Abstract
Objective
The objective of this study was to examine the effect of Cs-4® (Cordyceps sinensis) on exercise performance in healthy elderly subjects.
Design
Twenty (20) healthy elderly (age 50–75 years) subjects were enrolled in this double-blind, placebo-controlled, prospective trial. The subjects were taking either Cs-4 333 mg or placebo capsules 3 times a day for 12 weeks.
Measurement
Subjects received baseline screening including physical examination and laboratory tests. Maximal incremental exercise testing was performed on a stationary cycle ergometer using breath-by-breath analysis at baseline and at the completion of the study.
Results
After receiving Cs-4 for 12 weeks, the metabolic threshold (above which lactate accumulates) increased by 10.5% from 0.83 ± 0.06 to 0.93 ± 0.08 L/min (p < 0.02) and the ventilatory threshold (above which unbuffered H+ stimulates ventilation) increased by 8.5% from 1.25 ± 0.11 to 1.36 ± 0.15 L/min. Significant changes in metabolic or ventilatory threshold were not seen for the subjects in the placebo group after 12 weeks, and there were no changes in V̇o2 max in either group.
Conclusion
This pilot study suggests that supplementation with Cs-4 (Cordyceps sinensis) improves exercise performance and might contribute to wellness in healthy older subjects.
Introduction
Cordyceps sinensis (Berk) Sacc is a natural herbal medicine that has been popular in China for centuries for invigoration, health preservation, and reduction of fatigue.1 Naturally occurring Cordyceps sinensis is a wild fungus found on the Qinghai-Tibetan Plateau of China at an altitude of about 10,000 feet. The fungus is parasitic and colonizes the larvae of moths until their inner body is filled with mycelium.2 Wild Cordyceps is a composite consisting of the stroma of the parasite together within the larva of the Hepialidae moths.3 Wild cordyceps is increasingly rare in its natural habitat, and the price is now completely out of reach for clinical practice.4 For this reason and because of the scarcity of natural sources, a refined standardized fermentation product, Cs-4,® was produced from the mycelial strain Paecilomyces hepiali Chen at Dai that was isolated from wild C. sinensis. A close similarity between this fermentation product and natural Cordyceps has been demonstrated with respect to their chemical constituents (Cs-4 contains not less than 0.14% adenosine and 5% mannitol) and pharmacologic properties.2,5
The mechanisms of action of Cordyceps and its fermentation product Cs-4 in improving general well-being and physical ability have yet to be fully investigated.6 Improvements in quality of life have been suggested in patients with chronic heart failure,7 renal failure,8 and chronic pulmonary disease.9 Cordyceps gained world attention in 1993 when Chinese female runners achieved records in 1500 m, 3000 m, and 10,000 m events.10 Their coach attributed their success to a diet containing Cordyceps. It was suggested that Cordyceps helped improve exercise capacity in these athletes via antioxidant effects. Despite these reports, the ability of Cordyceps or Cs-4 to enhance aerobic capacity has not been tested objectively. Because of the popularity of Cordyceps among the older population in China,2 but recognizing the advantages of Cs-4, we chose to conduct a pilot study to examine the effect of Cs-4 on aerobic capacity in healthy elderly volunteers.
Materials and Methods
Subjects
Twenty (20) individuals, male and female, age 50–75 years, were recruited by flyers placed around the campus of the University of California, Los Angeles (UCLA) and also by advertising in the local newspapers. A medical history, physical examination, and routine blood tests were obtained to ensure that the subjects were healthy, without apparent cardiovascular, pulmonary, or musculoskeletal disease.
Study design
This was a double-blinded, placebo-controlled, prospective study. The subjects were randomized in a 1:1 fashion. The subjects were assigned using a predetermined randomization code via a random-number generator.
Supplementation
Subjects were provided with the commercially available formulation of Cordyceps, Cs-4 (CordyMax™), or identical placebo capsules. The Cs-4 was manufactured by Jiangxi GuoYao Company, Jiangxi, China and was supplied by Pharmanex, Inc. (Provo, UT). The active and placebo treatments were prepared in the UCLA Center for Human Nutrition by filling identical capsules with either Cs-4 or starch, respectively. The active components of Cs-4 were adenosine (0.14%), adenine, uracil, uridine, mannitol (5%), β-sitosterol, oligosaccharides, polysaccharides (0.5%), 18 common amino acids, zinc, potassium, manganese, phosphorus, selenium, vitamin B1, vitamin B2, and vitamin E. The subjects were provided with 756 capsules in a box and were asked to return any that were unused at the end of the trial. They were instructed to take three Cs-4 capsules (each 333 mg) or three placebo capsules 3 times a day with water or food for a total of 12 weeks.
Procedures
The protocol was approved by the UCLA Human Subjects in Research Protection Committee. All patients provided written informed consent prior to their participation in the study. Exercise performance was evaluated on a cycle ergometer before and after 12 weeks of taking supplements. Aerobic capacity and physical fitness were assessed by measuring maximal oxygen uptake (V̇o2 max) and the gas exchange metabolic threshold (V̇o2θ) during a symptom-limited, incremental work rate exercise test.
Maximal exercise performance was assessed at the screening visit using a symptom-limited incremental exercise protocol on a cycle ergometer (Ergoline 900S; Sensormedics Corp, Loma Linda, CA). The external work rate was continuously incremented in “ramp” fashion by computer control. The rate of work rate incrementation was calculated for each individual subject by considering their predicted V̇o2 max and level of habitual exercise activity with the intention of obtaining an exercise phase of 8–12 minutes before exhaustion.12 The subjects breathed through a mouthpiece and wore a nose clip.
Minute ventilation was measured using a mass flow meter, and expired fractional concentrations of oxygen and carbon dioxide were continuously monitored by paramagnetic oxygen analyzer and nondispersive infrared CO2 analyzer, respectively (2900; SensorMedics Corp., Loma Linda, CA). Gas exchange (i.e., oxygen uptake and carbon dioxide output) was calculated breath-by-breath using standard algorithms.13
A 12-lead electrocardiogram was obtained at rest and every 2 minutes throughout exercise (Quinton 5000; Seattle, WA), and heart rate was monitored continuously by rhythm strip from the same instrument. Peripheral oxygenation was monitored by a pulse oximeter (Ohmeda 3700; Datex-Ohmeda, Madison, WI) attached to a finger. If a subject was unable to complete the exercise test protocol during screening, they were not included in the study.
Detection of the metabolic threshold for lactate accumulation by noninvasive gas exchange measurements is inevitably subject to the possibility of observer error. In order to overcome this difficulty, we separately coded each of the sets of gas exchange data and presented them to two experienced exercise physiologists who were blinded to the study design. A standardized approach to interpretation was agreed on beforehand by these observers and has been previously validated.13 Essentially, this approach involved using the plot of V̇co2 versus V̇o2 to detect the threshold and then attempting to verify this by plots and tabulated data for ventilatory equivalents. Ventilatory threshold was obtained using the plot of V̇E versus V̇co2 to obtain the inflection point where unbuffered H+ stimulates ventilation via the carotid bodies.
The subjects were instructed to keep their lifestyles relatively unchanged during the trial, including dietary habits, exercise, and other routine activities, and not to take any other medicinal herbs and drugs during the trial. They were also instructed to report any adverse events to the investigators during the treatment period. In addition, brief interim histories and physical examinations at follow-up visits were used to inquire about adverse events and to check the well-being of the subjects. No adverse events occurred.
Statistical analysis
Descriptive statistics were expressed as means and standard deviations. Baseline data and 12-week changes from baseline data were compared using a univariate Student's t test for two independent samples. A χ2 test was used to compare categorical data. Overall differences between the groups were compared for key outcome measures using multivariable analysis of variance and Hotelling-Lawley trace tests. The level of statistical significance was set at a p value of <0.05.
Results
Twenty (20) subjects were recruited and randomized for this study in 1:1 fashion. After randomization, 3 subjects dropped out of the placebo group and 2 dropped from the Cs-4 group for schedule conflict (2 subjects), personal reason (2 subjects), and 1 was lost in follow-up. Two (2) subjects started on placebo and 1 started on Cs-4 before they dropped out of the study. A total of 15 subjects completed the study. Eight (8) were randomized to receive Cs-4 and 7 to receive placebo. The baseline characteristics of the subjects are summarized as mean (standard deviation) in Table 1. The subjects in both groups had comparable BMI, 27.6 kg/m2 in the placebo group versus 25.5 kg/m2 in the experimental group. (Table 1).
Table 1.
Baseline Characteristics of the Subjects
| Cs-4® | Placebo | |
|---|---|---|
| Number | 8 | 7 |
| Age (years) | 59.6 (8.8) | 56.3 (6.1) |
| BMI (kg/m2) | 25.5 (2.8) | 27.6 (5.6) |
| Male:female | 3:4 | 3:5 |
| Ethnicity | ||
| White | 4 | 6 |
| African American | 2 | 1 |
| Native American | 1 | 0 |
| Asian | 1 | 0 |
| Maximum work rate (W) | 135 (15) | 155 (24) |
| Work efficiency (mL/min/W) | 10.0 (0.0) | 10.1 (0.0) |
| V̇o2 max (L/min) | 1.95 (0.31) | 2.05 (0.30) |
| V̇o2 max (mL/kg/min) | 26.4 (3.9) | 26.2 (5.1) |
| Metabolic threshold (L/min) | 0.84 (0.17) | 1.03 (0.33) |
| Work rate at metabolic threshold (W) | 52.1 (14.1) | 67.1 (24.6) |
| Maximum heart rate (/min) | 137 (6) | 145 (11) |
| Ventilatory threshold (L/min) | 1.25 (0.32) | 1.63 (0.67) |
| Maximum ventilation (L/min) | 70.6 (6.4) | 72.6 (6.2) |
Values are mean (standard deviation). No statistically significant differences were found between groups in any of these variables.
BMI, body–mass index; V̇o2 max, maximal oxygen uptake.
Owing to the fact that we assessed several physiologic variables, we ran a multivariable analysis of variance and used the Hotelling-Lawley trace test to adjust for multiple comparisons. Using these methods, we did not find any overall differences between the two groups either at baseline or after 12 weeks of treatment (Cs-4 or placebo). However, noting trends that suggested a difference between in Cs-4 and placebo in key variables, and given that this was a small exploratory study, we proceeded to perform univariate analysis on a limited number of these variables. In doing so, we recognize that these statistical methods have limitations and therefore we have been very conservative in the conclusions we have drawn from these data.
The aerobic capacities measured by maximum oxygen uptake (V̇o2 max) were comparable between the two groups at baseline (placebo group 2.05 ± 0.30 L/ versus Cs-4 group 1.95 ± 0.31 L/min). When measured in mL/kg/min, baseline V̇o2 max was also comparable between groups (placebo group 26.2 ± 5.1 mL/kg/min versus Cs-4 group 26.4 ± 3.9 mL/kg/min). The baseline metabolic threshold in the placebo group was 1.03 ± 0.33 L/min and the Cs-4 group showed 0.84 ± 0.17 L/min. We observed similar results for the ventilatory threshold, which is the moment during incremental exercise when unbuffered H+ stimulates ventilation via the carotid bodies. This moment was detected from an inflection in the plot of minute ventilation (V̇E) versus carbon dioxide output (V̇co2). The placebo groups achieved 1.63 ± 0.67 L/min for their ventilatory threshold, while the Cs-4 group showed 1.25 ± 0.32 L/min for their ventilatory threshold (Table 2).
Table 2.
Changes in Selected Exercise Parameters with Cs-4® or Placebo
| |
Cs-4 |
Placebo |
||
|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | |
| V̇o2 max (L/min) | 1.95 (0.00) | 1.94 (0.00) | 2.05 (0.01) | 2.04 (0.01) |
| V̇o2 max (mL/kg/min) | 26.4 (1.4) | 26.3 (1.4) | 26.2 (1.9) | 26.1 (1.9) |
| Metabolic threshold (L/min) | 0.83 (0.06) | 0.93 (0.08)* | 1.03 (0.13) | 0.99 (0.11) |
| Work rate at metabolic threshold (W) | 52.1 (8.7) | 55.2 (13.3) | 67.1 (8.2) | 60.5 (5.1) |
| Ventilatory threshold (L/min) | 1.25 (0.11) | 1.36 (0.15)** | 1.62 (0.11) | 1.10 (0.11) |
| Work rate at ventilatory threshold (W) | 91.1 (11.3) | 97.2 (12.0) | 126.1 (20.7) | 123.7 (22.9) |
Values are mean (standard error of the mean).
V̇o2 max, maximal oxygen uptake.
Significance: *p = 0.022 compared with baseline. **p = 0.031.
The maximum heart rate achieved at baseline for the placebo group was 145 ± 11 beats/minute and was 137 ± 6 beats/minute for the Cs-4 group. Maximum work rate was also comparable at baseline. The placebo group reached 155 ± 24 watts and the intervention group reached 135 ± 15 watts. The placebo group had a maximum exercise ventilation of 72.6 ± 6.2 L/min while Cs-4 group was at 70.6 ± 6.4 L/min (Table 2).
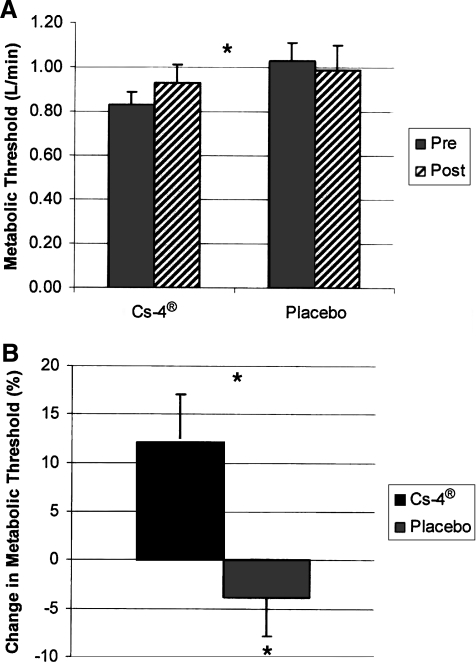
After 12 weeks of supplementation of Cs-4, the mean (standard error of the mean) metabolic threshold increased from 0.84 ± 0.06 L/min at baseline to 0.93 ± 0.08 L/min. This is an increase of 10.5% (p = 0.022). The placebo group started with a metabolic threshold of 1.03 ± 0.13 L/min at baseline and ended up at 0.99 ± 0.08 L/min, which was a decrease of 3.9% (p = 0.182) (Fig. 1).
FIG. 1.
Effect of Cs-4® and placebo on the metabolic threshold (V̇02θ) above which lactate accumulates during incremental exercise. Panel (A) shows absolute changes in V̇o2θ expressed as levels of oxygen uptake in L/min. Panel (B) shows percentage changes from baseline. *Statistical significance p = 0.022.
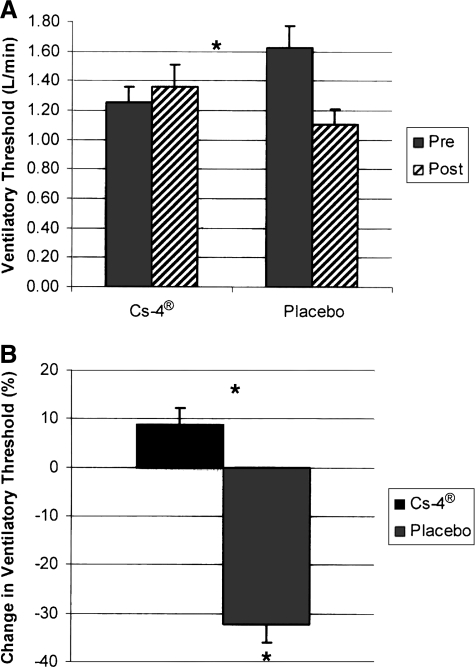
We observed similar results for the ventilatory threshold, which is the moment during incremental exercise when unbuffered H+ stimulates ventilation via the carotid bodies. This moment was detected from an inflection in the plot of minute ventilation (V̇E) versus carbon dioxide output (V̇co2). The intervention group achieved 1.25 ± 0.11 L/min at baseline and increased to 1.36 ± 0.11 L/min. This was an increase of 8.5% (p = 0.031). The ventilatory threshold in the placebo group fell from 1.63 ± 0.11 L/min at baseline to 1.10 ± 0.11 L/min; a decrease of 32.5% (p = 0.414) (Fig. 2).
FIG. 2.
Effect of Cs-4® and placebo on the ventilatory threshold (V̇Eθ) above which unbuffered hydrogen ion stimulates the carotid body during incremental exercise. Panel (A) shows absolute changes in V̇Eθ expressed as levels of oxygen uptake in L/min. Panel (B) shows percentage changes from baseline. *Statistical significance p = 0.031.
At 12 weeks, there was no significant change in V̇o2 max in either group compared with the baseline measurement. Also, we did not observe significant differences in maximum heart rate, maximum work rate, or maximum ventilation in either group as compared with the baseline testing results. Blood pressure, blood pressure recovery, lactic acid recovery, lactic acid peak level, and heart rate recovery were recorded. There were no significant differences in these parameters between baseline and week 12 in either group.
Discussion
Cordyceps sinensis has been and is taken by millions of aging Chinese for invigoration and relief of fatigue as well as various medical problems.3 The present study was designed to test the hypothesis that the invigorating effect of C. sinensis would improve aerobic capacity as determined by measurement of maximum oxygen uptake during incremental exercise testing in older adults. In this study, we used Cs-4 as opposed to natural/wild Cordyceps for reasons important to clinician and patient. If Cs-4 can be used interchangeably with natural Cordyceps fungus, it can be an affordable, sustainably produced, and chemically standardized preparation. Furthermore, natural Cordyceps is often adulterated due to its increasing scarcity and very high market price.
Changes in V̇o2 max were not observed in the group who received supplementation with Cs-4 nor in the group who received placebo. However, 12 weeks of treatment with Cs-4 resulted in an increase in metabolic threshold of 10.5% and increase in ventilatory threshold of 8.5%. These threshold values are submaximal, non–effort-dependent measures of exercise performance. They were selected by the metabolic measurement system and verified by two independent investigators. A higher metabolic threshold for lactate accumulation indicates better aerobic performance, and that is a subject could perform a higher level of exercise without fatigue.14 Similarly, a higher ventilatory threshold reflects postponement of lactate accumulation and more effective buffering of any lactic acid that accumulates.15 The consistent pattern of changes in aerobic capacity as well as metabolic and ventilatory thresholds, albeit small, is consistent with improved aerobic performance in the subjects receiving Cs-4. Furthermore, if these changes are confirmed by larger studies they could be interpreted as meaningful improvements in terms of activities of daily living in older subjects. There were no reported changes in diet, activity, and exercise during the study period. Therefore, these improvements in aerobic performance are best explained by the supplementation with Cs-4.
Naturally occurring Cordyceps sinensis and its fermentation product Cs-4 contain a broad range of chemical constituents, as summarized by Zhu et al.2 Early investigation showed that Cordyceps species produce two principal constituents: cordycepin and cordycepic acid. Cunningham et al.,16 in 1951, isolated cordycepin and demonstrated its chemical composition. Later, cordycepin, produced by Cordyceps militaris, was identified as 3′-deoxyadenosine.17 Cordycepic acid is now known to be d-mannitol.18 The work of Cunningham represented one of the first isolations of a naturally occurring nucleoside.16 Small quantities of 3′-deoxyadenosine and 2′-deoxyadenosine have also been found in natural Cordyceps and Cs-4.19 These nucleotides and their precursor, adenosine, have been purported to have beneficial effects on the cardiovascular system.20 These are potential methods by which Cordyceps may improve aerobic performance.
Manabe et al.21 investigated the effects of a mycelial extract of cultured C. sinensis on hepatic energy metabolism in mice using 31P MRS. These investigators observed an increase in the ratio of ATP to inorganic phosphate (ATP:Pi) in the treated mice representing an increase in cellular energy state. Another study using water-soluble extracts of Cs-4 also showed an increase of 45–55% in ATP:Pi ratios in the liver of mice treated with Cs-4 compared with placebo.22 More recently, Manabe et al.,23 confirming their earlier findings, suggested that any increase in hepatic energy metabolism might be due to increased hepatic blood flow. Whatever the mechanism, if a similar improvement in cellular energy state occurred in muscle, this suggests a potential mechanism for the changes in aerobic performance observed in the present study.
Conclusions
This small pilot study shows a pattern of physiologic changes during incremental exercise testing that lends support to the hypothesis that Cs-4, the standardized fermentation product of naturally occurring C. sinensis, enhances aerobic performance in older human subjects. These findings support the belief, long held in China, that Cordyceps sinensis has the potential to improve exercise capacity and resistance to fatigue. An additional study involving more subjects would be needed to provide convincing evidence of post-treatment differences between the two groups. Also, endurance exercise testing at a constant submaximal work rate would be necessary to verify a truly ergogenic effect.
Acknowledgments
Funding for this study was provided in part through a National Institutes of Health training grant DK0718033. The Cs-4 was provided by Pharmanex, Inc., USA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bao TT. Further study of pharmacological functions on Jin Shui Bao. J Admin Trad Chinese Med. 1995;5(suppl6) [Google Scholar]
- 2.Zhu JS. Halpern GM. Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis. Part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 3.Commission CP, editor. Cordyceps. Pharmacopoeia of the People's Republic of China. Beijing: People's Medical Publishing House; 2005. p. 36. [Google Scholar]
- 4.Winkler D. Seventh International Congress on Traditional Asian Medicine, 2008. Thimphu, Bhutan: Institute for Traditional Medicine Services; 2008. Panel abstracts: Yartsa Gunbu (Cordyceps sinensis): An ancient medicinal fungus transforming rural Tibet; pp. 7–11. [Google Scholar]
- 5.Zhu JS. Halpern GM. Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis. Part II. J Altern Complement Med. 1998;4:429–457. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 6.Chen K. Li C. Recent advances in studies on traditional Chinese anti-aging materia medica. J Tradit Chin Med. 1993;13:223–226. [PubMed] [Google Scholar]
- 7.Chen DG. Effects of Jing Shui Bao capsule on quality of life of patients with chronic heart failure. J Admin Traditional Chin Med. 1995;5:40–43. [Google Scholar]
- 8.Jiang JC. Gao YF. Summary of treatment of 37 chronic renal dysfunction patients with Jin Shui Bao. J Admin Traditional Chin Med. 1995;5:23–24. [Google Scholar]
- 9.Lei M. Wang JP. Jin Shui Bao capsule as adjuvant treatment for acute stage pulmonary heart disease: Analysis of therapeutic effect of 50 clinical cases. J Admin Traditional Chin Med. 1995;5:28–29. [Google Scholar]
- 10.Division HMS, editor. Cordyceps. The Korean Herbal Pharmacopoeia (KHP) Seoul, Korea: Korea Food and Drug Administration; 2002. p. 59. [Google Scholar]
- 11.Brownell S. Beijing's Games: What the Olympics Mean to China. Rownam & Littlefield. 2008 [Google Scholar]
- 12.Buchfuhrer MJ. Hansen JE. Robinson TE, et al. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol. 1983;55:1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 13.Braith RW. Graves JE. Leggett SH. Pollock ML. Effect of training on the relationship between maximal and submaximal strength. Med Sci Sports Exerc. 1993;25:132–138. doi: 10.1249/00005768-199301000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman K. Stringer WW. Casaburi R, et al. Determination of the anaerobic threshold by gas exchange: Biochemical considerations, methodology and physiological effects. Z Kardiol. 1994;83(suppl 3):1–12. [PubMed] [Google Scholar]
- 15.Cooper CB. Storer TW. Exercise Testing and Interpretation: A Practical Approach. New York: Cambridge University Press; 2001. [Google Scholar]
- 16.Cunningham KG. Manson W. Spring FS. Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 17.Kaczka EA. Trenner NR. Arison B, et al. Identification of cordycepin, a metabolite of Cordyceps militaris, as 3'-deoxyadenosine. Biochem Biophys Res Commun. 1964;14:456–457. doi: 10.1016/0006-291x(64)90086-5. [DOI] [PubMed] [Google Scholar]
- 18.Kiho T. Hui J. Yamane A. Ukai S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol Pharm Bull. 1993;16:1291–1293. doi: 10.1248/bpb.16.1291. [DOI] [PubMed] [Google Scholar]
- 19.Chen SZ. Chu JZ. NMR and IR studies on the characterization of cordycepin and 2″-deoxyadenosine. Zhong Guo Kangshengsu Zazhi. 1996;21:9–12. [Google Scholar]
- 20.Yue D. Feng X. Liu H. Bao TT. Advanced study for traditional Chinese herbal medicine. Beijing Medical University and Peking Union Medical University Press; 1995. Cordyceps sinensis; pp. 91–113. [Google Scholar]
- 21.Manabe N. Sugimoto M. Azuma Y, et al. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn J Pharmacol. 1996;70:85–88. doi: 10.1254/jjp.70.85. [DOI] [PubMed] [Google Scholar]
- 22.Dai G. Bao T. Xu C, et al. CordyMax Cs-4 improves steady-state bioenergy status in mouse liver. J Altern Complement Med. 2001;7:231–240. doi: 10.1089/107555301300328106. [DOI] [PubMed] [Google Scholar]
- 23.Manabe N. Azuma Y. Sugimoto M, et al. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. Br J Nutr. 2000;83:197–204. doi: 10.1017/s0007114500000258. [DOI] [PubMed] [Google Scholar]