Abstract
In the beginning the surface of the Earth was extremely hot, because the Earth as we know it is the product of a collision between two planets, a collision that also created the Moon. Most of the heat within the very young Earth was lost quickly to space while the surface was still quite hot. As it cooled, the Earth's surface passed monotonically through every temperature regime between silicate vapor to liquid water and perhaps even to ice, eventually reaching an equilibrium with sunlight. Inevitably the surface passed through a time when the temperature was around 100°C at which modern thermophile organisms live. How long this warm epoch lasted depends on how long a thick greenhouse atmosphere can be maintained by heat flow from the Earth's interior, either directly as a supplement to insolation, or indirectly through its influence on the nascent carbonate cycle. In both cases, the duration of the warm epoch would have been controlled by processes within the Earth's interior where buffering by surface conditions played little part. A potentially evolutionarily significant warm period of between 105 and 107 years seems likely, which nonetheless was brief compared to the vast expanse of geological time.
The present Earth-Moon system is generally believed to have formed in the aftermath of a collision between two planets 4.45–4.5 billion years ago (e.g., ref. 1). If the gravitational potential and kinetic energy of the impact all were converted into heat, the energy would have been sufficient to vaporize much of the Earth and exterminate any life present on either body. In practice one must expect vaporization and even melting to be incomplete, so that some rocks would survive the initial impact and some of the survivors enter into orbit about the sun. In principle life also might survive in such rocks to be returned later once Earth became habitable; we will not pursue these speculations here. It is obvious that an initially hot Earth implies that the surface conditions on the Earth passed through those associated with modern thermophilic microbes (around 100°C) before becoming more conventionally clement. This is interesting because the putative thermophilic root of the tree of life is compatible with life's origin while the surface was still near 100°C (2). In this paper we will focus on the hypothesis that the Earth was hot everywhere when life began. Alternative explanations for a thermophylic root are (i) origin of life within hydrothermal systems (3) and (ii) extinction of all early mesophiles by large asteroid impacts at later times (refs. 4 and 5 and refs. therein).
In the hot Earth scenario, the time scale and mechanism by which the Earth reached clement conditions and the time interval over which surface temperatures lingered near 100°C are important parameters. Also relevant is the gross chemistry of seawater at that time. It is convenient to divide the time after the moon-forming impact into two epochs distinguished by different controlling physics. In the first, surface temperatures are directly maintained by heat vented from the Earth's interior. In the second, surface temperatures are maintained by a solar-heated greenhouse as on the present Earth. We treat both possibilities and find that the implications of both histories to biology are similar. We use physical constraints moving forward in time from the moon-forming impact and back in time from the present state of the Earth. We obtain robust general conclusions that are independent of the many unknown details.
Direct evidence now comes only from very old zircons preserved in younger sediments. Extensive surface water of indeterminate temperature and long-lived continental crust were present by 4.4 billion years ago (6). Surficial weathering by liquid water between 0 and 100°C occurred by 4.2 billion years ago (7).
Moon-Forming Impact and an Atmosphere Heated from Below
The moon-forming collision was violent enough that conservation of energy gives a first appraisal of the initial consequences. The impact supplied ≈4 × 1031 J. This is equivalent to 7 × 106 J⋅kg−1 if distributed over the mass of the Earth. This energy density is comparable to the low-pressure heat of vaporization of rock 6–14 × 106 J⋅kg−1 (8, 9). Evidently a fair fraction of the Earth-Moon materials were vaporized, and most of the impact energy was invested in latent heat of vaporization. Thick and probably supercritical silicate vapor atmospheres would have gathered about both bodies, although owing to the Moon's small mass such an atmosphere would not have been gravitationally bound to it (10). By contrast Earth's rock vapor atmosphere would have been strongly bound.
Most of the impact's energy escaped the Earth as thermal radiation at this time and was unavailable to heat the surface later when conditions became favorable to life. The surface heat flow Q is determined by the physics of black body radiation
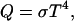 |
1 |
where σ = 5.67 × 10−8 W⋅m−2⋅K−4 is the Stefan-Boltzmann constant, and T is the effective radiating temperature. The first significant thermal buffer encountered by the cooling Earth occurred when rock vapor began to condense at the top of the atmosphere to form an optically thick aerosol layer. Subsequently the aerosols coagulated and fell to the surface. Eventually all the rock vapor condensed and fell out. While partly condensed rock vapor existed, the effective radiating temperature was around 2,300 K and the surface heat flow was 1.6 × 106 W⋅m−2 (11). At this rate of cooling, the rock-vapor epoch lasted less than 2,000 years.
Once the rock vapor was gone an atmosphere of water vapor and other common volatiles including CO2 remained. Heat transfer to space was controlled by the temperature of the molten rock at the surface. A vigorous global convection system continued at least until the adiabat was cool enough that significant solid froze to form a rind on the magma (10). As the low-pressure liquidus of the mantle is estimated to be 2,036 K (correcting a typo in ref. 12), a tenuous solid rind first formed around 2,000 K. The heat removed was comparable to the latent heat of melting plus the specific heat for cooling a few hundred kelvin, together around 106 J⋅kg−1. In the absence of an optically significant atmosphere, direct radiation of molten rock to space would rapidly cool the Earth's interior. For example, an effective radiating temperature of 1,500 K implies a surface heat flow of 0.9 × 106 W⋅m−2, which would remove all the remaining available heat in about 400 years. More likely a massive atmosphere blanketed the Earth. Still cooling was geologically rapid. A runaway water greenhouse provides a reasonable model for the thermally blanketed early Earth. The critical surface heat flow, 150 W⋅m−2 (13), is the difference between the critical greenhouse threshold for a water-rich atmosphere and the heat supplied by the sun. Heat loss at this rate would have globally removed 106 J⋅kg−1 in 2.5 million years (Myr).
The Earth became potentially habitable once it developed a significant solid lid to partition the hot interior from a cooler surface environment featuring liquid water. This first happened when the interior was only several hundred kelvin hotter than the present interior temperature. Only the heat remaining in the Earth at this time and the additional heat subsequently generated by radioactivity are relevant to habitability. To quantify this amount of heat, we introduce the concept of potential temperature, which is the temperature extrapolated to the surface ignoring latent heat effects of melting. This concept allows considering only temperature changes in the deep interior, where most of the heat capacity of the Earth resides, without having to explicitly consider the details of melting in the heat balance. In practice, the physics of melting of ascending material needs to be explicitly considered to determine when a solid lid can form. From refs. 10 and 12, this potential temperature is about 2,500 K. The current mantle potential temperature is obtained by considering the physics of the melting that produces magmas at modern ridge axes. It is about 1,600 K. The average early Archean potential temperature is not precisely constrained from studies of Archean volcanic rock, but was around 1,800 K (14). Cooling of about 700 K from the potential temperature where a solid lid first formed provided the energy that was extracted before the Archean by surface heat flow. The amount is 700 K, multiplied by the present specific heat of the core and mantle, about 6.25 × 1027 J⋅K−1, or 4 × 1030 J.
Once the silicate Earth was solid enough to form a significant cool rind, convection became sluggish. If the massive atmosphere were not already optically thick, the opacity of water vapor would have made it so once the effective temperature of the surface dropped below 1,500 K (13). Then, after a brief transition, more gradual cooling with a heat flow comparable to the present solar flux occurred through a massive water (runaway greenhouse) atmosphere. A hot runaway greenhouse maintained by interior temperatures could have existed only for a modest geological time. For example, a critical runaway greenhouse would cool the Earth's interior 700 K in 1.8 Myr.
Eventually, the heat flow from the interior dropped below that needed to maintain a runaway greenhouse, and from this point the surface temperature declined. The duration of the epoch where heat flow from the Earth's interior continued to have influence on global climate is crudely obtained by noting that heat flow must have been a significant fraction of solar heating. An atmosphere without major amounts of CO2 (or other greenhouse gases) provides a quantitative example. Allotting the whole 700 K of potential temperature change to maintain a heat flow of 100 W⋅m−2 gives a maximum duration of 2.7 Myr. For comparison, a surface heat flow of 70 W⋅m−2 would have provided clement 30°C surface conditions on the early Earth in this case (13).
However, it is quite difficult for the Earth to linger in the temperature range inhabited by modern thermophiles for a long period because the surface temperature is quite sensitive to the internal heat flow. There is no obvious mechanism to stabilize the global average heat flow precisely within this range. At these high heat flows, the thickness of the rind of solid rock above the molten magma would have been quite small and not mechanically stable. For example, using a surface heat flow of 100 W⋅m−2 and a thermal conductivity of 2.4 W⋅m−1⋅K−1 implies that molten rock would be encountered at 20-m depth. Hydrothermal circulation would have transferred heat through a thicker more realistic rind, but the problem of maintaining the global average heat flow within a narrow range while the Earth's interior cooled by a few 100 K (to supply the heat) remains because the transfer of heat from the interior is expected to have become less vigorous as cooling made the mantle and its erupted magmas more viscous and reduced the fraction of melting along ascending adiabats. To continue the duration estimate, we represent the magma ocean by a linear viscous fluid, which is admittedly a poor model as both melt and mostly crystalline mush transport heat. The heat flow scales to the interior viscosity to the −1/3 power (15). Typically viscosity increases by at least a factor of 10 for 100 K cooling, implying that heat flow decreases by at least a factor of 2.15 for 100 K of cooling. The 20% change in heat flow given above would require 23°C of cooling, implying a duration of only 0.09 Myr.
Gradual Cooling and Volatile Exchange on the Early Earth
In the above example, we assumed that a massive CO2 atmosphere did not exist when the interior heat flow waned to insignificance. We now consider the alternative scenario, where a massive CO2 atmosphere maintained high surface temperatures within a solar heated greenhouse after the heat flow from the interior ceased to be significant. We review physical constraints on this situation by returning to the initial aftermath of the moon-forming impact. We do not attempt to consider the effects of the greenhouse gases methane and hydrogen, which were conceivably present after the moon-forming impact.
The mode of exchange of volatiles between the silicate Earth and the atmosphere evolved after the moon-forming impact. At first, traditional volatiles were gaseous components of a well-mixed rock vapor atmosphere, which was continuous with an underlying supercritical rock fluid. Later ascending melt partially vaporized and rock rain fell into a boiling molten magma ocean from rock clouds. It would be expected that most of the traditional volatiles would partition into the rock vapor and not fall in the rock rain. Once all the rock vapor had condensed, a surface chemical equilibrium between molten rock and the atmosphere was approached. This equilibrium favored partitioning of traditional volatiles into the atmosphere. As noted above these hot epochs lasted only thousands of years, too brief for much hydrogen or anything else to escape to space.
Vigorous convective transfer of both heat and volatiles is implied by both the rock vapor atmosphere and rapidly convecting molten rock. More heat loss occurred then than during all the subsequent history of the Earth. Most of the Earth's mantle needed to convect turbulently through the photosphere of the rock-vapor atmosphere and later through the surface of the molten rock to cool the mantle.
Less vigorous mantle convection is implied by a runaway greenhouse lasting on the order of a Myr. Still, much of the mantle ascended to the surface to cool. The duration of this epoch was long enough for any remaining metallic iron to sink to the core. The nearly quantitative depletion of 36Ar from the Earth's mantle is an indication of the efficacy of degassing during these epochs.
Midoceanic Ridge Analogy
Once the atmosphere became significantly cooler than erupting molten rock, a dynamic balance between degassing of lava at high temperatures and alteration of cooled lava by the atmosphere (and ocean) followed by crustal foundering and return of the cooled lava to the interior of the Earth became relevant. This exchange has persisted in various forms to the present with subducting slabs delivering oceanic sediments and altered oceanic crust to the mantle.
Modern midoceanic ridge axes thus provide useful analogies for such water-rock interaction on the early Earth. In that case, the rock partially dissolves causing the circulating water to become saturated relative to minerals formed from the major constituents of the rock. In the case of the early Earth, eruption repeatedly brought rock into contact with the ocean maintaining saturation with respect to the major elements in the rock. Venus is a modern hot atmosphere reacting with rocks.
One analogy with the ridge axis involves the initial cooling of the runaway greenhouse. As seawater penetrates deeper into oceanic crust, it reacts with rock at progressively higher temperatures until small amounts of it encounter molten magma. The runaway water-vapor atmosphere experienced these conditions except that it started at high temperatures and then cooled. The pressures in both cases are similar (a few hundred bars) as they are supplied by the weight of the global ocean of water. The chemistry of rocks altered at high temperatures at modern ridges has been studied. A significant difference is that CO2 was an abundant volatile in the early runaway atmosphere but not in modern seawater. This volatile-rock reaction during cooling differs from the classical approach where volatiles react with rock in a low-temperature environment (e.g., ref. 16).
We discuss volatiles in the order that they first condense, beginning with chlorine. Depending on the pressure, solid NaCl may be in equilibrium with a water-rich gas or a dense NaCl-rich brine may be in equilibrium with a water-rich gas (17). The transition temperature from two fluids to fluid plus solid NaCl is 481°C at 300 bar. At this pressure and seawater composition, a single fluid phase exists below 410°C. In addition, the chloride-bearing mineral amphibole forms in the rock below 750°C beneath modern ridge axes (18). NaCl, as a dissolved component in water or in a solid, is the stable chlorine species in equilibrium with the rock. Only trace amounts of HCl exist in the hot atmosphere of Venus because Cl is buffered by surface rocks (19).
Mass balances indicate that there was plenty of Na within the rock to form NaCl. Both Cl and Na should strongly partition into voluminous high-temperature melts. Their ratio within a melt was similar to the bulk Earth Na:Cl of about 60 by mass or 92 by atom (table 13 of ref. 20). An alternative way of viewing the excess of Na over Cl is that all the Na in the modern ocean could be supplied by a global layer of basalt only 1/2 km thick.
Hydrous silicates began to form around 500°C when a NaCl-rich brine condensed. As further cooling occurred and the temperature approached the critical temperature of water, the brine and most of the water vapor in the atmosphere condensed into a dilute brine similar to the modern ocean. The details of reactions involving NaCl and of those involving hydrous silicates are beyond the scope of this paper as our objective is the fate of a warm greenhouse. Still the situation, once the ocean had condensed, was not drastically different from deep hydrothermal systems of a modern ridge axis.
In the presence of a massive CO2 atmosphere, carbonates first formed when the surface cooled below about 450°C. The highlands of Venus are a possible modern analogy where far less H2O is present in the gas phase (19).
In general, reactions that sequester volatiles within rocks require both efficient access of the volatiles to the rocks at shallow depths and favorable reaction kinetics. Modern ridge axes provide a good indication that both these requirements would have been satisfied on the early Earth. The bulk of the alteration in new oceanic crust occurs quickly when the rock is near the axis where water readily circulates through the shallow crust. To quantify our analogy, we compare the average heat flow through oceanic crust younger than a given age to the heat flow estimate for epochs on the ancient Earth.
The axial zone of modern ridge axes provides an analogy to crust above a vigorous magma ocean. High-temperature (350°C) vents and hence much of the high temperature water-rock reaction occur within about a kilometer of the ridge axis. The heat balance of a fast ridge axis is easily constrained by using the data from ref. 21. The heat is supplied by the latent heat of molten rock, which freezes as fast as it is supplied from the mantle to the magma chamber. At any one time, only a thin lens of magma at the top of the chamber is fully molten (21). The rest of the chamber is filled with mostly crystalline mush formed by cooling of the magma at the top of the lens. Here the lens is about a kilometer wide and the full spreading rate is 155 mm⋅yr−1. This implies that it takes crust about 6,000 yr to traverse the width of the lens. About a 5-km thickness of basalt is partially frozen by heat loss from the top of the magma lens and eventually carried laterally from the axis at the spreading rate. Using a latent heat of 1.5 × 109 J⋅m−3 (22), the heat flow through the top of the lens is around 40 W⋅m−2. Our best estimate for the average heat flow through the top of the axial crust is somewhat less than this. The area of recharge for hydrothermal flow needs to be included in the axial zone. This is poorly constrained but may be about twice as wide as the lens itself. Also the mush forms beneath the molten lens is not fully frozen so the effective value of the latent heat is somewhat less than used above. Even taking these effects into account, the axial heat flow is well above 10 W⋅m−2. The data from ridges with various spreading rates provide a constraint on the physics governing the vigor heat flow from magma chambers. The lens width does not strongly depend on spreading rate (21), implying that the axial heat flow computed in this way is linearly proportional to spreading rate. That is, the limiting factor for heat flow appears to be the rate at which seafloor spreading moves the newly formed mush aside to make room for more magma and the rate that solid-state convection supplies material to the upwelling zone. Similarly, the vigor of solid-state convection in the mantle beneath a magma ocean imposes a long-term limit on the heat flow.
The axial region out to about 1 Myr age provides an analogy to crust above a sluggish magma ocean where the heat flow is 1 W⋅m−2. The typical near-axis hydrothermal water is warm, 20–60°C (23, 24). In the modern region, heat is supplied by conductive cooling of rocks down to about 10-km depth. The bulk of water-rock chemical reactions occur within crust less than 1 Myr old, implying that the alteration of crust that endures at least 1 Myr is similar to that of modern crust.
Returning to the global heat budget of the Earth's interior, the available heat on the early Earth was equivalent to that needed to cool the interior by a few hundred kelvin. We obtained above that a heat flow of 100 W⋅m−2 could be maintained globally for no more than 2.7 Myr. Alternatively, the heat flow for global conditions analogous to those at a fast ridge axis could persist on the order of 27 Myr, and the heat flow analogous to that from crust younger than 1 Myr could persist for 270 Myr (we use the same maximum available heat to get upper limits). The persistence and detailed properties of this magma ocean mode of convection are uncertain (10). The Moon provides an example of gradual cooling through a thick static lid. The chemistry of Martian samples indicates that a thick static lid did not exist there (25). Fast ridge axes provide examples of thin lids that are so sufficiently unstable that the lower part of the lid often collapses back into the chamber. The upper part of the lid is buried by lava flows. At the modern ridge axis, both processes cease once the crust has spread away from the axial magma lens. In a global magma ocean, parts of the cool lid (with their altered rocks) would have foundered into the interior magma ocean in a manner crudely analogous to modern subduction. We do not attempt to examine this process in detail.
Fate of Massive CO2-Dominated Greenhouse
We continue the evolution of the atmosphere to the time where a massive CO2 atmosphere existed above a hot ocean in contact with silicate rocks. The quasi-steady temperature of this ocean was controlled by the balance of incoming and outgoing radiation in a water-CO2 greenhouse.
To make the greenhouse as hot as possible, we will assume that the entire global inventory of CO2 was in the air. (The amount dissolved in the ocean is not large enough to affect the mass balance; see Table 2, which is published as supplemental material on the PNAS website, www.pnas.org.) Zhang and Zindler (26) estimate that the CO2 inventories in the crust and mantle sum to 25,000 × 1018 mol, which is equivalent to a partial pressure of 215 bars. This CO2 pressure would have resulted in a 230°C surface with a condensed ocean, as presumed above, rather than Venus-like conditions once insolation dominated the heat balance (27). (At this temperature only 40 bars of water were in the air, compatible with our assumption that the atmosphere was mostly CO2.)
A massive CO2 greenhouse no longer exists. A necessary (but not sufficient) condition for its demise is that the CO2 could react with exposed silicates to form carbonates. As the major volatiles in the early atmosphere were quite voluminous, we need to consider only the major constituents of the rocks that reacted with the atmosphere. Probable reactable rocks range from basalt, the most common volcanic rock on the modern Earth's surface, to ultramafic rocks approaching bulk mantle composition. For quick mass balances, basalt may be represented as a mixture (by mass) of 3% Na2O; 10% each of MgO, CaO, and FeO; 15% Al2O3; and 50% SiO2 with some TiO2 and other minor and trace elements. The bulk of ultramafic rocks is magnesium silicates. We do not need to speculate beyond this on the nature of the earliest igneous rocks.
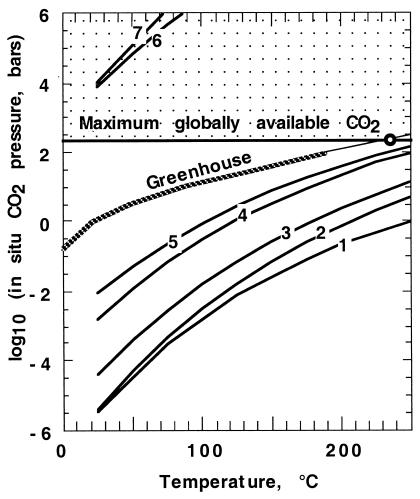
Equilibrium CO2 partial pressure-temperature curves for model rock reactions are shown in Fig. 1 along with the greenhouse curve, which gives surface temperature at a given pCO2. All the curves involving Ca and Mg silicates lie below the greenhouse curve. Reactions in the shallow crust thus will take CO2 into the rock to form carbonates. At the high temperature end of the curves, it can be expected that the kinetics are fast and that accessible Ca, Mg, and Fe within the rock would get used up in that order.
Figure 1.
Equilibrium curves 1–7 for carbonatizing reactions of basalt altered by seawater are shown as functions of temperature and the partial pressure of CO2. The idealized mineral species and references to the data used to obtain the equilibrium curves in Table 1. The greenhouse curve (27) defines possible global states for the earliest Earth; the thin part of the curve is extrapolated from their results. The CO2 pressure must lie below that which would occur if the available CO2 now in the crust and mantle was degassed. The dotted region above this curve is forbidden. The circle indicts the initial state, which would have occurred if all the Earth's CO2 started in the air. Curves 1–5 are for Ca, Mg, and Fe carbonates. These reactions are 1) leonhardite + albite + CO2 = calcite + paragonite + 4 quartz + 2.5 H2O; 2) leonhardite + CO2 = calcite + kaolinite + 2 quartz + 1.5 H2O; 3) clinochlore-14A + 5 calcite + 5 CO2 = 5 dolomite + kaolinite + quartz + 2 H2O; 4) clinochlore-14A + 5 CO2 = 5 magnesite + kaolinite + quartz + 2 H2O; and 5) daphnite-14A + 5 CO2 = 5 siderite + kaolinite + quartz + 2H2O. The carbonate species are stable as the curves lie below the greenhouse curve. Equilibrium curves for Na-carbonates 6–7 lie in the forbidden region and represent reactions, which cannot occur in a global ocean. The reactions are 6) 2 albite + CO2 =3 thermonatrite + kaolinite + 4 quartz and 7) paragonite + CO2 + 5 H2O = thermonatrite + 3 kaolinite. More detail is given in Table 2.
Mass balance constraints indicate that the solid lid of the magma ocean cannot store most of the global inventory of CO2. In analogy, carbonation of the modern oceanic crust is limited to the upper 500 m where significant permeability and porosity exist (see refs. 32 and 33 for discussion of the thickness of this layer). Using up all of the Ca, Mg, and Fe available in that layer would produce an equivalent thickness of 300 m of carbonates, containing globally 4,100 × 1018 mol, which is significantly smaller than the global crust plus mantle inventory of 25,000 × 1018 mol (26). Thus a second necessary condition for the demise of a massive CO2 atmosphere exists: the rocks carbonatized near the surface need to get returned to depths in the magma ocean without immediately venting their CO2 back to the surface. Organized foundering of the solid crust back into the magma ocean in a manner analogous to subduction and local foundering of blocks are possibilities. A magma ocean lid formed by repeated eruption of lava flows to the surface could conceivably store more CO2 than the modern reactable layer of oceanic crust, but the problem of venting the CO2 and other volatiles upon foundering remains.
Foundering of small blocks occurs efficiently at the fastest modern ridge axes where the magma chamber is relatively near the surface, ≈1 km, and is evident from the chemistry of Cl and water (34). Water- and Cl-bearing amphiboles formed at temperatures below 750°C are the modern contaminant (18). The situation is basically similar to that in ancient times in that water and NaCl were significant components of the circulating fluid. At modern ridge axes, water in the magma builds up to a few 0.1% by mass (18). Such assimilation of altered rock would have had major consequences within a thick magma ocean. For example, if water built up to 0.3% by mass (or 1% by low-temperature volume), the entire modern ocean could be incorporated within a 250-km-thick magma chamber.
The modern ridge does not provide much information on CO2 assimilation because only minor amounts of it are present within the modern ocean and only relatively shallow rocks are carbonatized. (From Fig. 1, carbonates are most stable at low temperatures when formed from basalt.) Still, assimilation of modest amounts of CO2 would have had significant effects on the sizes of reservoirs. For example, inclusion of 0.25% by mass CO2 to 250-km depth would incorporate the present crust plus mantle reservoir. Modern eruptions provide some calibration to the efficacy that CO2 (at present derived from the mantle) is retained (once present) in a magma even at shallow depths where it is not very soluble. (CO2 is soluble below a few 10 s of kilometers depth.) For example, the trapped CO2 in some modern submarine basalts has a high enough concentration, ≈1.5% by mass, that the rocks pop when brought onto the deck of a ship (e.g., refs. 35 and 36). This also implies that, although chemical equilibration between bubbles and lava is quick, bubbles do not necessarily escape from the liquid magma.
We present a generalized model that is also applicable to other volatiles and modern plate tectonics. We consider processes in which CO2 is vented to the surface or atmosphere to be sources and those that incorporate CO2 into the magma ocean or mantle to be sinks. The relevant sink is formation of carbonates by the alteration of shallow rocks near the ambient ocean temperature. The mass per time removed by the sink is proportional to the global rate, which new crust is formed or, more precisely, to the volume of reactable crust produced per unit time. We assume that CO2 is present in excess of that needed to carbonatize the reactable crust. The carbonatized crust is soon subducted. A fraction f of the CO2 within it is returned to the mantle and a fraction (1-f) of it is vented back to the surface. The formation of oceanic crust acts as a CO2 source that depends on the global rate that new crust is produced and on the abundance of CO2 in the source region, and hence varies with the size of the mantle or magma ocean CO2 reservoir.
A mass balance equation for the change in size of the mantle and surface reservoirs is easily obtained for this simple model. Recalling that the mantle material must be vigorously churned through the surface to lose its heat, we treat the magma ocean (or mantle) as a well-mixed reservoir. For simplicity, we ignore the CO2 that at any one time resides in the crust. With these assumptions the growth rate of the atmospheric reservoir was
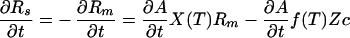 |
2a |
or
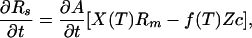 |
2b |
where t is time, Rs is the size of the surface reservoir, Rm is the size of the mantle reservoir, ∂A/∂t is the area of new crust produced per unit time, X is the ratio of the thickness Dg of degassed mantle at upwellings to the volume of the magma ocean Vm, T is mantle temperature, f(T) is the fraction of the CO2 subducted to great depths, Z is the thickness of reactable crust, and c is the number of mol of reactable divalent cations per volume in the crust. The total available CO2 reservoir is RT = Rm + Rs.
The behavior of the reservoirs is first considered as a succession of steady states because the lifetime of individual batches of crust is brief (≈1 Myr) and the amount of CO2 that can be added to a global layer of crust is a modest fraction of the total reservoirs. At equilibrium, the size of the mantle reservoir is
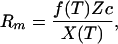 |
3 |
which is independent of the crustal production rate. This equation can be simplified somewhat by letting the amount of carbonate at any one time in the oceanic crust be Rb = ZcAE, where AE is the surface area of the Earth. The volume Vm of the magma ocean is its thickness Dm times AE. With these substitutions, the surface reservoir becomes
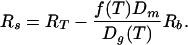 |
4 |
As the mantle cools with time, the deep subduction efficency function f increases while the source degassing depth Dg decreases. The size Rb (here 4,100 × 1018 mol) of the oceanic crustal reservoir does not change much. The variation of the effective thickness of the magma ocean (or now the mantle) is not obvious.
The steady-state size of the mantle reservoir in Eq. 3 increased as the Earth cooled because f increased and Dg decreased. Conversely, the surface reservoir Rs decreased eventually to the point that the amount predicted by Eq. 4 was negative. No steady-state solution of Eq 2 is then possible. Rather, the CO2 in the air and ocean decreased toward zero.
This completed the demise of the warm greenhouse. When temperate conditions with little CO2 in the air were reached the assumption in Eq. 2 that c was constant (all the available divalent cations reacted) broke down. Kinetics depending on the ocean/atmosphere temperature and on the amount of dissolved CO2 in the ocean became important and have remained so to the present. Mathematically, this situation can be represented by including other reservoirs for CO2, including the continental crust, and by making the amount subducted dependent on the available reactant in seawater (see ref. 37).
While the mantle remained hot enough to render subduction inefficient, the bulk of Earth's CO2 remained in the atmosphere. This phase of Earth's history may have lasted tens or even hundreds of millions of years, ending only when the upper mantle had cooled enough for subduction to be efficient. During this time Earth would have been too hot (T > 230°) for life as we know it. Owing to the limited solubility of carbonate minerals in equilibrium with basalt at 230° (note that the cation content of the seawater is not limited to the reactable 300 m of the crust because seawater is not subducted), only a small part (< 10 bars; see Table 2) of the CO2 could have been partitioned into the ocean. At the lower temperatures where thermophiles flourish the solubility is smaller still, and partitioning into the atmosphere would be stronger.
The length of time Earth spent with surface temperatures in the 60°C to 110°C range of thermophilic organisms would have been short. To maintain such temperatures required that 5–25 bars CO2 (600–2,900 × 1018 mol) were in the air (27). At this point the remaining > 90% of the CO2 were in the water ocean, or already in the magma ocean or mantle. To partition CO2 a change in the fraction in Eq. 4 by 9% would have moved the surface temperature through the range of thermophile organisms. Two characteristic time scales exist in the system. The first is the time scale for crust to be subducted or, equivalently, the time scale to approach steady state in Eq. 2. This is around a Myr for a magma ocean lasting over 100 Myr, as discussed above, and 10,000 yr for a magma ocean behaving like a fast ridge axis. All the carbonates formed from 20 bars of CO2 could fit into less than one global layer of oceanic crust and vanish into the interior over one crustal turnover time.
The second time scale involves the rate at which the magma ocean or Earth's mantle cools and the mass balances of degassing. This is the time scale implied by considering the succession of steady states implied by Eqs. 3 and 4. At the time that the surface reservoir predicted by Eq. 4 vanishes, the ratios Rb/Rm and fDm/Dg were about 1/6. In geological terms, these ratios tend to become small when the magma ocean was thick and well mixed in CO2 (large Dm), degassing at upwelling was insufficient (small Dg), and deep subduction was efficient (large f). All of these criteria involve deep processes where the details of the surface conditions play little part.
Some constraint on the time scale over which f varies is obtained from analogy to modern subduction. This is done by finding change in mantle temperature needed to sufficiently change degassing of foundered material without having to know the magma ocean temperature at which this change occurred. As with modern subduction, melts need to ascend to shallow depths to degas because CO2 is not very soluble in magma. For example, the amount of CO2 in the magma ocean discussed above (0.25% by mass) is soluble in molten rock below a depth of 15 km (figure 2 of ref. 38). Foundered material was heated by conduction from the surrounding magma ocean. When the magma ocean was hot enough, they either decomposed liberating CO2 or melted, forming a CO2-rich melt. Modern subducted crust does not usually get hot enough for either process to occur but some CO2 is extracted from the slab in hydrous flows that react with the overlying mantle to form arc volcanics (39).
Continuing with the analogy with subduction, the temperature near the surface of a foundered block is about the mean of the surface temperature and the magma ocean temperature. An equivalent statement is that the temperature in foundered blocks changes at half the rate that the magma ocean temperature changes (40, 41). Phase diagrams for idealized rock systems indicate that 100-K temperature changes in the foundered blocks, and hence 200-K differences in the magma ocean, would greatly affect carbonate stability (from 50% to 0% retention in rock) and therefore significantly change f (39, 42–44). This amount of cooling would require a few tens of Myr for the sluggish magma ocean discussed above and a few Myr for a vigorous magma ocean. The minor change in f to move the greenhouse through the temperature range of thermophiles would require no more than a few 10-K cooling of the magma ocean. Overall, this reasoning indicates that conditions favorable to thermophile organisms at the end of a massive CO2 greenhouse would have persisted somewhat less than 1 Myr with an upper limit of around 20 Myr.
It is useful to compare the behavior of CO2 with that of other elements. During the early history of the Earth, the efficacy of deep subduction and degassing (as expressed in Eqs. 3 and 4) may have controlled the crust-mantle partition of CO2. At present, there is too little CO2 in the ocean to fully carbonatize the reactable oceanic crust and much CO2 is sequestered in continental carbonates. The modern mantle can hold (over long times) only a certain mass of water because hydration of oceanic crust is limited by the amount of available rock, as assumed in Eq. 4, and not strongly dependent on the amount of water in the ocean. Eq. 4 also applies to 36Ar as a trivial example where only degassing occurs.
Early Ocean Chemistry
We used the modern midoceanic ridge axis to show the water-rock reactions produced a NaCl brine grossly similar to modern seawater. However, an ocean saturated with sodium carbonate or bicarbonate minerals in analogy to modern rift valley lakes has been considered a model for the ancient Earth (45, 46). Given that there is a lot of Na in basalt and potentially a lot of available CO2 the hypothesis seems plausible. Sodium carbonate species persist if the available divalent cations already have formed carbonates. Sodium carbonate once formed is expected to build up in surface reservoirs, as it cannot be deeply subducted. It is the first significant material to melt if subduction occurs. Pure Na2CO3 melts at a low temperature, 850°C. Modern carbonatite lavas, which form by liquid immiscibility from cooling silicate magmas, are a more useful analog. These lavas erupt at temperatures below 600°C (47).
However, consideration of chemical equilibrium with basalt shows that sodium carbonates cannot form in a global ocean on the Earth. The partial pressure of CO2 needed to form them is far greater than that which can be produced by the available CO2 (Fig. 1). The mass balance reason for this is that an assemblage with albite NaAlSi3O8 plus a more aluminum-rich Na silicate form when basalt is altered. Soluble sodium silicates and hence sodium carbonate minerals can form only if the atomic Na:Al is greater than 1. The mantle ratio on the Earth is about 1:5 (table 13 of ref. 20) and voluminous mantle-derived volcanic rocks are moderately enriched in sodium, with ratios between the mantle value and 1:3 (see ref. 48 for numerous tabulated analyses). Sodium-rich igneous rocks do occur but they are rare on a global basis. That rock-water reactions limited the build-up of Na in the ocean on the early Earth was a feature of classical models of volatiles and rock weathering (16).
Evaporation and the different mobility of Na and Al in water contribute to the local occurrence of alkaline lakes (but not the ocean) on the Earth. Sodium is leached from rocks over a broad region and then concentrated while its associated Al stays behind. Evaporation, however, does nothing to a global ocean composition because the water rains out elsewhere. The basaltic rocks, the most abundant reactants, remain in contact with the water.
A sodium carbonate or bicarbonate ocean cannot be ruled out within outer solar system satellites, like Europa. These bodies formed form the condensation of rock and ice at temperatures far below that where terrestrial planets formed. Carbon dioxide is expected to be an abundant component of ice. Rock-forming elements, including Na and Al, are not volatile at these conditions and are expected to have solar atomic ratios, about 5.7:8.5 in this case (table 1 of ref. 20). Igneous processes, which concentrated Na relative to Al by moderate amounts, could bring the global ratio in reactable crust above 1:1. There is thus far no direct evidence as to whether this happened.
Conclusions and Evolutionary Implications
We have investigated the climate on the earliest Earth to see how long conditions around 100°C persisted as the surface and interior cooled after the moon-forming impact. Only gross constraints such as conservation of energy in the planetary interior, chemical equilibrium between volatiles and rock, and heat transfer through a massive CO2 atmosphere were considered to obtain the essence of the result without obscuration by detail. More precise constraints would be nice, but at present would not modify the shopping list for biologists interested in the origin and earliest evolution of life.
The answer is that the time was geologically brief but not instantaneous. The available internal heat within the Earth can maintain ≈100°C surface conditions for at most a couple of Myr under contrived circumstances and more likely for much less than a Myr. The lifetime of a massive CO2 greenhouse also is determined by internal conditions in the planet, mainly the relative efficiencies with which CO2 is vented to the surface and with which it is subducted into the deep interior. CO2 reacts with basalt to form carbonates under reasonable greenhouse conditions and the greenhouse gradually evolves to lower temperatures as the deep interior cools, making the latter a progressively more effective CO2 reservoir. Surface temperatures around 100°C might be maintained in this way for at most 20 Myr, with a best estimate on the order of 1 Myr. Rock-water reactions within the seafloor maintained earthlike ocean chemistry.
The biological implications of a brief but finite period with a surface near 100°C are evident. Thermophilic life might have originated during that epoch (2), but a climate in the range preferred by thermophile organisms did not exist for most of the early history of the Earth. This leaves other hypotheses on the table for a tree of life rooting in a thermophilic ancestor, as hydrothermal environments have existed throughout the rest of geologic time. For example, subsurface high-temperature organisms are the likely survivors of an ocean boiling asteroid impact that otherwise sterilized the planet (5, 49, 50).
Supplementary Material
Table 1.
Idealized mineral formulas
Acknowledgments
This topic arose from discussions with Norman Pace. We thank the National Aeronautics and Space Administration's exobiology and astrobiology programs and the National Science Foundation (Grant EAR-0000743) for support. P.S.N. was supported by Grant ACS-PRF 31742-AC2 from the Petroleum Research Fund of the American Chemical Society and by a graduate fellowship from the U.S. Environmental Protection Agency.
Abbreviation
- Myr
million years
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 27, 1999.
References
- 1.Cameron A G W. Icarus. 1997;126:126–137. [Google Scholar]
- 2.Pace N R. Cell. 1991;65:531–533. doi: 10.1016/0092-8674(91)90082-a. [DOI] [PubMed] [Google Scholar]
- 3.Shock E L, Schulte M D. J Geophys Res. 1998;103:28513–28527. [Google Scholar]
- 4.Sleep N H, Zahnle K, Kasting J F, Morowitz H. Nature (London) 1989;342:139–142. doi: 10.1038/342139a0. [DOI] [PubMed] [Google Scholar]
- 5.Sleep N H, Zahnle K. J Geophys Res. 1998;103:28529–28544. [Google Scholar]
- 6.Wilde S A, Valley J W, Peck W H, Graham C M. Nature (London) 2001;409:175–178. doi: 10.1038/35051550. [DOI] [PubMed] [Google Scholar]
- 7.Mojzsis S T, Harrison T M, Pidgeon R T. Nature (London) 2001;409:178–181. doi: 10.1038/35051557. [DOI] [PubMed] [Google Scholar]
- 8.Melosh H J, Vickery A M. Nature (London) 1989;338:487–489. doi: 10.1038/338487a0. [DOI] [PubMed] [Google Scholar]
- 9.Melosh H J. In: Origin of the Earth. Newsom H E, Jones J H, editors. Oxford: Oxford Univ. Press; 1990. pp. 69–98. [Google Scholar]
- 10.Abe Y. Phys Earth Planet Int. 1997;100:27–39. [Google Scholar]
- 11.Zahnle K, Sleep N H. In: Comets and the Origin and Evolution of Life. Thomas P J, Chyba C F, McKay C P, editors. New York: Springer; 1996. pp. 175–208. [Google Scholar]
- 12.Nisbet E U, Cheadle M J, Arndt N T, Bickle M J. Lithos. 1993;30:291–307. [Google Scholar]
- 13.Kasting J F. Icarus. 1988;74:472–494. doi: 10.1016/0019-1035(88)90116-9. [DOI] [PubMed] [Google Scholar]
- 14.Abbott D L, Burgess L, Longhi J, Smith W H F. J Geophys Res. 1994;99:13835–13850. [Google Scholar]
- 15.Turcotte D L, Schubert G. Geodynamics: Application of Continuum Physics to Geological Problems. New York: Wiley; 1982. [Google Scholar]
- 16.Lafon G M, Mackenzie F T. In: Studies of Paleo-oceanography. Hay W W, editor. Tulsa, OK: Soc. Econ. Paleontologists and Mineralogists; 1974. , Spec. Pub. 20, pp. 205–218. [Google Scholar]
- 17.Bischoff J L. Am J Sci. 1991;291:309–338. [Google Scholar]
- 18.Michael P J, Schilling J G. Geochim Cosmochim Acta. 1989;53:3131–3143. [Google Scholar]
- 19.Fegley B, Jr, Treiman A H. Venus and Mars: Atmospheres, Ionospheres, and Solar Wind Interactions. Washington, DC: Am. Geophys. Union; 1992. , Geophysical Monograph 66, pp. 7–71. [Google Scholar]
- 20.Newsom H E. In: Global Earth Physics: A Handbook of Physical Constants, AGU Reference Shelf 1. Ahrens T J, editor. Washington, DC: Am. Geophys. Union; 1995. pp. 159–189. [Google Scholar]
- 21.Kent G M, Harding A J, Orcutt J A, Detrick R S, Mutter J C, Buhl P. J Geophys Res. 1994;99:9097–9116. [Google Scholar]
- 22.Kojitani H, Akaogi M. Geophys Res Lett. 1995;22:2329–2332. [Google Scholar]
- 23.Schultz A, Elderfield H. Philos Trans R Soc London A. 1997;355:387–425. [Google Scholar]
- 24.Schultz A, Elderfield H. In: Mid-Ocean Ridges: Dynamics of Processes Associated with Creation of New Oceanic Crust. Cann J R, Elderfield H, Laughton A, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 171–209. [Google Scholar]
- 25.Blichert-Toft J, Gleason J D, Telouk P, Albarede F. Earth Planet Sci Lett. 1999;173:25–39. [Google Scholar]
- 26.Zhang Y, Zindler A. Earth Planet Sci Lett. 1993;117:331–345. [Google Scholar]
- 27.Kasting J F, Ackerman T P. Science. 1986;234:1383–1385. doi: 10.1126/science.11539665. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J W, Oelkers E H, Helgeson H C. Comput Geosci. 1992;18:899–947. [Google Scholar]
- 29.Saccocia P J, Seyfried W J., Jr Am Mineral. 1993;78:607–611. [Google Scholar]
- 30.Neuhoff P S. Ph.D. dissertation. Stanford, CA: Stanford University; 2000. [Google Scholar]
- 31.Robie R A, Hemingway B S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures. Washington, DC: U.S. Geological Survey; 1995. , Bulletin B2131. [Google Scholar]
- 32.Hooft E E E, Schouten H, Detrick R S. Earth Planet Sci Lett. 1996;142:289–309. [Google Scholar]
- 33.Carlson R L. J Geophys Res. 1998;103:7069–7077. [Google Scholar]
- 34.Michael P J, Cornell W C. J Geophys Res. 1998;103:18325–18356. [Google Scholar]
- 35.Javoy M, Pineau F. Earth Planet Sci Lett. 1991;107:598–611. [Google Scholar]
- 36.Pinneau F, Javoy M. Earth Planet Sci Lett. 1994;123:179–198. [Google Scholar]
- 37.Sleep N H, Zahnle K. J Geophys Res. 2001;106:1373–1399. [Google Scholar]
- 38.Kuramoto K, Matsui T. J Geophys Res. 1996;101:14909–14932. [Google Scholar]
- 39.Huang W-L, Wyllie P J, Nehru C E. Am Mineral. 1980;65:285–301. [Google Scholar]
- 40.Peacock S M, Rusher T, Thompson A B. Earth Planet Sci Lett. 1994;121:227–244. [Google Scholar]
- 41.Peacock S M. In: Subduction Top to Bottom, Geophysical Monograph 96. Bebout G E, Scholl D W, Kirby S H, Platt J P, editors. Washington, DC: Am. Geophys. Union; 1996. pp. 19–36. [Google Scholar]
- 42.Luth R W. In: Mantle Petrology: Field Observations and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd. Fei Y, Bertka C M, Mysen B O, editors. Houston, TX: Geochem. Soc.; 1999. , Spec. Pub. 6, pp. 297–316. [Google Scholar]
- 43.Olafsson M, Eggler D H. Earth Planet Sci Lett. 1983;64:305–315. [Google Scholar]
- 44.Kerrick D M, Connolly J A D. Geology. 1998;26:375–378. [Google Scholar]
- 45.Kempe S, Degens E T. Chem Geol. 1985;53:95–108. [Google Scholar]
- 46.Kempe S, Kazmierczak J. Bull l'Institut Océanographique, Monoco. 1994;13:61–117. [Google Scholar]
- 47.Dawson J B. In: Carbonatites Genesis and Evolution. Bell K, editor. London: Unwin Hyman; 1989. pp. 255–277. [Google Scholar]
- 48.Basaltic Volume Study Project. Basaltic Volcanism on the Terrestrial Planets. New York: Pergamon; 1981. [Google Scholar]
- 49.Gogarten-Boekels M, Hilario E, Gogarten J P. J P Origin Life Evol Biosphere. 1995;25:251–264. doi: 10.1007/BF01581588. [DOI] [PubMed] [Google Scholar]
- 50.Stevens T O. In: The Microbiology of the Terrestrial Deep Subsurface. Amy P S, Haldeman D L, editors. Boca Raton, FL: CRC; 1997. pp. 202–222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.