Abstract
Neutrophils play an important role in immunological function. Neutropenic patients are vulnerable to infection, and except fever is present, inflammatory reactions are scarce in many cases. Additionally, because infections can worsen rapidly, early evaluation and treatments are especially important in febrile neutropenic patients. In cases in which febrile neutropenia is anticipated due to anticancer chemotherapy, antibiotic prophylaxis can be used, based on the risk of infection. Antifungal prophylaxis may also be considered if long-term neutropenia or mucosal damage is expected. When fever is observed in patients suspected to have neutropenia, an adequate physical examination and blood and sputum cultures should be performed. Initial antibiotics should be chosen by considering the risk of complications following the infection; if the risk is low, oral antibiotics can be used. For initial intravenous antibiotics, monotherapy with a broad-spectrum antibiotic or combination therapy with two antibiotics is recommended. At 3-5 days after beginning the initial antibiotic therapy, the condition of the patient is assessed again to determine whether the fever has subsided or symptoms have worsened. If the patient's condition has improved, intravenous antibiotics can be replaced with oral antibiotics; if the condition has deteriorated, a change of antibiotics or addition of antifungal agents should be considered. If the causative microorganism is identified, initial antimicrobial or antifungal agents should be changed accordingly. When the cause is not detected, the initial agents should continue to be used until the neutrophil count recovers.
Keywords: Practice guideline, Neutropenia, Fever, Korea
INTRODUCTION
Background and purpose
The neutrophil is an important component of the innate immune system. Neutrophils primarily defend the body against microorganisms, and a low number of neutrophils indicates that a person is vulnerable to infection. Additionally, because neutropenic patients lack the leukocytes needed to develop an inflammatory response, common inflammatory manifestations that are observed in patients within the normal range of leukocytes are rarely found. Thus, except in the presence of a fever, an accurate diagnosis is difficult and the most appropriate time for treatment may be missed. Thus, febrile neutropenic patients should be treated differently from other febrile non-neutropenic patients [1].
Many countries, including the US and Europe, have developed and reported guidelines on approaches to and treatments for febrile neutropenic patients. However, the pattern of neutropenic fever has changed over the last 20 years, and the distribution and resistance rate of causative microorganisms are known to differ by region, antibiotic prophylaxis, and the use of catheters [2].
The aim of this study was to investigate the epidemiology of infectious diseases and the patterns of resistance and antibiotic therapy in febrile neutropenic patients, and to develop and suggest empirical treatment guidelines for neutropenic fever that fit the circumstances in Korea through both a foreign literature review and a multidisciplinary study. These guidelines are for adults and refer to data published in Korea. These guidelines are also applicable to other diseases associated with neutropenia, anticancer therapy of malignant tumors, and hematopoietic stem cell transplantation (HSCT) recipients.
Organization of a guideline-development committee
In June 2009, the committee for the development of "Guidelines for the Empirical Therapy of Neutropenic Fever Patients based on Literature in Korea" was organized by receiving recommendations from committee members from eight academic societies under the supervision of the National Evidence-based Healthcare Collaborating Agency (NECA): the Korean Society of Infectious Diseases (KSID), the Korean Society for Immunocompromised Host Infections (KSIHI), the Korean Cancer Association (KCA), the Korean Society of Clinical Microbiology (KSCM), the Korean Society of Blood and Marrow Transplantation (KSBMT), the Korean Society of Hematology (KSH), the Korean Society for Chemotherapy (KSC), and the Korean Society of Clinical Oncology (KSCO). The committee consists of five infectious diseases physicians, four hematology-oncology physicians, one laboratory medicine physician, one NECA internist, and one methodologist.
Literature search
For a systematic literature review, the latest guidelines of Infectious Diseases Society of America (IDSA) [2], National Comprehensive Cancer Network (NCCN) [3], the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) [4-13], the First European Conference on Infections in Leukaemia (ECIL-1) [14-18], Asia-Pacific [19], and Japan [20-27] were collected. To search the literature published after the publication of the IDSA guidelines (2002), which are relatively widely used, the PubMed (www.pubmed.gov) search engine was used. The search period was from January 2002 to October 2009. Search entries for neutropenia were "neutrop*nia," "granulocytop*nia," and "leu?op*nia." The search entries for tumor were "cancer," "malignancy," "neoplasm," "leukemia," "lymphoma," "hematolog*" and the combination of "(stem or marrow) AND transplantation." Literature regarding fever and antibiotic therapy were searched by combining "fever or febrile," "anti-infect*," "anti-bacteri*," "anti-microb*," "anti-bio*," "anti-fung*," and "anti-vir*." To find Korean studies published in foreign journals, the Korean literature was also searched through the PubMed engine.
Major reports published in Korea over the last 10 years were searched through the database of Korean Studies Information (http://kiss.kstudy.com) and KoreaMed (http://www.koreamed.org). Search entries were combination of "neutrophil" or "granulocyte," "fever" and "infection" by Korean letters. Reports before 2000 were collected if they were considered to be related to the development of this treatment guideline. Related literature was added by searching references of the collected literature, manually if necessary. The searched Korean literature totaled 39 reports (4 review articles and 35 original articles). In total, 218 references are cited; 27 were from the Korean literature.
Formulation of key questions
To create empirical treatment guidelines for febrile neutropenic patients, the following major categories were selected: definition of neutropenia and fever, initial evaluation and risk of infection, antibiotic prophylaxis, initial antibiotic therapy for febrile neutropenic patients, re-evaluation after 3-5 days and change of antibiotics, use of glycopeptides, catheter-related infections, and antifungal therapy.
The subcommittee of infectious diseases specialists formulated key questions in each area. Key questions were determined by reviewing foreign treatment guidelines and recommendations that could cause problems in Korean circumstances.
Consensus
Recommended answers to the key questions were based on major guidelines and literature, and the final version of these recommendations was made by a consensus of the guideline development committee.
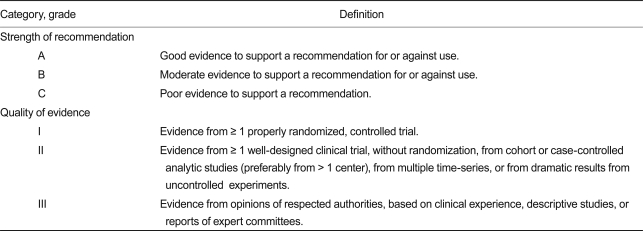
Strength of recommendations and quality of evidence
For strength of recommendations and quality of evidence, the methods used in the latest guidelines of IDSA were accepted (Table 1) [28]
Table 1.
Definition of strength of recommendation and quality of evidence
Adapted from the Canadian Task Force on the Periodic Health Examination [28].
Evaluation by external specialists
Questionnaire survey
To evaluate the key questions and recommended answers given by a consensus of the committee for the development of these guidelines, a questionnaire survey on the guidelines was performed. The questionnaire asked whether each recommendation could be accepted in Korea and whether the strength of each recommendation was graded appropriately.
The subjects of the questionnaire survey were infectious diseases physicians and hematology-oncology physicians to enhance its specialty, and physicians in general hospitals operating HSCT centers around the nation to ensure representativeness.
Symposia of related academic societies
The final treatment guidelines, which reflected opinions from the internal review and the questionnaire survey, were presented in symposia of major related societies through 2010. Additionally, its revision and spread are planned after acceptance of opinions of and evaluations by various specialist groups.
DEFINITION OF NEUTROPENIA AND FEVER
Fever is defined as an increase in body temperature to over 38.0℃, using a tympanic thermometer, or to over 37.5℃, using an axillary thermometer. If the tympanic or axillary temperature is thought to be inaccurate or the oral temperature is mainly measured, fever is defined as an increase in a single oral temperature to over 38.3℃ or to over 38.0℃ for more than 1 hour.
Neutropenia is defined as an absolute neutrophil count less than 500/mm3 or expected to be less than 500/mm3 within 2-3 days.
Major foreign guidelines, including those of IDSA and NCCN, define fever as an increase in oral temperature to over 38.3℃ once or to 38.0℃ for more than 1 hour [2,3,11,19,22]. In the questionnaire survey conducted with 33 medical staff members in 28 hospitals in Korea, 79% of the respondents answered that fever was defined as an increase in body temperature in two locations to over 38.0℃ or an increase in body temperature to over 38.0℃ for 1-2 hours [29]. Only two respondents (6%) measured oral temperature, and 31 (94%) said that they measured axillary or tympanic temperature [29]. Thus, the definition of fever using tympanic or axillary temperature, as stated in the guidelines developed by a consensus of specialists in Asia-Pacific countries, is more pragmatic in Korea [19]. The correlation between tympanic and core temperatures has been well studied [30,31], and a correlation in febrile neutropenic patients has also been reported [32]. Notably, even in patients with no or only mild fever, the oral temperature may read higher than the actual temperature in the presence of oral mucositis [33].
When the neutrophil count is reduced to less than 500/mm3, the risk of infection is increased [34]. In a study on the neutrophil count, measured at the time of fever, and the frequency of infection in leukemia patients undergoing chemotherapy, over 70% of febrile patients showed a neutrophil count of less than 500/mm3; furthermore, fever with a lower neutrophil count was caused by infection in more cases [35]. A questionnaire survey involving Korean medical institutions also revealed that 31 (94%) of the respondents used the same aforementioned definition [29].
INITIAL EVALUATION
Fever in neutropenic patients can be caused not only by bacterial or fungal infection, but also by non-infectious causes, such as drugs, blood transfusions, and the use of granulocyte colony stimulating factor. Because infection in neutropenic patients proceeds rapidly and symptoms or signs of an inflammatory response are rarely observed even in cases of infection, a close initial evaluation is necessary [36].
The initial evaluation of suspected febrile neutropenic patients should focus on determining possible causative sites or microorganisms. As soon as a patient is admitted to a hospital, a history should be taken, a physical examination should be conducted, and blood and other specimens should be collected for bacterial and fungal cultures.
A thorough history should include information on associated disease (s), currently used drug (s), the latest anticancer therapy, and whether a family member currently has an infectious disease. Decisions should be made regarding whether to hospitalize the patient and/or to use prophylactic antibiotics.
The physical examination should evaluate common sites of infection, such as the oral mucosa, paranasal sinuses, ear, chest, abdomen, skin, nails, groin, anal and vaginal areas, vascular catheter insertion sites, and bone marrow biopsy sites [2,3]. It is important to pay attention to even small symptoms and signs, including mild pain or tenderness at these sites [2]. Initial blood tests should include a complete blood cell count, differential blood count, blood urea nitrogen, creatinine, electrolytes, total bilirubin, and a liver function test. If necessary, based on symptoms, an arterial blood gas analysis or urinalysis should also be conducted.
Microbiological cultures should be performed before the administration of antibiotics. At least two pairs of blood cultures should be conducted. When a central venous catheter is present, culture of blood collected through the catheter is recommended. Some specialists insist that unless the differential time to positivity is calculated, specimens from a central venous catheter alone can be cultured without peripheral venous samples because catheter-related infection may occur [3].
In cases with no sign or symptom of infection, specimens from the nasal cavity, oropharynx, urine, stool, and rectum do not need to be cultured, except for the purpose of hospital-related infection control [2,3]. However, stool cultures and Clostridium difficile toxin assays can be conducted for patients with diarrhea, and rotavirus or norovirus infections can be checked in the winter and during epidemic periods. Urine culture is recommended when there are symptoms of urinary tract infection, when a urethral catheter has been inserted, or when a urinalysis reveals abnormal findings. Although a colony-stimulating factor (CSF) examination is not absolutely necessary, it should be conducted in cases with symptoms of central nervous system infection. The presence of hemorrhagic tendencies and thrombocytopenia should be evaluated and, if necessary, appropriate interventions, such as transfusions, should be performed before the examination. For newly observed skin lesions or those of unknown causes, biopsies should be conducted and the results of microbiological cultures and histopathological findings should be evaluated. In cases with bullous lesions on the mucous membranes or skin, the presence of herpes simplex virus (HSV) infection should be determined. If a respiratory manifestation is present, a chest X-ray should be taken. Additionally, even with no symptoms, basal chest X-rays are recommended for comparison with future images when respiratory symptoms are present. Although there may be no abnormality on chest X-rays because there is no inflammatory response in neutropenic patients, approximately half of these patients can show evidence of pulmonary infiltration on chest computed tomography (CT) images [2,37].
RISK OF INFECTION
To determine the risk of serious infectious diseases in febrile neutropenic patients, the risk index of the Multinational Association for Supportive Care in Cancer (MASCC) can be used. A patient with a total score of 21 points or above is classified as low-risk [38]. Since the 1980s, many studies identifying patients who can be treated with oral antibiotics or as outpatients have been conducted by classifying their risk [38-41]. The MASCC risk index was developed through a prospective study by scoring weights based on factors influencing the prognosis of neutropenic fever using various factors, such as age, gender, underlying disease (s), the therapeutic condition of a cancer, associated disease (s), history of treatments for previous infectious diseases, and blood test results, in 1,139 subjects from 15 countries [38]. When the MASCC risk index score of 21 points or above was classified as the low-risk group, the positive and negative predictive values of no serious complications of neutropenic fever were 94% and 39%, respectively. NCCN differentiates between low- and high-risk groups by adding clinically important factors not included in the MASCC risk index [3]. A study analyzing the risk of severe complications or death caused by infection in Koreans has also been reported. Among the factors that could be initially assessed in febrile neutropenic patients visiting the emergency department, the risk factors of a continuous fever lasting 3 or more days were a visit within 10 days after the last anticancer therapy and newly observed pulmonary infiltration. Risk factors of septic shock were a change in consciousness and a creatinine clearance of less than 75 mL/min, and those of death were tachycardia, reduced creatinine clearance, a change in consciousness, and an associated pathogenic condition. Additionally, duration of neutropenia was significantly related with the mortality rate and incidence rate of septic shock [42]. The risk factor of death due to acute leukemia during a hospital stay in patients undergoing anticancer therapy was a previous or current fungal infection [43].
ANTIBIOTIC PROPHYLAXIS
Is antibiotic prophylaxis necessary for expected febrile neutropenic patients?
1. Antibiotic prophylaxis is recommended for patients at intermediate-to-high risk of infection (A-I).
2. Fluoroquinolones are recommended as prophylactic antibacterial agents (A-I).
Because neutropenic patients have a high risk of infection, antibiotic prophylaxis can be helpful. However, if antibiotic prophylaxis is applied to all neutropenic patients, including those at a relatively low risk of infection who do not need it, antibiotic-resistant bacteria may emerge and excessive medical costs may be incurred. Thus, it is important to determine which patients will be most helped by antibiotic prophylaxis and the appropriate period for prophylaxis. Patients for whom prophylactic antibacterial, antifungal, or antiviral treatment is recommended are shown in Table 2 [3].
Table 2.
Overall infection risk in cancer patients by type of disease or therapy
HSV, herpes simplex virus; HSCT, hematopoietic stem cell transplantation; 2-CdA, 2-chlordexoyadenosine (also known as cladribine).
While past studies on antibiotic prophylaxis mainly used sulfamethoxazole/trimethoprim, many studies conducted since the late 1990s have used fluoroquinolones. According to a meta-analysis on the use of prophylactic antibacterial agents [44], the group using prophylactic antibacterial agents showed a lower mortality rate following infection and a lower total mortality rate compared with those not using prophylactic antibiotics or using placebo; the effects in the fluoroquinolones group were particularly evident. Another meta-analysis reported that fever, infection caused by Gram-negative bacteria, microbiologically documented infection, and total infection occurred less in patients using prophylactic fluoroquinolones than in those using sulfamethoxazole/trimethoprim or placebo. However, even prophylaxis using fluoroquinolones did not reduce Gram-positive bacterial infection, fungal infection, or the mortality rate [45]. Additionally, although there was concern about the emergence of antibiotic-resistant bacteria in the group using prophylactic antibacterial agents, resistance was not increased in the fluoroquinolone group. However, some reports have stated that resistant Gram-negative bacteria increased during fluoroquinolone prophylaxis, and this tendency improved after discontinuation of the prophylaxis; close attention to this is necessary [46]. That is, evidence for the use of prophylactic antibacterial agents such as fluoroquinolones exist in intermediate-to-high risk groups, but the long-term effects of antibiotic prophylaxis have not yet been fully determined; the emergence of resistant bacteria should be continuously monitored and antibiotic prophylaxis should be optimized in each hospital.
Ciprofloxacin or ofloxacin, which were widely used for prophylaxis in the past, had good antimicrobial activities against Gram-negative bacteria but relatively poor activities against Gram-positive bacteria. Studies using levofloxacin showed outstanding antimicrobial activity against Gram-positive bacteria as a prophylactic antibacterial agent for solid tumor and lymphoma patients [47] and solid tumor, lymphoma, and acute leukemia patients [48]. Both were large-scale studies that included over 300 subjects in each group. Infection caused by Gram-positive bacteria or bacteremia that was not prevented by fluoroquinolones decreased in the levofloxacin group. Although infection caused by Gram-negative bacteria was also significantly decreased, the total mortality rate and the mortality rate due to infection were not significantly different between the two groups.
In Korea, a study was performed on the effects of prophylactic antibacterial agents in acute leukemia patients undergoing anticancer therapy [49]. The study used ciprofloxacin and roxithromycin for prophylaxis, and the patients using these prophylactic antibacterial agents showed fewer Gram-negative bacterial infections, but more Gram-positive bacterial infections. Additionally, the total infection rate and the mortality rate following infection did not differ between the two groups. As of 2010, the hospital does not use prophylactic roxithromycin.
Until when should antibiotic prophylaxis be used?
3. Antibacterial prophylaxis is administered until neutrophil recovery (absolute neutrophil count 500-1,000/mm3) (B-III).
Because there has been no prospective clinical study comparing the effect of the same drug for different periods to determine the proper administration period of prophylactic antibacterial agents, it is difficult to determine the appropriate end point of antibacterial prophylaxis. Most previous studies that showed effective outcomes of antibacterial prophylaxis used antibacterial agents from the beginning of anticancer therapy or within 48-72 hours after anticancer therapy until recovery of the absolute neutrophil count, and there was no difference in the preventive effects based on the administration period [48,50-53].
Is antifungal prophylaxis necessary?
4. Antifungal prophylaxis is recommended to prevent fungal infections in patients whose neutropenia is expected to last for more than 7 days. Appropriate antifungals for this purpose include posaconazole (A-I), fluconazole (B-I), itraconazole oral solution (B-I), low-dose amphotericin B deoxycholate (B-I), and low-dose liposomal amphotericin B (C-II).
5. Antifungal prophylaxis is recommended to prevent fungal infections in allogeneic HSCT recipients. Appropriate antifungals for this purpose include posaconazole (A-I), fluconazole (A-I), micafungin (B-I), and itraconazole intravenous injection followed by itraconazole oral solution (B-I).
When prolonged neutropenia is expected, such as in patients undergoing remission induction therapy or maintenance/consolidation therapy due to a hematologic malignancy or those receiving allogeneic HSCT, antifungal prophylaxis is recommended [2,3]. Azoles have been widely used as prophylactic antifungal agents because of their favorable costs, adverse reaction profiles, and they allow the selection of other therapeutic antifungal agents for breakthrough fungal infections during antifungal prophylaxis.
A meta-analysis on antifungal prophylaxis revealed that the total mortality rate (relative risk [RR], 0.84; 95% confidence interval [CI], 0.74 to 0.95), fungus-related mortality rate (RR, 0.55; 95% CI, 0.41 to 0.74), invasive fungal infection, definite invasive fungal infection, definite invasive Candida infection, and the use of empirical antifungals decreased in the group using prophylactic antifungal agents compared with the groups not using prophylaxis or using a placebo [54]. Although fluconazole as a prophylactic antifungal agent tended to increase invasive aspergillosis, antifungal agents with specificity for filamentous fungi, such as itraconazole, resulted in less invasive aspergillosis infections [54].
Many studies have been performed on fluconazole, which has been shown to be very effective. Prophylaxis using 400 mg fluconazole per day reduced invasive fungal infections and the infection-related mortality rate in allogeneic HSCT recipients [55,56]. However, some reports stated that fluconazole did not significantly prevent invasive fungal infection in acute leukemia or autologous HSCT patients [57-59]. Additionally, studies using less than 400 mg prophylactic fluconazole per day did not find any significant difference in invasive fungal infections or mortality rates [60-62].
Itraconazole has antimicrobial activity against Aspergillus species and its preventive effect is thought to be superior to that of fluconazole; however, a study that directly compared fluconazole and itraconazole showed no significant differences in the all-cause mortality rate, fungus-related mortality rate, definite invasive fungal infection, invasive Candida infection, or superficial fungal infection [63-69]. When the efficacy of itraconazole was compared with that of fluconazole, limiting the studies to those using itraconazole oral solution, not the capsule, invasive fungal and Candida infections were decreased significantly [65-69]. The combination of itraconazole with vincristine or cyclophosphamide should be avoided because of the potential for drug interactions, and the administration of itraconazole should be performed cautiously in patients with a history of heart failure or lower cardiac output because of its cardiotoxicity [70] (intravenous itraconazole is not approved as a prophylactic antifungal agent by the Korea Food and Drug Administration [KFDA] as of 2009).
Posaconazole oral solution has a wide range of antifungal activity against Candida and filamentous fungi [71,72]. An assessment of the effect of prophylactically administered posaconazole in acute myelogenous leukemia and myelodysplastic syndrome patients found that proven or probable invasive fungal infection and invasive aspergillosis were observed less and the survival rate was significantly higher in the posaconazole group than in the fluconazole or itraconazole groups. However, a higher frequency of adverse reactions was found, including increases in bilirubin and liver enzyme levels [73] (posaconazole is approved as a prophylactic antifungal agent for neutropenic fever by the KFDA, but is not on the market and is purchased through the Korea Orphan Drug Center as of 2009).
A study that compared micafungin and fluconazole as prophylactic agents in 882 allogeneic or autologous HSCT recipients reported that the success rate of prophylaxis (80.0% vs. 73.5%; 95% CI, 0.9 to 12) was higher and the frequency of invasive aspergillosis was lower in the micafungin group; however, the mortality rate was not significantly different between the two groups [74]. Moreover, no significant difference was found in the frequency of adverse reactions or the discontinuation rate between the two groups. However, a limitation of this study was that over 70% of the subjects were autologous HSCT or low-risk allogeneic HSCT recipients. A recent study insisted that the incidence of fungal infection was not significantly different between micafungin and fluconazole groups [75].
Although low-dose amphotericin B deoxycholate (0.2 mg/kg/day or 0.5 mg/kg 3 times per week) showed much better preventive effects than fluconazole, it is difficult to use in many cases because of its toxicity [76,77]. Because the amphotericin B lipid formulation has less toxicity than amphotericin B deoxycholate, studies on its use to prevent neutropenic fever have been conducted. Although a preventative effect of 50 mg (low-dose) liposomal amphotericin B was not observed in previous small-scale studies [78-80], recent large-scale studies found that it decreased invasive fungal infection and infection-related mortality rates [81]. Inhalation of amphotericin B deoxycholate has also been attempted to prevent pulmonary fungal infection [82-84].
In Korea, a study investigated the effects of itraconazole oral solution and fluconazole as prophylactic agents and showed that the preventative effects of the two drugs were not significantly different [85]. However, administration compliance was lower due to gastrointestinal adverse reactions in the itraconazole group.
Until when should antifungal prophylaxis be used?
6. Use of prophylactic antifungal agents should be considered at least until neutrophil recovery (absolute neutrophil count 500-1,000/mm3) (B-III).
7. Use of prophylactic antifungal agents should be considered until the discontinuation of immunosuppressants if immunosuppressants are used after allogeneic HSCT (B-III).
Although it is difficult to find studie s with reliable evidence for the determination of the end point of antifungal prophylaxis, they are generally administered until recovery of absolute neutrophil counts occurs [55,57,59,62,79,81,86-93]. However, allogeneic HSCT recipients may require antifungal prophylaxis even after neutrophil recovery, and NCCN recommends continuing prophylaxis until 75 days after HSCT [3]. Additionally, when graft versus host disease (GVHD) is observed, the period of antifungal prophylaxis can be extended. A recent large-scale study reported that an average of 112-day prophylactic posaconazole therapy effectively prevented invasive fungal infection in patients with GVHD [94].
Does Pneumocystis jirovecii need to be prevented?
8. Prophylaxis against P. jirovecii is recommended in allogeneic HSCT recipients (A-I).
9. Use of prophylaxis against P. jirovecii should be considered in cases of autologous HSCT, high-dose corticosteroid therapy (e.g., the equivalent of 20 mg/day or more of prednisone for 4 weeks or more), administration of T-cell-depleting agents, such as fludarabine (B-II) or anticancer therapy due to acute leukemia (e.g., acute lymphocytic leukemia) (B-III).
10. Use of sulfamethoxazole/trimethoprim (A-I) is recommended for prevention of P. jirovecii. If the patient is intolerant to the drug, consider using dapsone or aerosolized pentamidine (B-II).
Sulfamethoxazole/trimethoprim can be used to prevent P. jirovecii in acute leukemia patients and HSCT recipients, and its preventive effect is excellent [95-97]. A meta-analysis on the prevention of P. jirovecii in immunocompromised patients (with the exception of human immunodeficiency virus [HIV] patients) showed that the P. jirovecii-related mortality rate was significantly reduced in the sulfamethoxazole/trimethoprim group (RR, 0.17; 95% CI, 0.03 to 0.94) [96,97]. Because P. jirovecii infection is known to increase in patients using alemtuzumab or fludarabine because of chronic lymphocytic leukemia or lymphoproliferative disorders [98-100], prevention of P. jirovecii can be considered. For this, 160/800 mg or 80/400 mg sulfamethoxazole/trimethoprim is administered, and if there is a concern about adverse events, such as bone marrow suppression, 160/800 mg is administered every other day. When the drug was used every other day, its preventive effect did not differ from that of daily administration (RR of pneumonia, 0.82; 95% CI, 0.61 to 1.09). Significantly more patients who took the drug daily discontinued it due to adverse reactions (RR, 2.14; 95% CI, 1.73 to 2.66) [101]. However, these results should be interpreted carefully because the study was performed not with neutropenic patients, but with HIV infection patients. If sulfamethoxazole/trimethoprim is difficult to administer because of leukopenia, dapsone or aerosolized pentamidine can be used [102]. However, dapsone and aerosolized pentamidine produce a weaker preventive effect against P. jirovecii than sulfamethoxazole/trimethoprim and can lead to additional infections and a higher mortality rate following infection [103].
Is antiviral prophylaxis necessary?
11. Antiviral prophylaxis against HSV is advised in HSV-seropositive patients in the case of allogeneic HSCT (A-I), autologous HSCT at high risk for mucositis (A-II), induction or re-induction therapy for acute leukemia (B-I), or the use of T-cell-depleting monoclonal antibodies (e.g., alemtuzumab) (B-II).
12. Consider using prophylactic antiviral agents in consecutive chemotherapy if HSV was reactivated in the previous chemotherapy (B-III).
13. Acyclovir or valacyclovir is recommended for the prevention of HSV (A-I).
If antiviral prophylaxis against HSV is not conducted for allogeneic HSCT recipients, approximately 62-80% of HSV IgG-seropositive patients show reactivation of the virus, while only 1-1.5% of HSV IgG-seronegative patients experience viral reactivation [104,105]. For autologous HSCT, a lack of antiviral prophylaxis leads to lesions caused by HSV in approximately 2-6% of cases [106-108]. Thus, antiviral prophylaxis is recommended for HSV-seropositive patients among allogeneic HSCT recipients and autologous HSCT recipients with a high risk of mucositis [3]. According to a study performed in the early 1990s, the antibody-positive rates of HSV type 1 were 100%, 91%, and 82% in populations aged over 30 years, in their 20s, and in their teens, respectively, in Korea. Antiviral prophylaxis is advised in most cases in this country [109].
A meta-analysis on studies using acyclovir to prevent reactivation of HSV revealed that lesions caused by HSV and its isolation rate were significantly decreased in the acyclovir group [110,111]. However, the mortality rate was reduced only when prophylactic antiviral agents were used during engraftment after allogeneic HSCT [111]. Recent studies using valaciclovir, which is more easily administered than acyclovir, reported that the development of HSV lesions was not significantly different between acyclovir and valaciclovir groups [112,113]. Antiviral prophylaxis is generally used until the completion of engraftment or the improvement of mucositis (approximately 30 days in most cases) [114,115].
Although varicella-zoster virus is frequently observed in patients undergoing anticancer therapy, it is not mentioned in these guidelines because it is beyond the scope of empirical therapy for neutropenic fever.
INITIAL ANTIBIOTIC THERAPY
Because infection proceeds rapidly and discrimination between the early stages of bacterial infection and noninfectious fever is difficult in neutropenic patients, empirical antibiotics should be initiated immediately after the development of fever in all neutropenic patients. Even when a fever is not present, symptoms and signs causing a reasonable suspicion of infection require empirical antibiotics, as in febrile patients.
What are the major etiological agents of neutropenic fever in Korea?
14. I n contrast to western countries, Gram-negative bacteria are the prevailing etiological agents of infections in neutropenic fever patients in Korea.
15. Adjustment of empirical antibiotics may be necessary depending on the resistance patterns in each hospital because the reported antimicrobial resistance rates of the bacteria causing neutropenic fever vary widely by hospital.
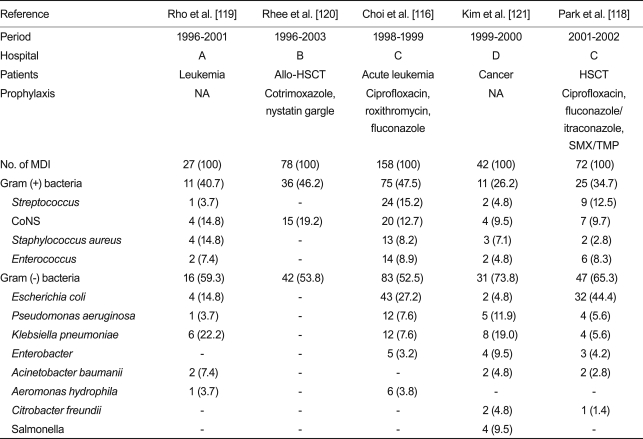
The distribution of etiological agents of neutropenic fever in studies published in Korea over the last 10 years is shown in Table 3. While Gram-positive bacteria account for 60-70% of microbiologically documented infection in Europe and America, Gram-negative bacteria were more frequently observed in studies in Korea until the early 2000s. This is a general characteristic in the Asia-Pacific region, including China, Taiwan, Thailand, and Malaysia [19]. Among Gram-positive bacteria, Streptococcus and coagulase-negative Staphylococcus are the most frequently observed, and Staphylococcus aureus and Enterococcus are next. Among Gram-negative bacteria, Escherichia coli is found most frequently, and Pseudomonas aeruginosa and Klebsiella pneumoniae follow it.
Table 3.
Distribution of bacterial organisms in patients with neutropenic fever in Korea
Values are presented as number (%).
HSCT, hematopoietic stem cell transplantation; NA, not available; SMX/TMP, sulfamethoxazole/trimethoprim; MDI, microbiologically defined infection; CoNS, coagulase-negative Staphylococcus.
Little data regarding antimicrobial susceptibility to etiological agents has been reported in Korea, and the reported resistance rates vary. The rate of methicillin-resistant S. aureus (MRSA) and those of fluoroquinolone-resistant and third-generation cephalosporin-resistant E. coli were 38-77%, 16-93%, and 0-7.0%, respectively [116-118]. Thus, each hospital needs to choose early empirical antibacterial agents by considering the types of frequently detected bacteria and their susceptibilities. For example, ciprofloxacin combination therapy is difficult to use as an early empirical antibacterial agent in hospitals showing a high fluoroquinolone resistance rate. Additionally, these guidelines do not recommend glycopeptides as early empirical antibacterial agents, but their partial use can be considered in hospitals with high MRSA rates. These guidelines describe general recommendations, and antibacterial agents not mentioned in these guidelines can also be empirically used according to types of detected bacteria and their susceptibilities in each hospital.
Outpatient oral antibiotics
When should oral antibiotics be used as the initial treatment for febrile neutropenic patients?
16. Oral antibiotics may be used for the initial treatment of febrile neutropenic patients if the risk of infectious complications is low (A-I).
Many randomized controlled studies have demonstrated that febrile neutropenic patients with low risks of complications may be treated with oral antibiotics [122-125]. Thus, if the risk of infectious complications is low, based on the risk index, oral antibiotics can be used for treatment. However, a survey conducted in Korea from 2005 to 2006 found that oral antibiotics were rarely used for the treatment of febrile neutropenic patients in Korea [29].
Outpatient treatments can be considered when febrile neutropenic patients meet the following conditions: a fever does not begin during the hospital stay, acute diseases are not associated, neutropenia is expected to improve within 7 days, the general condition is good (ECOG 0-1), the serum creatinine level is less than 2.0 mg/dL, the liver function level is within 3 times the normal range, and the MASCC risk index is 21 points or more [3]. Additionally, access to a medical institution needs to be secured for patients to ensure early outpatient treatment.
Which oral antibiotics can be used empirically for the initial treatment of febrile neutropenic patients?
17. The combination of ciprofloxacin and amoxicillin/clavulanic acid is recommended as oral antibiotics for febrile neutropenic patients (A-I).
18. The combination of ciprofloxacin and clindamycin is an acceptable alternative as oral antibiotics for penicillin-allergic patients (A-II).
19. However, ciprofloxacin-based oral antibiotic regimens are not recommended for patients recently treated with fluoroquinolone prophylaxis (B-II).
Well-designed randomized studies have demonstrated that the combination of ciprofloxacin and amoxicillin/clavulanic acid was effective as empirical oral antibiotic therapy in febrile neutropenic patients in the low-risk group [122,124]. Penicillin-allergic patients can be treated with a combination of ciprofloxacin and clindamycin [126]. A randomized study found that ofloxacin was also effective in low-risk febrile neutropenic patients [125]. Levofloxacin was also estimated to have similar effects. Additionally, there are some reports that moxifloxacin can be effective in low-risk patients [127].
Although some small studies reported that ciprofloxacin monotherapy was acceptable [128,129], it has also been associated with the risk of serious infection caused by viridans streptococci and thus should be used carefully [130]. Fluoroquinolone-based oral antibiotic therapy is not recommended if fluoroquinolones have been used for antibiotic prophylaxis.
There are almost no studies on other oral antibiotics, such as cephalosporins, for the initial treatment of neutropenic fever, but they can be used according to frequently reported etiological bacteria and their susceptibilities. If the etiological bacteria are determined, various oral antibiotics can be appropriately used, based on their antimicrobial susceptibilities.
Intravenous antibiotics
Empirical antibiotics as initial therapy should be chosen by considering the susceptibilities of the bacteria detected in each hospital. For hospitals with high rates of resistant bacteria, such as MRSA and multiple-drug-resistant Gram-negative bacteria, appropriate antibiotics should be used based on the circumstances in each hospital. Additionally, these guidelines suggest generally recommended antibiotics, and antibiotics not mentioned in these guidelines can also be used properly if their effects are demonstrated.
Use of antibiotics against Pseudomonas is commonly recommended as an initial empirical antibiotic therapy. Other factors that should be considered in choosing initial empirical antibiotics for febrile neutropenic patients include the infection site (s), history of MRSA infection or colonization, organ dysfunction, history of the use of antibiotics, and bactericidal effects of antibiotics.
Which intravenous antibiotics can be used as the initial monotherapy for febrile neutropenic patients?
20. Cefepime, imipenem/cilastatin, meropenem, or piperacillin/tazobactam is recommended as empirical monotherapy if the febrile neutropenic patient has no complications of infection (A-I).
21. Ceftazidime can be considered as empiric monotherapy if the febrile neutropenic patient has no complications of infection, but clinicians should be aware of the possibility of breakthrough infections (from Gram-positive bacteria or drug-resistant Gram-negative bacteria) (B-II).
No significant difference between antibiotic monotherapy and antibiotic combination therapy has been observed in febrile neutropenic patients without complications of infection in many randomized studies [131-140]. Antibiotics recommended for antibiotic monotherapy are cefepime, ceftazidime, imipenem/cilastatin, meropenem, and piperacillin/tazobactam [141,142]. Because ceftazidime is not effective against Gram-positive bacteria, such as viridans streptococci or pneumococci, and is vulnerable to extended-spectrum β-lactamase and type 1 β-lactamase, it should be used carefully [143]. Additionally, a clinical study found that the clinical effect of ceftazidime was lower than that of meropenem in cancer patients with neutropenic fever [144,145]. Thus, some professionals recommended the addition of cefazolin to ceftazidime to enhance the antibacterial activity against Gram-positive bacteria [146].
In a nationwide survey from 2005 to 2006, cefepime was used most frequently as a single antibacterial agent for neutropenic patients in Korea [29]. A recent meta-analysis revealed that cefepime could increase the mortality rate in neutropenic patients [147,148]. However, the US FDA found that the mortality rate of cefepime was not significantly different from that of the control group in an additional analysis. In Korea, a study compared cefepime monotherapy and ceftazidime + tobramycin combination therapy in 90 solid cancer patients with neutropenic fever; no significant difference was found in effects or complications between the two groups [149]. Moreover, a comparison between cefepime and ceftazidime monotherapy in 40 Koreans with cancer associated with neutropenic fever found no significant difference in the treatment success rate [150].
Because cefepime and ceftazidime can be used without dose adjustment in cases of mild or intermediate renal inadequacy, they are relatively safe for patients taking other drugs to treat renal toxicity. Aminoglycoside monotherapy is not generally recommended as an initial antibacterial monotherapy for febrile neutropenic patients [2].
Other antibiotics can be added to antibacterial monotherapy regimens according to clinical outcomes; thus, clinical responses to antibiotics, secondary infection, adverse reactions, and resistant bacteria should be evaluated carefully.
Which intravenous antibiotics (with the exception of glycopeptides) can be used as the initial combination therapy for febrile neutropenic patients?
22. An aminoglycoside + anti-pseudomonal penicillin (± β-lactamase inhibitor), or ciprofloxacin + anti-pseudomonal penicillin are recommended as the initial intravenous combination therapy for febrile neutropenic patients (A-I).
23. An aminoglycoside + an extended-spectrum cephalosporin (cefepime or ceftazidime) is also recommended as the initial intravenous combination therapy for febrile neutropenic patients (A-II).
Empirical combination therapy using an aminoglycoside and anti-pseudomonal β-lactam antibiotic (ticarcillin/clavulanic acid, piperacillin/tazobactam, ceftazidime, or cefepime), with the exception of glycopeptides, is recommended. A combination of a fluoroquinolone and anti-pseudomonal β-lactam antibiotic can be administered to patients not treated with prophylactic fluoroquinolones [151-155].
According to a survey in Korea, the most common antibiotic combination therapy for neutropenic patients was the combination of a third- or fourth-generation cephalosporin (ceftazidime or cefepime) and an aminoglycoside [29]. However, inclusion of an aminoglycoside can increase adverse reactions, such as renal toxicity, ototoxicity, and hypokalemia. Taking an aminoglycoside once daily is an alternative to maintain its therapeutic effect and to help reduce these adverse events [156,157]. However, for treatment of meningoencephalitis or endocarditis, administration of an aminoglycoside once per day is not recommended. When a patient has poor renal function, it is necessary to measure the blood aminoglycoside level and maintain it at an appropriate therapeutic level.
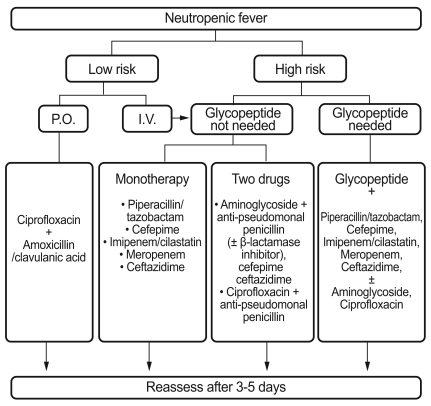
In cases associated with resistant bacteria or complications, such as hypotension, combination therapy, rather than monotherapy, is recommended. In particular, clinically unstable febrile neutropenic patients with hypotension, tachypnea, newly developed or deteriorating tachycardia, changes in consciousness, decreased urine amounts, or organ dysfunction may require a combination of broad-spectrum β-lactam antibiotics (imipenem/cilastatin, meropenem, or piperacillin/tazobactam) and an aminoglycoside to extend the antibacterial spectrum and to obtain an synergistic effect against some Gram-negative bacteria. A study in Korea found that in 35 febrile neutropenic patients with shock, the most frequently observed etiological microorganism was Gram-negative bacteria (27 subjects, 77%); of these, E. coli was the most common [158]. Fig. 1 presents the algorithm for the initial management of febrile neutropenic patients.
Figure 1.
Algorithm for initial management of febrile neutropenic patients.
RE-EVALUATION AFTER 3-5 DAYS AND CHANGE OF ANTIBIOTICS
To evaluate the effect of initial antibiotics, 3-5 days are needed [159]. At this time, future treatments are determined according to whether the patient has bacteremia or pneumonia, whether fever has improved, or whether the condition of the patient has deteriorated. If a patient's condition deteriorates within 3 days, the evaluation of empirical antibiotics can be advanced. However, because many studies have suggested that the period to defervescence in febrile neutropenic patients is 2-7 days (median 5 days), we can wait until 5 days have passed without changing the initial antibiotics if a bacterium is not grown in cultures and fever continues to be observed.
Patients without fever in 3-5 days
What should be done when initial empirical antibiotics are effective within 3-5 days?
24. If the causative organism is not found and initial empirical antibiotics seem to be effective after 3-5 days, the initial empirical antibiotics should be maintained until neutrophil recovery (A-II).
25. Maintain intravenous antibiotics until absolute neutrophil count recovery for patients who were in the high-risk group at the beginning of the administration of empirical antibiotics. For those in the low-risk group, consider changing to oral antibiotics (B-II).
When a patient's fever improves, symptoms and signs of infection are stable or improved, and hemodynamic levels such as blood pressure or pulse rates are stable, the initial antibiotics are considered to be effective [3]. Under the circumstances, if a causative organism is identified, more appropriate antibiotics can be used to decrease adverse reactions and treatment costs. However, to prevent newly developed bacteremia, it is recommended to maintain a broad antibacterial spectrum [2]. Antibiotics should be maintained for at least 7 days, and it is recommended to continue treatment until the causative organism is removed in cultures, until infections of all sites are cured, or until symptoms and signs in the patient are eliminated. Changing to oral antibiotics after intravenous antibiotics for the first 72 hours can be considered. A study in Korea reported that when ciprofloxacin was orally administered to 40 patients showing no clear evidence of infection, an increasing absolute neutrophil count and defervescence in 72 hours indicated successful treatment in 39 cases (98%) [160].
Although it is desirable to discontinue antibiotics after the recovery of neutrophils to an absolute count of > 500/mm3, the discontinuance of antibiotics can be considered even in the cases of absolute neutrophil counts of < 500/mm3 if neutropenia is maintained without symptoms or signs of infection. However, this approach is available only when a patient can be monitored carefully, the mucous membranes and skin are normal (no inflammation of the mucous membranes, ulcers, evidence of catheter site infections, or hemorrhage), and neither invasive intervention nor anticancer therapy has been planned [2].
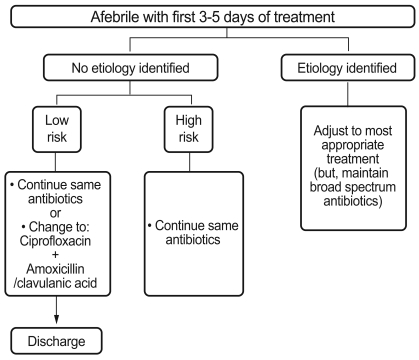
If fever is eliminated after 3-5 days but etiological bacteria are not identified, it is generally recommended to maintain the initial antibiotics until the recovery of neutrophils to an absolute count of > 500/mm3. In cases with specific infection sites, the administration of antibiotics for an appropriate period based on the site is recommended. However, if there is no clear infection (pneumonia, enteritis, endocarditis, catheter-related infection, or skin or soft tissues infection) or no cultured bacteria, and if a patient is in the low-risk group at the beginning of the therapy, intravenous antibiotics for over 2 days can be replaced with oral antibiotics if clinically necessary [122,124]. However, patients in the high-risk group should continue intravenous antibiotics (Fig. 2).
Figure 2.
Algorithm for management of patients who become febrile in the first 3-5 days of initial antibiotic therapy.
Patients with fever in 3-5 days
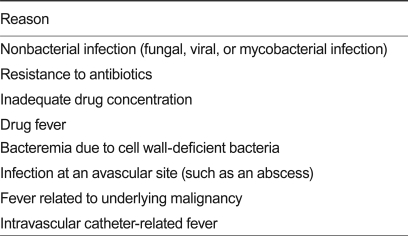
When fever persists even after 3-5 days of antibiotic therapy and neither infection sites nor causative organisms are detected, reasons shown in Table 4 can be considered. Re-evaluation of the following is necessary: complete blood cell count, general chemistry, electrolyte test, C-reactive protein (CRP), urinalysis, results of all cultures, a close physical examination, chest X-ray, evaluation of any vascular catheter, additional cultures of blood and specimens from specific infection site (s), imaging studies on sites suspected to have infection (if possible), blood antibiotic levels (particularly aminoglycosides), and ultrasonography or CT for patients with pneumonia, paranasal sinusitis, or enteritis.
Table 4.
Reasons for persistent fever 3-5 days after initiating antibiotic therapy
The current blood culture system can detect 90-100% of bacteria in blood within 48 hours of blood culture. Thus, it is recommended to repeat blood cultures at 48-hour intervals, as necessary.
What should be done if fever persists after 3-5 days?
26. If fever persists after 3-5 days of antibiotic therapy and reassessment does not yield a cause, continue administration of the same antibiotics when the patient's condition is clinically stable (B-II).
27. However, if the patient is in an unstable condition, consider expanding the antibacterial spectrum to cover anaerobes, drug-resistant Gram-negative bacteria, or drug-resistant Gram-positive bacteria (B-II).
28. If the fever persists even after the use of empirical antibacterials, consider using antifungal agents, depending on the risk of infection (A-II).
If fever persists even after 3-5 days of the initial antibiotic therapy and its cause is not identified, one of following three measures can be taken (Fig. 3). First, if the condition of a patient is not unstable and no additional relevant information is obtained from re-evaluation, the initial antibiotics can continue to be administered. In particular, for patients who are expected to show recovery of neutrophils within 5 days, it may be appropriate to maintain the initial antibiotics. It is not recommended to change antibiotics when fever persists in a patient in stable condition.
Figure 3.
Algorithm for management of patients who have a persistent fever after 3-5 days of initial antibiotic therapy. CBC, complete blood count; CRP, C-reactive protein.
Second, antibiotics can be changed or added. When a disease proceeds or complications or adverse drug reactions are observed with newly found or deteriorating abdominal pain or lesions of the mucous membranes, infection around a catheter, or changed mucous membrane flora, changing the initial antibiotics or adding another antibiotic should be considered. For these cases, cooperation with the infectious diseases specialists is recommended [3].
Third, antifungal agents can be added while changing or maintaining antibacterials. Generally, if fever persists after several days of empirical antibacterial use, it is necessary to consider the use of antifungal agents (see EMPIRICAL ANTIFUNGAL THERAPY section for details).
USE OF GLYCOPEPTIDES
Should glycopeptides be included in an empirical antibiotic regimen?
29. Glycopeptides should not be routinely added to an initial empirical antibiotic regimen (A-I).
Eighteen randomized studies have investigated whether glycopeptides should be added to an initial empirical antibiotic regimen. Of them, only two were double-blind randomized trials [17]. The largest was a multi-center study that included 747 subjects [161], and the smallest had only 46 subjects [162]. When the 747 neutropenic patients in the largest study were randomly divided into addition of vancomycin to ceftazidime + amikacin and no-addition groups, the vancomycin addition group showed faster responses in patients who were found to have bacteremia caused by Gram-positive bacteria; however, the addition group was not significantly different in terms of defervescence and mortality rate compared with the no-addition group. Furthermore, no patient died in the first 3 days among the patients with bacteremia caused by Gram-positive bacteria. However, renal toxicity following the use of antibiotics occurred in 2% of patients in the no-vancomycin group, but was significantly higher (6%) in the vancomycin group (p = 0.02) [161]. Recently, the results of two meta-analyses on the need for the administration of vancomycin as an initial empirical antibiotic regimen were reported [163,164]. One meta-analysis examined a total of 2413 patients by including 14 of 18 randomized studies [163]. It revealed that the addition of a glycopeptide did not significantly reduce the total mortality rate (odds ratio [OR], 0.67; 95% CI, 0.42 to 1.05). In particular, an analysis of 405 patients that included only six studies using the same broad-spectrum antibiotic showed the same finding (OR, 1.05; 95% CI, 0.52 to 2.00). The other meta-analysis investigated a total of 2392 patients by including 13 randomized studies [164]. It also found that the additional administration of a glycopeptide did not significantly decrease the total mortality rate (RR, 0.96; 95% CI, 0.58 to 1.26). For breakthrough infection, the first meta-analysis did not show any significant association with the use of glycopeptides with an OR of 1.18 (95% CI, 0.81 to 1.98) [163], while the second one found that breakthrough infection caused by Gram-positive bacteria was reduced, with a RR of 0.28 (95% CI, 0.11 to 0.37) [164]. However, these findings should be interpreted carefully. All of the studies in the analysis were conducted from 1985 to 1993, before the emergence of vancomycin-resistant enterococci (VRE); thus, VRE breakthrough infection during the administration of vancomycin was not reflected [17]. Moreover, because viridans streptococci bacteremia can deteriorate rapidly, to streptococcal toxic shock syndrome, in neutropenic patients, there is a suggestion that the addition of vancomycin to an initial antibiotic regimen is favorable in hospitals with high penicillin resistance of viridans streptococci [165]. However, most β-lactam antibiotics (e.g., cefepime, imipenem/cilastatin, meropenem, piperacillin/tazobactam), except ceftazidime, have good antibacterial activity against viridans streptococci, so vancomycin is not likely to be of additional help unless ceftazidime monotherapy is used [166].
Although the frequencies of MRSA and VRE are high in Korea, there has been no randomized study on this issue. Only one retrospective study on MRSA bacteremia in not only neutropenic patients, but also others, reported that the addition of vancomycin to an initial antibiotic regimen did not significantly affect the prognosis [167]. According to data from an analysis of 457 febrile neutropenic patients in a university hospital for 10 years [158], S. aureus was identified in 10 (6%) of 172 patients with proven bacteremia, and 77% of them had MRSA. Although data on the rate of viridans streptococci bacteremia in febrile neutropenic patients are insufficient in Korea, approximately 5-7% of the total bacteremia was reported to show viridans streptococci [117,158]. The data on penicillin-resistance of viridans streptococci are also insufficient. One study reported that with the exception of pneumococcus isolated from neutropenic patients, 7 (36%) of 19 streptococci strains were penicillin-resistant [116]. While some researchers have reported that the antimicrobial susceptibility tests of 103 strains of viridans streptococci isolated from various clinical specimens in a university hospital found no penicillin resistance (although they were not from neutropenic patients) [158,168], others have insisted that of 45 strains isolated from blood, 27% were not susceptible to penicillin [169].
However, S. aureus bacteremia is rarely found as a causative organism of bacteremia for the first fever in neutropenic cancer patients after cytotoxic chemotherapy. Its rate was reportedly 1-2% in large-scale clinical studies [132,138,144]. Furthermore, the frequency of resistant bacteria, such as MRSA, is low for the first fever; bacteremia caused by Gram-positive bacteria does not deteriorate rapidly in cases of late initial treatment, unlike bacteremia caused by Gram-negative bacteria, and indiscriminate use of glycopeptides can lead to the emergence of resistant bacteria and nephrotoxicity. Thus, there is insufficient evidence to support the routine inclusion of glycopeptides in the initial antibiotic therapy for febrile neutropenic patients in Korea. Based on these findings, it is recommended not to routinely add vancomycin to the treatment regimen for febrile neutropenic patients in whom the cause has not been clearly determined (A-I).
30. When fever persists or recurs 3-5 days after the initiation of the empirical treatment, glycopeptides should not be routinely added to the empirical treatment (B-I).
Two randomized studies investigated whether the addition of a glycopeptide was effective if fever persisted for 3-4 days after beginning initial antibiotic therapy [170,171]. One study randomly administered vancomycin or placebo to 165 of 763 patients whose fever had not improved within 3-4 days after the empirical use of piperacillin/tazobactam. The two groups were not significantly different in terms of defervescence, mortality rate, breakthrough infection, or the frequency of the use of amphotericin B deoxycholate [170]. This result was consistent with that of a recent meta-analysis (RR of treatment failure, 0.61; 95% CI, 0.18 to 2.09) [164]. Based on these findings, it is recommended not to routinely add glycopeptides when fever persists or recurs after 3-5 days (B-I).
31. The use of glycopeptides as empirical antimicrobial therapy is recommended if the patient's blood cultures are positive for Gram-positive bacteria, a catheter-related infection is suspected, there is colonization with MRSA or a history of MRSA infection, the patient has severe sepsis or shock pending the results of cultures, or the patient has a skin or soft tissue infection (A-II).
Studies on the detailed indications of glycopeptides as an initial empirical antibiotic regimen are not sufficient, but the indications consistently suggested by most specialists, including those who contributed to the IDSA and NCCN guidelines, are as follows:
Positive for Gram-positive bacteria in blood culture (A-II)
Suspected catheter-related infection (A-II)
History of MRSA and penicillin-resistant S. pneumoniae colonization or infection (A-II)
Severe sepsis or shock following sepsis (A-II)
Skin or soft tissue infection (A-II)
The following indications remain controversial among specialists:
Risk of viridans streptococci bacteremia (B-III)
Severe damage to the mucous membrane due to anticancer therapy (B-III)
Prophylaxis using sulfamethoxazole/trimethoprim or fluoroquinolone (B-III)
However, even after glycopeptides are administered according to the indications, the discontinuance of glycopeptides is recommended if bacteremia caused by resistant Gram-positive bacteria is not observed in blood cultures (A-I).
Are the efficacy and the adverse reactions of teicoplanin identical to those of vancomycin when a glycopeptide is used as an empirical antibiotic. regimen for neutropenic patients?
32. The use of teicoplanin can be considered as empirical antibiotic therapy for neutropenic patients because it has equivalent efficacy and lower adverse reactions, such as nephrotoxicity, compared with vancomycin (B-I).
As of 2009, 18 randomized studies comparing the efficacy and adverse reactions of teicoplanin and vancomycin had been reported. Of these studies, 13 were performed with neutropenic patients. A meta-analysis on these randomized studies revealed that the mortality rates (RR, 0.95; 95% CI, 0.74 to 1.21) and clinical failures (RR, 0.92; 95% CI, 0.81 to 1.05) were not significantly different between teicoplanin and vancomycin [172]. Additionally, an analysis limited to the studies conducted in neutropenic patients found no statistically significant difference in the mortality rates (RR, 0.95; 95% CI, 0.68 to 1.34) or clinical failures (RR, 0.98; 95% CI, 0.83 to 1.16) between teicoplanin and vancomycin [172]. However, fewer adverse reactions were observed in the teicoplanin group compared with the vancomycin group (RR, 0.61; 95% CI, 0.50 to 0.74). In particular, nephrotoxicity was lower in the teicoplanin group than in the vancomycin group (RR, 0.44; 95% CI, 0.32 to 0.61) [172]. However, teicoplanin is not yet approved by the US FDA as an empirical antibiotic for MRSA infection and neutropenia, and clinical experience and research data on it in severe infections (e.g., endocarditis and encephalomeningitis) are not sufficient compared with those for vancomycin. Moreover, previous randomized studies did not include many patients with MRSA infection itself [172]. As the minimum inhibitory concentration (MIC) of vancomycin against S. aureus has risen, treatment failures have been reported [173], and the vancomycin MIC and levels need to be measured in some cases. Data regarding the association between teicoplanin MIC and treatment failure are insufficient, and levels are difficult to examine; thus, it should be used carefully. Additionally, most studies have stated that the efficacy of teicoplanin was identical to that of vancomycin by taking it once daily after a loading dose [172], but some researchers recommend administering a high dose for infections with complications. Thus, the appropriate dose remains controversial [174].
DISCONTINUATION OF ANTIBIOTICS
When can antibiotic therapy be discontinued?
33. If the origin of the fever is unclear, it is recommended to continue antimicrobials until the absolute neutrophil count reaches 500/mm3 or higher (A-II).
34. I f the causative organism or infection site has been identified, treatment duration is adjusted to the specific infectious disease in line with the recovery of neutrophils (A-II).
Duration of antibiotic therapy should be determined by considering the infection site, causative organism, general condition of the patient, treatment response, and neutrophil recovery [3]. If the origin of fever is unclear, antimicrobials should be maintained until the absolute neutrophil count reaches 500/mm3 or higher [3]. When the causative organism or the infection site has been identified, treatment duration should be adjusted to the specific infectious disease by considering the neutrophil recovery [3]. Because there is insufficient evidence regarding treatment duration for these clinical circumstances, this committee suggests general recommendations.
If the cause of fever is unclear
When the absolute neutrophil count is over 500/mm3, the fever has improved, and the patient is in a clinically stable condition, empirical antibiotic therapy should be discontinued (A-II). If the absolute neutrophil count is over 500/mm3, fever is persistent, and the patient is clinically stable, then the patient should be monitored for approximately 5 days and empirical antibiotic therapy should be discontinued for a differntial diagnosis (e.g., drug fever, hepatosplenic candidiasis). If the absolute neutrophil count is less than 500/mm3 but the fever has improved and the patient is in a clinically stable condition, antibiotic therapy should be continued until neutrophil recovery (A-II). Patients in the low-risk group can be changed to oral antibiotics and therapy can be maintained until recovery of the absolute neutrophil count to over 500/mm3 occurs (A-II). However, some experts say that for a clinically stable patient whose absolute neutrophil count is expected not to recover, the cause of fever is not clear, and the fever does not persist for more than 7-14 days, empirical antibiotic therapy can be discontinued carefully (B-III).
If the cause of infection is identified microbiologically or clinically
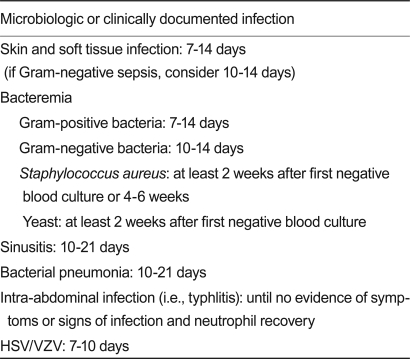
For a microbiologically or clinically documented infection, antibiotic therapy for the treatment duration of a specific infection should be conducted as shown in Table 5.
Table 5.
Suggested duration of therapy for documented infection
HSV, herpes simplex virus; VZV, varicella-zoster virus.
CATHETER-RELATED INFECTIONS
Which examination is useful in the diagnosis of catheter-related infection?
35. If a catheter-related infection is suspected, a skin swab for culture from the exit site of the catheter and blood cultures from the catheter may be obtained (B-II).
36. The differential time to positivity is a useful diagnostic tool for detecting catheter-related infection (A-II).
Catheter-related infection is a common complication frequently observed in neutropenic patients [3]. Exit-site infection is defined as a local flare or induration within 2 cm of the exit of a catheter and tunnel infection is defined as a local flare or induration > 2 cm from the exit of a catheter, pus from the exit, or a local flare or induration along the tunnel [175]. If catheter-related infection is suspected, a skin swab for culture from the exit of the catheter should be obtained, and blood cultures from the catheter itself should be conducted [3]. Because the skin swab culture from the exit site has a low specificity for catheter-related infection, but shows a high sensitivity, it can be useful for the exclusion of certain diagnoses [176]. Additionally, a study reported that blood cultures from both central venous catheters and peripheral blood, when performed by the automated blood culture systems used by many hospitals, could also measure the time for bacteria to grow initially. Furthermore, the differential time to positivity (DTP) or the difference between the two times was helpful for the diagnosis of catheter-related infection [3]. That is, when > 120 min of DTP was designated as a cutoff value, its sensitivity and specificity were high for the diagnosis of long-term catheter-related infection in recent studies [177-181]. However, because the studies were not performed with long-term catheters, such as the Hickman catheter, and because the specificity of the examination was lower for patients already treated with antibiotics, the results should be interpreted carefully.
Most catheter-related infections are caused by Gram-positive bacteria, and coagulase-negative Staphylococcus is most frequently isolated [182]. Thus, if catheter-related infection is clinically suspected, a glycopeptide, such as vancomycin, can be used (A-II). Because linezolid was found to increase the mortality rate when it was routinely administered to patients with suspected catheter-related infection in a randomized trial [183], it is not routinely recommended for the treatment of suspected catheter-related infection except in patients with catheter-related infection confirmed to have been caused by Gram-positive bacteria (A-I).
When should a catheter be removed?
37. Catheter removal is recommended for patients with bloodstream infections caused by fungi, non-tuberculous mycobacteria, Bacillus spp., Corynebacterium jeikeium, S. aureus, Acinetobacter, P. aeruginosa, Stenotrophomonas maltophilia, and vancomycin-resistant Enterococcus (A-II).
38. If the catheter has not been removed because the presence of a catheter-related infection is clinically uncertain, catheter removal may be considered if the same bacteria are identified in the consecutive blood culture at 48 72 hours after beginning appropriate antibacterial agents (B-II). However, immediate removal of the catheter is necessary if a catheter-related infection is suspected and the patient is clinically unstable (A-II).
Most catheter-related infections can be improved by antibiotic therapy without removal of the catheter [3]. In particular, the catheter salvage rate of coagulase-negative Staphylococcus reaches 70-80% with intravenous antibiotics alone; thus, the use of antibiotics without catheter removal is generally recommended [182,184] (A-II). However, for catheter-related infection caused by fungi (yeasts or molds) or non-tuberculous mycobacteria (e.g., Mycobacterium chelonae, M. abscessus, or M. fortuitum), the catheter should be removed immediately [3]. Bacillus spp., C. jeikeium, S. aureus, Acinetobacter, P. aeruginosa, S. maltophilia, and VRE can be difficult to treat with antibiotic therapy alone [3]. Thus, catheter-related infections caused by these microorganisms also require initial catheter removal (A-II). In cases with severe inflammation of the mucous membranes, intestinal bacteria flora, such as VRE and Candida, can cause infection through the blood; DTP is helpful in discriminating these cases from those of catheter-related infection [3]. Moreover, when the catheter is not removed because the presence of catheter-related infection is unclear, but the same bacteria are identified in consecutive blood cultures 48-72 hours after beginning appropriate antibiotics, catheter removal should be considered [3,182] (B-II). However, if catheter-related infection is suspected and a patient is clinically unstable, the catheter requires immediate removal [3] (A-II). In cases of bacteremia caused by S. aureus, when catheter-related infection is suspected, catheter removal is generally recommended, because the success rate is low [185] (A-II). However, if the fever of a patient with a catheter and unclear cause of infection improves in 48-72 hours after beginning proper antibiotics and the blood culture result is negative, the process can be closely monitored while maintaining the catheter [184] (B-II). General indications for catheter removal are presented in Table 6.
Table 6.
Suggested indication for catheter removal
All of the recommendations are level A-II with the exception of recommendation 7.
EMPIRICAL ANTIFUNGAL THERAPY
When should empirical antifungal therapy be considered if empirical antibacterial agents are not effective?
39. Empirical antifungal therapy is recommended in patients who are expected to maintain neutropenia for a longer period (> 10 days), when the fever dose not resolved within 3-5 days of initial empirical administration of antibacterial agents (A-II).
40. Regardless of fever, empirical antifungal therapy is recommended in patients who have a history of invasive fungal infection, fungal colonization with neutropenia, symptoms (pleuritic chest pain, blood tinged sputum, or hemoptysis) or signs that suggest newly developed pneumonia, tenderness, or edema around the paranasal sinuses or orbital area, ulcerating lesions or eschar in the nose (A-II).
Empirical antifungal therapy is a standard treatment when broad-spectrum antibacterials are not effective in neutropenic fever patients, based on clinical studies conducted in the 1980s [2,3,11,14,186-190]. Although characteristics of the patients, medications, and epidemiology of fungi may differ compared with those of 20-30 years ago, a lack of antifungal therapy for continuous neutropenic fever can increase invasive fungal infection (IFI) and lead to a higher mortality rate following IFI. Thus, empirical antifungal therapy is recommended in these cases (A-II) [3,14,191].
Currently, 40-50% of neutropenic patients classified as high-risk are known to take empirical antifungal agents [190]. According to a study that analyzed patients after HSCT and anticancer therapy in a single center in Korea from March 2000 to February 2001, 122 of 318 (38.4%) patients used empirical antifungal agents, and 74 (23.8%) among them had IFIs that were caused by Aspergillus and Candida species in most cases (6, 46, and 22 proven, probable, and possible IFIs, respectively) [192]. A foreign study also stated that approximately 15-45% of patients with a continuous neutropenic fever were estimated to have IFI [190]. Other reasons that antifungal therapy is used before a definitive diagnosis of infection are 1) because IFI is difficult to diagnose during the neutropenic period, 2) the delay of antifungal therapy to definitive diagnosis can easily provoke disseminated infection, and 3) in the autopsy of patients who died of neutropenic fever, IFI (Candida or Aspergillus species in most cases) that had not been clinically documented was found, and a continuous fever was the only initial sign of IFI [14,188-190].
If fever persists or recurs even after the administration of antibacterials, empirical antifungal therapy should be conducted [2]. When to begin the empirical antifungal therapy can differ according to the degree of risk. Patients in the low-risk group do not need to start antifungal therapy before diagnosis, while those in the intermediate-risk group are recommended to begin antifungal therapy when fever persists after 6-8 days of beginning broad-spectrum antibacterials due to continuous neutropenic fever and neutropenia. For patients in the high-risk group with over 10 days of neutropenia, empirical antifungal therapy should be started quickly when neutropenic fever persists or recurs after 3-5 days of beginning broad-spectrum antibacterials or when the clinical condition deteriorates [11,193]. When neutropenia is expected to persist for a relatively short period (< 10 days) or estimated to have been resolved for several days from the decision of whether to use empirical antifungal therapy, empirical antifungal therapy is not routinely considered, unless there is a symptom or sign causing suspicion of invasive fungal infection or a history of invasive fungal infection (B-III).
Which antifungal agents can be used empirically?
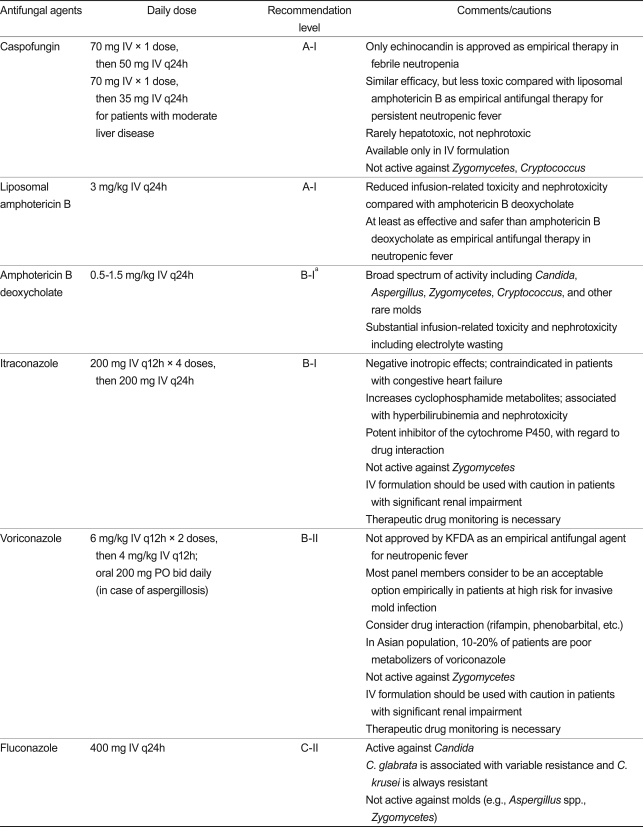
41. The following antifungal agents are recommended or can be considered for empirical antifungal therapy: caspofungin (A-I), liposomal amphotericin B (A-I), amphotericin B deoxycholate (B-I), itraconazole (B-I), and voriconazole (B-II). Amphotericin B deoxycholate should not be considered in the presence of risk factors for nephrotoxicity (B-I).
42. Azoles may not be considered as empirical antifungals if prophylaxis with fluconazole or itraconazole has already been administered (B-II).
Because many types of antifungal agents have been developed over the last 10 years and antifungal prophylaxis has been widely conducted in the high-risk group, filamentous fungi, as opposed to yeasts, such as Candida, have been found more frequently in IFIs of febrile neutropenic patients. Moreover, the rate of non-albicans species in candidiasis has increased, and fluconazole resistance has become a problem [193-196]. Thus, empirical antifungal agents need to meet the following conditions: 1) appropriate antifungal activity (having susceptibility to prevalent fungi in a region and in a hospital, or at least to Candida and Aspergillus species), 2) acceptable results in randomized controlled studies, 3) recommendations in currently published guidelines, 4) superior tolerance and less adverse reactions, and 5) a reasonable price [197].
To assess the effect of empirical antifungal therapy, the following five composite endpoints are comprehensively considered: 1) resolution of fever during neutropenia, 2) successful treatment of any baseline fungal infection, 3) absence of any breakthrough fungal infection during therapy or within 7 days after completion of therapy, 4) no premature discontinuation of therapy because of drug-related toxicity or lack of efficacy, and 5) survival for 7 days after the completion of therapy. If the therapy does not satisfy any of these endpoints, it is considered to be ineffective [198-200]. When toxicity is observed after initial empirical antifungal therapy, other antifungal agents can be used early in treatment. However, the time to determine whether it is effective remains a controversial issue. For amphotericin B deoxycholate, which is still widely used in Korea, it is recommended to change to other antifungal agents if there is no effect within 3-5 days. When an empirical antifungal agent is changed, a different class of antifungals should be considered first (B-III).
Because amphotericin B deoxycholate has been used as an empirical antifungal for decades and various antifungal agents have since been developed, their efficacy, safety, and adverse reactions have been compared. In Europe and the US, fluconazole, itraconazole, liposomal amphotericin B (not amphotericin B deoxycholate), caspofungin, and voriconazole have been administered empirically (voriconazole is not used as an empirical antifungal agent without the approval of KFDA as of 2009) [14,29,195,201].
Recommended empirical antifungal agents are summarized in Table 7, including doses and characteristics.
Table 7.
Recommendation of empirical antifungal agents in neutropenic fever
KFDA, Korea Food and Drug Administration.
aPanels do not recommend amphotericin B deoxycholate in the presence of risk factors for renal toxicity (B-I) (e.g., impaired renal function at baseline, nephrotoxic co-medication including cyclosporin or tacrolimus in allogeneic hematopoietic stem cell transplantation recipients, aminoglycosides antibiotics, old age, or history of previous toxicity).
Although amphotericin B deoxycholate has been widely used in Korea due to the accumulated experience with its use and its broad antifungal spectrum, the rate of adverse reactions, such as infusion-related toxicity and nephrotoxicity, which is reportedly as high as 50%, is a limitation. ECIL-1 does not recommend amphotericin B deoxycholate in patients 1) with underlying renal impairment, 2) taking co-medications with nephrotoxicity (e.g., taking an immunosuppressant, such as cyclosporin or tacrolimus after allogeneic HSCT, or antibiotics with nephrotoxic potential, such as aminoglycosides), and 3) with a previous history of toxicity. Additionally, current guidelines and reports do not recommend amphotericin B deoxycholate as an empirical agent because of its low efficacy and frequent and severe adverse events [3,14,190,193,195,197,201]. Therefore, although considering the accumulated experiences with its use, broad-spectrum activity, and low price, this committee does not suggest amphotericin B deoxycholate for patients with underlying renal impairment, taking co-medications with nephrotoxicity after allogeneic HSCT, or with a risk of renal failure, such as the elderly (B-I).
Liposomal amphotericin B produces an effect similar to that of amphotericin B deoxycholate (50% vs. 49%), but the frequencies of proven breakthrough fungal infection and adverse reactions (infusion-related toxicity and nephrotoxicity) of the five composite endpoints were significantly lower for liposomal amphotericin B [3,14,190,198,202]. In particular, the frequencies of infusion-related toxicity (17% vs. 44%) and nephrotoxicity (19% vs. 34%) were significantly lower [198]. This committee recommends liposomal amphotericin B for antifungal therapy as A-I.
The overall success rate of caspofungin does not differ from that of liposomal amphotericin B (33.9% vs. 33.7%), but its survival rate for > 7 days after the end of therapy was higher and the mortality rate in underlying fungal infection patients was significantly lower. Moreover, discontinuation of a drug due to drug-related toxicity and adverse reactions was observed less frequently for caspofungin [3,14,190,200]. Thus, this committee recommends caspofungin and liposomal amphotericin B of the same strength (A-I). However, only the intravenous type is available, and it is not effective against Zygomycetes or Cryptococcus species.
Itraconazole in capsule form shows a different absorption rate according to patients, and its absorption rate is improved in the oral and intravenous forms when combined with cyclodextrin. When intravenous itraconazole and amphotericin B deoxycholate were compared, they had similar efficacies and the toxicity of intravenous itraconazole was lower [3,14,190,203,204]. In a recent study, itraconazole provoked fewer adverse reactions and its efficacy was superior to that of amphotericin B deoxycholate, but the time to response and the length of fever were longer [205]. However, its potential for drug interaction should be monitored carefully, and it should be particularly avoided in patients with congestive heart failure.
Although voriconazole shows less breakthrough invasive fungal infection compared with liposomal amphotericin B in neutropenic fever patients (p = 0.02), its overall success rate is lower (26% vs. 31%), "non-inferiority" has not been proven, and it is not approved as an empirical antifungal agent by the FDA [3,14,190,199]. However, it is recommended as a primary therapeutic agent for aspergillosis, with a recommendation strength of A-I, and is used for off-label indications as an empirical antifungal agent due to low rates of breakthrough fungal infection and nephrotoxicity in the US and other countries [193,195,201,206]. Thus, this committee suggests the use of voriconazole as an empirical antifungal agent only in the high-risk group with a high risk of invasive aspergillosis.
Although fluconazole has been reported not to differ from amphotericin B deoxycholate, it has limitations as an empirical antifungal agent because it is not effective against filamentous fungi, such as Aspergillus species, and is used prophylactically [3,14,190,195,207]. A questionnaire survey conducted in HSCT centers of Korea reported that antifungal prophylaxis was administered to 57.6% of anticancer therapy and 90.9% of HSCT patients [29]; thus, fluconazole can be used as an initial empirical antifungal agent for patients not undergoing antifungal prophylaxis and in hospitals with a high rate of Candida infection.
The current trend of prophylaxis with antifungal agents effective against filamentous fungi is thought to largely influence the choice of empirical antifungal agents. When fluconazole or itraconazole is included in antifungal prophylaxis, there is an opinion that it should not be considered an empirical antifungal agent [195]. Future clinical studies to determine which empirical antifungal agents are appropriate according to types of antifungal prophylaxis are expected to be performed.
Which additional examination(s) can be conducted to diagnose fungal infection after examinations have been performed in the early stages of fever?
43. Periodic radiological examinations such as chest X-rays and CT, fungal cultures, non-culture based microbiological tests, such as galactomannan and β-D-glucan, and sputum or nasal swab surveillance are useful for the early diagnosis of fungal infections (B-I).
44. Active efforts, such as bronchoscopy, bronchoalveolar lavage, tissue biopsy, and culture, are necessary (B-II).
The Mycoses Study Group of the European Organization for Research and Treatment of Cancer (EORTC/MSG) revised the definition of invasive fungal infection in 2008 to include various methods [208]. However, the definitions were made for communication between clinical researchers, researchers of epidemiological studies, and clinicians, and were not an actual standard practice applied to patients clinically. That is, even when a case does not meet the definition, it does not mean every IFI can be excluded. As clinical characteristics and radiological and laboratory markers have been developed and repeatedly tested, they have been used to identify IFIs and to treat them early. These efforts enhance the survival rate of IFI by treating it early with only a small number of antifungals and reduce unnecessary use, adverse reactions, and medical costs by administering antifungals only to patients in need of them [209]. Additionally, regarding beginning empirical antifungal therapy, there are many efforts to diagnose and treat IFI early and to determine the treatment duration by using one or a combination of repeated fungal cultures, simple X-ray, CT, and non-culture-based diagnostic tests, even when IFI is not clinically suspected [209-211].
In patients with a sustained neutropenic fever, CT is helpful to diagnose IFI, especially invasive aspergillosis. The halo sign or haziness around a nodule or infiltration is a characteristic chest CT finding of infection caused by angio-invasive microorganisms, and it is useful for the suspicion of invasive aspergillosis observed in long-term neutropenic patients; however, it is not found in all patients. Although there is no disease-specific radiological finding characteristic of IFI, empirical antifungal therapy (mold-active therapy) should begin when severe neutropenia persists with symptoms such as fever, cough, and chest pain and pulmonary nodules or infiltration are newly observed on CT images. Additionally, the results of radiological examinations (e.g., CT, MRI) of the abdomen, paranasal sinuses, and head and neck are considered in combination with patients' symptoms and signs and laboratory findings. If there are any abnormalities in the examinations, active efforts, such as bronchoscopy and biopsy, are made to lead to confirmation of the diagnosis and to determine the appropriate use of antifungals through targeted therapy [2,3,12,209].
Galactomannan (GM) is a component of the cell wall of fungi and is used for double-sandwich enzyme-linked immunosorbent assays. It is examined in the plasma, serum, bronchoalveolar lavage fluid, and cerebrospinal fluid, and is known to be helpful for the diagnosis of invasive aspergillosis. Its sensitivity and specificity differ according to the study, and its cutoff value varies according to the type of underlying disease and antifungal prophylaxis [209,212-214]. When antibiotics such as piperacillin/tazobactam or amoxicillin/clavulanic acid are used, a false-positive result may be observed, and antifungals that are currently effective against filamentous fungi can provoke a false-negative result [195,213,215]. Except as an auxiliary measure for diagnosis, GM is used for surveillance in the high-risk group (for early diagnosis before symptoms) and monitoring of responses after antifungal therapy. A recent meta-analysis reported that the sensitivity and specificity of GM analysis were 71% (95% CI, 68 to 74) and 89% (95% CI, 88 to 90), respectively [216], but a study using it clinically found that its sensitivity was < 50% [217]. In particular, because false-negative results can be observed when using antifungals, results should be interpreted carefully.
Moreover, another component of the cell wall of fungi, (1→3)-β-D-glucan, is currently used as an auxiliary measure to diagnose IFI. Unlike GM, it is detected in not only Aspergillus, but also Candida, Fusarium, Trichosporon, P. jirovecii, Acremonium, and Saccharomyces; however, it has not been identified in Zygomycetes [213,215].
Although the polymerase chain reaction (PCR) method has been developed, it has not been standardized and results on clinical validity do not exist. Thus, EORTC/MSG does not recommend it as an auxiliary measure for diagnosis. Despite this, real-time quantitative PCR has been attempted for many genera and species of fungi [208,213,215]. Nucleic acid sequence-based amplification (NASBA) that manufactures a primer with a preserved 18S rRNA base sequence and nucleic acid and performs isothermal amplification has also been developed [218].
Antifungal therapy using described surrogate markers is called "preemptive therapy" and has been recently studied [211], but this committee believes that it is too early to recommend it for routine preemptive therapy in Korea.
Until when should empirical antifungal therapy be used?
45. Treatment duration is usually determined by defervescence, recovery of the absolute neutrophil count, and a clinically stable condition. Empirical antifungals may be discontinued early if defervescence is achieved, neutropenia has recovered, and fungi have not been identified. However, if invasive fungal infection is identified during empirical therapy, the proper treatment duration for the respective disease should be followed (B-III).
When fungal infection is confirmed during the use of empirical antifungal therapy, the treatment duration is determined by any underlying disease(s) and the type and spectrum of fungal infection [2,193,194]. If fungal infection is not confirmed, there is no accurate standard by which to determine the empirical treatment duration, but in previous studies, the duration was an average of 7-14 days (range, 1 to 113) [198-200,203-205]. The treatment duration was limited mainly to experience with amphotericin B deoxycholate. Amphotericin B deoxycholate may be discontinued when neutropenia is improved, the patient's condition is stable, and there are no abnormalities on chest and abdominal CT. If neutropenia persists but the patient's condition is clinically stable, amphotericin B deoxycholate can be discontinued when it has been used for 2 weeks and there are neither suspicious lesions on physical examination nor abnormalities on CT. For stable patients in the high-risk group, continuation of amphotericin B deoxycholate is considered during the period of neutropenia [2]. GM can be helpful to determine the discontinuation of antifungal therapy with no evidence of fungal infection in clinical or radiological examinations despite continuous neutropenia because its negative predictive value is high. On the contrary, when the level is positive, additional examinations, such as CT, are necessary [209,210,213,215]. Moreover, if fever persists even after the recovery of neutropenia, active efforts, such as radiological examinations, biopsy, and cultures, are needed to determine the cause of the fever.
CLOSING REMARKS
These guidelines represent an approximately 6-month effort. There were more studies in Korea than expected, but most were retrospective surveys conducted at a single hospital. These guidelines suggest drugs actually used as of December 2009 and their evidence, and if related Korean literature exists, it is also described. Although most questionnaire surveys performed in hospitals in Korea focused on clinical experiences based on the standard of reimbursement benefits, these guidelines attempt to provide an academic background for antibiotic therapy for febrile neutropenic patients. Thus, the contents of these guidelines may differ from the current notification of the Ministry of Health and Welfare and the standards of review of HIRA. In particular, the use and doses of antibiotics and the use of empirical antifungal therapy differ. Regarding the assessment of this guideline by specialists, the manuscript was revised by accepting opinions after presentations at five academic conferences in early 2010 and after the questionnaire survey had been conducted with related physicians in Korea. Of 40 recommendations, 36 indicated appropriateness and 11 indicated the need for adjustment in over 10% of the respondents. Among them, seven were related to antibiotic prophylaxis. With the exception of these, there were opinions that some oral antibiotics, the use of glycopeptides, catheter-related infections, and empirical antifungal therapy need to be modified. This is believed to be because therapeutic strategies, including experiences, prophylaxis, distribution of causative organisms, and the current situation of resistance, differ according to hospitals. In future, we hope that clinical experiences in Korea help patients and are consistent with international standards through much discussion.
This committee has attempted to develop guidelines that are helpful for clinicians who examine and have concern for patients. However, the current guidelines do not mention tuberculosis or chronic hepatitis, the incidence rates of which are expected to be characteristically high in Korea. Moreover, various guidelines for the diagnosis and treatment of common diseases (e.g., invasive aspergillosis) in febrile neutropenic patients and prevention of infectious complications in specific groups (e.g., HSCT recipients) are considered necessary. We hope that many researchers obtain the necessary additional data through prospective studies and suggest future directions. Finally, we hope that these guidelines are widely used and periodically revised every few years by related societies to provide the latest treatment information based on literature from Korea and other countries.
Acknowledgments
This study was completed as part of the Clinical Practice Guideline development project (project no. NA2009-013), funded by NECA, in Korea. The results of this project underwent an appraisal process involving hematologists, infectious diseases specialists, medical oncologists, medical microbiologists, and methodologists.
The following committee members participated in the review and appraisal process of the guidelines: Chang-Ki Min (Division of Hematology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea), Yeon-Joon Park (Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea), Joung-Soon Jang (Division of Hematology Oncology, Department of Internal Medicine, Chung-Ang University College of Medicine), Jun Ho Jang (Division of Hematology Oncology, Department of Internal Medicine, Sungkyunkwan University School of Medicine), Jong Youl Jin (Division of Hematology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea).
Footnotes
No potential conflict of interest relevant to this article was reported.
These guidelines will be also published in Infection and Chemotherapy in the Korean language. This secondary publication has been allowed by the editors of both journals.
These guidelines were made by the committee "Guidelines for the empirical therapy of neutropenic fever patients based on literature in Korea" under the supervision of the National Evidence-based Healthcare Collaborating Agency. These could be different from those of Korean Society of Internal Medicine.
References
- 1.The Korean Society of Infectious Diseases. Infectious Disease. Seoul: Koonja Publishing Inc.; 2007. [Google Scholar]
- 2.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 3.Segal BH, Baden LR, Casper C, et al. NCCN Clinical Practice Guidelines in Oncology Prevention and Treatment of Cancer-Related Infections (v.2.2009) Fort Washington: National Comprehensive Cancer Network; 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bertz H, Auner HW, Weissinger F, et al. Antimicrobial therapy of febrile complications after high-dose chemo-/radiotherapy and autologous hematopoietic stem cell transplantation-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S167–S174. doi: 10.1007/s00277-003-0771-5. [DOI] [PubMed] [Google Scholar]
- 5.Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Bohme A, Ruhnke M, et al. Treatment of invasive fungal infections in cancer patients: recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2009;88:97–110. doi: 10.1007/s00277-008-0622-5. [DOI] [PubMed] [Google Scholar]
- 6.Bohme A, Ruhnke M, Buchheidt D, et al. Treatment of fungal infections in hematology and oncology-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S133–S140. doi: 10.1007/s00277-003-0767-1. [DOI] [PubMed] [Google Scholar]
- 7.Buchheidt D, Bohme A, Cornely OA, et al. Diagnosis and treatment of documented infections in neutropenic patients: recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S127–S132. doi: 10.1007/s00277-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, Bohme A, Buchheidt D, et al. Prophylaxis of invasive fungal infections in patients with hematological malignancies and solid tumors-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S186–S200. doi: 10.1007/s00277-003-0773-3. [DOI] [PubMed] [Google Scholar]
- 9.Einsele H, Bertz H, Beyer J, et al. Infectious complications after allogeneic stem cell transplantation: epidemiology and interventional therapy strategies-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S175–S185. doi: 10.1007/s00277-003-0772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatkenheuer G, Buchheidt D, Cornely OA, et al. Central venous catheter (CVC)-related infections in neutropenic patients-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S149–S157. doi: 10.1007/s00277-003-0769-z. [DOI] [PubMed] [Google Scholar]
- 11.Link H, Bohme A, Cornely OA, et al. Antimicrobial therapy of unexplained fever in neutropenic patients-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society) Ann Hematol. 2003;82(Suppl 2):S105–S117. doi: 10.1007/s00277-003-0764-4. [DOI] [PubMed] [Google Scholar]
- 12.Ruhnke M, Bohme A, Buchheidt D, et al. Diagnosis of invasive fungal infections in hematology and oncology-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S141–S148. doi: 10.1007/s00277-003-0768-0. [DOI] [PubMed] [Google Scholar]
- 13.Sandherr M, Einsele H, Hebart H, et al. Antiviral prophylaxis in patients with haematological malignancies and solid tumours: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Oncology (DGHO) Ann Oncol. 2006;17:1051–1059. doi: 10.1093/annonc/mdj132. [DOI] [PubMed] [Google Scholar]
- 14.Marchetti O, Cordonnier C, Calandra T. Empirical antifungal therapy in neutropaenic cancer patients with persistent fever. Eur J Cancer Suppl. 2007;5:32–42. [Google Scholar]
- 15.Maertens JA, Frere P, Lass-Florl C, Heinz W, Cornely OA. Primary antifungal prophylaxis in leukaemia patients. Eur J Cancer Suppl. 2007;5:43–48. [Google Scholar]
- 16.Drgona L, Paul M, Bucaneve G, Calandra T, Menichetti F. The need for aminoglycosides in combination with β-lactams for high-risk, febrile neutropaenic patients with leukaemia. Eur J Cancer Suppl. 2007;5:13–22. [Google Scholar]
- 17.Cometta A, Marchetti O, Calandra T. Empirical use of anti-Gram-positive antibiotics in febrile neutropaenic cancer patients with acute leukaemia. Eur J Cancer Suppl. 2007;5:23–31. [Google Scholar]
- 18.Bucaneve G, Castagnola E, Viscoli C, Leibovici L, Menichetti F. Quinolone prophylaxis for bacterial infections in afebrile high risk neutropenic patients. Eur J Cancer Suppl. 2007;5:5–12. [Google Scholar]
- 19.Jun HX, Zhixiang S, Chun W, et al. Clinical guidelines for the management of cancer patients with neutropenia and unexplained fever. Int J Antimicrob Agents. 2005;26(Suppl 2):S128–S132. doi: 10.1016/j.ijantimicag.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Ohno R. Current antimicrobial usage for the management of infections in leukemic patients in Japan: results of a survey. Clin Infect Dis. 2004;39(Suppl 1):S11–S14. doi: 10.1086/383044. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Ohno R. Antimicrobial prophylaxis in febrile neutropenia. Clin Infect Dis. 2004;39(Suppl 1):S65–S67. doi: 10.1086/383058. [DOI] [PubMed] [Google Scholar]
- 22.Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. Clin Infect Dis. 2004;39(Suppl 1):S53–S55. doi: 10.1086/383055. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K, Imajo K, Akiyama N, et al. Randomized trial of cefepime monotherapy or cefepime in combination with amikacin as empirical therapy for febrile neutropenia. Clin Infect Dis. 2004;39(Suppl 1):S15–S24. doi: 10.1086/383046. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K. Initial empirical antimicrobial therapy: duration and subsequent modifications. Clin Infect Dis. 2004;39(Suppl 1):S59–S64. doi: 10.1086/383057. [DOI] [PubMed] [Google Scholar]
- 25.Ohyashiki K. Monotherapy versus dual therapy based on risk categorization of febrile neutropenic patients. Clin Infect Dis. 2004;39(Suppl 1):S56–S58. doi: 10.1086/383056. [DOI] [PubMed] [Google Scholar]
- 26.Masaoka T. Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis. 2004;39(Suppl 1):S49–S52. doi: 10.1086/383054. [DOI] [PubMed] [Google Scholar]
- 27.Kanamaru A, Tatsumi Y. Microbiological data for patients with febrile neutropenia. Clin Infect Dis. 2004;39(Suppl 1):S7–S10. doi: 10.1086/383042. [DOI] [PubMed] [Google Scholar]
- 28.The periodic health examination. Canadian task force on the periodic health examination. Can Med Assoc J. 1979;121:1193–1254. [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SM, Park SH, Lee DG, Choi JH, Yoo JH, Shin WS. Current antimicrobial usage for the management of neutropenic fever in Korea: a nationwide survey. J Korean Med Sci. 2008;23:941–947. doi: 10.3346/jkms.2008.23.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HS, Song MG. Comparison of rectal temperature and tympanic membrane. Nurs Sci. 1998;10(2):22–30. [Google Scholar]
- 31.Fulbrook P. Core body temperature measurement: a comparison of axilla, tympanic membrane and pulmonary artery blood temperature. Intensive Crit Care Nurs. 1997;13:266–272. doi: 10.1016/s0964-3397(97)80425-9. [DOI] [PubMed] [Google Scholar]
- 32.Dzarr AA, Kamal M, Baba AA. A comparison between infrared tympanic thermometry, oral and axilla with rectal thermometry in neutropenic adults. Eur J Oncol Nurs. 2009;13:250–254. doi: 10.1016/j.ejon.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Ciuraru NB, Braunstein R, Sulkes A, Stemmer SM. The influence of mucositis on oral thermometry: when fever may not reflect infection. Clin Infect Dis. 2008;46:1859–1863. doi: 10.1086/588290. [DOI] [PubMed] [Google Scholar]
- 34.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 35.Bodey GP, Rodriguez V, Chang HY, Narboni Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978;41:1610–1622. doi: 10.1002/1097-0142(197804)41:4<1610::aid-cncr2820410452>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715–719. [PubMed] [Google Scholar]
- 37.Heussel CP, Kauczor HU, Heussel GE, et al. Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: use of high-resolution computed tomography. J Clin Oncol. 1999;17:796–805. doi: 10.1200/JCO.1999.17.3.796. [DOI] [PubMed] [Google Scholar]
- 38.Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038–3051. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 39.Talcott JA, Finberg R, Mayer RJ, Goldman L. The medical course of cancer patients with fever and neutropenia: clinical identification of a low-risk subgroup at presentation. Arch Intern Med. 1988;148:2561–2568. [PubMed] [Google Scholar]
- 40.Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: a prospective, two-center validation of a prediction rule. J Clin Oncol. 1992;10:316–322. doi: 10.1200/JCO.1992.10.2.316. [DOI] [PubMed] [Google Scholar]
- 41.Talcott JA, Whalen A, Clark J, Rieker PP, Finberg R. Home antibiotic therapy for low-risk cancer patients with fever and neutropenia: a pilot study of 30 patients based on a validated prediction rule. J Clin Oncol. 1994;12:107–114. doi: 10.1200/JCO.1994.12.1.107. [DOI] [PubMed] [Google Scholar]
- 42.Jung JS, Kwon KY, Kim KS, et al. Risk prediction factors in febrile neutropenic patients. J Korean Soc Emerg Med. 2000;11:305–315. [Google Scholar]
- 43.Yoo JH, Choi SM, Lee DG, et al. Prognostic factors influencing infection-related mortality in patients with acute leukemia in Korea. J Korean Med Sci. 2005;20:31–35. doi: 10.3346/jkms.2005.20.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142:979–995. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]
- 45.Engels EA, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta-analysis. J Clin Oncol. 1998;16:1179–1187. doi: 10.1200/JCO.1998.16.3.1179. [DOI] [PubMed] [Google Scholar]
- 46.Ito JI, Tegtmeier BR, O'Donnell MR. Antibacterial prophylaxis in patients with cancer and neutropenia. N Engl J Med. 2006;354:90–94. [PubMed] [Google Scholar]
- 47.Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353:988–998. doi: 10.1056/NEJMoa050078. [DOI] [PubMed] [Google Scholar]
- 48.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–987. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 49.Lee DG, Choi SM, Choi JH, et al. Selective bowel decontamination for the prevention of infection in acute myelogenous leukemia: a prospective randomized trial. Korean J Intern Med. 2002;17:38–44. doi: 10.3904/kjim.2002.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas X, Troncy J, Belhabri A, et al. Effectiveness of combined vancomycin and pefloxacine in gastrointestinal decontamination for preventing infections after chemotherapy-induced bone marrow aplasia: a randomized double-blind study. Presse Med. 2000;29:1745–1751. [PubMed] [Google Scholar]
- 51.Talbot GH, Cassileth PA, Paradiso L, Correa-Coronas R, Bond L. Oral enoxacin for infection prevention in adults with acute nonlymphocytic leukemia: the Enoxacin Prophylaxis Study Group. Antimicrob Agents Chemother. 1993;37:474–482. doi: 10.1128/aac.37.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lew MA, Kehoe K, Ritz J, et al. Prophylaxis of bacterial infections with ciprofloxacin in patients undergoing bone marrow transplantation. Transplantation. 1991;51:630–636. doi: 10.1097/00007890-199103000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Karp JE, Merz WG, Hendricksen C, et al. Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1987;106:1–7. doi: 10.7326/0003-4819-106-1-1. [DOI] [PubMed] [Google Scholar]
- 54.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- 55.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 56.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation: a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 57.Rotstein C, Bow EJ, Laverdiere M, Ioannou S, Carr D, Moghaddam N. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy: the Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis. 1999;28:331–340. doi: 10.1086/515128. [DOI] [PubMed] [Google Scholar]
- 58.Winston DJ, Chandrasekar PH, Lazarus HM, et al. Fluconazole prophylaxis of fungal infections in patients with acute leukemia: results of a randomized placebo-controlled, double-blind, multicenter trial. Ann Intern Med. 1993;118:495–503. doi: 10.7326/0003-4819-118-7-199304010-00003. [DOI] [PubMed] [Google Scholar]
- 59.Schaffner A, Schaffner M. Effect of prophylactic fluconazole on the frequency of fungal infections, amphotericin B use, and health care costs in patients undergoing intensive chemotherapy for hematologic neoplasias. J Infect Dis. 1995;172:1035–1041. doi: 10.1093/infdis/172.4.1035. [DOI] [PubMed] [Google Scholar]
- 60.Young GA, Bosly A, Gibbs DL, Durrant S. A double-blind comparison of fluconazole and nystatin in the prevention of candidiasis in patients with leukaemia: Antifungal Prophylaxis Study Group. Eur J Cancer. 1999;35:1208–1213. doi: 10.1016/s0959-8049(99)00102-1. [DOI] [PubMed] [Google Scholar]
- 61.Philpott-Howard JN, Wade JJ, Mufti GJ, Brammer KW, Ehninger G. Randomized comparison of oral fluconazole versus oral polyenes for the prevention of fungal infection in patients at risk of neutropenia: Multicentre Study Group. J Antimicrob Chemother. 1993;31:973–984. doi: 10.1093/jac/31.6.973. [DOI] [PubMed] [Google Scholar]
- 62.Menichetti F, Del Favero A, Martino P, et al. Preventing fungal infection in neutropenic patients with acute leukemia: fluconazole compared with oral amphotericin B. Ann Intern Med. 1994;120:913–918. doi: 10.7326/0003-4819-120-11-199406010-00003. [DOI] [PubMed] [Google Scholar]
- 63.Annaloro C, Oriana A, Tagliaferri E, et al. Efficacy of different prophylactic antifungal regimens in bone marrow transplantation. Haematologica. 1995;80:512–517. [PubMed] [Google Scholar]
- 64.Huijgens PC, Simoons-Smit AM, van Loenen AC, et al. Fluconazole versus itraconazole for the prevention of fungal infections in haemato-oncology. J Clin Pathol. 1999;52:376–380. doi: 10.1136/jcp.52.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glasmacher A, Cornely O, Ullmann AJ, et al. An open-label randomized trial comparing itraconazole oral solution with fluconazole oral solution for primary prophylaxis of fungal infections in patients with haematological malignancy and profound neutropenia. J Antimicrob Chemother. 2006;57:317–325. doi: 10.1093/jac/dki440. [DOI] [PubMed] [Google Scholar]
- 66.Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 67.Morgenstern GR, Prentice AG, Prentice HG, Ropner JE, Schey SA, Warnock DW. A randomized controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies: U.K. Multicentre Antifungal Prophylaxis Study Group. Br J Haematol. 1999;105:901–911. doi: 10.1046/j.1365-2141.1999.01465.x. [DOI] [PubMed] [Google Scholar]
- 68.Oren I, Rowe JM, Sprecher H, et al. A prospective randomized trial of itraconazole vs fluconazole for the prevention of fungal infections in patients with acute leukemia and hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2006;38:127–134. doi: 10.1038/sj.bmt.1705418. [DOI] [PubMed] [Google Scholar]
- 69.Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients: a multicenter, randomized trial. Ann Intern Med. 2003;138:705–713. doi: 10.7326/0003-4819-138-9-200305060-00006. [DOI] [PubMed] [Google Scholar]
- 70.Slordal L, Spigset O. Heart failure induced by non-cardiac drugs. Drug Saf. 2006;29:567–586. doi: 10.2165/00002018-200629070-00003. [DOI] [PubMed] [Google Scholar]
- 71.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole. A broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45:1610–1617. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 72.Schering-Plough. PR Newswire Association; 2006. [cited 2011 May 15]. Schering-Plough announces FDA approval of NOXAFIL(R) (posaconazole) for treatment of oropharyngeal candidiasis (OPC) [Internet] Available from: http://www2.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/10-23-2006/0004456852&EDATE= [Google Scholar]
- 73.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 74.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 75.Hiramatsu Y, Maeda Y, Fujii N, et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2008;88:588–595. doi: 10.1007/s12185-008-0196-y. [DOI] [PubMed] [Google Scholar]
- 76.Wolff SN, Fay J, Stevens D, et al. Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplant. 2000;25:853–859. doi: 10.1038/sj.bmt.1702233. [DOI] [PubMed] [Google Scholar]
- 77.Bodey GP, Anaissie EJ, Elting LS, Estey E, O'Brien S, Kantarjian H. Antifungal prophylaxis during remission induction therapy for acute leukemia fluconazole versus intravenous amphotericin B. Cancer. 1994;73:2099–2106. doi: 10.1002/1097-0142(19940415)73:8<2099::aid-cncr2820730814>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 78.Tollemar J, Ringden O, Andersson S, Sundberg B, Ljungman P, Tyden G. Randomized double-blind study of liposomal amphotericin B (Ambisome) prophylaxis of invasive fungal infections in bone marrow transplant recipients. Bone Marrow Transplant. 1993;12:577–582. [PubMed] [Google Scholar]
- 79.Kelsey SM, Goldman JM, McCann S, et al. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant. 1999;23:163–168. doi: 10.1038/sj.bmt.1701543. [DOI] [PubMed] [Google Scholar]
- 80.Tollemar J, Hockerstedt K, Ericzon BG, Sundberg B, Ringden O. Fungal prophylaxis with AmBisome in liver and bone marrow transplant recipients: results of two randomized double-blind studies. Transplant Proc. 1994;26:1833. [PubMed] [Google Scholar]
- 81.Penack O, Schwartz S, Martus P, et al. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single-center trial. Ann Oncol. 2006;17:1306–1312. doi: 10.1093/annonc/mdl128. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz S, Behre G, Heinemann V, et al. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial. Blood. 1999;93:3654–3661. [PubMed] [Google Scholar]
- 83.Alexander BD, Dodds Ashley ES, Addison RM, Alspaugh JA, Chao NJ, Perfect JR. Non-comparative evaluation of the safety of aerosolized amphotericin B lipid complex in patients undergoing allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2006;8:13–20. doi: 10.1111/j.1399-3062.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 84.Rijnders BJ, Cornelissen JJ, Slobbe L, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008;46:1401–1408. doi: 10.1086/586739. [DOI] [PubMed] [Google Scholar]
- 85.Choi SM, Lee DG, Choi JH, et al. Itraconazole oral solution versus fluconazole syrup for prevention of invasive fungal infections in patients receiving hematopoietic stem cell transplantation: prospective, randomized, comparative clinical trial. Infect Chemother. 2005;37:71–78. [Google Scholar]
- 86.Menichetti F, Del Favero A, Martino P, et al. GIMEMA Infection Program; Gruppo Italiano Malattie Ematologiche dell' Adulto. Itraconazole oral solution as prophylaxis for fungal infections in neutropenic patients with hematologic malignancies: a randomized, placebo-controlled, double-blind, multicenter trial. Clin Infect Dis. 1999;28:250–255. doi: 10.1086/515129. [DOI] [PubMed] [Google Scholar]
- 87.Nucci M, Biasoli I, Akiti T, et al. A double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patients. Clin Infect Dis. 2000;30:300–305. doi: 10.1086/313654. [DOI] [PubMed] [Google Scholar]
- 88.Akiyama H, Mori S, Tanikawa S, Sakamaki H, Onozawa Y. Fluconazole versus oral amphotericin B in preventing fungal infection in chemotherapy-induced neutropenic patients with haematological malignancies. Mycoses. 1993;36:373–378. doi: 10.1111/j.1439-0507.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 89.Riley DK, Pavia AT, Beatty PG, et al. The prophylactic use of low-dose amphotericin B in bone marrow transplant patients. Am J Med. 1994;97:509–514. doi: 10.1016/0002-9343(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 90.Kaptan K, Ural AU, Cetin T, Avcu F, Beyan C, Yalcin A. Itraconazole is not effective for the prophylaxis of fungal infections in patients with neutropenia. J Infect Chemother. 2003;9:40–45. doi: 10.1007/s10156-002-0207-5. [DOI] [PubMed] [Google Scholar]
- 91.Kern W, Behre G, Rudolf T, et al. German AML Cooperative Group. Failure of fluconazole prophylaxis to reduce mortality or the requirement of systemic amphotericin B therapy during treatment for refractory acute myeloid leukemia: results of a prospective randomized phase III study. Cancer. 1998;83:291–301. [PubMed] [Google Scholar]
- 92.Lass-Florl C, Gunsilius E, Gastl G, et al. Fungal colonization in neutropenic patients: a randomized study comparing itraconazole solution and amphotericin B solution. Ann Hematol. 2003;82:565–569. doi: 10.1007/s00277-003-0666-5. [DOI] [PubMed] [Google Scholar]
- 93.Van Delden C, Lew DP, Chapuis B, Rohner P, Hirschel B. Antifungal prophylaxis in severely neutropenic patients: how much fluconazole is necessary? Clin Microbiol Infect. 1995;1:24–30. doi: 10.1111/j.1469-0691.1995.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 94.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 95.Hughes WT, Rivera GK, Schell MJ, Thornton D, Lott L. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1987;316:1627–1632. doi: 10.1056/NEJM198706253162604. [DOI] [PubMed] [Google Scholar]
- 96.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 97.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2007;(3):CD005590. doi: 10.1002/14651858.CD005590.pub2. [DOI] [PubMed] [Google Scholar]
- 98.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–249. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 100.Sudhoff T, Arning M, Schneider W. Prophylactic strategies to meet infectious complications in fludarabine-treated CLL. Leukemia. 1997;11(Suppl 2):S38–S41. [PubMed] [Google Scholar]
- 101.El-Sadr WM, Luskin-Hawk R, Yurik TM, et al. A randomized trial of daily and thrice-weekly trimethoprim-sulfamethoxazole for the prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected persons: Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) Clin Infect Dis. 1999;29:775–783. doi: 10.1086/520433. [DOI] [PubMed] [Google Scholar]
- 102.Vasconcelles MJ, Bernardo MV, King C, Weller EA, Antin JH. Aerosolized pentamidine as pneumocystis prophylaxis after bone marrow transplantation is inferior to other regimens and is associated with decreased survival and an increased risk of other infections. Biol Blood Marrow Transplant. 2000;6:35–43. doi: 10.1016/s1083-8791(00)70050-4. [DOI] [PubMed] [Google Scholar]
- 103.Souza JP, Boeckh M, Gooley TA, Flowers ME, Crawford SW. High rates of Pneumocystis carinii pneumonia in allogeneic blood and marrow transplant recipients receiving dapsone prophylaxis. Clin Infect Dis. 1999;29:1467–1471. doi: 10.1086/313509. [DOI] [PubMed] [Google Scholar]
- 104.Meyers JD, Flournoy N, Thomas ED. Infection with herpes simplex virus and cell-mediated immunity after marrow transplant. J Infect Dis. 1980;142:338–346. doi: 10.1093/infdis/142.3.338. [DOI] [PubMed] [Google Scholar]
- 105.Schubert MM, Peterson DE, Flournoy N, Meyers JD, Truelove EL. Oral and pharyngeal herpes simplex virus infection after allogeneic bone marrow transplantation: analysis of factors associated with infection. Oral Surg Oral Med Oral Pathol. 1990;70:286–293. doi: 10.1016/0030-4220(90)90142-f. [DOI] [PubMed] [Google Scholar]
- 106.Barton T, Collis T, Stadtmauer E, Schuster M. Infectious complications the year after autologous bone marrow transplantation or peripheral stem cell transplantation for treatment of breast cancer. Clin Infect Dis. 2001;32:391–395. doi: 10.1086/318491. [DOI] [PubMed] [Google Scholar]
- 107.Herrmann RP, Trent M, Cooney J, Cannell PK. Infections in patients managed at home during autologous stem cell transplantation for lymphoma and multiple myeloma. Bone Marrow Transplant. 1999;24:1213–1217. doi: 10.1038/sj.bmt.1702044. [DOI] [PubMed] [Google Scholar]
- 108.Seropian S, Nadkarni R, Jillella AP, et al. Neutropenic infections in 100 patients with non-Hodgkin's lymphoma or Hodgkin's disease treated with high-dose BEAM chemotherapy and peripheral blood progenitor cell transplant: out-patient treatment is a viable option. Bone Marrow Transplant. 1999;23:599–605. doi: 10.1038/sj.bmt.1701610. [DOI] [PubMed] [Google Scholar]
- 109.Lee JT, Kim YT. Prevalence of antibody to herpes simplex virus. Korean J Dermatol. 1993;31:38–46. [Google Scholar]
- 110.Glenny AM, Fernandez Mauleffinch LM, Pavitt S, Walsh T. Interventions for the prevention and treatment of herpes simplex virus in patients being treated for cancer. Cochrane Database Syst Rev. 2009;(1):CD006706. doi: 10.1002/14651858.CD006706.pub2. [DOI] [PubMed] [Google Scholar]
- 111.Yahav D, Gafter-Gvili A, Muchtar E, et al. Antiviral prophylaxis in haematological patients: systematic review and meta-analysis. Eur J Cancer. 2009;45:3131–3148. doi: 10.1016/j.ejca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 112.Liesveld JL, Abboud CN, Ifthikharuddin JJ, et al. Oral valacyclovir versus intravenous acyclovir in preventing herpes simplex virus infections in autologous stem cell transplant recipients. Biol Blood Marrow Transplant. 2002;8:662–665. doi: 10.1053/bbmt.2002.v8.abbmt080662. [DOI] [PubMed] [Google Scholar]
- 113.Warkentin DI, Epstein JB, Campbell LM, et al. Valacyclovir versus acyclovir for HSV prophylaxisin neutropenic patients. Ann Pharmacother. 2002;36:1525–1531. doi: 10.1345/aph.1A434. [DOI] [PubMed] [Google Scholar]
- 114.Wade JC, Newton B, Flournoy N, Meyers JD. Oral acyclovir for prevention of herpes simplex virus reactivation after marrow transplantation. Ann Intern Med. 1984;100:823–828. doi: 10.7326/0003-4819-100-6-823. [DOI] [PubMed] [Google Scholar]
- 115.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface. Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 116.Choi SM, Lee DG, Park YH, et al. Infections in patients with acute leukemia: comparison of induction chemotherapy group and reinduction chemotherapy group. Infect Chemother. 2003;35:78–85. [Google Scholar]
- 117.Kim HB, Park SW, Kim US, et al. Infections in patients with acute leukemia (1993~1996) Korean J Hematol. 1999;34:359–365. [Google Scholar]
- 118.Park SH, Choi SM, Lee DG, et al. Current trends of infectious complications following hematopoietic stem cell transplantation in a single center. J Korean Med Sci. 2006;21:199–207. doi: 10.3346/jkms.2006.21.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rho YH, Lee YJ, Lee J, et al. Clinical aspects and prognostic factors of neutropenic fever in leukemic patients: 1996~2001. Korean J Infect Dis. 2002;34:152–159. [Google Scholar]
- 120.Rhee JY, Jang EH, Kim ST, et al. Profiles of infectious complications on the outcomes for the recipients of allogeneic hematopoietic stem cell transplantation. Korean J Med. 2007;72:200–208. [Google Scholar]
- 121.Kim JS, Oh JM. The patterns of infection and therapy in cancer patients receiving chemotherapy. J Korean Soc Health Syst Pharm. 2000;17:481–500. [Google Scholar]
- 122.Freifeld A, Marchigiani D, Walsh T, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341:305–311. doi: 10.1056/NEJM199907293410501. [DOI] [PubMed] [Google Scholar]
- 123.Hidalgo M, Hornedo J, Lumbreras C, et al. Outpatient therapy with oral ofloxacin for patients with low risk neutropenia and fever: a prospective, randomized clinical trial. Cancer. 1999;85:213–219. [PubMed] [Google Scholar]
- 124.Kern WV, Cometta A, De Bock R, Langenaeken J, Paesmans M, Gaya H. Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1999;341:312–318. doi: 10.1056/NEJM199907293410502. [DOI] [PubMed] [Google Scholar]
- 125.Malik IA, Khan WA, Karim M, Aziz Z, Khan MA. Feasibility of outpatient management of fever in cancer patients with low-risk neutropenia: results of a prospective randomized trial. Am J Med. 1995;98:224–231. doi: 10.1016/s0002-9343(99)80367-2. [DOI] [PubMed] [Google Scholar]
- 126.Rolston KV, Rubenstein EB, Freifeld A. Early empiric antibiotic therapy for febrile neutropenia patients at low risk. Infect Dis Clin North Am. 1996;10:223–237. doi: 10.1016/s0891-5520(05)70297-x. [DOI] [PubMed] [Google Scholar]
- 127.Rolston KV, Frisbee-Hume SE, Patel S, Manzullo EF, Benjamin RS. Oral moxifloxacin for outpatient treatment of low-risk, febrile neutropenic patients. Support Care Cancer. 2010;18:89–94. doi: 10.1007/s00520-009-0634-2. [DOI] [PubMed] [Google Scholar]
- 128.Aquino VM, Herrera L, Sandler ES, Buchanan GR. Feasibility of oral ciprofloxacin for the outpatient management of febrile neutropenia in selected children with cancer. Cancer. 2000;88:1710–1714. [PubMed] [Google Scholar]
- 129.Giamarellou H, Bassaris HP, Petrikkos G, et al. Monotherapy with intravenous followed by oral high-dose ciprofloxacin versus combination therapy with ceftazidime plus amikacin as initial empiric therapy for granulocytopenic patients with fever. Antimicrob Agents Chemother. 2000;44:3264–3271. doi: 10.1128/aac.44.12.3264-3271.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerr KG, Armitage HT, McWhinney PH. Activity of quinolones against viridians group streptococci isolated from blood cultures of patients with haematological malignancy. Support Care Cancer. 1999;7:28–30. doi: 10.1007/s005200050219. [DOI] [PubMed] [Google Scholar]
- 131.De Pauw BE, Deresinski SC, Feld R, Lane-Allman EF, Donnelly JP The Intercontinental Antimicrobial Study Group. Ceftazidime compared with piperacillin and tobramycin for the empiric treatment of fever in neutropenic patients with cancer: a multicenter randomized trial. Ann Intern Med. 1994;120:834–844. doi: 10.7326/0003-4819-120-10-199405150-00004. [DOI] [PubMed] [Google Scholar]
- 132.Cometta A, Calandra T, Gaya H, et al. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer: the International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto Infection Program. Antimicrob Agents Chemother. 1996;40:1108–1115. doi: 10.1128/aac.40.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rubinstein E, Lode H, Grassi C. Ceftazidime monotherapy vs. ceftriaxone/tobramycin for serious hospital-acquired gram-negative infections: Antibiotic Study Group. Clin Infect Dis. 1995;20:1217–1228. doi: 10.1093/clinids/20.5.1217. [DOI] [PubMed] [Google Scholar]
- 134.Winston DJ, Ho WG, Bruckner DA, Champlin RE. Beta-lactam antibiotic therapy in febrile granulocytopenic patients: a randomized trial comparing cefoperazone plus piperacillin, ceftazidime plus piperacillin, and imipenem alone. Ann Intern Med. 1991;115:849–859. doi: 10.7326/0003-4819-115-11-849. [DOI] [PubMed] [Google Scholar]
- 135.Pizzo PA, Hathorn JW, Hiemenz J, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315:552–558. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- 136.Behre G, Link H, Maschmeyer G, et al. Meropenem monotherapy versus combination therapy with ceftazidime and amikacin for empirical treatment of febrile neutropenic patients. Ann Hematol. 1998;76:73–80. doi: 10.1007/s002770050366. [DOI] [PubMed] [Google Scholar]
- 137.Bohme A, Shah PM, Stille W, Hoelzer D. Piperacillin/tazobactam versus cefepime as initial empirical antimicrobial therapy in febrile neutropenic patients: a prospective randomized pilot study. Eur J Med Res. 1998;3:324–330. [PubMed] [Google Scholar]
- 138.Del Favero A, Menichetti F, Martino P, et al. A multicenter, double-blind, placebo-controlled trial comparing piperacillin-tazobactam with and without amikacin as empiric therapy for febrile neutropenia. Clin Infect Dis. 2001;33:1295–1301. doi: 10.1086/322646. [DOI] [PubMed] [Google Scholar]
- 139.Akova M, Akan H, Korten V, et al. Meropenem Study Group of Turkey. Comparison of meropenem with amikacin plus ceftazidime in the empirical treatment of febrile neutropenia: a prospective randomised multicentre trial in patients without previous prophylactic antibiotics. Int J Antimicrob Agents. 1999;13:15–19. doi: 10.1016/s0924-8579(99)00096-5. [DOI] [PubMed] [Google Scholar]
- 140.Yamamura D, Gucalp R, Carlisle P, Cimino M, Roberts J, Rotstein C. Open randomized study of cefepime versus piperacillin-gentamicin for treatment of febrile neutropenic cancer patients. Antimicrob Agents Chemother. 1997;41:1704–1708. doi: 10.1128/aac.41.8.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Freifeld AG, Walsh T, Marshall D, et al. Monotherapy for fever and neutropenia in cancer patients: a randomized comparison of ceftazidime versus imipenem. J Clin Oncol. 1995;13:165–176. doi: 10.1200/JCO.1995.13.1.165. [DOI] [PubMed] [Google Scholar]
- 142.Bow EJ, Rotstein C, Noskin GA, et al. A randomized, open-label, multicenter comparative study of the efficacy and safety of piperacillin-tazobactam and cefepime for the empirical treatment of febrile neutropenic episodes in patients with hematologic malignancies. Clin Infect Dis. 2006;43:447–459. doi: 10.1086/505393. [DOI] [PubMed] [Google Scholar]
- 143.Johnson MP, Ramphal R. Beta-lactam-resistant Enterobacter bacteremia in febrile neutropenic patients receiving monotherapy. J Infect Dis. 1990;162:981–983. doi: 10.1093/infdis/162.4.981. [DOI] [PubMed] [Google Scholar]
- 144.Feld R, DePauw B, Berman S, Keating A, Ho W. Meropenem versus ceftazidime in the treatment of cancer patients with febrile neutropenia: a randomized, double-blind trial. J Clin Oncol. 2000;18:3690–3698. doi: 10.1200/JCO.2000.18.21.3690. [DOI] [PubMed] [Google Scholar]
- 145.Vandercam B, Gerain J, Humblet Y, et al. Meropenem versus ceftazidime as empirical monotherapy for febrile neutropenic cancer patients. Ann Hematol. 2000;79:152–157. doi: 10.1007/s002770050571. [DOI] [PubMed] [Google Scholar]
- 146.Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, editors. Cancer Management: A Multidisciplinary Approach. 12th ed. Manhasset, NY: CMP Medica; 2009. [Google Scholar]
- 147.Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:338–348. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 148.Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2006;57:176–189. doi: 10.1093/jac/dki448. [DOI] [PubMed] [Google Scholar]
- 149.Jung HW, Chae JW, Kang MR, et al. Comparative efficacy and safety of cefepime alone versus ceftazidime plus tobramycin in cancer patients with febrile neutropenia. Infect Chemother. 2004;36:341–349. [Google Scholar]
- 150.Lee DH, Kim CK, Ko JY, et al. A comparative study of cefepime versus ceftazidime monotherapy as empirical therapy for febrile episodes in neutropenic patients. J Korean Soc Chemother. 2002;20:243–251. [Google Scholar]
- 151.Flaherty JP, Waitley D, Edlin B, et al. Multicenter, randomized trial of ciprofloxacin plus azlocillin versus ceftazidime plus amikacin for empiric treatment of febrile neutropenic patients. Am J Med. 1989;87(5A):278S–282S. doi: 10.1016/0002-9343(89)90080-6. [DOI] [PubMed] [Google Scholar]
- 152.Cometta A, Zinner S, de Bock R, et al. Piperacillin-tazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer: the International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Antimicrob Agents Chemother. 1995;39:445–452. doi: 10.1128/aac.39.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cordonnier C, Herbrecht R, Pico JL, et al. The French Cefepime Study Group. Cefepime/amikacin versus ceftazidime/amikacin as empirical therapy for febrile episodes in neutropenic patients: a comparative study. Clin Infect Dis. 1997;24:41–51. doi: 10.1093/clinids/24.1.41. [DOI] [PubMed] [Google Scholar]
- 154.Peacock JE, Herrington DA, Wade JC, et al. Ciprofloxacin plus piperacillin compared with tobramycin plus piperacillin as empirical therapy in febrile neutropenic patients: a randomized, double-blind trial. Ann Intern Med. 2002;137:77–87. doi: 10.7326/0003-4819-137-2-200207160-00005. [DOI] [PubMed] [Google Scholar]
- 155.Sanz MA, Lopez J, Lahuerta JJ, et al. Cefepime plus amikacin versus piperacillin-tazobactam plus amikacin for initial antibiotic therapy in haematology patients with febrile neutropenia: results of an open, randomized, multicentre trial. J Antimicrob Chemother. 2002;50:79–88. doi: 10.1093/jac/dkf087. [DOI] [PubMed] [Google Scholar]
- 156.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Torfoss D, Høiby EA, Tangen JM, et al. Tobramycin once versus three times daily, given with penicillin G, to febrile neutropenic cancer patients in Norway: a prospective, randomized, multicentre trial. J Antimicrob Chemother. 2007;59:711–717. doi: 10.1093/jac/dkm003. [DOI] [PubMed] [Google Scholar]
- 158.Choi SM, Lee DG, Kim MJ, et al. Clinical features and risk factors of the septic shock in patients with neutropenic fever. Infect Chemother. 2003;35:370–376. [Google Scholar]
- 159.Elting LS, Rubenstein EB, Rolston K, et al. Time to clinical response: an outcome of antibiotic therapy of febrile neutropenia with implications for quality and cost of care. J Clin Oncol. 2000;18:3699–3706. doi: 10.1200/JCO.2000.18.21.3699. [DOI] [PubMed] [Google Scholar]
- 160.Kim WS, Lee H, Ki HK, et al. The efficacy and safety of oral ciprofloxacin therapy for low-risk febrite patients with neutropenia during cancer chemotherapy. J Korean Soc Chemother. 2000;18:29–38. [Google Scholar]
- 161.European Organization for Research and Treatment of Cancer (EORTC) International Antimicrobial Therapy Cooperative Group and the National Cancer Institute of Canada-Clinical Trials Group. Vancomycin added to empirical combination antibiotic therapy for fever in granulocytopenic cancer patients. J Infect Dis. 1991;163:951–958. [PubMed] [Google Scholar]
- 162.Micozzi A, Venditti M, Amadori S, Pulsoni A, Tirindelli C, Martino P. Teicoplanin in the treatment of gram-positive bacteraemia in neutropenic patients. Br J Haematol. 1990;76(Suppl 2):19–23. doi: 10.1111/j.1365-2141.1990.tb07930.x. [DOI] [PubMed] [Google Scholar]
- 163.Vardakas KZ, Samonis G, Chrysanthopoulou SA, Bliziotis IA, Falagas ME. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic patients: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5:431–439. doi: 10.1016/S1473-3099(05)70164-X. [DOI] [PubMed] [Google Scholar]
- 164.Paul M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram-positive infections for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2005;55:436–444. doi: 10.1093/jac/dki028. [DOI] [PubMed] [Google Scholar]
- 165.Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 166.Glasmacher A, von Lilienfeld-Toal M, Schulte S, Hahn C, Schmidt-Wolf IG, Prentice A. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clin Microbiol Infect. 2005;11(Suppl 5):17–23. doi: 10.1111/j.1469-0691.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 167.Kim SH, Park WB, Lee KD, et al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2004;54:489–497. doi: 10.1093/jac/dkh366. [DOI] [PubMed] [Google Scholar]
- 168.Uh Y, Hwang GY, Jang IH, Kwon O, Kim HY, Yoon KJ. Antimicrobial susceptibility patterns and macrolide resistance genes of beta-hemolytic viridians group streptococci in a tertiary Korean hospital. J Korean Med Sci. 2007;22:791–794. doi: 10.3346/jkms.2007.22.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Uh Y, Hwang GY, Jang IH, Yoon KJ, Kim HY. Antimicrobial susceptibilities of viridans streptococci isolated from blood cultures during recent period. J Lab Med Qual Assur. 2002;24:225–230. [Google Scholar]
- 170.Cometta A, Kern WV, De Bock R, et al. Vancomycin versus placebo for treating persistent fever in patients with neutropenic cancer receiving piperacillin-tazobactam monotherapy. Clin Infect Dis. 2003;37:382–389. doi: 10.1086/376637. [DOI] [PubMed] [Google Scholar]
- 171.Erjavec Z, de Vries-Hospers HG, Laseur M, Halie RM, Daenen S. A prospective, randomized, double-blinded, placebo-controlled trial of empirical teicoplanin in febrile neutropenia with persistent fever after imipenem monotherapy. J Antimicrob Chemother. 2000;45:843–849. doi: 10.1093/jac/45.6.843. [DOI] [PubMed] [Google Scholar]
- 172.Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 174.Brink AJ, Richards GA, Cummins RR, Lambson J Gauteng Understanding Teicoplanin Serum levels (GUTS) study group. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsis. Int J Antimicrob Agents. 2008;32:455–458. doi: 10.1016/j.ijantimicag.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 175.Press OW, Ramsey PG, Larson EB, Fefer A, Hickman RO. Hickman catheter infections in patients with malignancies. Medicine (Baltimore) 1984;63:189–200. doi: 10.1097/00005792-198407000-00001. [DOI] [PubMed] [Google Scholar]
- 176.Cercenado E, Ena J, Rodriguez-Creixems M, Romero I, Bouza E. A conservative procedure for the diagnosis of catheter-related infections. Arch Intern Med. 1990;150:1417–1420. [PubMed] [Google Scholar]
- 177.Blot F, Nitenberg G, Chachaty E, et al. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet. 1999;354:1071–1077. doi: 10.1016/s0140-6736(98)11134-0. [DOI] [PubMed] [Google Scholar]
- 178.Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004;140:18–25. doi: 10.7326/0003-4819-140-1-200401060-00007. [DOI] [PubMed] [Google Scholar]
- 179.Krause R, Auner HW, Gorkiewicz G, et al. Detection of catheter-related bloodstream infections by the differential-time-to-positivity method and gram stain-acridine orange leukocyte cytospin test in neutropenic patients after hematopoietic stem cell transplantation. J Clin Microbiol. 2004;42:4835–4837. doi: 10.1128/JCM.42.10.4835-4837.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abdelkefi A, Achour W, Ben Othman T, et al. Difference in time to positivity is useful for the diagnosis of catheter-related bloodstream infection in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2005;35:397–401. doi: 10.1038/sj.bmt.1704773. [DOI] [PubMed] [Google Scholar]
- 181.Blot F, Schmidt E, Nitenberg G, et al. Earlier positivity of central-venous- versus peripheral-blood cultures is highly predictive of catheter-related sepsis. J Clin Microbiol. 1998;36:105–109. doi: 10.1128/jcm.36.1.105-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Updateby the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48:203–212. doi: 10.1086/595686. [DOI] [PubMed] [Google Scholar]
- 184.Kim SH, Kang CI, Kim HB, et al. Outcomes of Hickman catheter salvage in febrile neutropenic cancer patients with Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2003;24:897–904. doi: 10.1086/502157. [DOI] [PubMed] [Google Scholar]
- 185.Dugdale DC, Ramsey PG. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am J Med. 1990;89:137–141. doi: 10.1016/0002-9343(90)90290-t. [DOI] [PubMed] [Google Scholar]
- 186.Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am J Med. 1982;72:101–111. doi: 10.1016/0002-9343(82)90594-0. [DOI] [PubMed] [Google Scholar]
- 187.EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989;86:668–672. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 188.Klastersky J. Empirical antifungal therapy. Int J Antimicrob Agents. 2004;23:105–112. doi: 10.1016/j.ijantimicag.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 189.Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis. 2004;39(Suppl 1):S38–S43. doi: 10.1086/383052. [DOI] [PubMed] [Google Scholar]
- 190.Goldberg E, Gafter-Gvili A, Robenshtok E, Leibovici L, Paul M. Empirical antifungal therapy for patients with neutropenia and persistent fever: Systematic review and meta-analysis. Eur J Cancer. 2008;44:2192–2203. doi: 10.1016/j.ejca.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 191.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–1184. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 192.Yoo JH, Choi JH, Lee DG, Choi S, Shin WS, Kim CC. Analysis of invasive fungal infection after hematopoietic stem cell transplantation or chemotherapy in patients with hematologic diseases. Infect Chemother. 2004;36:40–45. [Google Scholar]
- 193.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 194.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Michallet M, Ito JI. Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J Clin Oncol. 2009;27:3398–3409. doi: 10.1200/JCO.2008.20.1178. [DOI] [PubMed] [Google Scholar]
- 196.Lee JS, Shin JH, Lee K, et al. Species distribution and susceptibility to azole antifungals of Candida bloodstream isolates from eight university hospitals in Korea. Yonsei Med J. 2007;48:779–786. doi: 10.3349/ymj.2007.48.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bal AM, Gould IM. Empirical antimicrobial treatment for chemotherapy-induced febrile neutropenia. Int J Antimicrob Agents. 2007;29:501–509. doi: 10.1016/j.ijantimicag.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 198.Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia: National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 199.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 200.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 201.Girmenia C, Barosi G, Aversa F, et al. Prophylaxis and treatment of invasive fungal diseases in allogeneic stem cell transplantation: results of a consensus process by Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Clin Infect Dis. 2009;49:1226–1236. doi: 10.1086/605665. [DOI] [PubMed] [Google Scholar]
- 202.Prentice HG, Hann IM, Herbrecht R, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997;98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 203.Boogaerts M, Winston DJ, Bow EJ, et al. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy: a randomized, controlled trial. Ann Intern Med. 2001;135:412–422. doi: 10.7326/0003-4819-135-6-200109180-00010. [DOI] [PubMed] [Google Scholar]
- 204.Park SH, Choi SM, Lee DG, et al. Intravenous itraconazole vs. amphotericin B deoxycholate for empirical antifungal therapy in patients with persistent neutropenic fever. Korean J Intern Med. 2006;21:165–172. doi: 10.3904/kjim.2006.21.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schuler U, Bammer S, Aulitzky WE, et al. Safety and efficacy of itraconazole compared to amphotericin B as empirical antifungal therapy for neutropenic fever in patients with haematological malignancy. Onkologie. 2007;30:185–191. doi: 10.1159/000100055. [DOI] [PubMed] [Google Scholar]
- 206.Segal BH. Aspergillosis. N Engl J Med. 2009;360:1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 207.Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. Am J Med. 2000;108:282–289. doi: 10.1016/s0002-9343(99)00457-x. [DOI] [PubMed] [Google Scholar]
- 208.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ben-Ami R, Lewis RE, Kontoyiannis DP. Invasive mould infections in the setting of hematopoietic cell transplantation: current trends and new challenges. Curr Opin Infect Dis. 2009;22:376–384. doi: 10.1097/QCO.0b013e32832db9f3. [DOI] [PubMed] [Google Scholar]
- 210.Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–1250. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]
- 211.Cordonnier C, Pautas C, Maury S, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48:1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 212.Yoo JH, Choi JH, Choi SM, et al. Application of nucleic acid sequence-based amplification for diagnosis of and monitoring the clinical course of invasive aspergillosis in patients with hematologic diseases. Clin Infect Dis. 2005;40:392–398. doi: 10.1086/427284. [DOI] [PubMed] [Google Scholar]
- 213.Mennink-Kersten MA, Verweij PE. Non-culture-based diagnostics for opportunistic fungi. Infect Dis Clin North Am. 2006;20:711–727. doi: 10.1016/j.idc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 214.Song KH, Lee S, Jang HC, et al. Diagnostic usefulness of galactomannan assay for invasive aspergillosis. Infect Chemother. 2009;41:82–89. [Google Scholar]
- 215.O'Shaughnessy EM, Shea YM, Witebsky FG. Laboratory diagnosis of invasive mycoses. Infect Dis Clin North Am. 2003;17:135–158. doi: 10.1016/s0891-5520(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 216.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 217.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. Utility of galactomannan enzyme immunoassay and (1,3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol. 2009;47:129–133. doi: 10.1128/JCM.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yoo JH, Choi SM, Lee DG, et al. Comparison of the real-time nucleic acid sequence-based amplification (RTi-NASBA) with conventional NASBA, and galactomannan assay for the diagnosis of invasive aspergillosis. J Korean Med Sci. 2007;22:672–676. doi: 10.3346/jkms.2007.22.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]