2 | 9–11 [9-10-11] | 50 [14-18-18] | 113.28 ± 10.53 92 – 139 |
109.64 ± 11.00 80 – 134 |
113.94 ± 12.48 88 – 145 |
| Group 3 | 12–14 [12-13-14] | 50 [20-16-14] | 112.80 ± 10.04 96 – 131 |
111.02 ± 11.14 87 – 132 |
111.54 ± 11.40 91 – 137 |
| Group 4 | 15–17 [15-16-17] | 50 [15-17-18] | 110.84 ± 9.35 92 – 129 |
110.42 ± 9.70 90 – 128 |
108.62 ± 10.35 86 – 131 |
Age is shown in years.
FS-IQ = full-scale intelligence quotient
P-IQ = performance intelligence quotient
V-IQ = verbal intelligence quotient
Intelligence Measures
Intelligence measures were obtained using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The WASI is nationally standardized, consists of four subtests (Vocabulary, Similarities, Block Design, Matrix Reasoning), and yields full-scale intelligence (FS-IQ), performance intelligence (P-IQ), and verbal intelligence (V-IQ) scores. FS-IQ provides an estimate of general intellectual ability; it is based on the subtests Matrix Reasoning and Vocabulary (described below). P-IQ provides an estimate of visual information processing and abstract reasoning skills (Matrix Reasoning), nonverbal concept formation, simultaneous processing, visual-motor coordination, visual perception and organization, etc. (Block Design). V-IQ provides an estimate of word knowledge, verbal concept formation, and fund of knowledge (Vocabulary), as well as verbal reasoning and concept formation (Similarities). Importantly, with respect to FS-IQ, P-IQ, and V-IQ, there were no significant sex differences in the overall sample (n=200) nor between males and females within the four age groups (G1–G4). Similarly, with respect to FS-IQ, P-IQ, and V-IQ, there were no significant differences between the four age groups (G1-G4) when males and females were combined. Group-specific descriptive statistics for intelligence measures are summarized in Table 1.
Image Acquisition
Images were obtained on 1.5 T systems from General Electric (GE) or Siemens Medical Systems (Siemens) using a 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence with the following parameters: TR = 22–25 ms, TE = 10–11 ms, excitation pulse = 30°, refocusing pulse = 180°, orientation: sagittal; field of view: AP = 256 mm; LR = 160–180 mm (whole head coverage), voxel size = 1 mm3, where the maximum number of slices on GE scanners was 124, and hence the slice thickness was increased to 1.5 mm (Evans, 2006). Imaging data from study participants was obtained on six different scanners (for details see Evans, 2006); each scanner contributed 13%-19% of the subjects in the overall sample, and 6%-24% of the subjects to each of the four age groups, where scanner information for five subjects was missing (see Table 2).
Table 2.
Scanner Distribution.
| S1 | S2 | S3 | S4 | S5 | S6 | Total | Missing | |
|---|---|---|---|---|---|---|---|---|
| Overall | n=34 | n=38 | n=37 | n=26 | n=34 | n=26 | n=195 | 5 |
| Group 1 | n=11 | n=6 | n=9 | n=8 | n=5 | n=11 | n=50 | 0 |
| Group 2 | n=8 | n=10 | n=11 | n=8 | n=8 | n=4 | n=49 | 1 |
| Group 3 | n=9 | n=11 | n=9 | n=3 | n=9 | n=7 | n=48 | 2 |
| Group 4 | n=6 | n=11 | n=8 | n=7 | n=12 | n=4 | n=48 | 2 |
S1–S6 = scanners
1–6 n indicates the number of subjects.
Preprocessing
We applied automated radio-frequency bias field corrections to correct image volumes for intensity drifts caused by magnetic field inhomogeneities (Shattuck et al., 2001). In addition, we placed all images volumes into the same standard space by co-registering them to the ICBM-152 template using automated 6-parameter rigid-body transformations (Woods et al., 1998). That is, images were corrected for differences in brain position and orientation while preserving their native dimensions. The corpus callosum was then outlined automatically based on the Chan-Vese model for active contours (Chan and Vese, 2001) using the LONI pipeline processing environment (Dinov et al., 2009; Rex et al., 2003). This resulted in two midsagittal callosal segments (i.e., the upper and lower callosal boundary) for each subject, as detailed elsewhere (Luders et al., 2006). Subsequently, each callosal segment was overlaid onto the MR image from which it had been extracted and visually inspected to ensure that automatically generated callosal outlines precisely followed the natural course and boundaries of the corpus callosum. Contours that did not match this criterion were corrected manually by one rater (E.L.).
Callosal Thickness Measurements
To obtain highly localized measures of callosal thickness, anatomical surface-based mesh modeling methods were employed (Thompson et al., 1996b; Thompson et al., 1996a). That is, the upper and lower callosal boundaries were resampled at regular intervals to render the discrete points comprising the boundaries spatially uniform. Then, a new segment (i.e., the medial core) was automatically created by calculating a spatial average 2D curve from 100 equidistant surface points representing the upper and lower callosal boundaries. Finally, the distances between 100 surface points of the medial core and the 100 corresponding surface points of both the upper and the lower callosal boundaries were computed. These regional distances indicate callosal thickness with a high spatial resolution (i.e., at 100 locations distributed evenly over the callosal surface). Of note, callosal geometry was not scaled or otherwise adjusted for overall brain size, to avoid confounding global and local associations.
Statistical Analyses
First, we mapped the correlations between intelligence (i.e., FS-IQ, P-IQ, and V-IQ) and callosal thickness measures at 100 equidistant surface points within the overall sample (n=200). For these analyses, correlation coefficients (r) and significance values (p) were projected onto the group-averaged callosal surface models. Correlation coefficients (r) were color-coded according to Cohen (1992) with respect to effect sizes: small (r ≥ 0.1), medium (r ≥ 0.3), and large (r ≥ 0.5). Significance values (p) were provided uncorrected (p≤0.05) as well as corrected for multiple comparisons using False Discovery Rate (FDR) thresholded at 0.05 (Benjamini and Hochberg, 1995).
Subsequently, we used interactional designs and tested whether correlations between intelligence measures and callosal thickness are different (a) between the four age groups (age interaction), (b) between males and females within the four age groups (age-specific sex interaction), and (c) between males and females within the overall sample (overall sex interaction). For these interaction analyses, uncorrected significance values (p≤0.05) were projected onto the group-averaged callosal surface models.
Finally, to follow up on the significant age interactions and the significant age-specific sex interactions1 we mapped (a) the correlations between intelligence and callosal thickness measures separately within the four age groups and (b) the correlations within males and females separately within each of the four age groups. For these analyses, correlation coefficients (r) and significance values (p), both uncorrected and FDR-corrected, were projected onto the group-averaged callosal surface models.
Supplemental Analysis I
FS-IQ is a composite of V-IQ and P-IQ; however V-IQ and P-IQ are usually correlated. In order to take this into consideration we first calculated the Person correlation between V-IQ and P-IQ, for the overall sample but also within each of the four age groups (G1-G4). Subsequently, we mapped the correlations between callosal thickness and P-IQ while co-varying for V-IQ, and also between callosal thickness and V-IQ while co-varying for P-IQ. These correlation analyses were conducted for the overall sample and in addition within the four age groups (G1-G4).
Supplemental Analysis II
Using MRI-derived measures from multiple scanners raises the question whether study outcomes are possibly biased by scanner effects. However, the intrinsically strong intensity contrast in the corpus callosum renders it not very susceptible to possibly small differences in image contrast due to different scanners. Thus, it is rather unlikely that the integrity of current findings is compromised. Nevertheless, in order to fully eliminate the possibility of scanner effects, we tested whether there were significant interactions between scanner x FIQ (as well as PIQ and VIQ, respectively) when examining the link between callosal thickness and intelligence. These analyses were conducted for the overall sample, while excluding the five cases where the scanner information was missing.
Results
Correlations between callosal thickness and intelligence
As shown in Figure 1, in children and adolescents aged 6–17 years (overall sample), negative correlations between callosal thickness and FS-IQ, P-IQ, and V-IQ dominate over positive correlations. More specifically, we detected clusters of positive correlations in the callosal anterior third within the rostrum (for FS-IQ, P-IQ, and V-IQ) and in the rostral body (for P-IQ)2. However, these positive correlations did not reach statistical significance. In contrast, negative correlations were evident across almost the entire callosal surface and became significant within the splenium (for FS-IQ, P-IQ, and V-IQ) and in the posterior midbody (for FS-IQ and P-IQ). FDR-corrections confirmed the findings within the splenium for FS-IQ (p ≤ 0.0043) and for V-IQ (p ≤ 0.0033).
Figure 1. Correlations between callosal thickness and intelligence (overall sample).
Top Panel: Illustrated are correlation coefficients (r-maps) color-coded with respect to effect sizes: small (r ≥ 0.1), medium (r ≥ 0.3), and large (r ≥ 0.5). Cyan and yellow indicate where negative and positive correlations remained below the threshold for relevant effect sizes. Bottom Panel: Illustrated are significance values (p-maps). The color bar encodes the uncorrected significance of negative correlations. White regions indicate significant positive correlations. The smaller callosal maps indicate in pink where significant correlations survived FDR-corrections. Gray indicates where no significant correlations were detected. The callosal posterior section is located on the left; the callosal anterior section points to the right. FS-IQ = full-scale IQ; P-IQ = performance IQ; V-IQ = Verbal IQ.
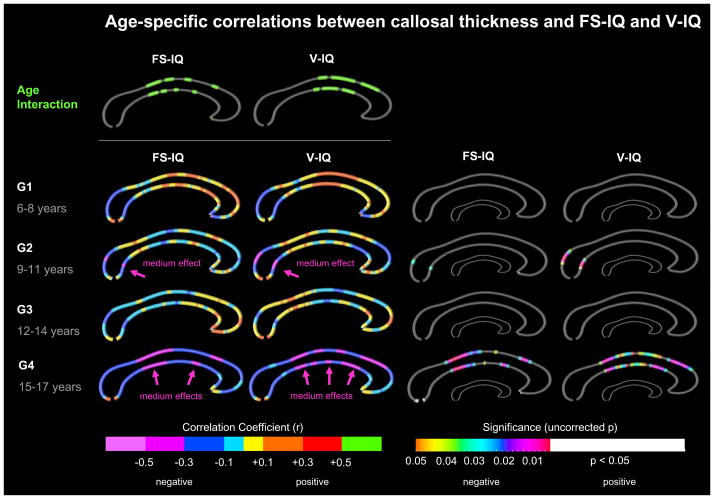
Age effects
As shown in Figure 2 (top panel), we detected significant differences between the four age groups for the correlations between callosal thickness and FS-IQ as well as V-IQ (age interaction). There were no significant age interactions for P-IQ.
Figure 2. Age-specific correlations between callosal thickness and intelligence.
Top Panel: Illustrated are significant age interactions (in green) at p≤0.05, uncorrected. Bottom Panels; left: Illustrated are correlation coefficients (r-maps) color-coded with respect to effect sizes. Medium effects are indicated by arrows. Bottom Panels, right: Illustrated are significance values (p-maps). The color bar encodes the uncorrected significance of negative correlations. White regions indicate significant positive correlations. The smaller callosal maps indicate where significant correlations survived FDR-corrections. Gray indicates where no significant correlations were detected. FS-IQ = full-scale IQ; V-IQ = Verbal IQ. G1–G4 = Groups 1–4.
Given the significant effects, we mapped correlations between callosal thickness and FS-IQ and V-IQ within the four age groups. As illustrated in the left panel, the significant age interactions in the rostral body can be mainly attributed to positive correlations at ages 6–14 years (G1-G3) and negative correlations at ages 15–17 years (G4). The significant age interactions in the callosal midbody can be mainly attributed to positive correlations at ages 6–8 years (G1) and negative correlations at ages 9–17 years (G2-G4). Altogether, there seems to be a developmental trend from mainly positive correlations in younger cohorts towards predominantly negative correlations in older cohorts. However, as illustrated in the right panel, there were no significant positive correlations in any of the four groups (right panel). In contrast, negative correlations with FS-IQ and V-IQ within the splenium became significant at age 9–11 years (G2), while negative correlations within the callosal midbody as well as the rostral body became significant at age 15–17 years (G4). Although these negative correlations constitute medium-sized effects (left panel), none of them survived FDR corrections (right panel).
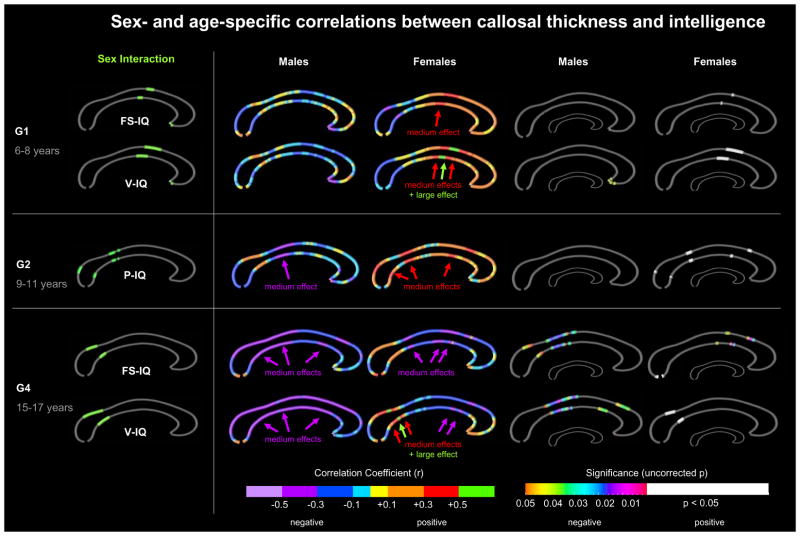
Age-specific sex effects
Finally, as shown in Figure 3 (left panel), we detected significant age-specific sex interactions for FS-IQ and V-IQ at 6–8 years (G1) and at 15–17 years (G4), as well as for P-IQ at 9–11 years (G2). There were no significant age-specific sex interactions at age 12–14 years (G3).
Figure 3. Sex- and age-specific correlations between callosal thickness and intelligence.
Left Panel: Illustrated are significant sex interactions (in green) at p≤0.05, uncorrected within age groups. Middle Panels: Illustrated are correlation coefficients (r-maps) color-coded with respect to effect sizes. Medium and large effects are indicated by arrows. Right Panels: Illustrated are significance values (p-maps). The color bar encodes the uncorrected significance of negative correlations. White regions indicate significant positive correlations. The smaller callosal maps indicate where significant correlations survived FDR-corrections. Gray indicates where no significant correlations were detected. FS-IQ = full-scale IQ; V-IQ = Verbal IQ. G1–G4 = Groups 1–4.
Given the significant interactions with sex within the different age groups, we mapped sex-specific correlations between callosal thickness and FS-IQ/V-IQ (for G1 and G4) and P-IQ (for G2). As illustrated in the middle and right panels, the significant sex interaction at age 6–8 years (G1) in the anterior midbody (for FS-IQ and V-IQ) was due to positive correlations in females and negative correlations in males. Effects sizes in females were medium to large, and reached statistical significance (uncorrected). As further demonstrated, the significant sex interaction at age 9–11 years (G2) in the posterior midbody and splenium (for P-IQ) was due to positive correlations in females and negative correlations in males. Effect sizes were medium in both sexes but reached significance (uncorrected) in females only. Additional significant positive correlations in females were detected in the rostral body. Finally, the significant sex interaction at age 15–17 years (G4) within the isthmus (for FS-IQ and V-IQ) was due to positive correlations in females and negative correlations in males, with medium to large effect sizes which reached significance (uncorrected) in both sexes (for FS-IQ in males; for V-IQ in females). In addition, significant negative correlations (medium effects) were observed in males within the posterior midbody (for FS-IQ and V-IQ) and the rostral body (for V-IQ). Interestingly, significant negative correlations (medium effects) were also detected in females within the anterior midbody and the rostral body (for FS-IQ). Surprisingly, none of the medium and large effects survived FDR corrections (right panel).
Supplemental Analysis I and II
The correlations between V-IQ and P-IQ are provided in Table 3. As shown, V-IQ and P-IQ are highly correlated, both within the overall sample and within each of the four age groups (G1-G4). Nevertheless, as illustrated in Supplemental Figure 1, co-varying for V-IQ (for P-IQ, respectively) revealed significance profiles (uncorrected at p≤0.05) similar to the ones calculated when examining links between callosal thickness and intelligence measures directly (i.e., without including any co-variates). Finally, with respect to possible bias introduced by different scanners, there were no significant scanner interactions (uncorrected at p≤0.05) for F-IQ, P-IQ, and V-IQ (maps not shown).
Table 3.
Correlations between V-IQ and P-IQ.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Discussion
Our goal was to map the link between intellectual abilities and callosal morphology in the developing brain in a large sample of healthy children and adolescents. We have taken great care to compose a well-balanced study design where analyses were not only conducted within the overall sample (n=200) but also within four equally-sized subgroups (n=50) defined by consecutive and relatively narrow age intervals of three years. Importantly, each subgroup was balanced for sex (25 males, 25 females) with comparative cognitive scores between the four age groups as well as between males and females within those age groups.
The Location of the Correlations
Significant negative correlations (i.e., the ones surviving FDR corrections) were confined to the splenium within the overall sample. Additional positive correlations constituting large effect sizes (albeit only significant at uncorrected thresholds) were detected within females and located in the isthmus and the anterior midbody. Based on recent fiber tractography studies (Hofer and Frahm, 2006; Park et al., 2008; Zarei et al., 2006), the anterior midbody appears to mainly connect (pre)frontal regions (including the anterior cingulate), the isthmus connects regions of the parietal and (superior) temporal cortex, and the splenium connects occipital, (inferior) temporal and parietal regions (including the posterior cingulate). Interestingly, previous studies in normative pediatric populations reported negative correlations between cognitive and cerebral measures in the temporal cortex (Shaw et al., 2006) and the parietal cortex (Wilke et al., 2003). Positive correlations were revealed in the cingulate (Frangou et al., 2004; Wilke et al., 2003), as well as in frontal (Frangou et al., 2004; Reiss et al., 1996; Schmithorst et al., 2005; Shaw et al., 2006) and parieto-occipital regions (Schmithorst et al., 2005; Shaw et al., 2006). Thus, there is some spatial correspondence between cortical associations revealed in those previous studies and the callosal regions implicated in the current study. While the direction of the correlations varies across studies (as discussed below), altogether these outcomes suggest the existence of a widely distributed intelligence network that already exists in the developing brain. This network seems to include frontal and parietal regions, but also areas within the temporal and occipital lobes as previously proposed (Jung and Haier, 2007).
The Direction of the Correlations
We observed both significant positive and negative correlations, but only negative correlations survived correction for multiple comparisons. These outcomes contrast with prior reports of positive correlations between cognitive functioning and callosal size (or DTI-based measures of callosal fractal anisotropy) in other pediatric populations. However, those studies were based on children (and adolescents) with hydrocephalus (Fletcher et al., 1992), mental retardation (Spencer et al., 2005), sickle-cell disease (Schatz and Buzan, 2006), spastic cerebral palsy (Kulak et al., 2007), fetal alcohol spectrum disorders (Wozniak et al., 2009), medulloblastoma and acute lymphoblastic leukemia (Aukema et al., 2009), and born preterm (Nosarti et al., 2004). Thus, their outcomes are not necessarily representative of the normal conditions or correlations that would also be found in the healthy developing brain. Although there is hardly any normative data available with respect to callosal measures, some previous studies investigated the link between callosal size and intelligence in healthy adults. Those studies revealed either no significant correlations (Tramo et al., 1998) or only positive correlations (Chiang et al., 2009; Luders et al., 2007). However, given the age ranges of 24–34 years (Tramo et al., 1998) and 16–44 years (Luders et al., 2007), as well as mean ages of 25.1 and 23.5 years (Chiang et al., 2009), those findings are representative of young adulthood rather than childhood and adolescence. Thus, the divergent findings are not necessarily surprising.
Nevertheless, the negative correlation in subjects aged 6–17 years, as observed in the current study, is intriguing. Of note, inverted correlations have also been reported in other studies of healthy children and adolescents relating cognitive measures to cortical thickness, gray matter volume, and gray matter density (Frangou et al., 2004; Shaw et al., 2006; Wilke et al., 2003). However, studies revealing negative correlations are extremely sparse - perhaps due to a potential publication bias favoring positive correlations as the interpretations of negative correlations is more difficult. Frangou et al. (2004) detected negative correlations between IQ and gray matter density within the caudate nucleus and suggested different developmental trajectories for cortical and subcortical gray matter (i.e., subcortical reductions in childhood preceding cortical reductions later during adolescence). They argue that even a “subtle deviation in the degree or timing of the maturational events that lead to basal ganglia changes during development may have a negative impact on IQ.”
The current findings with respect to the corpus callosum possibly imply that more intelligent individuals (at least during childhood and adolescence) possess more efficient neuronal networks (Haier et al., 1988; Li et al., 2009; Neubauer and Fink, 2009). This either may be the consequence of fewer inter-hemispheric fibers or possibly the cause of seemingly less established (but optimal) inter-hemispheric connections. Moreover, the current findings may suggest that intelligence is linked to hemispheric specialization with higher levels of intelligence in more lateralized brains. A similar relationship between specific cognitive abilities and lateralization of the arcuate fasciculus has been reported recently in children aged 5–13 years (Lebel and Beaulieu, 2009). Since more lateralized brains rely on less inter-hemispheric communication (but perhaps on more intra-hemispheric cross-talk), callosal dimensions in more intelligent children and adolescents may be decreased. However, this assumption requires testing in future studies relating intelligence to callosal morphology in association with structural and functional lateralization.
Possible Implications for Maturational Changes
The observed negative correlations of the current study suggest thinner corpora callosa in more intelligent children and adolescents. Taken together with reported positive correlations later in life (Luders et al., 2007), the current outcomes may imply that callosal dimensions in more intelligent individuals increase at a greater rate than in less intelligent individuals (accelerated rates of myelination and/or diminished rates of pruning in general, where the exact underlying mechanisms remain to be established. In support of intelligence-specific growth rates (albeit with respect to cortical thickness), a longitudinal study reported that “more intelligent resulting in thicker corpora callosa in more intelligent adults). Accelerated growth rates in more intelligent individuals might be due to children demonstrate a particularly plastic cortex, with an initially accelerated and prolonged phase of cortical increase” (Shaw et al., 2006). Clearly, only additional longitudinal studies addressing callosal microstructure will resolve the true nature of developmental changes.
An alternative hypothesis is that intelligence changes over time though this assumption is rather unlikely to be true given that IQ is shown to be a relatively stable measurement. However, a different explanation could be that the observed negative correlations are driven by other, non-cognitive processes requiring inter-hemispheric transfer. These processes may be confined to identical callosal regions like higher-order cognitive functions and underlie the same developmental mechanisms (thus causing significant correlations) but develop in an opposing manner (thus causing negative correlations). For example, non-cognitive processes may account for the significant negative correlation between callosal thickness and FS-IQ within the posterior midbody in the overall sample. Since this particular callosal region has been suggested to mainly connect sensory-motor regions (Park et al., 2008), it seems to have no immediate relevance with respect to intelligence measures. Perhaps, only later towards the end of adolescence, when callosal development and cortical maturation is almost complete, the direct link between intelligence and callosal thickness becomes the driving force and powerful determinant leading to positive correlations between brain structures and individual cognitive capacities in adults (Jung and Haier, 2007; Luders et al., 2009). Albeit only speculative at this point, this possibly explains why even positive correlations between cerebral and intelligence measures remain below the threshold of significance in early years of development. This phenomenon was not only apparent in the current study with respect to callosal thickness but also reported by others with respect to a number of cerebral measures, including callosal mean diffusivity and fractional anisotropy (Karama et al., 2009; Shaw et al., 2006; Wilke et al., 2003; Wozniak et al., 2009).
Altogether, the observed negative correlations in our subjects aged 6–17 years suggest that callosal growth and fiber reorganization may persist into adolescence, as also recently demonstrated (Luders et al., 2010). It is unknown when the transition from negative to positive correlations occurs, but similar developmental shifts have already been reported. For example, Shaw et al. (2006) detected a primarily negative correlations between intelligence and cortical thickness in subjects aged 3.8–8.4 years contrasting mainly positive correlations in subjects aged 8.6–29 years. Similarly, Wilke et al. (2003) illustrated that both positive and negative correlations were apparent in children and adolescents aged 5–19 years, but only positive correlations became significant after the age of 12 years. Moreover, Karama et al. (2009) reported highly significant positive correlations in adolescents (12–18.3 years), while significant correlations in children (6–11.9 years) were absent. These observations imply highly dynamic developmental processes in the young brain, not only related to the anatomical substrates per se (e.g., apoptosis, pruning, dendritic branching, axonal redirection) but also the resulting functional implications (e.g., changes in how higher-order cognitive information is processed). Thus, giving the complex nature of developmental processes, it is likely that developmental shifts affect not only the direction of the correlation (i.e., from negative to positive) but also the callosal region where correlations are detectable (Wilke et al., 2003). That is, callosal regions associated with IQ may only partly overlap between different stages during neurodevelopment (as observed in our different age groups) or when compared to outcomes in adult samples (Davatzikos and Resnick, 1998; Luders et al., 2007).
Sex Effects
Although a significant overall sex interaction was absent, significant differences between boys and girls became evident when mapping the correlation between callosal thickness and intelligence within the different age groups. Normative adult studies support the assumption of sex-specific relationships between cognitive measures and various cerebral measures (Gur et al., 1999; Haier et al., 2005; Jung et al., 2005; Narr et al., 2006; Pfleiderer et al., 2004; Witelson et al., 2006), including measures of the corpus callosum (Davatzikos and Resnick, 1998; Luders et al., 2007). Thus, our findings may add support to the growing body of literature that the neural substrates of intelligence differ between the sexes, even in the absence of sex differences in intellectual abilities (Haier et al., 2005). Moreover, they seem to agree with the assumption of sex-specific developmental processes underlying intelligence during childhood and adolescence (Schmithorst et al., 2008; Schmithorst and Holland, 2006; Schmithorst and Holland, 2007; Schmithorst and Yuan, 2010). The more so, as the positively correlated regions in females partly resemble callosal regions showing positive correlations in adulthood in both women and men (Luders et al., 2007).
Opposite correlations in males (negative) and females (positive), as observed in the current study, are intriguing. To our knowledge, there is currently no comparable gender data with respect to intelligence-callosal thickness associations in healthy children and adolescents. However, a recent study in young adults revealed that the integrity of inter-hemispheric connections was positively correlated to some intelligence factors in females but negatively correlated in males (Tang et al., 2010). Nevertheless, none of those correlations survived correction for multiple comparisons (Tang et al., 2010) and neither did the sex-specific correlations in the current study. Furthermore, negative correlations in males younger than 15–17 years were not even significant at uncorrected thresholds. Similarly, testing for an overall sex interaction did not reveal any significant findings. Future studies involving even more subjects will reveal whether non-significance was due to a lack of sufficient statistical power. At this point, however, it may be fair to conclude that sex effects on links between callosal thickness and intelligence are rather weak in general during childhood and adolescence. This conclusion is supported by outcomes from a different pediatric study which revealed no significant sex differences when analyzing the link between intelligence and the thickness of the cortex (Shaw et al., 2006).
Supplementary Material
Supplemental Figure 1. Correlations between callosal thickness and intelligence. Left Panel:Illustrated are significant correlations with P-IQ, with and without co-varying for V-IQ. RightPanel: Illustrated are significant correlations with V-IQ, with and without co-varying for P-IQ.The color bar encodes the uncorrected significance (p) of negative correlations. White indicatessignificant positive correlations. Gray indicates where no significant correlations were detected.None of the correlations passed FDR corrections for multiple comparisons. The callosalposterior section is located on the left; the callosal anterior section points to the right. P-IQ =performance IQ; V-IQ = Verbal IQ.
Acknowledgments
This study was supported by the NIH grants U54 RR021813, P41 RR013642, M01 RR000865, R01 EB007813, R01 EB008281, R01 EB008432, AG016570, EB01651, LM05639, and RR019771. Moreover, data used in the preparation of this article were obtained from the Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development. This is a multi-site, longitudinal study of typically developing children, from ages newborn through young adulthood, conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Footnotes
There were no significant overall sex interactions.
The small yellow clusters at the very tip of the splenium indicating positive correlations are likely to be an artifact due to the splitting point of the callosal top and bottom segments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aboitiz F. Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol Res. 1992;25:51–61. [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Atkinson DS, Jr, Abou-Khalil B, Charles PD, Welch L. Midsagittal corpus callosum area, intelligence, and language dominance in epilepsy. J Neuroimaging. 1996;6:235–239. doi: 10.1111/jon199664235. [DOI] [PubMed] [Google Scholar]
- Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AY. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Pratical and Powerful Approach to Multiple Testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cereb Cortex. 1998;8:635–640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- Dinov ID, Van Horn JD, Lozev KM, Magsipoc R, Petrosyan P, Liu Z, Kenzie-Graham A, Eggert P, Parker DS, Toga AW. Efficient, Distributed and Interactive Neuroimaging Data Analysis Using the LONI Pipeline. Front Neuroinformatics. 2009;3:22. doi: 10.3389/neuro.11.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ, Davidson KC, Thompson NM, Miner ME. Cerebral white matter and cognition in hydrocephalic children. Arch Neurol. 1992;49:818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Peak J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with Positron Emission Tomography. Intelligence. 1988;11:199–218. [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of Intelligence: Converging Neuroimaging Evidence. Behavioral and Brain Sciences. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, Sibbitt WL, Brooks WM. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26:965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagh Y, Haier RJ, Deary IJ, Lyttelton O, Lepage C, Evans A Brain Development Cooperative Group. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Kubas B, Walecki J. Corpus callosum size in children with spastic cerebral palsy: relationship to clinical outcome. J Child Neurol. 2007;22:371–374. doi: 10.1177/0883073807300537. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30:3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Toga AW. Neuroanatomical Correlates of Intelligence. Intelligence. 2009;37:156–163. doi: 10.1016/j.intell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The Development of the Corpus Callosum in the Healthy Human Brain. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.5122-09.2010. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and Regional Cortical Gray Matter Thickness in Healthy Adults. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, Michael N. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience. 2004;123:1053–1058. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119 (Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc. 2006;12:24–33. doi: 10.1017/S1355617706060085. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35:406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Gibson RJ, Moorhead TW, Keston PM, Hoare P, Best JJ, Lawrie SM, Johnstone EC. Qualitative assessment of brain anomalies in adolescents with mental retardation. AJNR Am J Neuroradiol. 2005;26:2691–2697. [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Wada J, Hunter M. Callosal morphology and performance on intelligence tests. J Clin Exp Neuropsychol. 1994;16:79–83. doi: 10.1080/01688639408402618. [DOI] [PubMed] [Google Scholar]
- Tang CY, Eaves EL, Ng JC, Carpenter DM, Mai X, Schroeder DH, Condon CA, Colom R, Haier RJ. Brain networks for working memory and factors of intelligence assessed in males and females with fMRI and DTI. Intelligence. 2010;38:293–303. [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci. 1996a;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996b;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. PsychologicalCorporation, Harcourt Brace and Company; San Antonio, TX: 1999. [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20:202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129:386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlations between callosal thickness and intelligence. Left Panel:Illustrated are significant correlations with P-IQ, with and without co-varying for V-IQ. RightPanel: Illustrated are significant correlations with V-IQ, with and without co-varying for P-IQ.The color bar encodes the uncorrected significance (p) of negative correlations. White indicatessignificant positive correlations. Gray indicates where no significant correlations were detected.None of the correlations passed FDR corrections for multiple comparisons. The callosalposterior section is located on the left; the callosal anterior section points to the right. P-IQ =performance IQ; V-IQ = Verbal IQ.