Abstract
Background/Aims
To compare the effect of levofloxacin and moxifloxacin on treatment outcomes among patients with multidrug-resistant tuberculosis (MDR-TB).
Methods
A retrospective analysis of 171 patients with MDR-TB receiving either levofloxacin or moxifloxacin was performed. Treatment responses were categorized into treatment success (cured and treatment completed) or adverse treatment outcome (death, failure, and relapsed).
Results
The median age of the patients was 42.0 years. Approximately 56% of the patients were male. Seventeen patients had extensively drug-resistant tuberculosis, and 20 had a surgical resection. A total of 123 patients (71.9%) received levofloxacin for a median 594 days, and 48 patients (28.1%) received moxifloxacin for a median 673 days. Other baseline demographic, clinical, and radiographic characteristics were similar between the two groups. The moxifloxacin group had a significantly higher number of resistant drugs (p < 0.001) and a higher incidence of resistance to ofloxacin (p = 0.005) in the drug sensitivity test. The treatment success rate was 78.9% in the levofloxacin group and 83.3% in the moxifloxacin group (p = 0.42). Adverse reactions occurred at similar rates in the groups (p = 0.44). Patients in the moxifloxacin group were not more likely to have treatment success than those in the levofloxacin group (adjusted odds ratio, 0.76; 95% confidence interval, 0.24 to 2.43; p = 0.65).
Conclusions
Both levofloxacin and moxifloxacin showed equivalent efficacy for treating MDR-TB.
Keywords: Levofloxacin; Moxifloxacin; Tuberculosis, multidrug-resistant; Quinolones; Tuberculosis
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), defined as in vitro resistance to at least isoniazid and rifampicins, is a growing health concern. An estimated 440,000 (95% confidence interval [CI], 390,000 to 510,000) cases of MDR-TB, which is 3.6% of all incident TB cases, emerge each year, causing 150,000 deaths worldwide [1].
Only a few effective second-line anti-TB drugs are available, and those at the forefront are fluoroquinolones (FQNs). FQNs show an encouraging in vitro pharmacokinetic profile for treating TB [2-5], and current guidelines for managing MDR-TB recommend that all patients be treated with FQNs if the strain is susceptible or if the agent is thought to have efficacy [5]. In particular, the minimum inhibitory concentrations of moxifloxacin are lower than those of levofloxacin [3,6], and moxifloxacin exhibits in vitro activity [6], and early bactericidal activity [7] that is comparable to that of isoniazid. Although several studies [8-11] have compared levofloxacin with other FQNs, such as ciprofloxacin or ofloxacin, studies comparing levofloxacin and moxifloxacin are lacking. This led us to conduct a retrospective case-control study of patients with MDR-TB who were treated with either levofloxacin or moxifloxacin and to compare their treatment outcomes.
METHODS
Study population
Patients with MDR-TB receiving either levofloxacin or moxifloxacin along with other second-line anti-TB medication from January 2002 through December 2008 were included. Patients were treated in one of three hospitals affiliated with Seoul National University College of Medicine in Korea: Seoul National University Hospital, Seoul Metropolitan Government Seoul National University Boramae Medical Center, or Seoul National University Bundang Hospital. Mycobacterium tuberculosis was identified by sputum culture, and all patients showed resistance to at least isoniazid and rifampicins in in vitro drug-susceptibility testing. Patients < 18 years of age, those treated with both levofloxacin and moxifloxacin for TB, and patients who received less than 3 months of levofloxacin or moxifloxacin were excluded from analysis. This study was approved by the ethics review committees of all three hospitals. Previous studies by our group were also based on part of this population of patients with MDR-TB [12-15].
The choice of FQNs was based on the preference of the attending physician, according to the drug-susceptibility test results. The other combined drugs included aminoglycosides, prothionamide, cycloserine, pyrazinamide, rifabutin, ethambutol, p-aminosalicylic acid, amoxicillin-clavulanic acid, clarithromycin, sulfamethoxazole, and trimethoprim. Drugs used before the diagnosis of MDR-TB were not included in the analysis.
Records were reviewed for age, gender, body mass index (BMI), underlying comorbidities, smoking history, family history of TB, primary drug resistance, laboratory test results, radiographic findings, FQN dose, the combination of extrapulmonary TB, nontuberculous mycobacteria (NTM) colonization, other anti-TB medications, duration of treatment, results of drug-susceptibility testing, and adverse events.
Definitions of terms and outcomes
Treatment outcomes were classified into the following groups in accordance with the suggested criteria of Laserson et al. [16]: cure, treatment completed, failure, death, default, and transferred out. Additionally, if patients were diagnosed with bacteriologically confirmed MDR-TB after being cured or after treatment was completed, they were considered relapse cases. Based on these classifications, treatment outcomes were further categorized into treatment success (cured and treatment completed) or adverse treatment outcome (death, failure, and relapse) to identify predictors of poor treatment response.
Extensively drug-resistant tuberculosis (XDR-TB) was defined as laboratory-confirmed resistance to all of the following: isoniazid, rifampins, any FQNs, and second-line injectable agents such as capreomycin, kanamycin, and amikacin. Although some patients with a poor response to treatment had subsequent resistance testing performed, they were classified according to the initial drug-susceptibility test results.
Statistical analysis
Data are expressed as median values with interquartile ranges (IQRs) or means ± standard deviations. The demographic characteristics, laboratory results, radiographic findings, and treatment outcomes were compared between the levofloxacin group and the moxifloxacin group using Pearson's χ2 test or Fisher's exact test for categorical variables and Student's t test for continuous variables. To understand the impact of choice between the levofloxacin and moxifloxacin group on treatment outcomes, we compared selected clinical variables between treatment success and failure through a univariate comparison and subsequent multiple logistic regression. All statistical analyses were performed with SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient demographic and clinical characteristics
Between January 2002 and December 2005, 171 patients received either levofloxacin or moxifloxacin to treat MDR-TB. In total, 123 patients (71.9%) were treated with levofloxacin and 48 patients (28.1%) received moxifloxacin. One-hundred nine patients were from Seoul National University Hospital, 26 were from Seoul Metropolitan Government Seoul National University Boramae Medical Center, and 36 patients were from Seoul National University Bundang Hospital. Except for one 38-year-old Chinese man, all 170 patients were Korean.
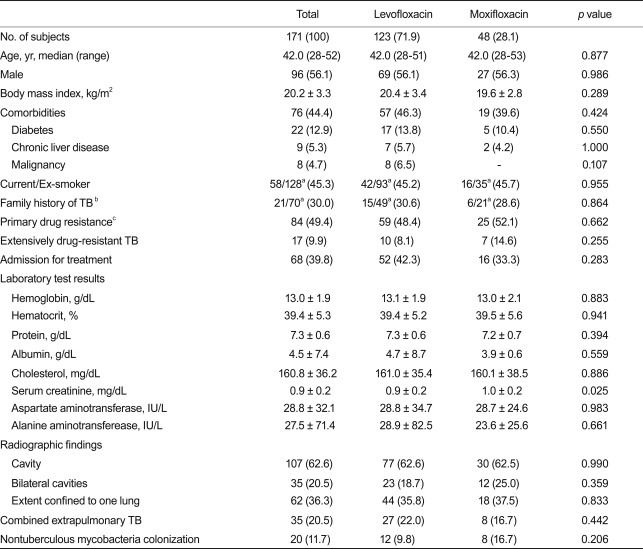
All subjects had radiographic and bacteriologic evidence of pulmonary TB, and 20.5% had combined extrapulmonary involvement. The most common extrapulmonary TB was tuberculous lymphadenopathy found in 17 patients (10.5%). The median age of the 171 patients was 42 years (IQR, 28 to 52). Ninety-six patients (56.1%) were male, and 84 patients (49.4%) had a history of TB treatment. Among the previously treated patients, 64.3% had been treated once, 28.6% twice, and 7.1% more than three times. No significant difference was found between patients treated with levofloxacin or moxifloxacin in terms of BMI, smoking history, family history of TB, primary drug resistance, proportion of XDR-TB, and radiographic findings (Table 1). However, serum creatinine was higher in the moxifloxacin group (0.9 ± 0.2 vs. 1.0 ± 0.2; p = 0.025) than in the levofloxacin group. Coinfection with the human immunodeficiency virus was rare, occurring in only one patient in the levofloxacin group. NTMs colonization was found in 20 patients (11.7%), and the most common NTM identified was Mycobacterium abscessus.
Table 1.
Baseline clinical characteristics of the patients who had multidrug-resistant tuberculosis (MDR-TB) treated with levofloxacin or moxifloxacin
Values are presented as the mean ± SD or number (%), unless otherwise indicated. p values were based on a comparison between patients on levofloxacin and patients on moxifloxacin.
aNo. of available data.
bHistory of tuberculosis among second-degree relatives.
cMDR-TB patients without prior treatment with anti-TB drugs were classified as having primary resistance.
Treatment and outcomes
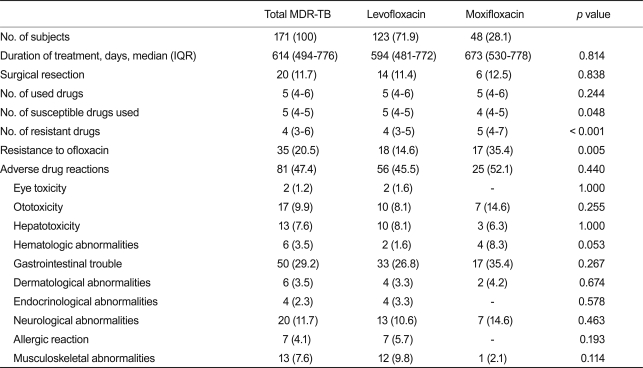
The median duration of treatment was 594 days (IQR, 481 to 772) in the levofloxacin group and 673 days (IQR, 530 to 778) in the moxifloxacin group. The usual prescribed daily dose of moxifloxacin was 400 mg, and the daily dose of levofloxacin varied from 300 to 1,000 mg. Sixty-eight patients (39.8%) were hospitalized at the initiation of treatment, and 20 patients had at least one surgical resection of a diseased lung as an adjunctive treatment for TB.
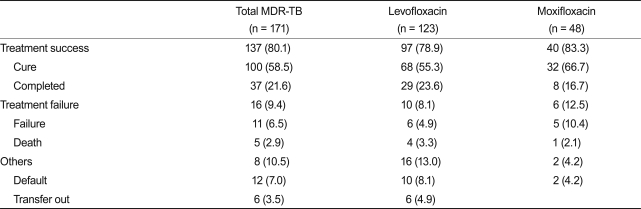
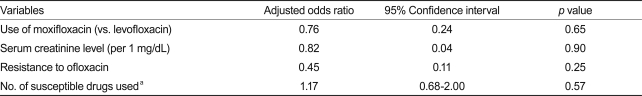
A median of five drugs (range, 4 to 6) were used in the MDR-TB treatment, and this was similar between the two groups (p = 0.244). However, the number of susceptible drugs used was significantly lower in the moxifloxacin group (4 vs. 5; p = 0.048) than in the levofloxacin group. Furthermore, according to the drug-susceptibility test results, the number of resistant drugs (4 vs. 5; p < 0.001) and the number of patients with ofloxacin resistance (14.6% vs. 35.4%; p = 0.005) was significantly higher in the moxifloxacin group than in the levofloxacin group. The use of ethambutol was more common in the levofloxacin group than in the moxifloxacin group (43.9% vs. 22.9%; p = 0.011) and the use of amoxicillin-clavulanate (24.4% vs. 45.8%; p = 0.006) was more common in the moxifloxacin group than the levofloxacin group (Table 2). The use of other TB drugs and the rates of adverse drug reactions were not different between the two groups. Isoniazid was used in 7.6% and rifamycins were used in 6.4% of the patients after being diagnosed with multidrug resistance. Among 171 patients, 137 (80.1%) were considered treatment successes and 16 (9.4%) were considered treatment failures. Eighteen patients (10.5%) were classified as default or transfer-out (Table 3). Treatment success was achieved among 97 patients (78.9%) in the levofloxacin group and 40 (83.3%) in the moxifloxacin group. Based on the variables included in the univariate comparison between the treatment success and failure groups, the final multiple logistic regression model included serum creatinine level, resistance to ofloxacin, and the number of susceptible drugs used. The patients in the moxifloxacin group were not more likely to have treatment success than those in the levofloxacin group (adjusted odds ratio, 0.76; 95% CI, 0.24 to 2.43; p = 0.65; Table 4).
Table 2.
Treatment modalities and adverse reactions among patients who had multidrug-resistant tuberculosis (MDR-TB) treated with levofloxacin or moxifloxacin
Values are presented as number (%). p values were based on a comparison between patients on levofloxacin and patients on moxifloxacin.
IQR, interquartile range.
Table 3.
Treatment outcomes among patients who had multidrug-resistant tuberculosis (MDR-TB) treated with levofloxacin or moxifloxacin
Values are presented as number (%).
Table 4.
Multivariate analysis of treatment success comparing moxifloxacin and levofloxacin
aOdds ratio for an increase of one susceptible drug used.
DISCUSSION
FQNs are one of the most promising classes of TB drugs and have been strongly recommended for treating MDR-TB [17-20]. As gatifloxacin was removed from most markets due to serious adverse drug reactions, levofloxacin and moxifloxacin are the two most frequently recommended FQNs for treating patients with MDR-TB [5,21]. Although clinical data comparing moxifloxacin to levofloxacin are scarce, previous in vitro and animal studies have reported favorable results for moxifloxacin compared to other FQNs [6,22-25]. However, this apparent superiority of moxifloxacin against TB was not corroborated in our study. A lack of superiority of moxifloxacin in our study may have been due to higher resistance to ofloxacin and fewer susceptible drugs used in the moxifloxacin group compared to the levofloxacin group. However, the superiority of moxifloxacin was not uncovered even after adjusting for these variables in a multivariate analysis.
The result can be explained by several factors. Although a variety of animals has been tested as animal models for pulmonary TB, research on animals has its limitations and may not adequately reflect human pulmonary TB [26]. Furthermore, most of the animal studies [22-24,27,28] comparing FQNs for treating TB involved mice. Together with the difference in lung pathology, the bacterial loads generally remain high in the lungs of infected mice, which is different from that in humans [29]. In fact, the effect of high-dose levofloxacin (1,000 mg/day) is comparable to moxifloxacin in terms of early bactericidal activity in patients with pulmonary TB [30].
The use of at least four susceptible drugs has been recommended to cure patients with MDR-TB [5,21]. Among the various drugs with antimycobacterial activities, injectables and FQNs, as well as ethambutol and pyrazinamide, are believed to be the most potent for patients with MDR-TB. Moreover, the impact of one of four or five drugs used to treat patients with MDR-TB may not make much difference in terms of outcome.
Lack of a difference in treatment outcomes between the levofloxacin and moxifloxacin groups may be attributable to low statistical power. Although the number of patients with MDR-TB was comparable to that of other studies, the small number of patients in the moxifloxacin group may have resulted in insufficient statistical power to detect real differences between the two groups. Prospective randomized studies enrolling a sufficient number of patients with MDR-TB could elucidate the relative efficacies among FQNs.
Treatment response was similar between patients who had MDR-TB treated with either levofloxacin or moxifloxacin. Further randomized prospective studies are warranted to compare the efficacy of FQNs used in a MDR-TB regimen.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.World Health Organization. Multidrug and extensively drug-resistant tuberculosis: 2010 global report on surveillance and response. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Berning SE. The role of fluoroquinolones in tuberculosis today. Drugs. 2001;61:9–18. doi: 10.2165/00003495-200161010-00002. [DOI] [PubMed] [Google Scholar]
- 3.van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53:849–862. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs. 2007;67:2077–2099. doi: 10.2165/00003495-200767140-00007. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2008. [Google Scholar]
- 6.Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3:432–442. doi: 10.1016/s1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 7.Pletz MW, De Roux A, Roth A, Neumann KH, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother. 2004;48:780–782. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MR. Activity of quinolones against mycobacteria. Drugs. 1999;58(Suppl 2):19–22. doi: 10.2165/00003495-199958002-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi N, Goh KS, Bryskier A, Devallois A. In vitro activities of levofloxacin used alone and in combination with first- and second-line antituberculous drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1610–1616. doi: 10.1128/aac.40.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yew WW, Chan CK, Leung CC, et al. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest. 2003;124:1476–1481. doi: 10.1378/chest.124.4.1476. [DOI] [PubMed] [Google Scholar]
- 11.Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–751. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SS, Kim HR, Kim HJ, et al. Impact of resistance to first-line and injectable drugs on treatment outcomes in MDR-TB. Eur Respir J. 2009;33:581–585. doi: 10.1183/09031936.00099608. [DOI] [PubMed] [Google Scholar]
- 13.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patient s with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 14.Kim DK, Park GM, Hwang YI, et al. Microarray analysis of gene expression associated with extrapulmonary dissemination of tuberculosis. Respirology. 2006;11:557–565. doi: 10.1111/j.1440-1843.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J. 2006;28:576–580. doi: 10.1183/09031936.06.00023006. [DOI] [PubMed] [Google Scholar]
- 16.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–645. [PubMed] [Google Scholar]
- 17.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 18.Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA. 1996;276:1229–1235. [PubMed] [Google Scholar]
- 19.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CY, Enarson DA, Yu MC, et al. Outcome of pulmonary multidrug-resistant tuberculosis: a 6-yr follow-up study. Eur Respir J. 2006;28:980–985. doi: 10.1183/09031936.06.00125705. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Coates AR, Mitchison DA. Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:653–657. doi: 10.1128/AAC.47.2.653-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki E, Miyazaki M, Chen JM, Chaisson RE, Bishai WR. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veziris N, Truffot-Pernot C, Aubry A, Jarlier V, Lounis N. Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2003;47:3117–3122. doi: 10.1128/AAC.47.10.3117-3122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–137. doi: 10.1016/s1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 27.JI B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007;51:576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn JL, Capuano SV, Croix D, et al. Non-human primates: a model for tuberculosis research. Tuberculosis (Edinb) 2003;83:116–118. doi: 10.1016/s1472-9792(02)00059-8. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–612. [PubMed] [Google Scholar]