Abstract
MicroRNAs (miRNAs) regulate gene expression by repressing target genes at the posttranscriptional level. Since miRNAs have unique expression profiles in different tissues, they provide pivotal regulation of many biological processes. The present study defined miRNA expression during murine myogenic progenitor cell (MPC) proliferation and differentiation to identify miRNAs involved in muscle regeneration. Muscle-related gene expression analyses revealed that the time course and expression of myosin heavy chain (MHC) and transcription factors (Myf5, MyoD, myogenin, and Pax7) were similar during in vitro MPC proliferation/differentiation and in vivo muscle regeneration. Comprehensive profiling revealed that 139 or 16 miRNAs were significantly changed more than twofold [false discovery rate (FDR) < 0.05] during MPC differentiation or proliferation, respectively; cluster analyses revealed five distinct patterns of miRNA expression during the time course of MPC differentiation. Not unexpectedly, the largest miRNA changes occurred in muscle-specific miRNAs (miR-1, -133a, and -499), which were upregulated >10-fold during MPC differentiation (FDR < 0.01). However, several previously unreported miRNAs were differentially expressed, including miR-10b, -335-3p, and -682. Interestingly, the temporal patterns of miR-1, -499, and -682 expression during in vitro MPC proliferation/differentiation were remarkably similar to those observed during in vivo muscle regeneration. Moreover, in vitro inhibition of miR-682, the only miRNA upregulated in proliferating compared with quiescent MPC, led to decreased MPC proliferation, further validating our in vitro assay system for the identification of miRNAs involved in muscle regeneration. Thus the differentially expressed miRNAs identified in the present study could represent new regulatory elements in MPC proliferation and differentiation.
Keywords: muscle regeneration, muscle differentiation, muscle regeneration, satellite cell
upon injury, skeletal muscle has the remarkable ability to initiate a rapid and extensive repair process that ultimately prevents the loss of muscle mass. Muscle regeneration is accomplished by a resident population of myogenic progenitor cells (MPC), also called satellite cells. In response to trauma, normally quiescent satellite cells become activated, giving rise to proliferating MPC, or myoblasts, that eventually differentiate and fuse to form multinucleated myotubes (7). Activation of satellite cells and the descendant myogenic precursors is a key element of muscle regeneration that is regulated via numerous genes that encode growth factors, regulatory proteins, receptors, and transcription factors (4, 29, 47). Studies in myogenic cell lines have provided much insight into the functional roles of these genes in the regulation of muscle regeneration.
Muscle regeneration resembles embryonic myogenesis in several ways including the de novo induction of the myogenic regulatory factors (MRFs), including Myf5, MyoD, and myogenin. Paired box proteins (Pax3 and Pax7) are involved in myogenic cell lineage determination and specification (25, 30, 32). In response to muscle damage, activated Pax7+/Myf5+ satellite cells upregulate MyoD expression and proliferate to generate myoblasts/MPC, whereas myogenin is expressed later during cell fusion and differentiation (29). A delicate balance between cell proliferation and exit from the cell cycle, differentiation, and fusion is required for muscle regeneration to occur normally.
MicroRNAs (miRNAs) are an increasingly important class of small, noncoding RNAs that regulate gene expression by binding to the 3′-untranslated region (UTR) of target mRNAs and inhibit initiation of translation and/or promote transcript cleavage (5). The miRNAs play essential roles in numerous biological processes such as development, cell proliferation, differentiation, and apoptosis (18, 28). Aberrant expression of miRNAs has been reported in a number of pathophysiological conditions (2, 22, 23, 43, 63). Functionally, a mutation in the target site of miR-1 and miR-206 in the myostatin gene was associated with muscle hypertrophy (11). Other studies have implicated miRNAs in control of human immunodeficiency virus (HIV) replication (54) and in coronary artery disease (61). Relative to the latter, the in vivo inhibition of specific miRNAs resulted in decreased plasma cholesterol in hypercholesterolemic mice and monkeys (17). Hence, disease-associated miRNAs could become viable targets for therapeutic intervention.
miRNAs are expressed in a tissue-specific manner. Muscle-specific miRNAs (miR-1, -133, -206, -208, and -499) have been identified and are involved in a range of processes including myogenesis (proliferation, differentiation, and fiber type specification), regeneration, hypertrophy, and muscular dystrophy (9, 36, 39, 55, 62). Current data on the roles of these miRNAs have been obtained largely from studies in cultured myoblast cell lines. miR-1 overexpression in cultured skeletal myoblasts promoted skeletal muscle differentiation, while miR-133 overexpression maintained myoblasts in a proliferative state and inhibited differentiation (9, 26). In addition, miR-499, which is encoded in the myosin gene, is involved in the regulation of myosin heavy chain (MHC) expression and fiber type (36, 55). These findings suggest that miRNAs may regulate muscle regeneration at multiple stages.
To investigate the function of miRNAs in muscle regeneration, we profiled miRNA expression during the course of murine MPC proliferation and differentiation. Importantly, these experiments were performed with newly established, low-passage primary cultures and well-defined proliferation and differentiation assays. Comprehensive analyses were performed to define the miRNA expression patterns during the time course of MPC proliferation and differentiation. To validate our in vitro systems, miRNAs that were differentially expressed in vitro were studied in regenerating mouse tibialis anterior muscle after cardiotoxin-induced injury. Moreover, the biological function of one of the differentially expressed miRNAs, miR-682, was studied during in vitro MPC proliferation. The miRNAs identified in the present study could represent new regulatory elements in muscle regeneration.
MATERIALS AND METHODS
MPC cell culture and transfection.
MPC isolations were performed according to the method of Lee et al. as previously described (31). Cells were isolated from the hindlimb muscles of 8- to 16-wk-old male C57BL/6J mice (Jackson Lab, Bar Harbor, ME); all muscles from the thigh and below the knee from two mice were used for each isolation. Briefly, muscles were minced into a coarse slurry and digested for 1 h with Pronase (650 U/ml, Calbiochem, San Diego, CA) in DMEM (American Type Culture Collection, Manassas, VA) containing 25 mM HEPES at 37°C with gentle agitation. The digest was mechanically dissociated by triturating the muscle slurry repeatedly and filtered through a 100-μm filter (Millipore, Bedford, MA). The filtered digest was centrifuged through an isotonic Percoll gradient (60% overlaid with 20%, GE Healthcare, Piscataway, NJ), which has been used widely in progenitor cell isolation (6, 35, 38, 44, 59). Cells were collected from the interface of the Percoll gradient and resuspended in primary growth medium (GM) containing Ham's F-10 (Invitrogen, Carlsbad, CA), 20% FBS (VWR, West Chester, PA), 10 ng/ml fibroblast growth factor-2 (FGF-2; Promega, Madison, WI), 100 U/ml penicillin G, and 100 μg/ml streptomycin (Invitrogen) and grown on type I collagen (0.1 mg/ml, Sigma-Aldrich, St. Louis, MO)-coated tissue culture plates. All analyses were performed with primary MPC cultures at passage 3. The cells in the passage 3 cultures were 96 ± 2.3% positive for MyoD by immunocytochemistry.
Locked nucleic acid (LNA)-enhanced antisense oligonucleotides against miR-682 were synthesized by Exiqon (Woburn, MA). Transfection was performed at a final concentration of 10 nM with Lipofectamine RNAiMAX reagent (Invitrogen), and the reverse transfection procedure was performed according to the manufacturer's instructions.
MPC proliferation assay and cell cycle analysis.
For MPC proliferation, cells were cultured in GM and plated on type I collagen-coated flasks at a density of 5 × 105 cells/T25 flask (Sigma, St. Louis, MO). Cell proliferation was evaluated by total cell counts with a hemacytometer and cell cycle analysis. For cell cycle analysis, MPC cultures from days 1–5, each time point performed in duplicate, were trypsinized, fixed in ice-cold 70% ethanol, and incubated with a propidium iodide (PI) solution (0.2 mg/ml RNase A, 0.1% Triton X-100, 20 μg/ml PI) for 30 min at room temperature to label DNA. A FACSCalibur (Beckman Instruments, Fullerton, CA) flow cytometer was used to measure the PI fluorescence. For each measurement, data from 10,000 single cell events were collected; cell doublets and aggregates were gated out of the analysis with a two-parameter histogram of FL2-Area versus FL2-Width. Cell cycle histograms were analyzed with ModFit LT 3.0 software packages (Verity Software House, Topsham, ME). All cell cycle analyses were confirmed to have a low coefficient of variation (CV) of the G0/G1 peak (CV < 5) and low reduced χ2 (RCS) values (RCS < 3), indications of how well the model described the observed data. Results are presented as the mean value of four independent assays; each assay used MPC cultures at passage 3 derived from different sets of mice.
MPC differentiation assay and immunocytochemistry.
For MPC differentiation, cells were seeded on entactin-collagen IV-laminin (ECL, 5 μg/cm2, Millipore)-coated plates at a density of 4 × 105 cells/60-mm dish, allowed to adhere for 24 h in GM (day 0), and switched to differentiation medium (DM) composed of 2% horse serum (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin in DMEM. Cultures were fixed and analyzed after switching to DM for days 1–5. Cell differentiation was evaluated by MHC immunolocalization; at least 1,500 nuclei from MHC-positive cells were counted from 10 random fields at ×10 magnification. The differentiation potential was calculated as (MHC-positive nuclei/total no. of nuclei) × 100, and the fusion index was calculated as (MHC-positive myotubes containing ≥2 nuclei/total no. of nuclei) × 100, as previously described (31). Proliferation was determined on the basis of the average number of nuclei per field in at least 10 random fields. All experiments were performed in quadruplicate with four different MPC cultures. Immunocytochemical analysis of MyoD and MHC were performed as previously described (52).
Cardiotoxin muscle injury mouse model.
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals as approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. C57BL/6J male mice were obtained from the Jackson Laboratory and used at 4–6 mo of age. Mice were anesthetized with inhalation of 1–2% isoflurane (Vedco, St. Joseph, MO) and placed on a warming pad to maintain body temperature during the procedure. Cardiotoxin (Calbiochem; 2.5 μM) was injected intramuscularly below the knee into the anterior (100 μl) compartment of both hindlimbs. Tibialis anterior muscle tissues were collected at baseline (no injections) and at days 1–5, 7, and 21 after cardiotoxin injection (n = 4 mice/time point). The tissues were weighed; half of the muscle was snap frozen in liquid nitrogen and stored in a −80°C freezer for RNA extraction; the other half was placed in 10% neutral buffered formalin (NBF) for histological examination to confirm the extent of muscle injury.
RNA isolation.
Total RNA was isolated from MPC cells with TRIzol (Invitrogen) and from skeletal muscle tissues with the miRNeasy Mini kit (Qiagen) according to manufacturer's instructions. RNA integrity was assessed with the Agilent 2100 Bioanalyzer (Agilent Technologies), and RNA samples with RNA integrity number (RIN) > 9 were used in the array studies.
Quantitative reverse transcriptase-polymerase chain reaction.
The mRNA expression of MHC and muscle regulatory factors including MyoD, myogenin, Myf5, and Pax7 was evaluated via quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Specific primers were designed based on published gene sequences (NCBI Entrez search system; Supplemental Table S1).1 The expression of miRNAs during in vivo muscle regeneration was determined by qRT-PCR with the TaqMan Universal PCR Master Mix and TaqMan MicroRNA Assays (Applied Biosystems, Foster City, CA). Expression of the amplified products for mRNAs and miRNAs was calculated relative to 18S rRNA and small nucleolar RNA (snoRNA)55, respectively.
TaqMan qRT-PCR microRNA array.
The stem-loop RT-PCR-based TaqMan Rodent MicroRNA Array A (Applied Biosystems) containing primers and probes of 335 murine miRNAs was used. RT-PCR reactions were performed as previously described (10). All reagents were obtained from Applied Biosystems. Briefly, 500 ng of total RNA was reverse-transcribed with Megaplex RT Primers and the TaqMan miRNA reverse transcription kit. cDNA templates were amplified with Megaplex PreAmp Primers and TaqMan PreAmp Master Mix. Quantitative real-time PCR was performed with the Applied Biosystems 7900HT system and a TaqMan Universal PCR Master Mix. Cycle threshold (Ct) values were calculated with SDS software v.2.3 using automatic baseline settings and a threshold of 0.2. The comparative Ct method was used to calculate the relative miRNA expression. The Ct value of an endogenous control gene [MammU6, small nuclear RNA (snRNA)] was subtracted from the corresponding Ct value for the target gene, resulting in the ΔCt value.
Data analysis.
Because a Ct value of 35 represents single-molecule template detection, Ct values > 35 were considered to be below the detection level of the assay (19). MiRNAs were included in analyses when at least 50% of the measurements were detectable (Ct ≤ 35) in an experiment (20): at least eight measurements with Ct ≤ 35 in differentiation assays and at least four measurements with Ct ≤ 35 in the proliferation assays. The LIMMA software package was used to estimate the log fold change and false discovery rate (FDR) (51). A miRNA was identified as differentially expressed when FDR < 0.05 and fold change > 2 and was selected for cluster analysis. Clustering was performed with the hierarchical method with average linkage and Euclidean distance metric (15). The log2-transformed intensity values were centered by subtracting the median log2 values across all the samples for individual miRNAs and used for cluster analysis to generate the heat map (Java Treeview) (46).
The MPC proliferation and differentiation data including the regulatory factor data were analyzed by repeated-measures analysis of variance with log transformation applied to MHC and Myf5. The multiple testing correction procedure utilized the SAS macro SimTests written by Peter Westfall (57) employing the Holm step-down method while incorporating the autocorrelation structure of the model. The macro was run with a simulation run set to 50,000 for a two-tailed test; in vivo transcription factor and miRNA expression were measured by analysis of variance on ranks (MHC, Pax7, and miR-682) or analysis of variance on log-transformed data (Myf5, myogenin, MyoD, miR-1, and miR-499) with Hochberg's correction for multiple testing. The effect of miR-682 antisense LNA on MPC proliferation and miR-682 levels was analyzed by paired t-test. A value of P ≤ 0.01 was considered as significant.
Identification of putative mRNA targets for differentially expressed miRNAs.
The mRNA targets for differentially expressed miRNAs were predicted with the computer program TargetScan, the database of conserved 3′-UTR miRNA targets (33). Gene Ontology (GO) term annotation and functional enrichment of the predicted targets were performed with the DAVID gene annotation tool (http://david.abcc.ncifcrf.gov/) at P < 0.01.
RESULTS
miRNA expression during MPC proliferation.
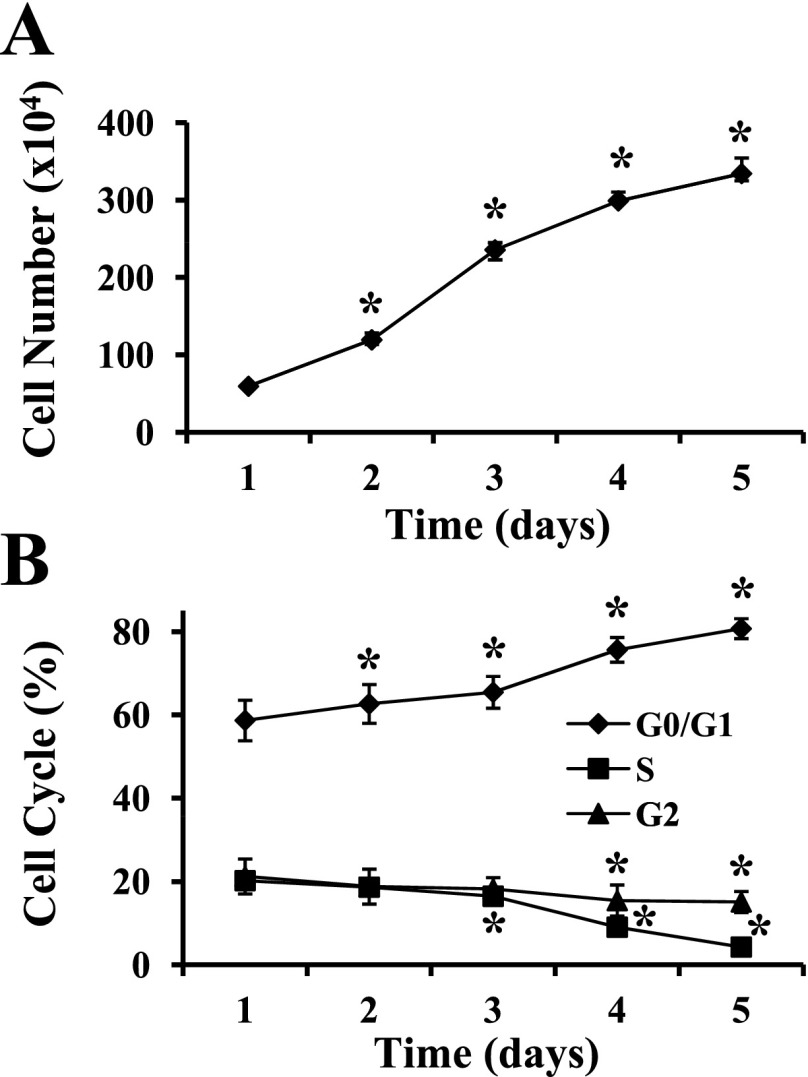
MPC proliferation was stimulated by serum and FGF-2 in GM; cell confluence was 20–30% at 1 day after subculture and reached >90% by day 5. The total number of MPC doubled every day for the first 3 days, with a decreasing proliferation rate on day 4, and approached a plateau phase at day 5 (Fig. 1A). The percentage of G0/G1-phase cells progressively increased such that 81 ± 2% were G0/G1 phase at day 5. In parallel, S- and G2-phase proliferating cells decreased (P ≤ 0.003) at days 3–5 and days 4–5, respectively, compared with day 1 (Fig. 1B). In combination, these results indicated that MPC were actively dividing in the first 3 days with a decreased proliferation rate at days 4 and 5.
Fig. 1.
Myogenic progenitor cell (MPC) proliferation in vitro. MPC were subcultured after the third passage and maintained in MPC growth medium on type I collagen-coated dishes. A: temporal changes in cell number during proliferation were determined manually. *P < 0.001 compared with proliferation day 1. B: the distribution of cells in different phases of the cell cycle was established by flow cytometry. *P ≤ 0.005 compared with proliferation day 1. Data represent means ± SD of 4 different MPC primary cultures.
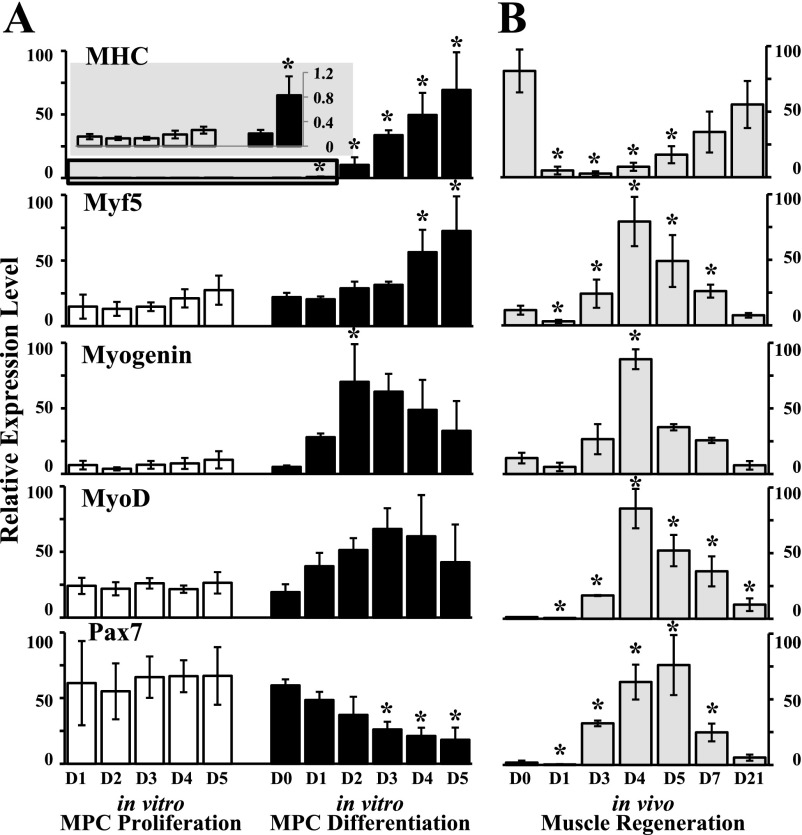
MPC can begin to differentiate when cells are confluent even in the presence of GM (8). However, no myotube formation was observed with phase-contrast microscopy during the 5-day proliferation assay (data not shown). The mRNA levels of MHC (a terminal muscle differentiation marker), Myf5, MyoD, myogenin, and Pax7 were similar throughout the 5-day proliferation time course (Fig. 2A), suggesting that the day 5 MPC had not started to differentiate despite being >90% confluent.
Fig. 2.
Muscle-specific gene expression in cultured MPC compared with in vivo muscle regeneration. Relative levels of mRNA were determined by quantitative real-time PCR in relation to 18S rRNA. A: gene expression in MPC was determined at various time points during proliferation and differentiation. D0, D1, D2, D3, D4, and D5, days 0, 1, 2, 3, 4, and 5, respectively. Myosin heavy chain (MHC) expression during MPC proliferation and at early time points during MPC differentiation is included on an expanded scale (gray-shaded inset). *P ≤ 0.002 compared with day 0. B: in vivo muscle regeneration at baseline (day 0) and at various days (day 1 through day 21) after cardiotoxin injection (D1–D21, respectively); *P ≤ 0.008 compared with day 0. Data represent means ± SD of 4 different MPC primary cultures or 4 mice per time point in vivo.
On the basis of the above, miRNA profiling was compared between proliferation on day 2 and day 5, with day 2 representing active proliferation while day 5 represented a relatively quiescent population of cells that had not started to differentiate. Of the 335 murine miRNAs tested by the TaqMan qRT-PCR array, 16 miRNAs (5%) changed significantly (>2-fold) between the proliferation time points (FDR < 0.05); of these, 12 (5%) changed more than fivefold (Table 1). Only 1 (1/16, 6%), miR-682, was upregulated in proliferating MPC at day 2 compared with quiescent cells at day 5. Data for all miRNA expression were submitted to the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) with the series accession number GSE16473.
Table 1.
Differentially expressed miRNAs during MPC proliferation
| Proliferation D2/D5 | |
|---|---|
| miR-598 | −22.6 |
| miR-499 | −20.1 |
| miR-208b | −11.9 |
| miR-98 | −11.6 |
| miR-486 | −9.9 |
| let-7f | −8.3 |
| miR-708 | −8.3 |
| miR-135a | −8.0 |
| miR-547 | −6.1 |
| miR-342-5p | −6.0 |
| miR-197 | −5.6 |
| let-7a | −5.4 |
| miR-1 | −4.2 |
| miR-450a-5p | −4.1 |
| miR-190 | −3.8 |
| miR-682 | 4.4 |
Data from proliferating myogenic progenitor cells (MPC) presented as fold change of microRNA (miRNA) expression at day 2 (D2) relative to that at day 5 (D5) in culture.
miRNA expression during MPC differentiation.
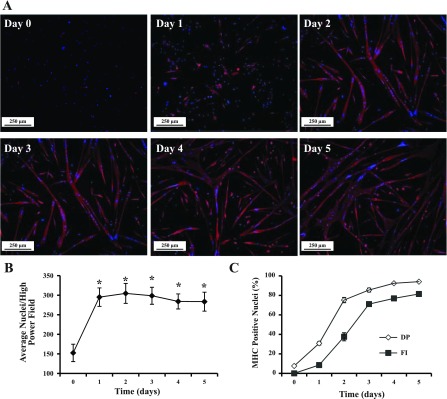
MPC differentiation was induced by exposure to a low serum concentration and FGF-2 withdrawal. In GM (day 0), MPC were small and round in appearance (Fig. 3A). After switching to DM (days 1–5), cell morphology became elongated and MHC-positive, multinucleated myotubes formed (Fig. 3A). Cell number was determined by counting nuclei per high-power field; the number of cells doubled during the first day in DM and were similar thereafter (Fig. 3B). Proliferation occurred during the first day in DM irrespective of plating densities from 1 × 105 to 1 × 106 per 60-mm dish (data not shown). Thus day 1 represented both proliferating and differentiating cells. MPC differentiation was assessed by the differentiation potential and the fusion index. After 1 day in DM, 31 ± 2% of the cells were differentiated as evidenced by MHC protein expression (Fig. 3C). Thereafter, the percentage of nuclei in myotubes continued to increase, reaching 94 ± 1% at day 5 (Fig. 3C). Enumeration of fused myocytes containing two or more nuclei, determined as the fusion index, revealed an increase in cell fusion; the fusion index of differentiated cultures was 82 ± 1% after 5 days (Fig. 3C). In parallel, increases (P ≤ 0.002) in MHC mRNA levels compared with day 0 were observed during MPC differentiation (Fig. 2A). The expression of myogenin increased at day 2 (P = 0.002) compared with day 0 and decreased thereafter (Fig. 2A). Although no statistical significance was observed, MyoD levels were 3.8 ± 1.0 times higher at differentiation day 3 than at day 0. Myf5 expression increased during late differentiation days 4–5. The transcription factor Pax7 was expressed in proliferating MPC, with peak expression occurring during differentiation day 0, and was downregulated beginning on day 3 (P < 0.001) and throughout the rest of the myogenic differentiation time course (Fig. 2A).
Fig. 3.
MPC differentiation in vitro. MPC were subcultured after the third passage and maintained in MPC differentiation medium on entactin-collagen IV-laminin-coated dishes. A: at various times in culture (day 0 to day 5), differentiated cells were identified by immunolocalization of MHC (red); nuclei were identified by DAPI (blue). B: cell proliferation during the differentiation assay. *P < 0.001 compared with day 0. C: differentiation potential (DP) and fusion index (FI) were determined on the basis of nuclei in MHC-positive cells and myotubes. Data represent means ± SD of 4 different MPC primary cultures.
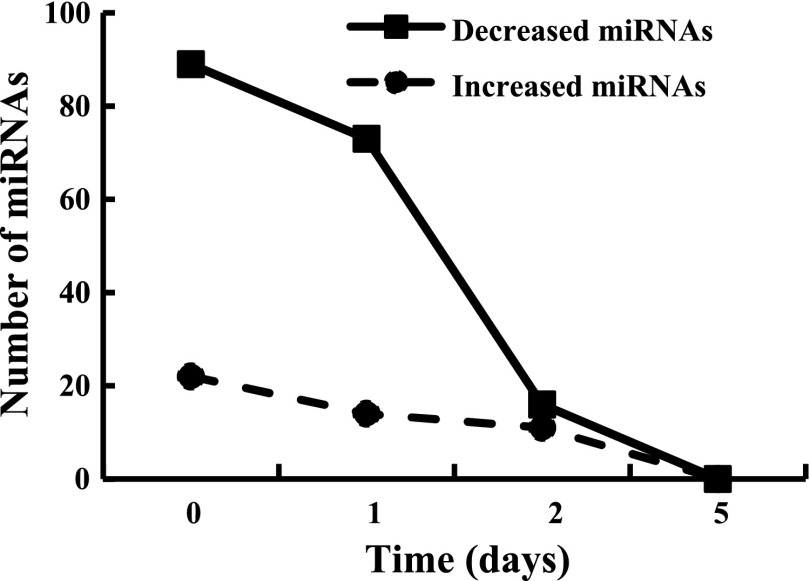
Four time points during MPC differentiation were selected for miRNA profiling. Day 0 represented proliferating cells, day 1 contained a combination of proliferating and differentiating cells, while day 2 contained both differentiating and fusing cells. Day 5 represented cells and myotubes that were predominantly differentiated. Array analysis revealed that 139 of 335 murine miRNAs (41%) were significantly differentially expressed (FDR < 0.05, fold change > 2). The number of differentially expressed miRNAs varied during the time course of MPC differentiation (Fig. 4). Compared with differentiated cells at day 5, a large number of miRNAs were downregulated at day 0 and day 1 (89 and 73 miRNAs, respectively); the number of differentially expressed miRNAs rapidly decreased by day 2 when cells began to fuse; only 27 miRNAs changed at day 2 versus 87 and 111 miRNAs at day 0 and day 1, respectively. Thus the patterns of miRNA expression were unique at various stages of MPC differentiation.
Fig. 4.
Differentially expressed microRNAs (miRNAs) during MPC differentiation. Results for a given miRNA were expressed in relation to MPC at differentiation day 5. Data are derived from 4 different MPC primary cultures with the cell differentiation assays initiated at passage 3.
Distinct miRNA expression patterns during MPC differentiation indicate potential role in regulation of muscle regeneration.
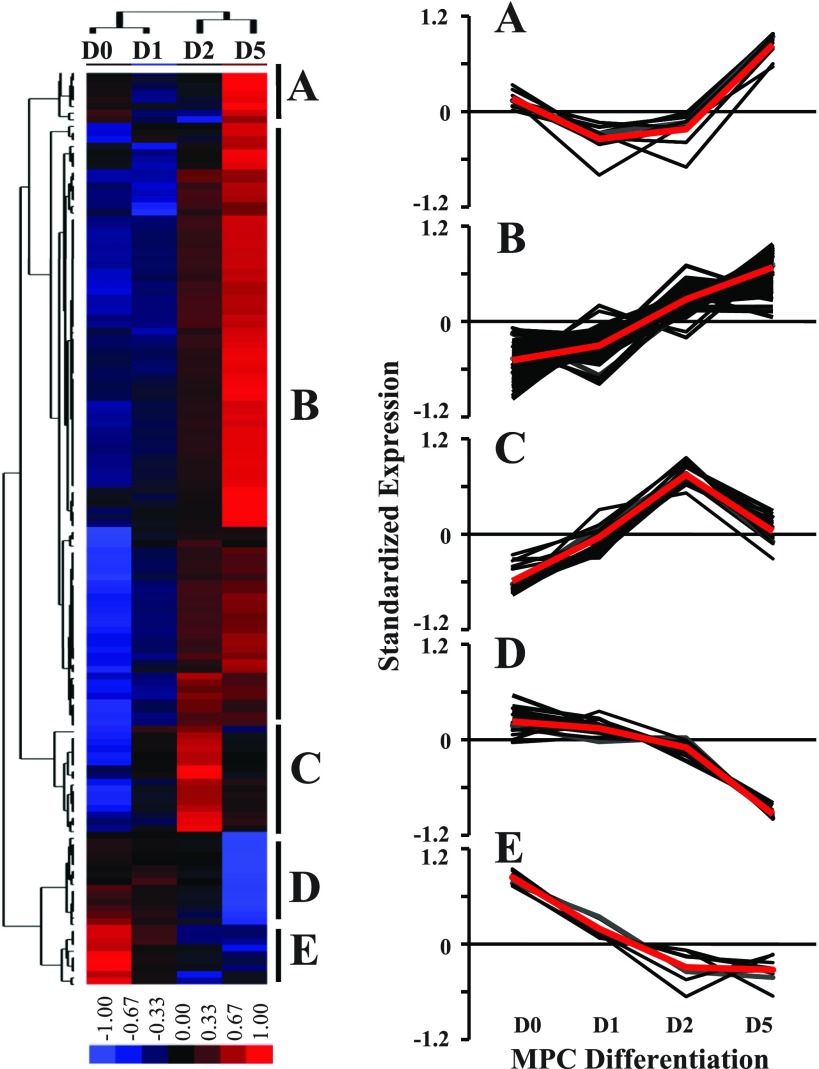
To further examine the expression patterns of miRNAs during the MPC differentiation assay, a hierarchical cluster analysis was performed for the 139 differentially expressed miRNAs. The miRNA expression patterns during the MPC differentiation assay clustered into five unique categories (Fig. 5): transient downregulation in the early phase of differentiation (cluster A; n = 9); progressive upregulation during MPC differentiation (cluster B; n = 91); transient upregulation in the early phase of differentiation (cluster C; n = 16); downregulation in the late phase of differentiation (cluster D; n = 14); and progressive downregulation during MPC differentiation (cluster E; n = 9). miRNAs in each cluster are listed in Supplemental Table S2.
Fig. 5.
Hierarchical cluster analysis of differentially expressed miRNAs during MPC differentiation. Cluster analysis was performed for 139 differentially expressed miRNAs after data adjustment (log transformation, median center, and normalization). The color codes of red, black, and blue represent high, average, and low expression levels, respectively. Five clusters were identified (A–E). For each cluster, a detailed view of expression changes is presented for individual miRNAs within that cluster; the average value of miRNA expression within each cluster is presented as a red line. Data were derived from 4 different MPC primary cultures with the cell differentiation assays initiated at passage 3.
These distinct expression patterns of miRNA suggest different regulatory roles during MPC proliferation and differentiation. For example, during differentiation days 1–2, mitogen deprivation stimulated cell cycle withdrawal, inducing MPC to differentiate. The 16 miRNAs in cluster C were transiently upregulated in the early stages of differentiation (days 1–2) but mostly downregulated in proliferating cells (Fig. 5), representing an important set of miRNAs for understanding the initial events for muscle differentiation. GO terms of the predicted targets of the miRNAs in cluster C were enriched with mRNAs important in protein metabolism, cell cycle regulation, and cell proliferation (P < 0.001).
Between differentiation days 2 and 5, MPC ceased proliferating and the majority fused into postmitotic myotubes. The differentiation time course contained miRNAs with the largest fold change ranging from −135 to +146. Among these, 13 miRNAs exhibited the most significant change with FDR < 0.01 and fold change > 10, including muscle-specific miR-1, -133a, and -499 (Table 2). Although probes and primers for another muscle-specific miRNA, miR-206, were not available in the present array analysis, miR-206 expression was upregulated during MPC differentiation on the basis of TaqMan RT-PCR assays (1.2-, 2.1-, and 10.4-fold upregulation at differentiation days 1, 2, and 5 compared with day 0, respectively). GO term annotation revealed that 14% (475/3,297) of the predicted targets of the 13 miRNAs were involved in cell differentiation. Some of these genes have been reported to inhibit myogenic differentiation, including sex determining region Y-box 6 (Sox6) (21), phosphatase and tensin homolog (Pten) (56), leukemia inhibitory factor (Lif) (1), and fibroblast growth factor receptor 1 (Fgfr1) (41), suggesting that these miRNAs promote MPC differentiation by regulating mRNA targets.
Table 2.
miRNAs with most significant change during MPC differentiation (FDR < 0.01, fold change > 10)
| Fold Change |
||||
|---|---|---|---|---|
| D0/D5 | D1/D5 | D2/D5 | Muscle Differentiation-Associated Targets | |
| miR-499 | −135.1 | −84.4 | −6.5 | Sox6 |
| miR-1 | −38.7 | −32.3 | −3.9 | Sox6, Myocd |
| miR-10b | −37.4 | −48.6 | −7.3 | Mbnl3, Pten |
| miR-98 | −36.9 | −9.2 | 1.2 | Ezh2, Nras, Utrn |
| miR-330 | −25.5 | −5.2 | −9.4 | Nras |
| miR-486 | −23.2 | −9.3 | −2.1 | Pten |
| miR-339-5p | −13.1 | −8.7 | −3.2 | ND* |
| miR-598 | −12.4 | −13.6 | −3.6 | Zeb2 |
| miR-133a | −11.8 | −9.1 | −2.5 | Fgfr1, Sirt1 |
| miR-139-5p | −11.2 | −8.0 | −2.5 | Unc45a |
| miR-504 | −10.9 | −3.9 | −1.3 | Lif |
| miR-375 | −5.7 | −10.3 | −11.9 | Sox6 |
| miR-335-3p | 145.5 | 247.4 | 76.6 | Epc1 |
Numbers with >10-fold change are indicated in bold. Epc1, enhancer of polycomb homolog 1; Ezh2, enhancer of zeste homolog 2; Fgfr1, fibroblast growth factor receptor 1; Lif, leukemia inhibitory factor; Mbnl3, muscleblind-like 3; Myocd, myocardin; Nras, neuroblastoma ras oncogene; Pten, phosphatase and tensin homolog; Sirt1, sirtuin 1; Unc45a, unc-45 homolog A; Sox6, sex determining region Y-box 6; Utrn, utrophin; Zeb2, zinc finger E-box binding homeobox 2; FDR, false discovery rate;
ND, not detectable.
miR-682 antisense inhibited MPC proliferation.
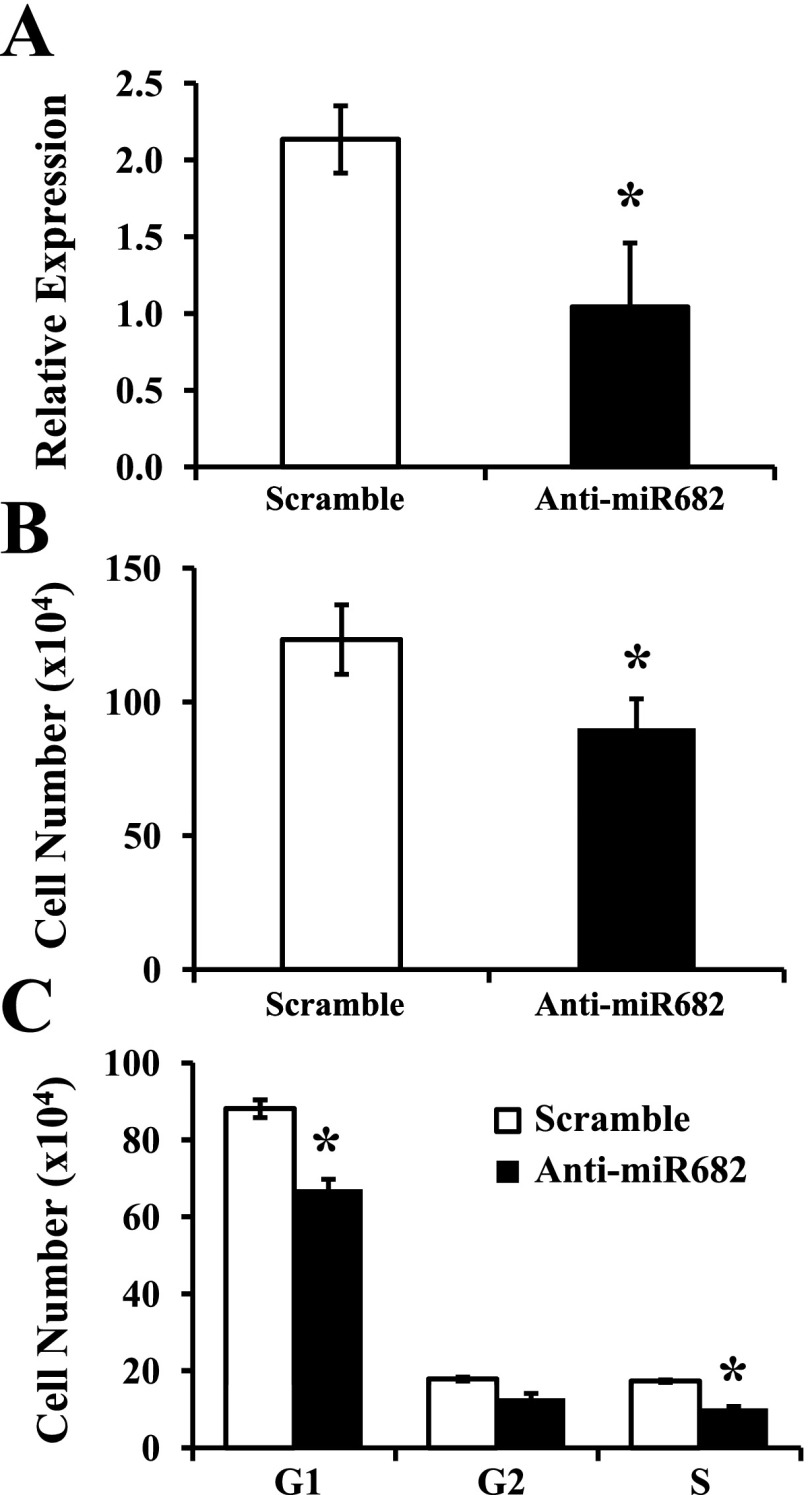
Although the evidence for regulation of miRNAs in MPC differentiation is growing, the roles of miRNAs in MPC proliferation remain largely unexplored. Only one miRNA, miR-682, was significantly upregulated in proliferating compared with quiescent MPC (Table 1). To determine whether miR-682 was involved in MPC proliferation, we performed loss-of-function assays using anti-miRNA oligonucleotides. A LNA-enhanced antisense oligonucleotide complementary to miR-682 abrogated (P = 0.005) miR-682 detection in real-time PCR compared with a scramble sequence used as a control at day 2 after transfection (Fig. 6A), most likely through sequestration of the target miRNA. Inhibition of endogenous miR-682 significantly reduced MPC proliferation at day 2 after transfection compared with the scrambled control (P = 0.01, Fig. 6B). Cell numbers at day 2 were similar in the scramble sequence (control) and the proliferation assay without transfection (Fig. 1A), suggesting that the transfection conditions did not change the kinetics of the proliferation assay. Cell cycle analysis revealed significant reductions of S-phase cells in miR-682 antisense-transfected cells (P < 0.001, Fig. 6C). However, no myotube formation or MHC expression level changes were observed in miR-682 antisense- and scramble-transfected cells when cultured in GM (data not shown). Taken together, these data functionally link miR-682 to MPC proliferation.
Fig. 6.
Effects of inhibition of miR-682 in cultured, proliferating MPC. MPC were subcultured after the third passage and maintained in MPC growth medium on type I collagen-coated dishes. Transfection with locked nucleic acid-enhanced antisense or scramble oligonucleotide was accomplished at subculture, and cells were allowed to grow for 2 days. A: miR-682 expression levels were measured by quantitative real-time PCR. B: total cell number was determined manually. C: the distribution of cells in different phases of the cell cycle was established by flow cytometry. Data represent means ± SD of 3 biological repeats. *P ≤ 0.01 compared with the scramble oligonucleotide.
Differentially expressed miRNAs in MPC proliferation and differentiation exhibited similar changes during in vivo muscle regeneration.
Although muscle proliferation and differentiation processes can be mimicked in vitro in tissue-cultured cells, in vivo MPC function is regulated by local signals from the environment. Thus interpretation of in vitro experiments needs to be relevant to in vivo function. To determine whether the expression of miRNAs during MPC proliferation and differentiation was similar in muscle regeneration, we assessed miRNA expression levels in a cardiotoxin-induced mouse muscle injury model. More than 80% of the tibialis anterior muscle was injured as evidenced by myocyte necrosis and inflammatory infiltrates at days 1–3 after cardiotoxin injection as previously described (40). Regenerated myofibers were readily identified by the presence of centrally located nuclei by day 4 and increased in size through day 21 (data not shown). The expression of several muscle-specific genes, including MHC, Myf5, myogenin, MyoD, and Pax7, in injured/regenerating muscle was studied by quantitative real-time PCR (Fig. 2B). The terminal muscle differentiation marker, MHC, was maximal at baseline (day 0), dramatically decreased after injury, and increased during the time course of muscle regeneration. The expression of myogenic transcription factors reached peak levels at days 4–5 after cardiotoxin injury and returned toward baseline levels thereafter. Thus both histology and gene expression data suggest that the in vivo time course of satellite cell activation and proliferation occurred from day 1 to day 3 and myoblast differentiation occurred at day 4 and thereafter, with overlap in these processes around the day 4 time point.
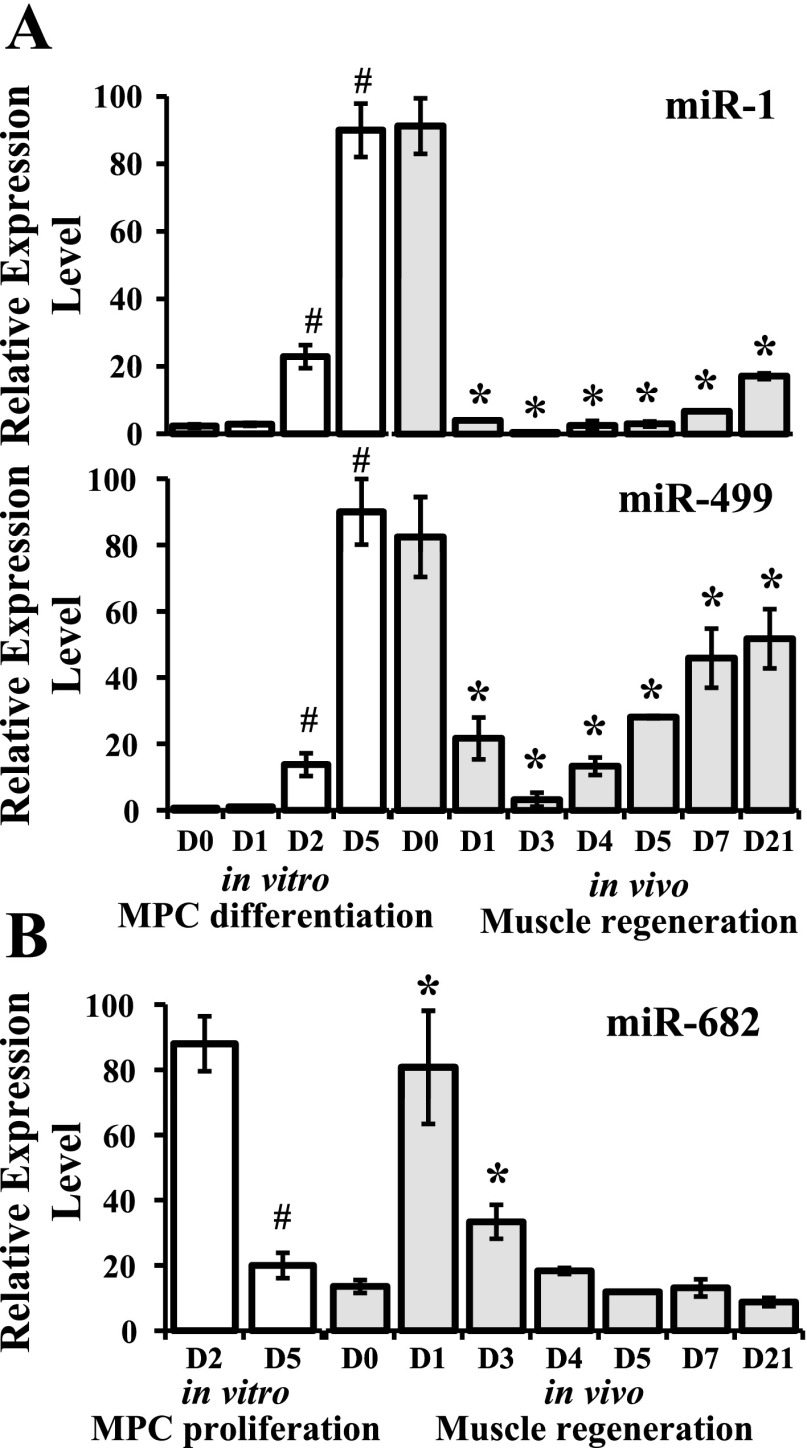
Concurrent expression of several miRNAs, determined by TaqMan real-time PCR, included miR-1, -499, and -682. These miRNAs were selected on the basis of significant differential expression during in vitro MPC proliferation or differentiation (Tables 1 and 2). The muscle-specific miRNAs, miR-1 and miR-499, exhibited in vivo patterns similar to MPC, with high expression in baseline and day 21 regenerated muscle and in day 5 differentiated MPC in vitro (Fig. 7A), suggesting potential roles in maintenance of differentiation of mature muscle fibers/myotubes. miR-682 was upregulated during MPC proliferation, with 4.4-fold increase at proliferation day 2 compared with day 5. Its expression reached peak levels immediately after cardiotoxin injury and decreased thereafter (Fig. 7B). This expression pattern is consistent with its potential role in the regulation of MPC proliferation.
Fig. 7.
Temporal patterns of miRNA expression in vitro and in vivo. In vitro miRNA expression in proliferating or differentiating MPC was determined by quantitative PCR array. In vivo miRNA expression was measured by quantitative real-time PCR at baseline (day 0) and at various days (day 1 through day 21) after cardiotoxin injection. A: expression of miR-1 and -499 during MPC differentiation in vitro (#P < 0.001 compared with day 0) and during in vivo muscle regeneration (*P ≤ 0.002 compared with day 0). B: expression of miR-682 during the proliferation of cultured MPC (#P = 0.02, day 2 compared with day 5) and during in vivo muscle regeneration (*P ≤ 0.003 compared with day 0). Data represent means ± SD of 4 different MPC primary cultures with the cell proliferation or differentiation assays initiated at passage 3 or 4 mice per time point during in vivo muscle regeneration.
DISCUSSION
The primary objective of this study was to comprehensively define the dynamic changes in the expression of miRNAs during murine MPC proliferation and differentiation in vitro. Given the well-recognized phenotypic changes of cells after prolonged culture and passaging (16, 34), we utilized only low-passage, primary MPC cultures; an in-depth characterization of our in vitro MPC culture system documented a consistent growth behavior and myogenic transcription factor gene expression in these cultured cells (Figs. 1–3). In parallel, distinct patterns of miRNA expression occurred during the time course of MPC proliferation and differentiation. Not surprisingly, muscle specific miRNAs, miR-1, -133a, and -499, exhibited the greatest changes and were significantly upregulated in association with MPC differentiation. However, of importance, we identified a number of differentially expressed miRNAs that have not previously been reported in MPC, e.g., miR-10b, -335-3p, and -682 (Tables 1 and 2). These newly identified miRNAs will be important targets for further study relative to their regulatory effects on MPC growth and function. Toward this goal, treatment of cultured MPC with anti-miR-682, the only miRNA upregulated in proliferating MPC in vitro, resulted in a significant decrease in MPC proliferation. Thus on-going studies are directed toward elucidating the gene targets for miR-682 in these cultured cells. It is also noteworthy that the temporal patterns of expression of myogenic transcription factors and miRNAs in cultured MPC were similar to those observed during the time course of muscle regeneration in vivo (Figs. 2 and 7). These findings further support the use of our in vitro assay system for the identification of essential miRNAs that may serve as new regulatory elements in myogenic cell proliferation and differentiation.
In skeletal muscle, the processes of proliferation and differentiation are mutually exclusive. The decision of whether myoblasts should continue to proliferate or switch into a differentiation pathway is regulated by the balance of positive and negative cell cycle regulators (14). Compared with differentiated MPC at day 5, a large number of miRNAs were changed at day 0 and day 1 when the cells were still proliferating; however, the number of differentially expressed miRNAs rapidly decreased by day 2 when cells began to fuse (Fig. 4). This expression pattern suggests different functions of these miRNAs during muscle differentiation and proliferation. A transient upregulation of a group of miRNAs in cluster C was observed at early differentiation days 1 and 2; however, most of the cluster C miRNAs were downregulated in proliferating MPC. This temporal expression pattern suggests possible roles for these miRNAs in controlling the transition from proliferation to differentiation. In fact, several miRNAs in this group have been shown to induce cell cycle arrest or suppress cell proliferation including miR-29b (42), miR-370 (37), miR-322, and miR-503 promoting cell cycle quiescence and muscle differentiation by downregulation of Cdc25A (48). In silico prediction and GO annotation indicated that these clusters of miRNAs may share functional similarity by targeting cell cycle-related genes. Therefore, these miRNAs, by modulating the balance between the antagonistic processes of MPC proliferation and differentiation, may be integral components of the regulatory circuitry for muscle regeneration.
Analysis of the 13 miRNAs with the most significant change during MPC differentiation indicated that many of these miRNAs may target mRNA critically involved in muscle differentiation (Table 2). Interestingly, several miRNAs targeted different sites in a given mRNA; miR-1, -499, and -375 targeted different sites in Sox6 mRNA, miR-10b and -486 targeted Pten, and miR-98 and -330 targeted neuroblastoma ras oncogene (Nras). The predicted targets of miR-98, Utrn and enhancer of zeste homolog 2 (Ezh2), were also repressed by myogenesis-related miR-206 and miR-26a, respectively (45, 58). Thus coordinated regulation of multiple miRNAs could be an efficient way to increase the specificity of target gene regulation and may also enhance the robustness of target gene expression levels against fluctuations in individual miRNA concentrations. Although the accuracy of the computational approaches for identification of mammalian miRNA targets is still limited, these results will advance hypothesis-driven functional studies of these miRNAs.
The sequential expression of MRFs has been studied in embryogenesis (24), muscle injury models (60), and cultured satellite cells (12, 50, 53). Consistent with previous reports, MyoD and myogenin exhibited transient induction during in vitro MPC differentiation and in vivo muscle regeneration, coincident with the initiation of muscle differentiation. This pattern is consistent with the ascribed roles of these genes in the differentiation of myogenic cells (29). The expression of Myf5 in satellite cells has been under debate since Myf5 activity occurs during specific stages of the cell cycle (27). Myf5 expression was observed in proliferating satellite cells but was absent upon differentiation and fusion into myotubes (27, 50). However, Myf5-driven reporter expression has shown that the Myf5 gene is active in resident satellite cells (13). We observed increased expression at the late phase of MPC differentiation in vitro and peak expression at day 4 during muscle regeneration following cardiotoxin-induced injury. The increased Myf5 expression may indicate the existence of quiescent residual cells in the late phase of MPC differentiation (3). Pax7 is known to regulate myogenic cell proliferation (4). In the present study, Pax7 was upregulated in proliferating MPC and downregulated during MPC differentiation. Thus the timed expression of the transcriptional network plays an important role for muscle lineage progression and muscle regeneration. Interestingly, a similar pattern was also suggested for miRNA expression. miR-1 and -499 have been extensively studied in cardiac and skeletal muscles and have been shown to regulate muscle cell differentiation (39, 49). Consistent with these roles, miR-1 and -499 were differentially regulated over the time course of both in vitro MPC differentiation and in vivo muscle regeneration. Compared with the large number of miRNAs differentially expressed during MPC differentiation, only 1 (miR-682) of 16 significantly changed miRNAs was upregulated in proliferating MPC. Importantly, miR-682 exhibited peak levels immediately at day 1 after muscle injury during in vivo muscle regeneration, raising the possibility of promoting MPC proliferation. Indeed, inhibition of endogenous miR-682 significantly reduced MPC proliferation. Although the mechanism of this regulation remains to be determined, these results validated our system in identifying miRNAs involved in muscle regeneration.
In conclusion, our results revealed distinct miRNA expression patterns during the time course of MPC proliferation and differentiation. We further confirmed similar miRNA expression between in vitro MPC and in vivo muscle regeneration and validated miR-682 involvement in MPC proliferation. These results will warrant further investigation to determine the regulatory roles of the differentially expressed miRNAs identified in the present study in controlling muscle regeneration.
GRANTS
These studies were supported, in part, by a Department of Veterans Affairs Merit Review grant and National Institutes of Health Grants R01-HL-074236, T32-HL-07446, and KL2-RR-025766.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Luiz F. Penalva and Suzanne Burns for access and technical support to the 2100 Bioanalyzer. Statistical analyses of the MPC proliferation and differentiation assays, as well as the mRNA levels of the myogenic regulatory factors and in vivo miRNAs, were graciously performed by Dr. Joel E. Michalek and Lee Ann Zarzabal.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Alter J, Rozentzweig D, Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem 283: 23224–23234, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Balaguer F, Link A, Lozano JJ, Cuatrecasas M, Nagasaka T, Boland CR, Goel A. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res 70: 6609–6618, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol 330: 530–533, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans 36: 1224–1231, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Chang Y, Hsieh PH, Chao CC. The efficiency of Percoll and Ficoll density gradient media in the isolation of marrow derived human mesenchymal stem cells with osteogenic potential. Chang Gung Med J 32: 264–275, 2009. [PubMed] [Google Scholar]
- 7. Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. BMC Genomics 10: 407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38: 813–818, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Day K, Paterson B, Yablonka-Reuveni Z. A distinct profile of myogenic regulatory factor detection within Pax7+ cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn 238: 1001–1009, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304: 246–259, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Falco G, Comes F, Simone C. pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene 25: 5244–5249, 2006. [DOI] [PubMed] [Google Scholar]
- 15. de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics 20: 1453–1454, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Eberli D, Soker S, Atala A, Yoo JJ. Optimization of human skeletal muscle precursor cell culture and myofiber formation in vitro. Methods 47: 98–103, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature 452: 896–899, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol 10: 116–125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guthrie JL, Seah C, Brown S, Tang P, Jamieson F, Drews SJ. Use of Bordetella pertussis BP3385 to establish a cutoff value for an IS481-targeted real-time PCR assay. J Clin Microbiol 46: 3798–3799, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hackstadt AJ, Hess AM. Filtering for increased power for microarray data analysis. BMC Bioinformatics 10: 11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagiwara N, Yeh M, Liu A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev Dyn 236: 2062–2076, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Haramati S, Chapnik E, Sztainberg Y, Eilam R, Zwang R, Gershoni N, McGlinn E, Heiser PW, Wills AM, Wirguin I, Rubin LL, Misawa H, Tabin CJ, Brown R, Jr, Chen A, Hornstein E. miRNA malfunction causes spinal motor neuron disease. Proc Natl Acad Sci USA 107: 13111–13116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hausler SF, Keller A, Chandran PA, Ziegler K, Zipp K, Heuer S, Krockenberger M, Engel JB, Honig A, Scheffler M, Dietl J, Wischhusen J. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br J Cancer 103: 693–700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikeda T, Kanazawa T, Otsuka S, Ichii O, Hashimoto Y, Kon Y. Expression of caspase family and muscle- and apoptosis-specific genes during skeletal myogenesis in mouse embryo. J Vet Med Sci 71: 1161–1168, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 19: 1426–1431, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174: 677–687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142: 1447–1459, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohlmaier A, Edgar BA. Proliferative control in Drosophila stem cells. Curr Opin Cell Biol 20: 699–706, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14: 82–91, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Lagha M, Brunelli S, Messina G, Cumano A, Kume T, Relaix F, Buckingham ME. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell 17: 892–899, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Shin HS, Shireman PK, Vasilaki A, Van Remmen H, Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med 41: 1174–1184, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460: 627–631, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 115: 787–798, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Machida S, Spangenburg EE, Booth FW. Primary rat muscle progenitor cells have decreased proliferation and myotube formation during passages. Cell Prolif 37: 267–277, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mau M, Oksbjerg N, Rehfeldt C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell Dev Biol Anim 44: 1–5, 2008. [DOI] [PubMed] [Google Scholar]
- 36. McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics 39: 219–226, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene 27: 378–386, 2008. [DOI] [PubMed] [Google Scholar]
- 38. Mesires NT, Doumit ME. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am J Physiol Cell Physiol 282: C899–C906, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med (September 14, 2009). doi:10.1111/j.1582-4934.2009.00898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Parakati R, Dimario JX. Repression of fibroblast growth factor receptor 1 gene expression by E2F4 in skeletal muscle cells. Dev Dyn 232: 119–130, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol 16: 23–29, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, Benham AL, Kim J, Soibam B, Harris RA, Coarfa C, Zariff A, Milosavljevic A, Batts LM, Kheradmand F, Gunaratne PH, Corry DB. Proinflammatory role for let-7 microRNAs in experimental asthma. J Biol Chem 285: 30139–30149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosca AM, Burlacu A. Isolation of a mouse bone marrow population enriched in stem and progenitor cells by centrifugation on a Percoll gradient. Biotechnol Appl Biochem 55: 199–208. [DOI] [PubMed] [Google Scholar]
- 45. Rosenberg MI, Georges SA, Asawachaicharn A, Analau E, Tapscott SJ. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol 175: 77–85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics 20: 3246–3248, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Sambasivan R, Tajbakhsh S. Skeletal muscle stem cell birth and properties. Semin Cell Dev Biol 18: 870–882, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Sarkar S, Dey BK, Dutta A. MiR-322/424 and -503 are induced during muscle differentiation and promote cell cycle quiescence and differentiation by down-regulation of Cdc25A. Mol Biol Cell 21: 2138–2149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sluijter JP, van Mil A, van Vliet P, Metz CH, Liu J, Doevendans PA, Goumans MJ. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol 30: 859–868, 2010. [DOI] [PubMed] [Google Scholar]
- 50. Smith CK, 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol 159: 379–385, 1994. [DOI] [PubMed] [Google Scholar]
- 51. Smyth GK. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor R, edited by Gentleman VC, Dudoit S., Irizarry R., Huber W. New York: Springer, 2005, p. 397–420. [Google Scholar]
- 52. Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomczak KK, Marinescu VD, Ramoni MF, Sanoudou D, Montanaro F, Han M, Kunkel LM, Kohane IS, Beggs AH. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J 18: 403–405, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315: 1579–1582, 2007. [DOI] [PubMed] [Google Scholar]
- 55. van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vaudin P, Dupont J, Duchene S, Audouin E, Crochet S, Berri C, Tesseraud S. Phosphatase PTEN in chicken muscle is regulated during ontogenesis. Domest Anim Endocrinol 31: 123–140, 2006. [DOI] [PubMed] [Google Scholar]
- 57. Westfall PH. Multiple testing of general contrasts using logical constraints and correlations. J Am Stat Assoc 92: 299–306, 1997. [Google Scholar]
- 58. Wong CF, Tellam RL. MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J Biol Chem 283: 9836–9843, 2008. [DOI] [PubMed] [Google Scholar]
- 59. Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol 119: 252–259, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003. [DOI] [PubMed] [Google Scholar]
- 61. Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13: 486–491, 2007. [DOI] [PubMed] [Google Scholar]
- 62. Yuasa K, Hagiwara Y, Ando M, Nakamura A, Takeda S, Hijikata T. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct 33: 163–169, 2008. [DOI] [PubMed] [Google Scholar]
- 63. Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial MiR-126 and other microRNAs in type 2 diabetes. Circ Res 107: 686–688, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.