Abstract
Serotonin and the 5HT1A receptor are expressed in a subset of taste receptor cells, and the 5HT3 receptor is expressed on afferent fibers innervating taste buds. Exogenous administration of the selective serotonin reuptake inhibitor, paroxetine, has been shown to increase taste sensitivity to stimuli described by humans as sweet and bitter. Serotonergic agonists also decrease food and fluid intake, and it is possible that modulations of serotonin may alter taste-based hedonic responsiveness; alternatively, or in combination, serotonin may interact with physiological state to impact ingestive behavior. In this study, the unconditioned licking of prototypical taste stimuli by rats in brief-access taste tests was assessed following paroxetine administration (0.3–10 mg/kg intraperitoneal). We also measured sucrose licking by rats in different deprivation states after paroxetine (5 mg/kg). In neither experiment did we find any evidence of an effect of paroxetine on licking relative to water to any of the taste stimuli in the brief-access test at doses that decreased food intake. However, in some conditions, paroxetine decreased trials initiated to tastants. Therefore, a systemic increase in serotonin via paroxetine administration can decrease appetitive behavior in brief-access tests but is insufficient to alter taste-guided consummatory behavior.
Keywords: appetitive, brief-access test, gustatory, hedonic, sweet
Introduction
The neurotransmitter serotonin (5HT) and its functional machinery have been identified in taste receptor cells on the tongue in multiple species (Herness and Chen 1997; Kaya et al. 2004; Herness et al. 2005; Roper 2006). The 5HT3 receptor subtype is located on the afferent fibers associated with Type III taste receptor cells, and the 5HT1A receptor subtype is found directly on Type II taste receptor cells (Kaya et al. 2004). It has been suggested that general activity of 5HT released from Type III cells acts in a paracrine fashion and, in the presence of ATP, signals negative feedback to Type II cells via 5HT1A receptors (Kaya et al. 2004; Tomchik et al. 2007; Huang et al. 2009).
Serotonin's presence and activity in the taste bud make it a likely candidate for modulating taste signals contributing to perceptual, affective, and physiological processes associated with gustatory stimulation. Indeed, drugs active at 5HT receptors influence food intake and ingestion of sweet fluids (for reviews, see Simansky 1996; Halford et al. 2007; Garfield and Heisler 2009). However, mutant mice lacking 5HT3A receptors show no obvious taste-related behavioral abnormalities (Finger et al. 2005). Although this suggests that the 5HT3 receptor is not necessary for the behavioral expression of normal taste function, it remains to be comprehensively tested what influence exogenous stimulation of 5HT3, as well as other 5HT receptor subtypes, would have on taste-guided behavior in the intact system. Recently, Heath et al. (2006) reported in healthy humans that paroxetine, a selective serotonin reuptake inhibitor (SSRI), decreased the sensory detection threshold for sucrose and quinine.
If a global increase in the activity of 5HT affects perceived sweetness and bitter taste, it may also impact hedonic responses to food and fluids, which may be what drives the effect of 5HT on food intake. Thus, in the first experiment of the present study, we assessed in a rat model the effect of multiple doses of paroxetine on unconditioned licking responses to sucrose, NaCl, citric acid, and quinine hydrochloride using a brief-access taste test. Brief-access tests allow some separation of appetitive and consummatory components of ingestive behavior, with the initiation of trials during the test representing the appetitive approach component and licking during the trials representing the final behavioral actions triggered by the contact of the stimulus with its appropriate receptors. Alternatively or in combination with its effect on affective taste processes, the influence of 5HT on ingestion may be due to its ability to alter physiological processes related to energy and fluid balance or satiation that modulate feeding and drinking. To this end, we also explored the interaction of a single dose of paroxetine with deprivation state on licking for sucrose.
Materials and methods
Experiment 1
Subjects
Forty male adult Sprague-Dawley rats that weighed ∼250–300 g at the start of procedures were used. All rats were housed individually in conventional polycarbonate shoebox cages in a vivarium in which temperature, humidity, and lighting (12:12 h lights on:lights off) were automatically controlled. All procedures were performed during the period of the day in which the lights were illuminated. Unless otherwise noted, rats received ad libitum access to standard maintenance chow (PMI 5001) and deionized water. All procedures were approved by the Animal Care and Use Committee of Florida State University.
Drug
The dose range for paroxetine maleate (Tocris) was chosen to encompass doses that decreased taste thresholds in humans (∼0.2 mg/kg, Heath et al. 2006), decreased food intake in rats (5 mg/kg, McCann et al. 1997), and affected motivated behavior of rats in other paradigms (3 and 7 mg/kg, Brimberg et al. 2007, 5 and 10 mg/kg, Sokolowski and Seiden 1999) but not those that completely ameliorated responding (15 mg/kg, Joel et al. 2004; 20 mg/kg, Sokolowski and Seiden 1999). Drug and vehicle solutions were prepared en masse and frozen in aliquots; the amount needed for injection each day was thawed that morning. For the majority of testing, paroxetine was dissolved in dimethyl sulfoxide (DMSO, Tocris) and rats were injected with vehicle and one of 5 doses (0, 0.3, 1, 3, 10 mg/kg, 1 ml/kg intraperitoneal [ip]). To reduce the possibility that stress from 100% DMSO injection was masking any effect, for the second round of testing with sucrose, paroxetine was dissolved in DMSO and then the final DMSO concentration was adjusted to 10% with the addition of deionized water. Rats were injected with this vehicle and one of the 5 doses listed above (1 ml/kg ip). Because precipitate was observed in the 10 mg/kg suspension when dissolved in the 10% DMSO vehicle shortly after the time of injection, this dose was not used in analysis. A washout period of at least 48 h occurred between each drug injection.
Test stimuli
All solutions were prepared daily with reagent-grade chemicals dissolved in deionized water and presented at room temperature. Test stimuli consisted of deionized water, sucrose (BDH Chemicals; 0.01, 0.03, 0.06, 0.1, 0.3 and 1 M), NaCl (Sigma-Aldrich; 0.03, 0.1, 0.2, 0.3, 0.5, and 1 M), citric acid (Fisher Scientific; 0.3, 1, 3, 10, 30, 100 mM), and quinine hydrochloride (Mallinckrodt Baker Inc.; 0.01, 0.03, 0.1, 0.3, 1, 3 mM) solutions. The concentrations of the taste compounds chosen reflect the dynamic range of responsiveness in the brief-access test (see Grobe and Spector 2008).
Apparatus
Rats were tested using a brief-access procedure (see Spector 2003) in a lickometer known as the Davis rig (Davis MS-160, DiLog Instruments; see Smith 2001) in which stimuli were presented from spouts attached to small reservoir tubes that held the solutions. The tubes and spouts moved horizontally on a computer-controlled motorized rack that positioned one spout at a time behind an access slot in the front panel of the test chamber. During some sessions, a single tube was presented for the entire 30-min session, whereas during other sessions, multiple tubes were presented individually in blocks of trials (randomized without replacement). A trial was initiated after one lick, and, after completion of a 10-s period, a shutter closed over the access slot and the rack moved such that a new stimulus tube was put into position. The shutter was then reopened and the rat was given the opportunity to initiate another trial.
Training and testing with water as the stimulus
Water bottles were removed from the home cages ∼23 h prior to the first training session. In 2 sessions across consecutive days, the rats were acclimated to the test chamber and allowed to lick water from one spout that remained stationary throughout the session. After completion of these sessions, water bottles were returned to the rats for ∼48 h.
After rehydration following stationary water training, the impact of paroxetine on general oromotor competence of licking was assessed when animals were water-deprived and presented with water from a stationary spout. Rats were injected 1 h prior to their session with vehicle on one day and with their assigned dose of paroxetine on another day. Water bottles were removed ∼23 h prior to and returned ∼1 h after the session on each test day. Individual rats were assigned to paroxetine dose groups based on their body weight and performance after vehicle injection.
After stationary water testing, 2 sessions across consecutive days were conducted in which the rats were trained to lick from multiple spouts delivering water; each spout was presented individually in 10-s trials. Water bottles were removed ∼23 h prior to training, returned ∼1 h after the second training session, and remained on the home cages for ∼48 h prior to further testing.
Testing with taste stimuli
For a total of 5 weeks, the rats were tested with a concentration series for a single compound in 3 sessions a week on Monday, Wednesday, and Friday in the following order: sucrose, NaCl, citric acid, quinine, and sucrose. For each session during which sucrose was the test stimulus, rats were partially food- and water-restricted by the provision of a ration of 10 g of chow and 20 ml of water on Tuesday, Thursday, and Sunday ∼23 h prior to testing. This was done to promote stimulus sampling without imposing a complete 24-h deprivation for water or food emulating a similar procedure that is effective with mice (see Glendinning et al. 2002). For each session with the other taste stimuli that are actively avoided by rats, water bottles were removed ∼23 h prior to each test session to generate a high rate of responding for water from which changes in licking could be revealed as the stimulus concentration was raised; food was available ad libitum. On the first session of each taste compound test phase, the rats did not receive any injection; on the second session, rats were injected with vehicle; and on the third session, rats were injected with their assigned dose of paroxetine. Injections were given 1 h prior to the test session. Food and/or water were returned ∼1 h after each test session.
Food intake
After the completion of brief-access testing, the effect of paroxetine on chow intake while the rats were ∼23 h food deprived was assessed. This was conducted to ensure that in our hands paroxetine would act in a manner similar to McCann et al. (1997), in which nondeprived rats injected twice daily with 5 mg/kg paroxetine ate less food after 24 h than rats injected with vehicle. In our study, we sought to determine if the drug was active within 1.5 h after administration, which is the time that brief-access testing had occurred; thus, we measured food of take when the rats were ∼23 h food deprived, which would ensure a high level of motivation to feed as soon as food was presented.
The rats were given at least 2 days to acclimate to the removal and replacement of the food bin, which was placed in the right rear of their home cage. Food bins were removed from the home cage ∼22 h prior to each session. Four sessions were conducted: on the first session, the rats were injected with the 100% DMSO vehicle twice, once at 10 AM and once at 6 PM; on the second session, they were injected with their assigned dose of paroxetine in 100% DMSO twice; on the third session, they were injected with the 10% DMSO vehicle twice; and on the fourth session, they were injected with their assigned dose of paroxetine in the 10% DMSO vehicle twice. Food bins were returned 1 h after injection and intake was measured at 0.5, 1, 1.5, 2, 7, and 24 h after chow presentation.
Data analysis
All statistical analyses were conducted using Systat software. Comparisons among groups were considered significant when P ≤ 0.05. Only significant results relative to the vehicle used in each phase are reported. The false discovery rate procedure (Benjamini and Hochberg 1995; Curran-Everett 2000) was used when necessary as a correction for multiple paired comparisons of drug dose groups after analyses of variance (ANOVAs) for water, trial, lick, and food intake data.
The average interlick interval (ILI) and the total number of licks taken to water during stationary water testing after injection of the paroxetine dose series was analyzed by one-way ANOVA with dose group as the factor. Only ILIs that were between 50 and 250 ms in duration were used in the analysis because values less than 50 ms were considered double licks and those greater than 250 ms were considered pauses between licking bursts.
Licks to each stimulus concentration were averaged and total number of trials was tallied on the third day of stimulus testing for each taste compound, which was when injections with the paroxetine dose series were given. Lick data for individual rats were included in the analysis only if at least 2 trials per concentration were taken; however, all rats were included in the analysis of trials initiated even if the minimum trial criterion was not met. Due to problems with air locks on the water tubes used to provide rations during the first phase of sucrose testing, it was not clear if all rats had been able to consume their entire ration prior to their 3rd test session. Thus, rats that had water remaining were injected and run in sessions, but their data were not included in either the lick or trial analyses. On occasion, computer malfunctions resulted in loss of data. The number of trials initiated was analyzed via one-way ANOVA with dose group as the factor. The lick average for each concentration was considered relative to the average licks to water during the session. For sucrose, a Lick Score was derived:
For all other stimuli, a Lick Ratio was calculated:
Lick Scores and Ratios within each stimulus phase were analyzed by 2-way ANOVA with dose group as a factor and test stimulus concentration as a repeated measure. Curves were fit to the lick data using a following 3-parameter logistic function:\
| (1) |
in which x = stimulus concentration; a = asymptotic lick response adjusted for water; b = slope; and c = log10 concentration at the inflection point.
Cumulative food intake at each time point after paroxetine injection was analyzed by one-way ANOVA. If substantial spillage was observed, the data for that rat were not included in analysis.
Experiment 2
Subjects
Thirty-two naive adult male Sprague-Dawley rats that weighed ∼250–300 g at the start of procedures were used and housed as described in Experiment 1.
Drug
Paroxetine maleate was dissolved in DMSO and then the final DMSO concentration was adjusted to 10%. Rats were injected with either vehicle or 5 mg/kg paroxetine (1 ml/kg ip).
Test stimuli
Sucrose and deionized water as described in Experiment 1 were used as test stimuli.
Apparatus
The rats were tested using the brief-access procedure described in Experiment 1, but in a modified (see Blonde et al. 2006) version of a gustometer (see Spector et al. 1990), rather than a Davis rig. In the gustometer, stimuli were delivered via computer-controlled solenoid valves to a spout connected with Teflon tubing to reservoirs positioned above the test chamber. At the start of the session, the motorized vertically oriented drinking spout was rotated behind a centrally positioned access slot in the front panel of the test chamber. During sessions in which the stimuli were presented in blocks of trials, a trial was initiated after the rat completed 2 dry licks on the spout within 250 ms upon which the drinking spout shaft was filled with solution and each subsequent lick deposited 5 μl (approximate lick volume for spout-drinking rats) of solution into the fluid column. After the trial was completed, the spout was rotated away from the access slot over a funnel where it was rinsed with water and dried with pressurized air. The spout was then rotated back behind the access slot and the rat was given the opportunity to initiate another trial.
Training and testing with water as the stimulus
The rats were trained to lick water from the stationary gustometer spout in 2 sessions using the training procedure described in Experiment 1. The rats were then divided into either the vehicle or the drug group in a balanced fashion based on their performance during the last stationary water training session. According to this assignment, rats were injected with either vehicle or 5 mg/kg paroxetine 1 h prior to a stationary water session. Water bottles were removed ∼23 h prior to and replaced ∼1 h after completion of the session.
The rats were then trained across 2 consecutive days to lick water from the gustometer spout when it was presented in 10-s trials, as described in Experiment 1. Water bottles were removed ∼23 h prior to the beginning of training and replaced ∼1 h after completion of the session where they remained for ∼48 h prior to any further manipulations.
After rehydration following training during which water was presented in 10-s trials, the effect of vehicle and paroxetine on licks and number of trials initiated to water was assessed. Rats were injected with either vehicle or 5 mg/kg paroxetine 1 h prior to this water test session. Water bottles were removed ∼23 h prior to and returned ∼1 h after completion the session.
Testing with the sucrose concentration series
Upon completion of water testing, the rats from each injection group were further assigned to begin testing in one of 4 deprivation states: ∼23 h water deprivation, ∼23 h food deprivation, partial food and water restriction (as described in Experiment 1), or no deprivation. The rats were assigned to testing starting points such that there were no statistical differences among the groups in body weight and lick performance during water testing. A Latin Square design was implemented such that all rats were tested in each condition starting with the one to which they were assigned and then progressing through the others in the order listed above; for example, if a rat was assigned to begin testing while partially food- and water-restricted, in the next phase it would be tested while nondeprived, and then while water-deprived, and finally while food-deprived. A testing phase for each deprivation condition consisted of 2 sessions: on the first, no injection was given; and on the second, drug or vehicle (based on group assignment established at the time of water testing) was injected 1 h prior to the test session.
Food intake
After brief-access testing in all deprivation states was complete, chow intake of the rats was measured. Food was removed from the rats ∼22 h prior to injection with vehicle or 5 mg/kg paroxetine. One hour after injection, food was returned and measured at 0.5, 1, 1.5, and 2 h after food presentation.
Data analysis
All statistical analyses were conducted using Systat software. Comparisons between drug and vehicle groups under different deprivation conditions were considered significant when P ≤ 0.05.
The average ILI and the total number of licks taken to water during stationary water testing after 5 mg/kg paroxetine or vehicle injection were analyzed as described in Experiment 1. The total number of trials and the average number of licks taken per water trial during testing in which water was presented in trials after drug or vehicle administration were compared by one-way ANOVA.
Licks to sucrose were averaged and total number of trials was tallied on the second day of testing during each deprivation condition, which was when injections with paroxetine or vehicle occurred. Lick data for individual rats were included in the analysis only if at least 2 trials per concentration were taken; however, all rats were included in the analysis of trials initiated, even if the minimum trial criterion was not met. The number of trials initiated was analyzed within deprivation group by one-way ANOVA with dose group as the factor. Lick Scores (as described in Experiment 1) were analyzed within deprivation group by 2-way ANOVA with dose group as factor and deprivation state as a repeated measure. On a few occasions, computer malfunctions resulted in loss of data. Curves were fit to the Lick Scores as described in Experiment 1.
Cumulative food intake at each time point after paroxetine injection was analyzed by one-way ANOVA. If substantial spillage was observed, the data for that rat were not analyzed.
Results
Experiment 1
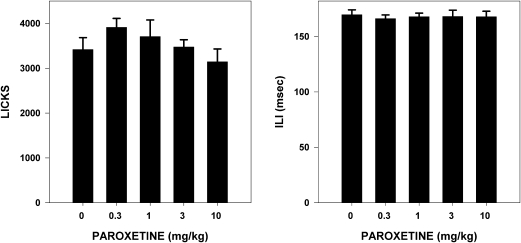
Paroxetine dose had no significant effect on the total number of licks taken to water (F4,35 = 1.217, P = 0.321) or basic oromotor performance as assessed through ILI (F4,35 = 0.077, P = 0.989) (Figure 1).
Figure 1.
Mean (± standard error) total number of licks taken by and ILI for water-deprived rats during stationary water testing (30 min) 1 h after injection of vehicle or a range of paroxetine doses (n = 8 per dose group). No differences (P ≤ 0.05) relative to vehicle (0 mg/kg paroxetine) were seen among groups.
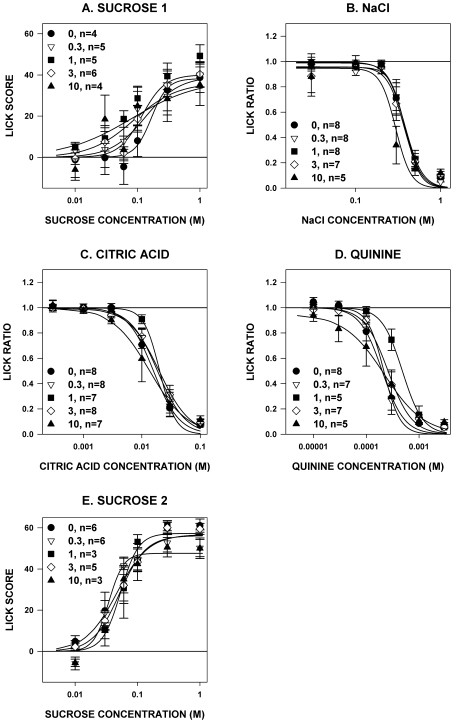
The Lick Scores during both test phases with sucrose and Lick Ratios during testing with NaCl and citric acid did not differ among paroxetine dose groups; there was a significant interaction among Lick Ratios during quinine testing, but none of it resulted from differences between paroxetine and vehicle and seemed to be attributable to the rats in the 1 mg/kg group displaying less lick suppressions to the 0.3 mM concentration (Table 1 and Figure 2). Thus, we found no evidence that the doses of paroxetine that we used had any effect on concentration-dependent licking of rats to prototypical taste stimuli when presented in 10-s trials.
Table 1.
Statistical comparisons of Lick Scores and Ratios among paroxetine dose groups across taste compounds from Experiment 1
| Phase | Dose | Stimulus concentration | Interaction |
| Sucrose 1a | F4,19 = 0.516, P = 0.725 | F5,95 = 55.771, P < 0.001 | F20,95 = 1.013, P = 0.455 |
| NaCl | F4,31 = 0.570, P = 0.686 | F5,155 = 159.304, P < 0.001 | F20,155 = 0.004, P = 0.856 |
| Citric acid | F4,33 = 0.397, P = 0.822 | F5,165 = 331.817, P < 0.001 | F20,155 = 1.081, P = 0.374 |
| Quinine | F4,27 = 1.520, P = 0.225 | F5,135 = 327.389, P < 0.001 | F20,135 = 2.697, P < 0.001 |
| Sucrose 2a | F3,16 = 0.138, P = 0.936 | F5,80 = 111.843, P < 0.001 | F15,80 = 1.247, P = 0.256 |
Sucrose 1 refers to tests after injection with paroxetine (0–10 mg/kg) in 100% DMSO vehicle and Sucrose 2 refers to tests after injection with paroxetine (0–3 mg/kg) in 10% DMSO.
Figure 2.
Mean (± standard error) Lick Scores to sucrose (panels A and E) or Lick Ratios to NaCl (panel B), citric acid (panel C), and quinine hydrochloride (panel D) for rats during brief-access tests after paroxetine injection (numbers in the legend denote mg/kg, followed by the number of rats that met analysis inclusion criteria). When tested with sucrose, the rats were partially food- and water-restricted, and when tested with all other stimuli, the rats were water-deprived. During the first phase of sucrose testing and for NaCl, citric acid, and quinine hydrochloride, paroxetine was dissolved in 100% DMSO. During the second phase of sucrose testing, paroxetine was dissolved in 10% DMSO. No significant differences relative to vehicle (0 mg/kg paroxetine) were seen among the groups.
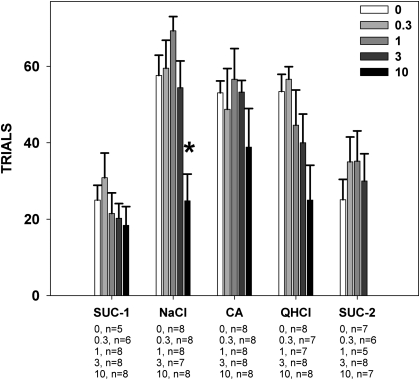
Paroxetine decreased the number of trials taken during NaCl testing (F4,34 = 7.603, P < 0.001; Figure 3), and paired comparisons revealed that the 10 mg/kg dose was significantly different from vehicle. Overall paroxetine decreased trials taken during citric acid (F4,33 = 2.944, P = 0.034; Figure 3) and quinine testing (F4,33 = 2.864, P = 0.038; Figure 3), but paired individual comparisons against vehicle did not reach significance. Paroxetine had no effect on the number of trials taken during testing with sucrose when 100% DMSO was the vehicle (F4,30 = 0.945, P = 0.452; Figure 3) or when 10% DMSO was the vehicle (F3,22 = 1.861, P = 1.660; Figure 3). These results suggest that under some conditions, paroxetine can suppress trial initiation in a brief-access test.
Figure 3.
Mean (± standard error) total number of trials initiated by rats during the brief-access tests described in the caption for Figure 2. The numbers under the tastants on the x axis represent group size at each dose. Rats injected with 10 mg/kg paroxetine took fewer trials to NaCl than those injected with vehicle. SUC-1, sucrose testing with 100% DMSO vehicle; CA, citric acid; QHCl, quinine hydrochloride; SUC-2, sucrose testing with 10% DMSO vehicle.
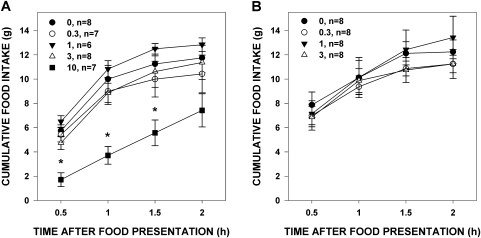
Paroxetine at 10 mg/kg in 100% DMSO decreased food intake 0.5–1.5 h after food presentation (Table 2 and Figure 4A). Paroxetine (0–3 mg/kg) in 10% DMSO had no effect on food intake (Figure 4B). This demonstrates that paroxetine at the highest dose was active in our hands and had the expected effect on food intake, albeit slightly different than that seen by McCann et al. (1997), who found an effect even at 24 h, but our rats were food deprived at the start of testing.
Table 2.
Statistical comparisons of cumulative food intake among paroxetine dose groups across time points from Experiments 1 and 2
| Experiment | Dose | 0.5 h | 1 h | 1.5 h | 2 h | 7 h | 24 h |
| 1 | 0–10 mg/kg in 100% DMSO | F4,31 = 9.892, P < 0.001 | F4,31 = 7.924, P < 0.001 | F4,31 = 3.881, P =0.018 | F4,31 = 2.033, P = 0.099 | F4,31 = 0.359, P = 0.836 | F4,31 = 1.833, P = 0.148 |
| 0–3 mg/kg in 10% DMSO | F3,27 = 0.202, P = 0.894 | F3,27 = 0.083, P = 0.969 | F3,27 = 0.588, P = 0.628 | F3,27 = 0.690, P = 0.566 | F3,27 = 1.266, P = 0.306 | F3,27 = 2.680, P = 0.067 | |
| 2 | 0 vs. 5 mg/kg in 10% DMSO | F1,30 = 1.610, P = 0.214 | F1,30 = 3.336, P = 0.078 | F1,30 = 6.142, P = 0.019 | F1,30 = 2.762, P = 0.107 | NA | NA |
NA, not applicable.
Figure 4.
Mean (± standard error) cumulative food intake of food-deprived rats 1 h after paroxetine injection (numbers in the legend denote mg/kg, followed by the number of rats that met analysis inclusion criteria). Panel (A): dose series of paroxetine in 100% DMSO; Panel (B): dose series of paroxetine in 10% DMSO. Rats injected with 10 mg/kg paroxetine ate less food 0.5–1.5 h after food presentation than rats injected with vehicle, which is noted by the presence of an asterisk over data points.
Experiment 2
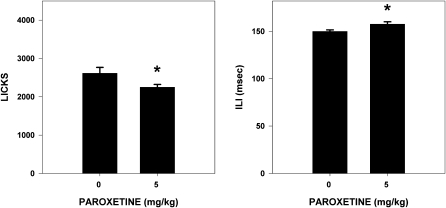
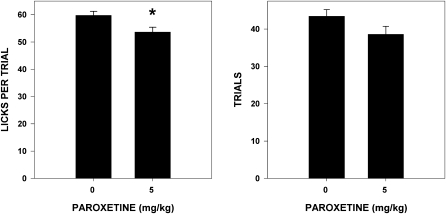
Paroxetine slightly but significantly decreased (14%) the total number of licks taken (F1,29 = 6.342, P = 0.018) and increased (9%) ILI (F1,29 = 4.579, P = 0.041) during stationary water testing (Figure 5). It is unclear why this effect was not seen in Experiment 1 but may be attributed to differences in the device used to measure licking and/or the vehicle in which paroxetine was dissolved. Paroxetine also decreased (10%) the number of licks taken per water trial during testing in which water was presented in trials (F1,30 = 7.149, P = 0.012), but did not significantly affect the number of trials taken (F1,30 = 3.957, P = 0.091) (Figure 6). The size of these effects was clearly modest at best.
Figure 5.
Mean (± standard error) total number of licks taken by and ILI for water-deprived rats during stationary water testing (30 min) after paroxetine injection (n = 15 in 0 mg/kg group; n = 16 in 5 mg/kg group). Rats injected with 5 mg/kg paroxetine took significantly fewer licks and had longer duration ILIs than rats injected with vehicle.
Figure 6.
Mean (± standard error [SE]) number of licks taken per trial and mean (± SE) total number of trials taken by water-deprived rats during rotating water testing in a brief-access test after paroxetine injection (n = 16 in both groups). Rats injected with 5 mg/kg paroxetine took significantly fewer licks per trial than rats injected with vehicle.
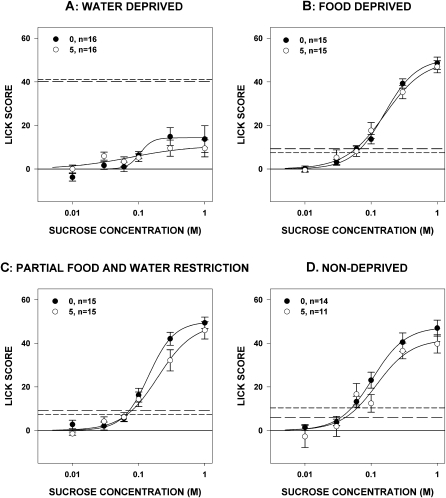
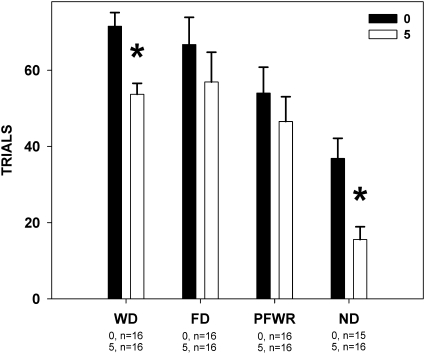
As seen in Experiment 1, paroxetine had no effect on concentration-dependent licking to sucrose, regardless of deprivation state (Table 2 and Figure 7). Deprivation state did, however, interact with paroxetine to impact the number of trials initiated. Rats injected with paroxetine took fewer trials than those injected with vehicle when water-deprived (F1,30 = 15.133, P = 0.001; Figure 8) or nondeprived (F1,29 = 11.193, P = 0.002; Figure 8), but not when they were food-deprived (F1,30 = 0.861, P = 435; Figure 8) or partially food- and water-restricted (F1,30 = 0.626, P = 0.435; Figure 8). This reflects our findings in Experiment 1 and suggests that despite multiple manipulations, there is no evidence of paroxetine having any effect on concentration-dependent licking during trials. In fact, we found no significant differences (all P values > 0.14) when we compared absolute licks to 1.0 M sucrose between drug and vehicle treatment within each deprivation state, suggesting that the drug did not appreciably affect motor competence. Also in line with Experiment 1, we saw that in some deprivation states, paroxetine decreased the number of times that the rats would approach the spout, which provides an assessment of the appetitive component of the licking behavior.
Figure 7.
Mean (± standard error) number of licks relative to water taken to sucrose by rats while in different deprivation states during brief-access tests after paroxetine injection (numbers in the legend denote mg/kg, followed by the number of rats that met analysis inclusion criteria). The broken line with large hatch marks in each graph indicates the mean number of licks to water that rats injected with vehicle took during the sessions; the broken line with small hatch marks indicates that of the 5 mg/kg group. No differences were seen between the groups.
Figure 8.
Mean (± standard error) total number of trials to sucrose initiated by rats during the brief-access tests described in Figure 7. The numbers under the deprivation states on the x axis represent group size at each dose. When tested while water deprived (WD) or when nondeprived (ND), the rats injected with 5 mg/kg paroxetine took fewer trials than the rats injected with vehicle. FD, food-deprived; PFWR, partial food and water restriction.
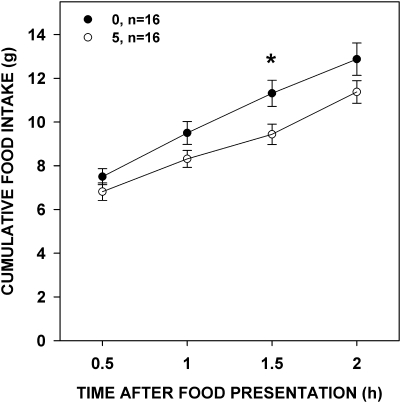
Paroxetine decreased food intake at 1.5 h after food presentation (Table 3 and Figure 9). This once again shows that our preparation of the drug gave us expected effects; however, in this experiment, we did not see an effect on food intake within the duration that the rats would have been in the brief-access tests. Despite this lack of effect, we saw an effect of paroxetine on trials taken, which is suggestive that it was active on taste-guided behavior during that time.
Table 3.
Statistical comparisons of Lick Scores between paroxetine dose groups across deprivation conditions from Experiment 2
| Phase | Dose | Stimulus concentration | Interaction |
| Water deprived | F1,30 < 0.001, P = 0.998 | F5,150 = 11.219, P < 0.001 | F5,150 = 1.587, P = 0.164 |
| Food deprived | F1,28 = 0.017, P = 0.897 | F5,140 = 127.832, P < 0 .001 | F5,140 = 0.671, P = 0.646 |
| Partially food and water restricted | F1,28 = 1.373, P = 0.251 | F5,140 = 127.771, P < 0.001 | F5,140 = 1.464, P = 0.205 |
| Nondeprived | F1,23 = 1.263, P = 0.273 | F5,115 = 79.363, P < 0.001 | F5,115 = 1.385, P = 0.235 |
Figure 9.
Mean (± standard error) cumulative food intake of food-deprived rats 1 h after paroxetine injection (numbers in the legend denote mg/kg, followed by the group size). Rats injected with 5 mg/kg paroxetine ate less food 1.5 h after food presentation than rats injected with vehicle.
Discussion
These findings demonstrated that an overall increase in 5HT transmission via systemic SSRI administration did not impact concentration-dependent unconditioned licking of taste compounds in rats during brief-access testing. Licking during brief-access trials can be considered primarily a consummatory behavior that is engaged upon contact of the stimulus with the appropriate receptors. The lack of effect was unexpected given the activity of serotonin within the taste bud and the effect of paroxetine on taste thresholds in humans (Heath et al. 2006). Although sensory thresholds and affective responding represent different domains of taste function (see Spector 2000; Glendinning and Spector 2009) and are dissociable in testing preparations, they are not necessarily mutually exclusive—and so it would follow that altered sensitivity to a taste could potentially impact hedonic evaluation. For example, rats in which the chorda tympani and glossopharyngeal nerves have been transected have higher detection thresholds for quinine and in turn show lower lick responsiveness (St John et al. 1994; St. John and Spector 1996). On the other hand, in humans, thresholds do not always correspond with suprathreshold intensity ratings (see Bartoshuk 1978).
That paroxetine did not affect consummatory taste-guided behavior is concordant with the findings of Finger et al. (2005), who demonstrated that 5HT3A knockout mice showed preference for sucrose, citric acid, and quinine, as well as other taste stimuli, similar to that of wild-type mice during 24-h 2-bottle tests. It remains to be tested whether these knockout mice show differences compared with wild-type mice in taste thresholds or in affective taste behavior in shorter term tests. Furthermore, the specific role of 5HT1A receptors, which act in a paracrine fashion in the taste bud and serve as autoreceptors in the central nervous system, regarding taste-guided behavior, remains to be addressed both pharmacologically and in knockout preparations.
Although our present findings show that alterations in 5HT signaling did not affect concentration-dependent consummatory behavior, we did demonstrate that under certain conditions paroxetine can impact appetitive behavior toward taste stimuli depending on internal state. In the brief-access tests, paroxetine decreased the number of times that rats approached the spout and initiated a trial to NaCl while water deprived and to sucrose while both water deprived and nondeprived. Because we did not observe any overt signs of malaise (e.g., diarrhea) in the rats after injection and/or during the tests, we have no reason to suspect that appetitive behavior was impacted by gastrointestinal effects of the SSRI, which have been reported in human patients (Spigset 1999; Pae and Patkar 2007). This suggests that under some circumstances, paroxetine decreases overall appetitive behavior but has no ostensible effect on the taste-elicited consummatory components of ingestion at least as measured by the brief-access test.
The range of doses we chose began near that which altered taste thresholds in humans (∼0.2 mg/kg, Heath et al. 2006) and increased to that which has been shown in other paradigms to affect motivated behavior (3–10 mg/kg, Sokolowski and Seiden 1999; Brimberg et al. 2007) without causing motor deficits (up to 12 mg/kg, Drapier et al. 2007); the timing of drug administration was also based on these reports. Our dose range also encompassed the dose clinically used in humans (Kelvin and Hackansson 1989), which is relevant since taste disturbances are at times reported with depression and as a side effect of SSRI use (see Settle and Amsterdam 1991), although to our knowledge this is not specific to treatment with paroxetine. At higher doses, paroxetine has been shown to completely suppress behavior (15 mg/kg, Joel et al. 2004; 20 mg/kg, Sokolowski and Seiden 1999), and so exploring the effects of higher doses on taste-guided behavior in the brief-access test would most likely not prove fruitful. We demonstrated that 5 and 10 mg/kg decreased feeding in food-deprived rats, and thus, in our hands paroxetine was, under some conditions, able to influence ingestive behavior but did not affect consummatory behavior toward taste solutions in brief-access tests. Furthermore, we saw little evidence that our dose range of paroxetine disrupted motor behavior as measured by oromotor competency during licks. In Experiment 2, 5 mg/kg paroxetine increased ILI during water tests, but by a very slight margin, and it was not observed in Experiment 1. Moreover, when water-deprived rats tested with aversive taste stimuli in Experiment 1, the Lick Ratio adjusts for any slight changes in licking rate. Also, during testing when nondeprived, rats responded at very high licking rates to 1.0 M sucrose regardless of drug treatment (F1,23 = 3.84, P = 0.542).
Because paroxetine was injected systemically and crosses the blood brain barrier (Cummings and Gjedde 1993; Uhr et al. 2003), it is unclear if these effects (and lack thereof) are central or peripheral in origin. The decrease in water intake seen in Experiment 2 suggests an impact on a central mechanism because peripherally injected 5HT, which does not cross the blood brain barrier, increases water intake (Montgomery and Burton 1986). It is also possible that the lack of effect seen on licking to the tastants was the result of a peripheral effect canceling out a central effect or vice versa. Furthermore, it is possible that by the time systemically administered drug reaches the taste bud, it is not concentrated enough to mimic the discrete paracrine activity that constitutively impacts receptors (Kaya et al 2004). Nevertheless, if peripheral versus central interactions affected hedonic processing of taste stimuli in rats, it might be expected to prevent an effect of paroxetine on taste thresholds in humans, but it did not (Heath et al. 2006). Finally, paroxetine has the highest potency among SSRIs to also block norepinephrine reuptake (Bourin et al. 2001; Frazer 2001), and although this potency is quite small compared with other drug classes (i.e., tricyclic antidepressants, reboxetine), possible noradrenergic interactions cannot be excluded. However, this is also unlikely because Heath et al (2006) demonstrated that specific pharmacological blockade of noradrenergic reuptake had little effect on taste thresholds in humans.
Using doses of paroxetine that are known to have effects on a range of behaviors including taste sensitivity, we were unable to demonstrate that the drug influences taste-guided unconditioned consummatory behavior in rats in a brief-access test. This was the case despite the inclusion of a panel of representative taste stimuli into the experimental design and, in the case of sucrose, systematic variation of physiological state. In some conditions, however, paroxetine treatment did significantly decrease number of trials initiated in the brief-access test, suggesting a global increase in 5HT signaling via SSRI administration can exert a suppressive effect on appetitive (approach), as opposed to consummatory (taste-elicited licking), responding. Such findings invite further scrutiny into the effect of modulation of serotonergic signaling on performance in other tasks explicitly designed to assess appetitive behavior, such as the progressive ratio procedure. Moreover, it is possible that a test of taste sensitivity in rats trained to perform a psychophysical task (see Spector 2003) would generate outcomes that match those seen in human subjects. Although the expression of serotonin in taste bud cells is well documented, its role in taste function remains to be fully understood.
Funding
This work was supported in part by the National Institute for Deafness and Other Communication Disorders at the National Institutes of Health [grant number 1F32DC010517-01 to C.M.M.].
Acknowledgments
We gratefully acknowledge the technical assistance of Ginger Blonde and Steven Janasik.
References
- Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr. 1978;31:1068–1077. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Blonde GD, Garcea M, Spector AC. The relative effects of transaction of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci. 2006;120(3):580–589. doi: 10.1037/0735-7044.120.3.580. [DOI] [PubMed] [Google Scholar]
- Bourin M, Chue P, Gullion Y. Paroxetine: a review. CNS Drug Rev. 2001;7(1):25–47. doi: 10.1111/j.1527-3458.2001.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimberg L, Flaisher-Grinberg S, Schilman EA, Joel D. Strain differences in ‘compulsive’ lever-pressing. Behav Brain Res. 2007;179(1):141–151. doi: 10.1016/j.bbr.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Cummings P, Gjedde A. Kinetics of the uptake of [3H]paroxetine in the rat brain. Synapse. 1993;15:124–129. doi: 10.1002/syn.890150204. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:1–8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Drapier D, Benue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann J-M. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176(2):202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Frazer A. Serotonergic and noradrenergic reuptake inhibitors: pre-clinical effects from in vitro potencies. J Clin Psychiatry. 2001;62(S12):16–23. [PubMed] [Google Scholar]
- Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587(Pt 1):49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27(5):461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Spector AC. Linking peripheral taste processes to behavior. Curr Opin Neurobiol. 2009;19(4):370–377. doi: 10.1016/j.conb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe CL, Spector AC. Constructing quality profiles for taste compounds in rats: a novel paradigm. Physiol Behav. 2008;95:413–424. doi: 10.1016/j.physbeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 2006;26(49):12664–12671. doi: 10.1523/JNEUROSCI.3459-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Chen Y. Serotonin inhibits calcium-activated K+ current in rat taste receptor cells. Neuroreport. 1997;8(15):3257–3261. doi: 10.1097/00001756-199710200-00014. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Shen T, Lu SG, Cao Y. Communication routes within the taste bud by neurotransmitters and neuropeptides. Chem Senses. 2005;30(Suppl 1):i37–i38. doi: 10.1093/chemse/bjh101. [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29(44):13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Ben-Amir E, Doljansky J, Flaisher S. Compulsive’ lever-pressing in rats is attenuated by the serotonin re-uptake inhibitors paroxetine and fluvoxamine but not by the tricyclic antidepressant desipramine or the anxiolytic diazepam. Behav Pharmacol. 2004;15(3):241–252. [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:649–658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kelvin AS, Hackansson S. Comparative acute toxicity of paroxetine and other antidepressants. Acta Psychiatr Scand Suppl. 1989;350:31–33. doi: 10.1111/j.1600-0447.1989.tb07165.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Yuan J, Hatzidimitriou G, Ricaurte GA. Selective serotonin reuptake inhibitors dissociate fenfluramine's anorectic and neurotoxic effects: importance of dose, species and drug. J Pharmacol Exp Ther. 1997;281:1487–1498. [PubMed] [Google Scholar]
- Montgomery AMJ, Burton MJ. Effects of peripheral 5-HT on consumption of flavoured solutions. Psychopharmacol. 1986;88:262–266. doi: 10.1007/BF00652252. [DOI] [PubMed] [Google Scholar]
- Pae CU, Patkar AA. Paroxetine: current status in psychiatry. Expert Rev Neurother. 2007;7(2):107–120. doi: 10.1586/14737175.7.2.107. [DOI] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell Mol Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settle RG, Amsterdam JD. Depression and the chemical senses. In: Getchell TV, et al., editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 851–862. [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Smith JC. The history of the "Davis Rig". Appetite. 2001;36(1):93–98. doi: 10.1006/appe.2000.0372. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Seiden LS. The behavioral effects of sertraline, fluoxetine, and paroxetine differ on the differential-reinforcement-of-low-rate 72-second operant schedule in the rat. Psychopharmacol. 1999;147(2):153–161. doi: 10.1007/s002130051155. [DOI] [PubMed] [Google Scholar]
- Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev. 2000;24(4):391–416. doi: 10.1016/s0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Spector AC. Psychophysical evaluation of taste function in nonhuman animals. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker, Inc.; 2003. pp. 861–879. [Google Scholar]
- Spector AC, Andrews-Labenski J, Letterio FC. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 1990;47:795–803. doi: 10.1016/0031-9384(90)90099-p. [DOI] [PubMed] [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20(3):277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- St. John SJ, Garcea M, Spector AC. Combined, but not single, gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci. 1994;108:131–140. doi: 10.1037//0735-7044.108.1.131. [DOI] [PubMed] [Google Scholar]
- St. John SJ, Spector AC. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus) Behav Neurosci. 1996;110(6):1456–1468. doi: 10.1037//0735-7044.110.6.1456. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27(40):10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr M, Grauer MT, Holsboer F. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry. 2003;54:840–846. doi: 10.1016/s0006-3223(03)00074-x. [DOI] [PubMed] [Google Scholar]