Abstract
Small, noncoding, microRNAs (miRNAs) have emerged as key mediators of posttranscriptional gene silencing in both pathogenic and pathological aspects of ischemic stroke biology. In stroke etiology, miRNA have distinct expression patterns that modulate pathogenic processes including atherosclerosis (miR-21, miR-126), hyperlipidemia (miR-33, miR-125a-5p), hypertension (miR-155), and plaque rupture (miR-222, miR-210). Following focal cerebral ischemia, significant changes in the miRNA transcriptome, independent of an effect on expression of miRNA machinery, implicate miRNA in the pathological cascade of events that include blood brain barrier disruption (miR-15a) and caspase mediated cell death signaling (miR-497). Early activation of miR-200 family members improves neural cell survival via prolyl hydroxylase mRNA silencing and subsequent HIF-1α stabilization. Pro- (miR-125b) and anti-inflammatory (miR-26a, -34a, -145, and let-7b) miRNA may also be manipulated to positively influence stroke outcomes. Recent examples of successfully implemented miRNA-therapeutics direct the future of gene therapy and offer new therapeutic strategies by regulating large sets of genes in related pathways of the ischemic stroke cascade.
Keywords: ischemia, brain, atherosclerosis, miRNA
the discovery of posttranscriptional gene silencing by small, noncoding, microRNA (miRNA) has led to an explosion of new mechanistic hypotheses in human disease. To date, over 940 human miRNA sequences have been recorded in the miRNA database “miRBase” alone, each with the potential for hundreds of evolutionarily conserved mRNA targets (4). Altogether, miRNAs have been suggested to regulate as much as one-third of protein coding genes in humans (37) and have been implicated in diverse biological processes including embryonic development, cellular differentiation, apoptosis, metabolism, and oncogenesis (30).
The etiological origins of focal cerebral ischemia as well as the resulting pathology are mediated by a multifaceted cascade of molecular mechanisms that are in part regulated by posttranscriptional activity. Given the complexity of the vertebrate central nervous system, it is not surprising that a number of brain-enriched miRNAs have emerged as potential regulators of homeostatic function and, under pathological conditions of stroke, as mediators of neurodegeneration and inflammation (9). While cloning and expression profiling studies have provided direct evidence of their presence in brain tissue, fewer studies have gone as far as biologically validating the effects of miRNAs beyond predicted mRNA targets. Therein lies the greatest challenge to the nascent field of miRNA biology: to determine the biological significance of miRNA under normal and pathological tissue conditions. Here, we present an overview of the current knowledge of miRNA in focal cerebral ischemia and highlight miRNA that have been biologically validated to regulate mRNA targets, encoded protein, and subsequent biological functions.
miRNA BIOLOGY AND LIMITATIONS OF PREDICTIVE TARGET ALGORITHMS
miRNAs are generated from long precursor primary transcripts (pri-miRNA) that are cleaved into ∼60- to 70-nucleotide stem-loop intermediates, referred to as pre-miRNA. This processing is enacted by the Drosha RNase III endonuclease, which cuts both strands of the stem at sites near the base of the primary stem-loop (2, 35). Pre-miRNA is then exported from the nucleus to the cytosol, where it is processed by yet another RNase III endonuclease, Dicer, to form mature 22- to 25-nucleotide miRNA. Mature miRNAs enact mRNA translational repression or cleavage via incorporation into a multiprotein complex termed RISC (RNA-induced silencing complex) and subsequent complete or incomplete binding to the 3′-untranslated region (UTR) of their respective target mRNAs (75). Several noteworthy reviews are available to provide greater detail on miRNA biogenesis and putative mechanisms of posttranscriptional mRNA regulation (2, 17, 75).
MiRNA nomenclature has been facilitated by the adoption of a standard naming scheme that was applied since the first large-scale miRNA discovery (21). MiRNAs are assigned sequential numerical identifiers with homologous miRNAs across species abbreviated with three or four letter prefixes. Nomenclature guidelines only require that novel miRNA genes are experimentally verified by cloning or with evidence of expression and processing. Validation of biological targets is not a prerequisite of miRNA discovery, naming, and publication.
Potential mRNA targets for miRNAs of interest are commonly predicted on the basis of computational software (i.e., PicTar - http://pictar.mdc-berlin.de/, miRanda - http://www.microrna.org/microrna/home.do, and TargetScan - http://www.targetscan.org/), which incorporate distinct algorithms to forecast the probability of a functional miRNA binding site within a given mRNA sequence (32). Such algorithms are primarily focused on programming alignment to identify complementary elements in the 3′-UTR of the target mRNA with the miRNA seed sequence; a region consisting of nucleotides 2–7 of the mature miRNA when read in the 5′ to 3′ direction. Additional considerations of computational algorithms may include the stability of the RNA-RNA duplex, target site conservation across species, and the presence of multiple target sites in the same gene (3, 44).
While there have been numerous candidate miRNA targets identified through computational algorithms, relatively few have been biologically validated in the context of ischemic stroke (66). Limitations of computational algorithms have previously been highlighted and underscore the need for stringent biological validation to limit false-positive and false-negative reporting (3, 4, 32, 44, 57). False-positive rates for early computational algorithms of miRNA/mRNA interaction were estimated to be greater than 20% (47). Conversely, a false-negative rate of >25% was recently reported for a set of experimentally validated miRNA interactions which were not predicted in several of the most commonly used miRNA target prediction programs due to requirements for evolutionary conservation of the miRNA target site across different species (44, 66).
Recent works have proposed guidelines to experimentally validate that a given miRNA regulates a predicted mRNA target (3, 32, 44). Biological validation approaches include, but are not limited to, 1) demonstration of the miRNA/mRNA binding interaction, 2) coexpression of the miRNA/mRNA in spatial and cell-specific resolution, 3) appreciable miRNA-induced changes in the amount of protein encoded by the target mRNA, and 4) a change in biological function as a result of miRNA-mediated regulation of a target mRNA (32). Given the complexity of posttranscriptional regulation, it stands to reason that not all of these criteria will be met under experimental conditions. To strengthen the current review, however, only miRNA that meet at least one of the aforementioned biological validation criteria are discussed.
ISCHEMIC STROKE INCIDENCE AND ETIOLOGY
Worldwide, 15 million people suffer stroke every year (43). Of these, stroke claims the lives of 5 million while another 5 million are left permanently disabled (43). In the United States alone, stroke is the third leading cause of death and the leading cause of serious long-term disability with ∼795,000 Americans afflicted by a new or recurring stroke event each year (40). Stroke is broadly defined by a cerebrovascular disruption of blood supply to brain tissue that is either ischemic or hemorrhagic in origin. Stroke pathology of ischemic origin accounts for 87% of all strokes subtypes presented clinically, while intracerebral and subarachnoid hemorrhagic constitute the remainder (10 and 3%, respectively) (40).
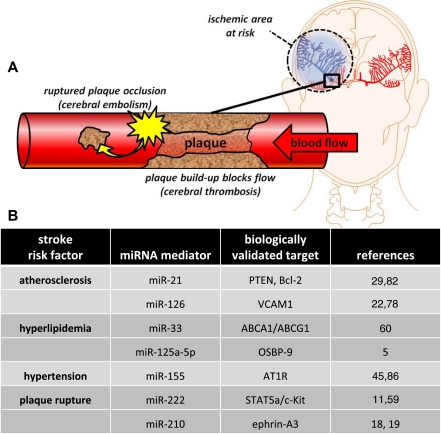
The etymology of the word ischemia is derived from the Greek words ischaimos, which means “to restrain” and haima meaning “blood” (61). In simple terms, ischemic stroke occurs when an artery is obstructed. The brain depends on continuous blood flow to deliver oxygen and nutrients (i.e.. oxygen, glucose) and to remove carbon dioxide and cellular waste. When brain tissue is unable to maintain homeostasis and subsequent trafficking of essential lipids, proteins, nutrients, and waste, the terminal result is energetic failure (ATP depletion) and cell death. Ischemic stroke has several etiological origins. The most common is due to the narrowing of the arteries in the neck or head. This is most often caused by atherosclerosis, a chronic disease process directly influenced by diet (13, 68). High-fat diets leading to elevated low-density lipoprotein (LDL) cholesterol and triglyceride levels are significant risk factors for atherogenesis. If cerebrovascular arteries become too narrow, blood cells collect and form atheromatous plaques. These plaques can block the artery where they are formed (thrombosis) or can dislodge and become trapped in smaller or more distant arteries of the brain (embolism) (Fig. 1).
Fig. 1.
Ischemic stroke etiology. A: a thrombotic stroke occurs when diseased or damaged cerebral arteries become blocked by the formation of atherosclerotic plaque within the brain. Clinically referred to as focal cerebral thrombosis or cerebral infarction, this classification of stroke is responsible for half of all clinically presented stroke cases. An embolic stroke is also caused by cerebrovascular occlusion of atherosclerotic plaque origin, but in this case the plaque (or emboli) ruptures from a distal site, travels in the blood, and occludes a distal point in the cerebrovascular system. B: biologically validated miRNA identified to play a role in ischemic stroke etiology.
miRNA IN ISCHEMIC STROKE ETIOLOGY
Atherosclerosis
Apart from its physiological effects, the vulnerability of an atheromatous plaque is of the utmost importance for its pathogenic role in stroke. Vulnerable plaques are composed of a large lipid core, enriched with >40% of LDL-filled foam cells, and a thin fibrous cap depleted of smooth muscle cells (25). An additional hallmark is the abundant infiltration of inflammatory macrophages that contribute to the vulnerability of plaque rupture and intraplaque hemorrhage. A number of genetic risk factors for atherosclerosis and restenosis have been identified (26, 36, 73). Only recently have these risk factors been studied in the light of miRNA-mediated posttranscriptional regulation (23).
Tissue-specific expression of miRNA is an importance determinant of biological activity. Indeed, one miRNA may be highly expressed in one tissue but have no or low expression in other tissues (33). Abundant miRNA expression has been reported in arteries where atherosclerotic plaques are known to accumulate. Of 180 miRNAs arrayed, 140 were found to be expressed in normal rat carotid arteries, with 49 of the 140 found to be highly expressed (29). It is well known that atherosclerosis manifests itself at certain predilection sites, such as side branches and curvatures in arteries. Studies over the past decade have provided evidence for a role of shear stress as a factor linking atherosclerotic plaque localization in such sites (7). Endothelial shear stress is a friction force, in plane with the cell body and induced by the movement of blood with respect to the endothelial layer. This force is transmitted to the cell nucleus, affecting the expression of several genes, including adhesion factors implicated in atheromatous plaque formation (7, 8). Recent work has described the induction of miR-21 in endothelial cells subjected to shear stress (82). Importantly, overexpression of miR-21 was associated with decreased phosphate and tensin homolog (PTEN) protein expression, and subsequent increasedendothelial nitric oxide synthase phosphorylation and nitric oxide production (82). Previous studies have demonstrated that PTEN reduces intimal hyperplasia in rat carotid artery and attenuates atherosclerotic lesion formation in high fat-fed rabbits (6, 53). miR-21 has also been shown to play a pivotal role in neo-intimal vascular smooth muscle cell (VSMC) proliferation following balloon injury in rodent carotid (29). While the molecular mechanism remains unclear, upregulation of miR-21 following angioplasty increases Bcl-2 expression, favoring VSMC survival and the subsequent proliferative phenotype.
New evidence also suggests miRNA play a distinct role in directing macrophage recruitment to atheromatous plaque. Vascular cell adhesion molecule-1 (VCAM1) is an immunoglobulin-like adhesion molecule expressed on activated endothelial cells (38). VCAM1 binds to α4β1-integrin, which is constitutively expressed on macrophages, mediating both rolling-type adhesion and firm adhesion. While not expressed under normal conditions, VCAM1 expression is rapidly induced by pro-atherosclerotic conditions in both mice and humans (52, 55). miR-126 has been biologically validated as a target of VCAM1. Decreased expression of miR-126 upregulates VCAM1 expression, which in turn enhances leukocyte adherence to the endothelium (22). miR-126 has also been reported to play essential roles in endothelial cells in maintenance of vascular integrity, angiogenesis, and wound repair (78). Indeed, targeted deletion of miR-126 causes leaky vessels, hemorrhage, and partial embryonic lethality due to a loss of vascular integrity and defects in endothelial cell proliferation, migration, and angiogenesis (78). A recent study revealed a distinct loss of endothelial cell specific miR-126 in patients with Type 2 diabetes (89). Considering the well-established link between Type 2 diabetes and an increased risk of atherosclerotic disease (i.e., stroke), miR-126 represents a strong candidate for microRNA mediated therapeutic intervention of atherosclerosis (27, 81).
Hyperlipidemia
Hyperlipidemia is widely recognized as a risk factor for decreased cerebral perfusion and stroke (50, 80). Studies during the late 1990s on the effects of 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) in patients with coronary artery disease have shown significant stroke preventive effects, culminating in the Food and Drug Administration including stroke prevention as an indication for the use of both Simvastatin and Pravastatin (64, 83). Because plasma high-density lipoprotein (HDL) levels show a strong inverse correlation with atherosclerosis, there is strong interest in therapeutically targeting HDL and macrophage cholesterol efflux pathways. It has recently been shown that miR-33 regulates two such pathways: 1) HDL biogenesis in the liver and 2) cellular cholesterol efflux from macrophages, the first step in the reverse cholesterol transport pathway (60). In mouse and human cells, miR-33 inhibits the expression of the adenosine triphosphate-binding cassette transporter, ABCA1, thereby attenuating cholesterol efflux to apolipoprotein 1. Antagonism and overexpression of miR-33 in macrophages was shown to significantly alter cholesterol efflux, a critical first step in the reverse cholesterol transport pathway for the delivery of excess cholesterol back to the liver (60). In mouse macrophages, miR-33 was biologically validated to inhibit ABCG1, reducing cholesterol efflux to nascent HDL.
MicroRNAs have also been implicated in the regulation of LDL uptake by atheroma-associated macrophages. Macrophages that have infiltrated the LDL-enriched plaque play important roles in the formation of atherosclerotic lesions by taking up oxidized LDL, which leads to their conversion into foam cells (56, 67). Once formed, foam cells secrete proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (58). Cholesterol-loaded foam cells eventually undergo secondary necrosis to form the lipid core of mature atherosclerotic plaques. When exposed by plaque disruption, this core triggers acute thrombotic events leading to stroke. miR-125a-5p has been shown to be significantly upregulated in macrophages following oxidized LDL exposure (5). A biologically validated target of miR-125a-5p is oxysterol binding protein (OSBP)-related protein 9, characterized by a COOH-terminal OSBP domain that binds cholesterol, ergosterol, and phospholipids(5). Inhibition of miRNA-125a-5p by a complimentary binding miRNA “antagomiR” significantly increased lipid uptake of macrophages and enhanced expression of the oxidized LDL receptor-1 (LOX-1). Indeed, LOX-1 contributes to plaque instability and development of acute coronary syndromes as demonstrated by LOX-1 deletion reducing proinflammatory signaling and attenuation of atherogenesis (48).
Hypertension
Elevated blood pressure, or hypertension, has long been recognized as a significant risk factor for stroke. There is increasing evidence that an angiotensin II type 1 receptor (AT1R) polymorphism and increased vascular oxidative stress contributes to sympathetic hypertension and vascular disease (10, 49). miRNAs have been directly implicated in mechanisms by which the AT1R polymorphism contributes to increased AT1R activity and associated hypertension (65). Prior to this discovery, and because the polymorphism occurs in the 3′-UTR of the human AT1R gene, the biological significance of the mutation has been questionable. Recently, however, it has been observed that the +1166 A/C polymorphism occurs in a cis-regulatory site that is recognized by miR-155 (45). When the +1166 C allele is present, base pairing complementarity is interrupted and the ability of miR-155 to interact with the cis-regulatory site is decreased. As a result, miR-155 no longer attenuates translation as efficiently. Importantly, mature miR-155 is abundantly expressed in the endothelial and vascular smooth muscle. Downregulation of miR-155 expression in human primary vascular smooth muscle cells induced endogenous human AT1R expression and angiotensin II-induced ERK1/2 activation. Compared with that of Wistar-Kyoto rats, miR-155 expression was decreased in aorta of adult spontaneously hypertensive rats and is negatively correlated with blood pressure, suggesting that miR-155 is possibly involved in the development and pathologic progression of hypertension (86). miR-155 expression was reported to be significantly lower in the aorta of 16-wk-old spontaneously hypertensive rats than in age-matched Wistar-Kyoto rats. Furthermore, miR-155 expression in the aorta is also negatively correlated with blood pressure and age.
Plaque Rupture
Rupture of arterial plaque and subsequent embolic occlusion is one of the leading causes of stroke. Angiogenesis plays a key role in atherothrombotic plaque vulnerability to rupture and is thought to be responsible for the majority of coronary and carotid artery symptomatic events (23, 54). In atherosclerotic intima, newly formed blood vessels are distributed irregularly but ubiquitously across zones of atheromatous plaque disruption. In most cases, these vessels are immature and leaky, permitting infiltration of inflammatory cells as well as an influx of blood and related factors (51, 71). A recent study identified signal transducer and activator of transcription 5a (STAT5a) as a target for miR-222 (11). Importantly, the upregulation of STAT5A due to IL-3/basic fibroblast growth factor-induced downregulation of miR-222 was shown to increase endothelial cell proliferation and migration and, therefore, intraplaque neovascularization during atherosclerosis (71). miR-222 has also been biologically validated to modulate c-Kit (59), a tyrosine kinase cytokine receptor expressed in mature endothelial cells and believed to play a role in microtubule formation and angiogenesis (46). In addition to miR-222, inhibition of miR-210 has also been shown to attenuate endothelial cell migration and angiogenesis (19). Specifically, decreased miR-210 expression inhibits hypoxia induced vessel formation and expression of ephrin-A3 (18). Ephrin-A3 modulation by miR-210 has been demonstrated to possess significant functional effects, including the prevention of tubulogenesis in endothelial cells.
miRNA IN ISCHEMIC STROKE PATHOLOGY
Poststroke miRNA Transcriptome
Cerebral ischemia triggers a cascade of pathological events that ultimately cause irreversible neuronal injury in stroke-affected brain tissue within minutes of stroke onset (14). The pathophysiological order of events includes excitotoxicity within minutes, a robust inflammatory response within hours, and programmed cell death (apoptosis) within hours and days of stroke onset. Preclinical studies in rodents have demonstrated that focal brain ischemia produced by the intraluminal thread model of middle cerebral artery occlusion (MCAO) induces a profound temporal change in the cerebral miRNA transcriptome (12, 28, 39). Table 1 collates differentially expressed miRNA common to preclinical studies in early (≤24 h) and late (24–72 h) phases associated with reperfusion injury following stroke. To date, none of these miRNA targets have been adequately validated in the context of stroke. Additionally, observed changes in the miRNA transcriptome in all three preclinical studies were at the tissue level. Newly developed laser capture microdissection techniques permit cell-specific resolution of miRNA transcription, which represents a powerful tool to strengthen biological validation of differentially expressed candidates (63, 79).
Table 1.
Temporal changes in the microRNA transcriptome following ischemic stroke
| miRNA | Early Phase ≤24 h | Late Phase 24–72 h | Authors | |
|---|---|---|---|---|
| Upregulated at one or both phases | 17 | up (J) | up (D) | D, J |
| 134 | up | up | D, J | |
| 140 | up | up | D, J | |
| 145 | up | up | D, J | |
| 206 | up | up | D, J | |
| 214 | up | up | D, J | |
| 290 | up | up | D, J | |
| 320 | up | up | D, J | |
| 330 | up | up | D, J | |
| 331 | up | up | D, J | |
| 292-5p | up (J) | up (D) | D, J | |
| 324-3p | up | up | D, J | |
| 324-5p | up | up | D, J | |
| 422b | up (J) | up (D) | D, J | |
| 223 | NC | up | D, J, L | |
| Mixed at both phases | 7a | down (J) | down (D), up (J) | D, J |
| 20a | down (J) | up (D) | D, J | |
| 25 | down (J) | up (D) | D, J | |
| 138 | up (J) | down (D) | D, J | |
| 151 | down (J) | up (D) | D, J | |
| 361 | down (J) | up (D) | D, J | |
| Downregulated at one or both phases | 26b | down | down | D, J |
| 27a | down (J) | down (D, J) | D, J | |
| 29b | down | down | D, J | |
| 29c | down | down | D, J | |
| 30e | down | down | D, J | |
| 98 | down | down | D, J | |
| 137 | down (J) | down (D, J) | D, J | |
| 218 | down (J) | down (D, J) | D, J | |
| 335 | down (J) | down (D, J) | D, J | |
| 664 | down (D, J) | down (D) | D, J | |
| 379 | NC | down | D, J | |
| 539 | NC | down | D, J |
Table collates microRNA common to at least 2 of 3 preclinical studies to perform transcriptome-wide analysis following focal cerebral ischemia in rat. Final column designates studies in agreement: D(12), J(28), L(39). NC, no change. Note: specific conditions of experimental MCAO differ across studies and likely contribute to variability in transcriptome changes. For example, D and J employed transient ischemia while L employed permanent ischemia. Refer to original manuscripts for detailed descriptions of experimental stroke procedures.
Of particular note, however, is that changes in the miRNA transcriptome following focal cerebral ischemia were found to occur independently of an effect on miRNA synthesis machinery. Specifically, focal ischemia produced no significant effect in the mRNA expression of the miRNA processing RNases Drosha and Dicer, Drosha cofactor Pasha, or the pre-miRNA transporter exportin-5 up to 24 h postreperfusion (12). Furthermore, changes in the miRNA transcriptome of stroke-affected cortical brain tissue have been observed as early as 3 h postreperfusion (12), at a time when posttranscriptional regulation by miRNA would influence acute phase molecular mechanisms associated with oxidative stress, inflammation, and apoptosis. Taken together, these important clues suggest that miRNA play a pivotal role in regulating the complex cascade of molecular signaling associated with stroke pathology and neuron cell death.
To date, only two miRNA have undergone extensive investigation to biologically validate a physiological effect following acute ischemic stroke: miR-15a and miR 497 (87, 88). miR-15a has recently been shown to contribute to the pathogenesis of ischemic vascular injury through direct inhibition of the antiapoptotic gene bcl-2 (87). Gain or loss of miR-15a significantly reduced or increased oxygen-glucose deprivation-induced cerebral vascular endothelial cell death, respectively. Of particular interest, miR-15a itself was found to be transcriptionally regulated by peroxisome proliferator-activated receptor δ (PPARδ). Intracerebrovascular infusion of a specific PPARδ agonist significantly reduced ischemia-induced miR-15a expression, increased bcl-2 protein levels, and attenuated caspase-3 activity, leading to decreased blood brain barrier (BBB) disruption and reduced cerebral infarction in mice after transient focal cerebral ischemia (87). Another bcl-2 targeting miR, miR-497 was found to be induced in mouse brain transient MCAO. miR-497 was demonstrated to directly hybridize to the predicted 3′-UTR target sites of bcl-2 and inhibit translation. Demonstrating the physiological significance of this effect, in vivo repression of miR-497 using antagomirs was found to effectively lower miR-497 levels, reduce MCAO-induced infarct, and improve neurological deficits with a corresponding increase in bcl-2 protein (87).
Neural Cell Survival
Acute focal cerebral ischemia causes energetic failure in hypermetabolic neural cells, resulting in loss of transmembrane potential, Ca2+ influx, glutamate release, subsequent excitotoxicity, and cell death. One strategy known to improve poststroke neural cell survival is to induce a sublethal threshold of ischemic insult to brain tissue prior to stroke, an approach termed ischemic preconditioning (IPC) (70, 74, 90). While the therapeutic relevance and clinical significance of IPC are questionable, this approach has contributed to the identification of a number of neuroprotective mechanisms in preclinical models of acute ischemic stroke (15). To date, the only miRNA directly implicated in poststroke neural cell survival have been identified through IPC screening (34). Members of the miR-200 family (miR-200a, miR-200b, and miR-429) were all found to be significantly upregulated 3 h after IPC in rodents. Transfection of these miRNA in Neuro-2a cells increased neural cell survival when subjected to oxygen glucose deprivation. Predicted mRNA binding targets of the miR-200 family include the untranslated 3′ region of prolyl hydroxylase 2 (PHD2). PHD2 is known to hydroxylate hypoxia inducible factor 1α (HIF-1α) marking it for proteosomal degradation under normoxic conditions. HIF-1α is a well-established transcription factor that is rapidly induced by hypoxia and accounts for transcriptional regulation of both prosurvival and prodeath genes including Bcl homology 3-only protein. The overall physiological effect of HIF-1α activation following stroke is believed to be toward prosurvival, however, as neuron-specific inactivation of HIF-1α has been demonstrated to increase brain injury following stroke (1). Indeed, miR-200 family overexpression was shown to increase HIF-1α expression, suggesting effective silencing of PHD2 target (34). Outcomes advocate strategies to enhance early upregulation of miR-200 family members to promote poststroke neural cell survival.
Stroke-mediated Inflammation
The cascade of molecular events following focal brain ischemia transforms the cerebrovascular endothelium from a quiescent to a proinflammatory state. Cytokine induction of cell adhesion molecules on the vascular endothelium promote BBB disruption and leukocyte recruitment (69). This exacerbates the proinflammatory state, with an increase in reactive oxygen species, cerebral edema, and release of cytotoxic enzymes. While no miRNA have been biologically validated in stroke-induced cytokine signaling, several have been implicated in other models of proinflammatory brain injury and serve as starting points for investigation.
IL-6 is a proinflammatory cytokine known to induce proinflammatory astrocytic scarring (astrogliosis) following stroke (76). After stroke, astrogliosis is particularly localized in regions of neural cell death. Astrogliotic phenotype and miR-125b levels were found to be increased in IL-6 stressed normal human astrocytes (42). AntagomiR-125b attenuated glial cell proliferation and increased mRNA and protein expression of putative mRNA target cyclin-dependent kinase inhibitor 2A (CDKN2A), a negative regulator of cell growth. CDKN2A expression is known to be downregulated in chronic neurodegenerative diseases associated with astrogliosis, such as Alzheimer's disease, suggesting a role in reactive astrocyte proliferation (62). Taken together, these findings support IL-6 mediated inflammation and miR-125b upregulation as a potential mediator of poststroke astrogliosis.
Interferon-beta (IFN-β) is a regulatory cytokine with anti-inflammatory properties that has been approved for the treatment of multiple sclerosis (MS), an inflammatory, demyelinating disease of the central nervous system. In rodents subjected to autoimmune encephalomyelitis, a preclinical model of human MS, IFN-β therapy strongly inhibited extravasation of proinflammatory blood-derived monocytes into the central nervous system by preventing upregulation of vascular cell adhesion molecule 1 (20). Several miRNAs targeting IFN-β have been biologically validated to silence IFN-β in monocytes-derived macrophages isolated from human subjects, including miR-26a, -34a, -145, and let-7b (85). Importantly, IFN-β therapy has already shown promise in stroke. In a preclinical model of focal cerebral ischemia, systemic IFN-β delivery attenuated infiltration of neutrophils and monocytes to brain tissue and reduced stroke-induced lesion volume by 70% compared with controls (77). A phase I clinical trial to test the dosing safety of IFN-β1a in patients with acute ischemic stroke was completed in 2008 (ClinicalTrials.gov Identifier: NCT00097318). Whether miRNA-26a, 34a, -145, and let 7-b could be augmented as a therapeutic strategy to improve IFN-β anti-inflammatory signaling and poststroke outcome remains to be seen.
miRNA THERAPEUTICS FOR PREVENTION AND TREATMENT OF ISCHEMIC STROKE
Examples of therapeutic opportunities for miRNA regulation already abound. Antagomirs, although incapable of silencing miRNA in the central nervous system when injected systemically, efficiently target miRNAs when injected locally in the mouse cortex (31). Furthermore, antagomir treatment has been shown to be effective in abolishing tumor growth in vivo, specifically in therapy-resistant neuroblastoma. The potential for antagomir therapy has also been proven in the context of acute ischemic stroke by the aforementioned regulation of miR-497 (88). In contrast to antagomir-directed miRNA silencing, miRNA overexpression strategies also exist in the form corrective synthetic miRNA delivery. miR-34a targets gene products that promote cell cycle progression and counteract apoptosis (24). In an oncogenic environment and in many forms of human cancer, homeostatic miR-34a expression is decreased (41, 72). Systemic delivery of miR-34a in a lipid-based delivery vehicle has been shown to block lung tumor growth in vivo (84).
Barriers to successful therapeutic intervention of miRNA activity include tissue and cell-specific delivery in vivo, degradation avoidance, and target specificity. Chemically modified miRNAs have shown significant promise in preclinical studies to overcome such hurdles. The locked nucleic acid-modified oligonucleotide antagomir (LNA-antimiR) construct contains a methylene bridge that connects the 2′-O-oxygen and the 4′-C atom of the ribose ring. This conformation increases the thermal stability of duplexes and confers resistance to exo- and endonuclease activity for improved in vivo stability. miR-122 is a liver expressed miRNA implicated in cholesterol and lipid metabolism that has been successfully targeted in vivo using a LNA-antimiR. Systemic administration of a phosphate-buffered solution formulated LNA-antimiR for miR-122 demonstrated potent antagonism of liver-expressed miR-122 in nonhuman primates (16). Biological validation of the LNA-antimiR action revealed cytoplasmic uptake in hepatocytes, formation of stable heteroduplexes between the LNA-antimiR and miR-122, and subsequent depletion of mature miR-122 with appreciable physiologic effect of lowering plasma cholesterol. Chemical modification and delivery systems for miRNA to efficiently cross the BBB and target brain tissue are the focus of ongoing investigation.
In a setting where one miRNA regulates more than one hundred gene targets, and one gene can be regulated by a number of miRNA, a critical barrier is to improve the knowledge of regulatory loops that govern miRNA-mRNA interaction and functional outcome (65). Robust biological validation of miRNA targets is moving us ever closer to better understanding the complex molecular mechanisms associated with pathological disorders such as ischemic stroke. An improved understanding of miRNA biology in disease will also direct new therapeutic strategies to modulate discordant gene expression toward a favorable outcome. Given the complexity of pathophysiological molecular signaling in the context of ischemic stroke, it comes as no surprise that targeting single genes for therapeutic intervention have failed in the clinic. Targeting noncoding genes such as miRNAs, which have the capacity to regulate large sets of evolutionary conserved coding genes, represents the future of gene therapy.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-42617.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27: 6320–6332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bentwich I. Prediction and validation of microRNAs and their targets. FEBS Lett 579: 5904–5910, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37: 766–770, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83: 131–139, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Chen WJ, Lin KH, Lai YJ, Yang SH, Pang JH. Protective effect of propylthiouracil independent of its hypothyroid effect on atherogenesis in cholesterol-fed rabbits: PTEN induction and inhibition of vascular smooth muscle cell proliferation and migration. Circulation 110: 1313–1319, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cheng C, de Crom R, van Haperen R, Helderman F, MousaviGourabi B, van Damme LC, Kirschbaum SW, Slager CJ, van der Steen AF, Krams R. The role of shear stress in atherosclerosis: action through gene expression and inflammation? Cell Biochem Biophys 41: 279–294, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Christensen M, Schratt GM. microRNA involvement in developmental and functional aspects of the nervous system and in neurological diseases. Neurosci Lett 466: 55–62, 2009 [DOI] [PubMed] [Google Scholar]
- 10. De Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. Am J Hypertens 23: 708–715, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol 30: 1562–1568, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29: 675–687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhawan SS, Avati Nanjundappa RP, Branch JR, Taylor WR, Quyyumi AA, Jo H, McDaniel MC, Suo J, Giddens D, Samady H. Shear stress and plaque development. Expert Rev Cardiovasc Ther 8: 545–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391–397, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26: 248–254, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature 452: 896–899, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell 132: 9–14, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fasanaro P, D'Alessandra Y, DiStefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, Capogrossi MC, Martelli F. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem 284: 35134–35143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Dopp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. J Neuroimmunol 127: 69–79, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–D144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105: 1516–1521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haver VG, Slart RH, Zeebregts CJ, Peppelenbosch MP, Tio RA. Rupture of vulnerable atherosclerotic plaques: microRNAs conducting the orchestra? Trends Cardiovasc Med 20: 65–71, 2010 [DOI] [PubMed] [Google Scholar]
- 24. He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellings WE, Peeters W, Moll FL, Pasterkamp G. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med 17: 162–171, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol 3: 227–235, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med 139: 824–834, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39: 959–966, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100: 1579–1588, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res 35: 2885–2892, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuhn DE, Martin MM, Feldman DS, Terry AV, Jr, Nuovo GJ, Elton TS. Experimental validation of miRNA targets. Methods 44: 47–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735–739, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke 41: 1646–1651, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Lee YW, Kuhn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol 33: 83–94, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest 107: 1209–1210, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 30: 92–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 7: 2591–2600, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem 101: 1265–1269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mackay J, Mensah GA, Mendis S, Greenlund K, World Health Organization The Atlas of Heart Disease and Stroke. Geneva: World Health Organization, 2004, p. 112 [Google Scholar]
- 44. Martin G, Schouest K, Kovvuru P, Spillane C. Prediction and validation of microRNA targets in animal genomes. J Biosci 32: 1049–1052, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J Biol Chem 282: 24262–24269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem 279: 18600–18607, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Maziere P, Enright AJ. Prediction of microRNA targets. Drug Discov Today 12: 452–458, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100: 1634–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Mettimano M, Romano-Spica V, Ianni A, Specchia M, Migneco A, Savi L. AGT and AT1R gene polymorphism in hypertensive heart disease. Int J Clin Pract 56: 574–577, 2002 [PubMed] [Google Scholar]
- 50. Meyer JS, Rogers RL, Mortel KF, Judd BW. Hyperlipidemia is a risk factor for decreased cerebral perfusion and stroke. Arch Neurol 44: 418–422, 1987 [DOI] [PubMed] [Google Scholar]
- 51. Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol 17: 548–555, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 18: 842–851, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res 102: 1036–1045, 2008 [DOI] [PubMed] [Google Scholar]
- 54. O'Brien ER, Garvin MR, Dev R, Stewart DK, Hinohara T, Simpson JB, Schwartz SM. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 145: 883–894, 1994 [PMC free article] [PubMed] [Google Scholar]
- 55. O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, Lobb R, Alpers CE. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest 92: 945–951, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev 83: 1069–1112, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Papadopoulos GL, Reczko M, Simossis VA, Sethupathy P, Hatzigeorgiou AG. The database of experimentally supported targets: a functional update of TarBase. Nucleic Acids Res 37: D155–D158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Persson J, Nilsson J, Lindholm MW. Cytokine response to lipoprotein lipid loading in human monocyte-derived macrophages. Lipids Health Dis 5: 17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108: 3068–3071, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328: 1570–1573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ 16: 378–385, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82: 21–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335: 1001–1009, 1996 [DOI] [PubMed] [Google Scholar]
- 65. Sen CK, Gordillo GM, Khanna S, Roy S. Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res 46: 527–540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods 3: 881–886, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des 11: 3061–3072, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 91: 502–509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Starzyk RM, Rosenow C, Frye J, Leismann M, Rodzinski E, Putney S, Tuomanen EI. Cerebral cell adhesion molecule: a novel leukocyte adhesion determinant on blood-brain barrier capillary endothelium. J Infect Dis 181: 181–187, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke 38: 680–685, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Suarez Y. Microregulation of plaque neovascularization. Arterioscler Thromb Vasc Biol 30: 1500–1501, 2010 [DOI] [PubMed] [Google Scholar]
- 72. Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6: 1586–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Trogan E, Fisher EA. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol Biol 293: 221–231, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Truettner J, Busto R, Zhao W, Ginsberg MD, Perez-Pinzon MA. Effect of ischemic preconditioning on the expression of putative neuroprotective genes in the rat brain. Brain Res Mol Brain Res 103: 106–115, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Van den Berg A, Mols J, Han J. RISC-target interaction: cleavage and translational suppression. Biochim Biophys Acta 1779: 668–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol 100: 124–139, 1999 [DOI] [PubMed] [Google Scholar]
- 77. Veldhuis WB, Derksen JW, Floris S, Van Der Meide PH, De Vries HE, Schepers J, Vos IM, Dijkstra CD, Kappelle LJ, Nicolay K, Bar PR. Interferon-beta blocks infiltration of inflammatory cells and reduces infarct volume after ischemic stroke in the rat. J Cereb Blood Flow Metab 23: 1029–1039, 2003 [DOI] [PubMed] [Google Scholar]
- 78. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15: 261–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang S, Wang L, Zhu T, Gao X, Li J, Wu Y, Zhu H. Improvement of tissue preparation for laser capture microdissection: application for cell type-specific miRNA expression profiling in colorectal tumors. BMC Genomics 11: 163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Warsch JR, Wright CB. Stroke: hyperlipidemia and cerebral small-vessel disease. Nat Rev Neurol 6: 307–308, 2010 [DOI] [PubMed] [Google Scholar]
- 81. Watson KE, PetersHarmel AL, Matson G. Atherosclerosis in type 2 diabetes mellitus: the role of insulin resistance. J Cardiovasc Pharmacol Ther 8: 253–260, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 393: 643–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. White HD, Simes RJ, Anderson NE, Hankey GJ, Watson JD, Hunt D, Colquhoun DM, Glasziou P, MacMahon S, Kirby AC, West MJ, Tonkin AM. Pravastatin therapy and the risk of stroke. N Engl J Med 343: 317–326, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 70: 5923–5930, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Witwer KW, Sisk JM, Gama L, Clements JE. MicroRNA regulation of IFN-beta protein expression: rapid and sensitive modulation of the innate immune response. J Immunol 184: 2369–2376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu CC, Han WQ, Xiao B, Li NN, Zhu DL, Gao PJ. [Differential expression of microRNAs in the aorta of spontaneously hypertensive rats.]. Sheng Li Xue Bao 60: 553–560, 2008 [PubMed] [Google Scholar]
- 87. Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci 30: 6398–6408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 38: 17–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial MiR-126 and other microRNAs in type 2 diabetes. Circ Res 107: 810–817, 2010 [DOI] [PubMed] [Google Scholar]
- 90. Zhang HX, Du GH, Zhang JT. Ischemic pre-conditioning preserves brain mitochondrial functions during the middle cerebral artery occlusion in rat. Neurol Res 25: 471–476, 2003 [DOI] [PubMed] [Google Scholar]