Abstract
Caloric restriction enhances N-methyl-D-aspartate (NMDA) receptor binding and upregulates messenger RNA expression of the GluN1 subunit during aging. Old growth hormone receptor knockout mice resemble old calorically restricted rodents in enhanced life span and brain function, as compared with aged controls. This study examined whether aged growth hormone receptor knockout mice also show enhanced expression of NMDA receptors. Six or 23- to 24-month-old male normal-sized control or dwarf growth hormone receptor knockout mice were assayed for NMDA-displaceable [3H]glutamate binding (autoradiography) and GluN1 subunit messenger RNA (in situ hybridization). There was slight sparing of NMDA receptor binding densities within aged medial prefrontal and motor cortices, similar to caloric restriction, but there were greater age-related declines in GluN1 messenger RNA in growth hormone receptor knockout versus control mice. These results suggest that some of the functional improvements in aged mice with altered growth hormone signaling may be due to enhancement of NMDA receptors, but not through the upregulation of messenger RNA for the GluN1 subunit.
Keywords: NMDA, GluN1, Laron mice, Prefrontal cortex, Hippocampus
DECLINES in brain functions during aging, including memory, strength, sensation, balance, and motor coordination, affect almost half of the human population over 65 years of age (1). Memory is one of the earliest of the cognitive functions to show declines during aging (2), and deficits are seen in aged humans and nonhuman primates (see reviews 3,4), dogs (5), and rodents (6–9). Humans and rodents both exhibit deficits in spatial memory during aging (7–17). Spatial memory normally involves brain regions such as the hippocampal formation and temporal and prefrontal cortices (18–23). Normal aging is also associated with a decline in memory retention for inhibitory avoidance (24–26). This task engages the prefrontal cortex, specifically the anterior cingulate and insular cortices, and the hippocampal formation (27–29). Age-related alterations in posture and movement are also a major problem for the elderly, resulting in injury and death (1,30,31). Skilled and purposeful movements are controlled by the motor cortices (1). Analyses of age-related changes in the cerebral cortex and hippocampal formation may provide clues for some of the important physiological changes seen during aging.
The N-methyl-D-aspartate (NMDA) receptors, a subtype of glutamate receptor, are found in the highest densities in the cerebral cortex and hippocampal formation (32). This receptor is particularly important in neuronal plasticity (33). NMDA antagonists impair formation of long-term memory, including spatial memory (34–37), and block the initiation of long-term potentiation in the hippocampal formation (37–39) and cerebral cortex (40). NMDA receptors also play a role in inhibitory avoidance memory (41–43). In addition, pharmacological and genetic manipulations of the NMDA receptor indicate that it is important for motor coordination (44–46). Thus, the NMDA receptor may be important in age-related declines in these functions.
Aging animals exhibit declines in NMDA receptor binding densities and functions. NMDA-stimulated release of transmitters is decreased with increasing age (47,48). Long-term potentiation is also altered in aged rodents (49,50). Age-related declines in binding of glutamate and/or [(±)-2-carboxypiperazin-4-yl] propyl-1-phosphonic acid to NMDA binding sites have been noted in multiple regions of the cerebral cortex and hippocampus in mice, rats, dogs, and monkeys (51–56). Humans also exhibit declines with age in [3H]MK801 binding to the NMDA receptor complex in the frontal cortex (57). This age-related decrease in NMDA binding site density within the frontal cortex (58) and hippocampal formation (54,58) of rodents has been correlated with poor performance in long-term spatial memory tasks, such as the Morris water maze. In addition, age-related reductions in binding of [3H]MK801 in the channel of the NMDA receptor are positively correlated with deficits in memory retention for inhibitory (passive) avoidance (26).
Some of the age-related changes in the NMDA receptor appear to be due to alterations in the expression of receptor subunits (for review, see 59). The GluN1 (previous nomenclature: mouse, ζ1; other species, NR1, 60) subunit has the same distribution as NMDA-displaceable [3H]glutamate binding (61–63). The effects of aging on the GluN1 subunit are variable between studies and species (59). Significant reductions during aging in the GluN1 subunit messenger RNA (mRNA) and/or protein have been seen in the cerebral cortex and/or hippocampal formation of C57BL/6 mice (55,64–66) and the hippocampal formation of Fischer 344 and Fischer 344 × Brown Norway F1 rats (67–71) and aged macaque monkeys (72). However, there are other studies using C57BL/6 mice (73–75) or Fischer 344 × Brown Norway F1, Wistar or Long–Evans rats that show no age-related change in the expression of the GluN1 subunit in frontal cortex and/or hippocampal formation (76–78). Correlation studies, regardless of whether or not there are age-related changes, suggest that GluN1 subunit expression in aged individuals contributes to spatial memory performance (66,73,76,79). The variability in the effects of aging may indicate that the changes that are observed in the expression of the GluN1 subunit are not due to a programmed decline that occurs across species, but rather are the result of environmental factors and experiences during aging.
In support of this, exposure to a behavioral testing experience can not only lead to an increase in the expression of mRNA for the GluN1-a splice variant in the prefrontal cortex of aged mice only but also exacerbate the age-related decline in the GluN1-3 splice variant (73). Calorie restriction, to 60% of ad-libitum intake beginning at 3 months of age, resulted in the maintenance of slightly higher levels of [3H]glutamate binding to the NMDA site in regions of the cerebral cortex and hippocampus in older C57Bl/6 mice (80,81). This effect in frontal cortical and hippocampal regions is associated with improved spatial memory performance in the water maze (58,81). Caloric restriction can also lead to significant increases in mRNA expression of the GluN1 subunit in medial and lateral frontal cortex in middle-aged and old mice, as compared with ad-libitum fed animals (81). Fischer 344 rats also show a positive effect of caloric restriction on GluN1 subunit protein expression in the aged hippocampal formation (68). Because caloric restriction is not a very practical intervention for human aging, it is important to understand the mechanisms underlying its effects.
In addition to positive effects on memory performance (82), including spatial (81,83,84) and passive avoidance (85,86) tasks, caloric restriction leads to extensions of the median and maximum life spans, reduction of adult body size, a delay in puberty, a reduction in fertility, and reduced plasma insulin-like growth factor-1 and insulin levels (87). Several lines of dwarf mice exhibit these same characteristics (88–90). Laron dwarf (growth hormone receptor knockout [GHRKO]) mice have a targeted disruption of the mouse growth hormone receptor or binding protein gene (90). In addition to the similarities to calorie restriction mentioned above, old GHRKO mice also show improved spatial memory, retention of inhibitory avoidance information, and locomotion relative to age-matched control siblings (24,91).
The present study was designed to determine whether alterations in the growth hormone signaling pathway could produce positive effects on NMDA receptor binding and GluN1 mRNA expression during aging, similar to those previously seen with caloric restriction in mice (81,92). In horizontal sections, the region identified as frontal cortex in the calorie restriction studies could have included insular and primary and secondary motor and somatosensory cortices (81,92). These rostral cerebral cortical regions were analyzed separately in the present study. The region labeled cingulate cortex in the calorie restriction study was located medially and could have included infralimbic, prelimbic, and cingulate cortices (81). This combined region will be referred to as medial prefrontal in the current study. Dorsal and intermediate hippocampal formations were analyzed separately because differences occur in the effects of aging between these regions in C57BL/6 mice (74).
METHODS
Animals and Tissue Handling
Male GHRKO mice and their control littermates were supplied by the breeding colony at Southern Illinois University. The colony was established by crossing 129Ola and BALB/c N (GHR+/–) animals with mice derived from crosses of C57BL/6 and C3H/J strains and has been maintained on this heterogeneous genetic background with minimal inbreeding by avoiding Brother × Sister matings. A heterogeneous strain background was chosen in order to reduce the likelihood of obtaining results that may be unique to only one inbred strain and to more closely resemble a natural population. The knockout dwarf (GHR–/–; GHRKO) and heterozygous, normal-sized (GHR+/–; control) mice used in the present study were obtained by crossing knockout (GHR–/–) males with heterozygous (GHR+/–) females. As littermates, the GHRKO and control mice were matched for both genetic background and environmental effects (e.g., intrauterine environment and maternal care). The knockout mice were differentiated from the controls based on phenotypic differences, that is, reduced body size, weight, and length, which have been previously described for GHRKO mice (89,90). Heterozygous mice are phenotypically similar to wild-type (GHR+/+) mice in body weight, body length, serum GH and insulin-like growth factor-1, and life span (89,90).
There were two age groups (6 and 23–24 months of age) and two genotypes (GHRKO and control) used in this study. The N for each group was 4 for 6-month-old controls, 7 for 6-month-old GHRKOs, and 5 for both the 23- to 24-month-old GHRKO and control mice. Animals were housed under 12:12 hour light:dark cycles, with controlled temperature (20–23°C) and access to pelletized food and water ad libitum at Southern Illinois University. Mice were anesthetized with pentobarbital (100 mg/kg intraperitoneally) and decapitated. The brains were quickly removed, frozen on dry ice, and shipped to the Magnusson laboratory. Coronal 20-μm sections were obtained through prefrontal, motor, and somatosensory cortices (between Bregma +2.22 and –0.94 mm, 93) and the dorsal hippocampal formation (from septal half of hippocampal formation, between Bregma –1.22 and –2.54 mm, 93) and horizontal 20-μm sections were obtained through the intermediate hippocampal formation (located between septal half and temporal quarter of hippocampal formation; approximately between 2.5 and 4 mm deep to the skull, 93) with the use of a Microm HM500 cryostat (Zeiss, Thornwood, NY). Sections were cold-mounted onto gelatin-coated glass slides. Due to technical difficulties, the CA1 and CA3 regions were missing from many of the intermediate hippocampal sections, so those regions were not analyzed. Each slide contained a section from each age and genotype group. The placement order of the groups on the slides was varied between assays to decrease variability due to slide handling.
NMDA-Displaceable [3H]glutamate Binding Assay
Binding was performed as previously described (55) with a modification in the incubation time. Slides were preincubated in cold (4°C) 50-mM Tris acetate buffer (pH 7.0) for 30 min, followed by 2 × 10 min incubations in cold Tris acetate buffer (4°C, pH 7.0). Sections were then incubated in a solution of 100-nM [3H]L-glutamate (New England Nuclear, Boston, MA), 1-μM kainate, 5-μM α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and 100-μM 4-acetamido-4’-isothiocyanotostilbene-2,2’-disulfonic acid for 45 min at 4°C. Slides were rinsed four times in Tris acetate buffer (4°C) for a total of 30 seconds and dried by a stream of compressed air at room temperature. Unlabeled 200-μM NMDA was added to the incubation solution for determining nonspecific binding.
Slides were allowed to dry overnight and were apposed to tritium sensitive film, Hyperfilm-3H (Amersham, Piscataway, NJ), along with tritium standards (Amersham) at –20°C for 2 months. Exposed films were developed in D-19 developer (Kodak, Rochester, NY). Brain and standard images were captured using a Macintosh G4 computer with a Powerlook 2100XL scanner (UMAX, Taiwan) and NIH Image software. Quantitative densitometry was performed on the images from four sections for total binding and two sections for nonspecific binding from each animal with the use of NIH Image software. Tritium standards were used to convert optical density measurements to femtomole per milligram protein for each brain region analyzed. Specific binding was determined by subtracting nonspecific binding from total binding.
In Situ Hybridization—GluN1 Subunit mRNA
In situ hybridization was performed as previously described (55). Oligonucleotides were commercially prepared (Macromolecular Resources, Colorado State University, Fort Collins, CO) for the GluN1 subunit (Probe sequence: 5’ GCACAGCGGGCCTGGT-TCTGGGTTGCGCGAGCGCGACCACCTCGC; complimentary to nucleotide residues –54 to –10). Probes were tailed with 33P-dATP (ICN, Costa Mesa, CA) using terminal deoxyribonucleotidyl transferase (New England Nuclear, Boston, MA) and separated from unbound label with Microspin G-25 columns (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). In situ hybridization was performed according to the method of Watanabe and coworkers (94). Each solution step was performed with gentle rotation, except for the fixation and hybridization steps. Slides were thawed, air dried, fixed in 4% paraformaldehyde in phosphate-buffered saline (pH 7.2, 25°C) for 15 minutes, placed in 2 mg/ml glycine in phosphate-buffered saline (pH 7.2, 25°C) for 20 minutes, and acylated in freshly prepared 0.25% acetic anhydride in 0.1-M triethanolamine (pH 8.0, 25°C) for 10 minutes. Slides were blocked for 2 hours in a prehybridization solution, which consisted of 50% formamide, 0.1-M Tris–HCl (pH 7.5), 4× saline and sodium citrate solution (SSC; 1× SSC = 150-mM NaCl and 15-mM sodium citrate), 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin, 2% sarkosyl, and 250 μg/ml salmon testes DNA. Chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Slides were then successively washed for 5 minutes each in 2× SSC and 70% and 100% ethanol and air dried for 15 minutes. Hybridization was performed by placing 150 μl of prehybridization solution plus 10% dextran sulfate and 2 picomoles 33P-labeled oligonucleotide probe per milliliter of prehybridization solution onto the slides, covering the sections with Parafilm (American National Can, Greenwich, CT) and incubating them for 18 hours in a 42°C oven humidified with 5× SSC. Parafilm was removed, and slides were rinsed for 40 minutes in 2× SSC and 0.1% sarkosyl (25°C) and twice for 40 minutes each in 0.1× SSC and 0.1% sarkosyl (55°C) and then air dried. Nonspecific hybridization was determined by addition of 20-fold excess unlabeled oligonucleotide to the hybridization solution on some slides. Sections were exposed to Kodak Biomax film (Eastman Kodak Co., Rochester, NY) for 3 days, along with 14C standards (American Radiolabeled Chemicals, Inc., St. Louis, MO). Brain images were captured and analyzed as described for receptor binding. The standards were used to convert optical density to picomoles 33P per milligram tissue. Specific signal was determined by subtracting nonspecific from total hybridization.
Statistical Analysis
The binding and mRNA data were normalized between groups, based on the 6-month-old control mice, as previously described (80), in order to decrease variability due to film apposition. Age-related differences in the densities of mRNA and receptor binding were analyzed separately for rostral cortex, dorsal hippocampal formation, and intermediate dentate gyrus by repeated-measures analysis of variane (Age × Genotype × Brain Region) followed by Fisher's protected least significant difference with the use of Statview software (SAS Institute, Inc., Cary, NC). The analyses of individual brain regions and within genotype age differences were part of the original experimental plan.
RESULTS
NMDA Receptor Binding
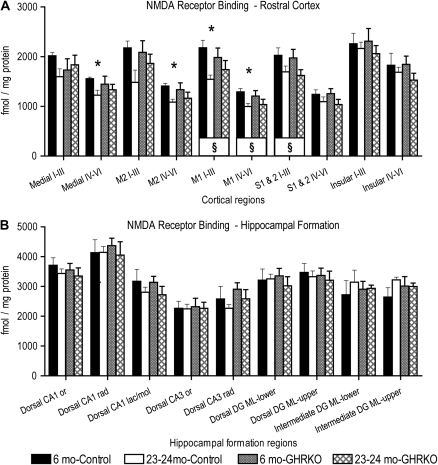
There was a near significant main effect of Age, F(1,17) = 3.4, p = .08, but no significant main effect of Genotype, F(1,17) = .003, p = .96, on NMDA-displaceable [3H]glutamate binding overall within the rostral cerebral cortical regions analyzed. There was, however, a significant interaction between Age, Genotype, and Brain Region, F(9,153) = 2.3, p = .02, on NMDA-displaceable [3H]glutamate binding within the cortical regions (Figures 1A–D, N and 2A). There was a significant main effect of Age, when data were collapsed across Genotype, on NMDA-displaceable [3H]glutamate binding in layers I–III of the primary motor (p = .02) and combined primary and secondary somatosensory (p = .04) cortices and layers IV–VI of the primary motor cortex (p = .03); 6-month-old mice exhibited higher binding densities than 23- to 24-month olds (Figure 2A). There were significant decreases in binding densities of NMDA-displaceable [3H]glutamate between 6 and 23–24 months of age in control mice in layers IV–VI of the medial prefrontal (p = .02), secondary motor (p = .006), and primary motor (p = .01) cortices and layers I–III of the primary motor cortex (p = .006; Figures 1A, B, N and 2A). In contrast, no significant effects of Age were noted in GHRKO mice in the cortical regions examined (Figures 1C, D, N and 2A).
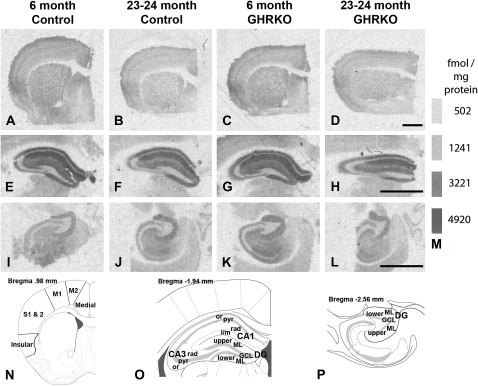
Figure 1.
Receptor autoradiography of [3H]glutamate binding to N-methyl-D-aspartate (NMDA) receptor binding sites. (A–L) Representative film images of [3H]glutamate binding to NMDA binding sites in frontal (A–D), dorsal hippocampal (E–H), and intermediate hippocampal (I–L) regions from 6-month-old control mice (normal-sized heterozygotes; A, E, I), 23- to 24-month-old control mice (B, F, J), 6-month-old growth hormone receptor knockout (GHRKO) mice (dwarfs; C, G, K), and 23- to 24-month-old GHRKO mice (D, H, L). (M) Standard bars showing femtomole per milligram protein equivalents for given gray levels. (N–P) Example diagrams (adapted from Franklin & Paxinos's Mouse Brain Atlas, 2007, 93) of subregions analyzed for the frontal cortex (N), dorsal hippocampus (O), and intermediate hippocampus (P) for receptor binding, in situ hybridization, or both. Millimeters (mm) in the upper left corner of each diagram indicate that sections are rostral (N), caudal (O), or ventral (P) to Bregma (point on the skull where the saggital suture intersects the coronal [bregmatic] suture). Medial, medial prefrontal cortex; M2, secondary motor cortex; M1, primary motor cortex; S1 & S2, primary and secondary somatosensory cortices; Insular, granular and agranular insular cortex; CA, cornu ammonis; or, stratum oriens; pyr, stratum pyramidale; rad, stratum radiatum; l/m, stratum lacunosum/moleculare; DG, dentate gyrus; ML, molecular layer; GCL, granule cell layer; upper, upper blade; and lower, lower blade. Bars = 1 millimeter.
Figure 2.
Effects of age and presence or absence of the growth hormone receptor on N-methyl-D-aspartate (NMDA)-displaceable [3H]-glutamate binding in the rostral cerebral cortex and hippocampal formation. Graphs showing binding densities for NMDA-displaceable [3H]-glutamate binding in femtomole per milligram protein within specific regions of the rostral cerebral cortex (A) and hippocampal formation (B) in two different ages of control (normal-sized heterozygotes) and growth hormone receptor knockout (GHRKO; dwarf) mice. §: p <.05 for differences between 6 and 23- to 24-month-old mice, when data were collapsed across genotypes (symbol located in box at base of bars). *: p <.05 for difference from 6-month-old control mice. Repeated-measures analysis of variance and Fisher's protected least significant difference post hoc tests. N = 4 for 6-month-old control, 6–7 for 6-month-old GHRKO, and 5 each for the 23- to 24-month-old groups for rostral cortex and dorsal hippocampal formation. N = 2 for 6-month olds and 3 for 23- to 24-month olds for both genotypes for intermediate hippocampal formation. Roman numerals indicate cortical layers. Medial, medial prefrontal cortex; M2, secondary motor cortex; M1, primary motor cortex; S1 & S2, primary and secondary somatosensory cortices; CA, cornu ammonis; or, stratum oriens; rad, stratum radiatum; lac/mol, stratum lacunosum/moleculare; DG ML, dentate gyrus molecular layer; upper, upper blade; lower, lower blade; and mo, months of age.
There was no significant main effect of Age, F(1,15) = .09, p = .77, or Genotype, F(1,15) = .19, p = .67, on NMDA-displaceable [3H]glutamate binding overall within the dorsal hippocampal formation (Figures 1E–H, O and 2B). There was also no significant main effect of Age, F(1,6) = .01, p = .92, or Genotype, F(1,6) = .92, p = .37, on NMDA-displaceable [3H]glutamate binding overall within the intermediate dentate gyrus (Figures 1I–L, P and 2B [far right]). No individual regions in the hippocampal formation showed any significant effects of Age or Genotype (p range = .14 to .96; Figure 2B). The N for the intermediate CA1 and CA3 regions was insufficient to report.
GluN1 mRNA
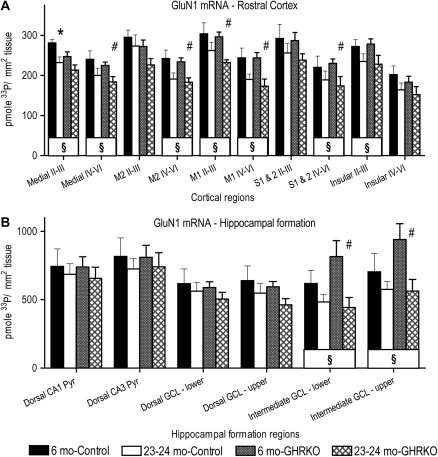
There was a significant main effect of Age, F(1,17) = 11.1, p = .004, but no main effect of Genotype, F (1,17) = 1.1, p = .3, on mRNA density for the GluN1 subunit of the NMDA receptor within the rostral cerebral cortex (Figures 3A–D and 4A). There was a significant main effect of Age on the density of mRNA for the GluN1 subunit in cortical layers II–III of the medial prefrontal (p = .01), insular (p = .02), and primary motor (p = .006) cortices and in layers IV–VI of the medial prefrontal (p = .02), secondary motor (p = .002), primary motor (p = .001), and combined primary and secondary somatosensory (p = .04) cortices; 23- to 24-month-old mice had lower mRNA densities than the 6-month-old mice when the data were collapsed across genotypes (Figure 4A). The 23- to 24-month-old control mice had significantly lower densities of mRNA for the GluN1 subunit in layers II–III of the medial prefrontal cortex, as compared with 6-month-old control mice (p = .03; Figures 3A,B and 4A). The 23- to 24-month-old GHRKO mice had significantly less mRNA density for the GluN1 subunit than the 6-month-old GHRKO mice in layers II–III of the primary motor cortex (p = .002) and in layers IV–VI of the medial prefrontal (p = .02), secondary motor (p = .006), primary motor (p = .007), and combined primary and secondary somatosensory (p = .04) cortices (Figures 3C, D and 4A).
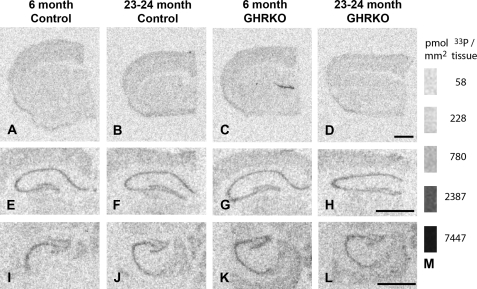
Figure 3.
In situ hybridization for the GluN1 subunit of the N-methyl-D-aspartate (NMDA) receptor. (A–L) Representative film images of 33P-dATP–labeled complementary DNA probes hybridized to messenger RNA for the GluN1 subunit of the NMDA receptor in frontal cortical (A–D), dorsal hippocampal (E–H), and intermediate hippocampal (I–L) regions from 6-month-old control mice (normal-sized heterozygotes; A, E, I), 23- to 24-month-old control mice (B, F, J), 6-month-old growth hormone receptor knockout (GHRKO) mice (dwarfs; C, G, K), and 23- to 24-month-old GHRKO mice (D, H, L). (M) Standard bars showing picomole 33P per square millimeter tissue equivalents for given gray levels. See Figure 1N–P for diagrams of regions analyzed. Bars = 1 mm.
Figure 4.
Effects of age and presence or absence of the growth hormone receptor on messenger RNA (mRNA) density for the GluN1 subunit of the N-methyl-D-aspartate receptor in the rostral cerebral cortex and hippocampal formation. Graphs showing mRNA densities for the GluN1 subunit, expressed as picomole 33P per square millimeter tissue, within rostral cerebral cortex (A) and the hippocampal formation (B) in two different ages of control (normal-sized heterozygotes) and growth hormone receptor knockout (GHRKO; dwarf) mice. §: p <.05 for differences between 6 and 23- to 24-month-old mice, when data were collapsed across genotypes (symbol located in box at base of bars). *: p <.05 for difference from 6-month-old control mice. #: p <.05 for difference from 6-month-old GHRKO mice. Repeated-measures analysis of variance and Fisher's protected least significant difference post hoc tests. N = 4 for 6-month-old control, 6–7 for 6-month-old GHRKO, and 5 each for the 23- to 24-month-old groups for rostral cortical regions and dorsal hippocampal formation. N = 3 for both 6-month-old groups, 5 for control, and 4 for GHRKO 23- to 24-month olds in the intermediate dentate gyrus. Roman numerals indicate cortical layers. Medial, medial prefrontal cortex; M2, secondary motor cortex; M1, primary motor cortex; S1 & S2, primary and secondary somatosensory cortices; CA, cornu ammonis; Pyr, pyramidal cell layer; GCL, granule cell layer; lower, lower blade; upper, upper blade; and mo, months of age.
There was no significant main effect of Age, F(1,16) = 1.2, p = .29, or Genotype, F(1,16) = .15, p = .70, on the mRNA density of the GluN1 subunit overall within the dorsal hippocampal formation or within the regions of the dorsal hippocampal formation (p range = .09 to .96; Figures 3E–H and 4B). There was a significant main effect of Age, F(1,11) = 9, p = .01, but no main effect of Genotype, F(1,11) = 1.3, p = .3, on GluN1 mRNA density in the upper and lower blades of the intermediate dentate gyrus (Figures 3I–L and 4B). The 6-month-old mice had higher mRNA densities for GluN1 than 23- to 24-month-old mice overall in the intermediate dentate gyrus (Figure 3I–L) and separately within the lower and upper blades of the intermediate dentate gyrus (Figure 4B), both when data were collapsed across genotypes and when the GHRKO mice were analyzed separately. The N for the intermediate CA1 and CA3 regions was insufficient to report.
DISCUSSION
There were three main findings from this study: (i) there were age-related changes in NMDA receptor binding density in motor and somatosensory cortices and in GluN1 subunit expression in many rostral cortical regions and intermediate dentate gyrus, when both GHRKO and control mice were averaged together; (ii) there was a slight sparing of NMDA receptor binding density within medial prefrontal and motor cortices of aged GHRKO mice; (iii) there was a greater age-related decline in the GluN1 mRNA expression in GHRKO mice in all of the same regions that showed the sparing in NMDA receptor binding density, as compared with controls.
Three out of ten rostral cortical regions showed a significant decline in NMDA-displaceable [3H]glutamate binding between 6 and 24 months of age in these mice, regardless of knockout-related genotype. Primary motor and somatosensory cortices appeared to be more susceptible to aging than prefrontal regions across both genotypes. An age-related decrease in NMDA binding site densities has also been demonstrated within the cortex and/or hippocampal formation of C57Bl/6 and BALB/c mice (55,80,95,96), Fischer 344 (52,53,97–101, but see 102), Long–Evans (54), Wistar (103), and Sprague–Dawley (104) rats, dogs (56), and rhesus monkeys (51). Declines in the binding within the channel of the NMDA receptor have also been described in the frontal cortex of humans during aging (57). The lack of age-related changes in glutamate binding within the hippocampal formation regions is consistent with a lesser effect of aging on NMDA binding sites in these regions as compared with the cerebral cortex in C57BL/6 mice (81,92). The mice in the current study, from a heterogeneous strain background, thus showed similar age-related changes in NMDA receptor binding density to many other strains and species. The heterogeneity in the effects of aging on NMDA receptor binding between different brain regions seen in the present study has also been documented in C57BL/6 mice (59,74).
There was a slight sparing of NMDA-displaceable [3H]glutamate binding in the old GHRKO dwarf mice (9–17% better maintenance of binding density across aging), as compared with control, normal-sized mice, within the deep layers of the medial prefrontal and secondary motor cortices and in all layers of the primary motor cortex. In these regions, normal-sized aged mice showed a significant decline in binding density (21–30%), as compared with the normal-sized young mice. The decreases in binding between young and old GHRKO mice (8–14% of young GHRKO binding) did not reach significance in the same regions. It is possible that the differences in percent of age-related decline in NMDA receptor binding would have been greater in magnitude if the GHRKO mice had been compared with wild-type mice, rather than heterozygotes. Caloric restriction also produced a similar pattern with respect to significant changes in horizontal sections of the rostral cerebral cortex; ad libitum-fed mice showed significant aging changes (13–22% reduction from young ad libitum-fed) but calorie-restricted mice did not (5–14% reduction from young ad libitum-fed; 58,81). Caloric restriction in aged mice led to 3–15% better maintenance of NMDA receptor binding densities within rostral cortex across aging, as compared with the old ad libitum-fed mice (58,81). Thus, alteration of the growth hormone signaling pathways led to a similar pattern to caloric restriction within rostral cortical regions with respect to improvement in glutamate binding to NMDA receptors in aged animals, both in the degree of reduction of the age-related change, as compared with old controls, and the percent decrease from binding densities found in the young of the same genotype.
Seven out of 10 of the cortical regions and both blades of the intermediate dentate gyrus showed a significant decline in mRNA expression of the GluN1 subunit of the NMDA receptor during aging when both GHRKO and control mice were considered together. The only regions that overlapped with the NMDA receptor binding changes across genotype were all layers of the primary motor cortex. Antagonism of NMDA receptors with MK801 can produce motor ataxias (105), so it is possible that receptor declines in motor cortex may contribute to movement problems in aged individuals (106). Age-related declines in GluN1 mRNA have also been seen within frontal cortex of C57BL/6 mice (55,65), but other studies with this same strain showed no aging changes in the mRNA for this subunit (73–75). Fischer 344 and Fischer 344 × Brown Norway F1 rats show declines in protein expression of the GluN1 subunit in the hippocampal formation during aging (67–71). Wistar and Long–Evans rats, however, show no age-related change in the expression of the GluN1 subunit in hippocampal formation (76,77). A decrease in the protein expression of the GluN1 subunit protein is also observed in the distal dendrites of the dentate granule cells in aged macaque monkeys, as compared with young adults (72). The mice in the current study, with a heterogeneous strain background, showed a strong effect of the aging process on mRNA expression of the GluN1 subunit of the NMDA receptor.
More significant effects of aging were seen in the intermediate dentate gyrus, as compared to the dorsal, regardless of knockout genotype. This pattern is also seen in the hippocampal formation of C57BL/6 mice with NMDA receptor binding and GluN2B subunit mRNA (74). Rat studies also show no significant effect of aging on NMDA receptor binding densities in the dorsal hippocampal formation (53,107). It is not known why NMDA receptors in the intermediate hippocampal formation should be more susceptible to aging than the dorsal. It is also not clear exactly what the functional consequences of this change are. Declines in NMDA receptor binding in the intermediate hippocampus of C57BL/6 mice show a significant correlation to spatial memory deficits during aging (58). NMDA receptors are important for the acquisition of spatial memories (8,108,109), but the dorsal hippocampal formation is sufficient for acquiring spatial learning (110). The intermediate hippocampal formation, along with the dorsal, is involved in retrieval of spatial memories (111), but NMDA receptors are not involved in retrieval, at least not in young animals (8,108,109). The role of NMDA receptors within the aged intermediate hippocampus in memory remains to be elucidated.
The deep layers of medial prefrontal, secondary motor, and somatosensory cortices and the whole thickness of the primary motor cortex exhibited a significant decrease in GluN1 mRNA in the GHRKO mice between young and old, but the changes were not significant in the control mice. Four of these five regions are the same regions that showed more significant age-related changes in NMDA receptor binding density in control mice than the GHRKO mice; the opposite pattern. The strain of Wistar-Kyoto rats show markedly higher binding of [3H]MK801 to NMDA receptors than the Wistar strain of rats (112) and C57BL/6 mice show higher surface expression of another NMDA receptor subunit, GluN2B, than the 129S6/SvEv strain (113). Thus, it is possible that the heterogeneous strain background, combined with a small N, may have produced variability between individuals with respect to the NMDA receptor that interfered with detecting significant differences in both binding and mRNA within the same regions within the present study. The fact that there appear to be differences in the magnitude of the change between binding and mRNA densities within the knockout and control groups, however, suggests that variability was not the major issue. Although C57Bl/6 and FVB/N mice do not show differences in basal expression of glutamate receptor subunits, they do show strain differences in the change in receptor expression following manipulation with kainate injections (114). It is possible that the manipulation of the GH receptor on a heterogeneous strain background may have differentially affected some of the knockouts with respect to the NMDA receptor.
There still remains the fact that within each genotype group, the age-related changes in NMDA receptor binding differed in magnitude from the changes in GluN1 mRNA (i.e., in the knockouts, when GluN1 mRNA decreased significantly with increased age, the NMDA-displaceable glutamate binding was not significantly different across ages and vice versa in the controls). Several possibilities might explain this opposing relationship: (i) the decline in GluN1 subunit expression could preferentially affect certain splice variants that could lead to an increase in agonist affinity for the receptor (115) in GHRKO mice, (ii) mRNA expression could be inversely related to the protein expression of the GluN1 subunit, or (iii) there may be other associated changes in the GluN2 family of NMDA receptor subunits that could have influenced either the number or the affinity of glutamate binding sites.
Growth hormone treatments in normal Sprague–Dawley rats lead to declines in GluN1 subunit mRNA in young rats and increases in middle-aged rats (116). In Brown Norway rats, growth hormone treatment enhances microvascular density and spatial working and reference memory (117). Aged Fischer 344 × Brown Norway F1 rats, however, show no influence of insulin-like growth factor-1 on GluN1 subunit expression, despite improvements in GluN2A and GluN2B subunits (78). These results suggest that growth hormone and its downstream factors can be beneficial during aging in rodents, but do not consistently act on the GluN1 subunit. The use of GH in aged humans is not currently recommended by the Growth Hormone Research Society, but they do encourage further study in model systems, such as the GHRKO mouse (118).
The enhanced decline in the GluN1 subunit mRNA within some regions of the rostral cerebral cortex in GHRKO mice compared with normal-sized control mice is the opposite of the effect of caloric restriction on this subunit mRNA. Within both medial and lateral rostral cortex, caloric restriction in middle-aged and old C57BL/6 mice resulted in an increase in GluN1 subunit mRNA as compared with middle-aged, old, and/or young ad libitum-fed mice (81). However, it is possible that behavioral testing experience is necessary to induce the increase (81). The mRNA for the GluN1-a splice variants was upregulated following a behavioral testing experience in the water maze (73).
Rodents undergoing caloric restriction resemble the GHRKO mice in many different ways. Both have increased life spans, decreased insulin-like growth factor-1 plasma expression, and show similar pathologies (119). In addition, caloric restriction was unable to further extend life span in the GHRKO mice, as it did in the Ames dwarf mice (120–123), suggesting that there is a shared mechanism. Although caloric restriction and GHRKO mice show many phenotypic similarities, there are many differences being discovered in the gene expression profiles that are altered by caloric restriction in control mice versus GHRKO mice (120,124,125). There are also differences in stress tolerance in fibroblasts and Akt phosphorylation in cardiac muscle cells between the diet intervention and the knockout mice (126,127). One major shared cellular mechanism between GHRKO and normal calorie-restricted mice appears to be increased insulin sensitivity (120,121,123).
Insulin can enhance NMDA receptor activity (128–130), at least in part via phosphorylation of the GluN2A and GluN2B subunits (128). The similar degree of sparing of NMDA receptor binding density in GHRKO mice, as compared with calorie-restricted aged mice, suggest that altered insulin sensitivity may have had some effect on NMDA receptor expression. The opposite effects of the two interventions on the mRNA expression of the GluN1 subunit suggest that it is unlikely that the effects of increased insulin sensitivity alone account for the GluN1 subunit expression enhancements of caloric restriction. As mentioned above, it may require an interaction between increased insulin sensitivity and behavioral experience to enhance GluN1 subunit expression.
The life span of C57BL/6 mice is extended by caloric restriction to a greater extent than DBA/2 mice (131,132). These two strains also show differences in metabolic and oxidative stress markers following caloric restriction (133,134). Although the DBA/2 strain was not part of the background of the heterogeneous strain mice, the differences seen in GluN1 subunit expression between caloric restriction in C57BL/6 and ad libitum-fed GHRKO mice could also be due to strain differences.
Aged GHRKO mice show enhanced spatial and inhibitory avoidance memory and better locomotor function, as compared with normal-sized mice (24,91). The small effect of the GHRKO genotype on NMDA-displaceable glutamate binding might suggest that the memory and motor improvements are unlikely to be related to NMDA receptors. However, the higher NMDA agonist binding that was seen in old, calorically restricted C57BL/6 mice showed a significant association with better reference memory with a similar degree of sparing of receptor binding density (58,81). It remains to be seen if the changes seen here in the NMDA receptor are associated with the memory and/or locomotor improvements in aged GHRKO mice. It is also possible that the reduction in GluN1 mRNA in the GHRKO mice might enhance memory performance in old mice. There is increasing evidence of a negative relationship between this subunit and memory within groups of old mice (59,79). Additional examples, involving antioxidant therapy, high blood pressure, and obesity, also suggest that not all changes that occur during aging are detrimental to health and longevity (135). The best learners among old Fischer 344 rats appear to be able to upregulate some transmitter or signaling molecules in the face of age-related declines in others (136). Therefore, it is possible that the improved function in the aged GHRKO mice might be due to an enhanced ability to compensate for other declines.
In conclusion, there was a slight sparing of NMDA receptor density in GHRKO mice, similar to that seen with caloric restriction in C57BL/6 mice. This suggests that the enhanced memory and locomotion of the old GHRKO mice may be due, at least in part, to an enhancement of NMDA receptors. However, in contrast to caloric restriction, there was a significant decline in mRNA expression of the major subunit, GluN1. These results suggest that alterations in growth hormone signaling pathways may enhance the expression of NMDA receptors in old age, but not through the upregulation of mRNA expression for the GluN1 subunit.
FUNDING
National Institute on Aging at the National Institutes of Health (AG016332 to K.R.M., AG019899 to A.B., and AG021215 to P.R.P.).
Acknowledgments
We would like to acknowledge the technical expertise of Xue Zhao in helping to conduct these experiments.
References
- 1.Timiras PS. The nervous system: functional changes. In: Timiras PS, editor. Physiological Basis of Aging and Geriatrics. 3rd ed. Boca Raton, FL: CRC Press; 2003. pp. 119–140. [Google Scholar]
- 2.Albert MS, Funkenstein HH. The effects of age: normal variation and its relation to disease. In: Asbury AK, McKhann GM, McDonald WI, editors. Diseases of the Nervous System: Clinical Neurobiology. 2nd ed. Philadelphia, PA: Saunders; 1992. pp. 598–611. [Google Scholar]
- 3.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Ann Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 5.Head E, Mehta R, Hartley J, et al. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- 6.Zyzak DR, Otto T, Eichenbaum H, Gallagher M. Cognitive decline associated with normal aging in rats: a neuropsychological approach. Learn Mem. 1995;2:1–16. doi: 10.1101/lm.2.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- 8.Gage F, Dunnett S, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 9.Rapp P, Rosenberg R, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Evans GWP, Brennan PLM, Skorpanich MAM, Held DB. Cognitive mapping and elderly adults: verbal and location memory for urban landmarks. J Gerontol. 1984;39:452–457. doi: 10.1093/geronj/39.4.452. [DOI] [PubMed] [Google Scholar]
- 11.Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older animals. Psychol Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- 12.Kirasic KC, Bernicki MR. Acquisition of spatial knowledge under conditions of temporospatial discontunity in young and elderly adults. Psychol Res. 1990;52:76–79. doi: 10.1007/BF00867215. [DOI] [PubMed] [Google Scholar]
- 13.Moore TE, Richards B, Hood J. Aging and the coding of spatial information. J Gerontol. 1984;39:210–212. doi: 10.1093/geronj/39.2.210. [DOI] [PubMed] [Google Scholar]
- 14.Sharps MJ, Gollin ES. Memory for object locations in young and elderly animals. J Gerontol. 1987;42:336–341. doi: 10.1093/geronj/42.3.336. [DOI] [PubMed] [Google Scholar]
- 15.Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- 16.Pelleymounter MA, Smith M, Gallagher M. Spatial learning impairments in aged rats trained with a salient configuration of stimuli. Psychobiology. 1987;15:248–254. [Google Scholar]
- 17.Magnusson KR, Scruggs B, Aniya J, et al. Age-related deficits in mice performing working memory tasks in a water maze. Behav Neurosci. 2003;117:485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Kandel ER, Kupfermann I, Iversen S. Learning and memory. In: Kandel ER, SChwartz J, Jessel T, editors. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000. pp. 1227–1246. [Google Scholar]
- 19.Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex. 1994;6:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- 20.Miotto EC, Bullock P, Polkey CE, Morris RG. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex. 1996;32:613–630. doi: 10.1016/s0010-9452(96)80034-7. [DOI] [PubMed] [Google Scholar]
- 21.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place-navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 22.Olton DS. Memory functions and the hippocampus. In: Seifert W, editor. Neurobiology of the Hippocampus. London: Academic Press; 1983. pp. 335–373. [Google Scholar]
- 23.Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- 24.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 25.Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- 26.Scheuer K, Stoll S, Paschke U, Weigel R, Muller WE. N-methyl-D-aspartate receptor density and membrane fluidity as possible determinants of the decline of passive avoidance performance in aging. Pharmacol Biochem Behav. 1995;50:65–70. doi: 10.1016/0091-3057(94)00254-g. [DOI] [PubMed] [Google Scholar]
- 27.Azami NS, Piri M, Oryan S, Jahanshahi M, Babapour V, Zarrindast MR. Involvement of dorsal hippocampal alpha-adrenergic receptors in the effect of scopolamine on memory retrieval in inhibitory avoidance task. Neurobiol Learn Mem. 2010;93:455–462. doi: 10.1016/j.nlm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learn Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Zheng XL, Li BM. The anterior cingulate cortex is involved in retrieval of long-term/long-lasting but not short-term memory for step-through inhibitory avoidance in rats. Neurosci Lett. 2009;460:175–179. doi: 10.1016/j.neulet.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12:155–162. doi: 10.1080/03610738608259454. [DOI] [PubMed] [Google Scholar]
- 32.Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. 2nd ed. Oxford: Oxford University Press; 1994. pp. 340–375. [Google Scholar]
- 34.Alessandri B, Battig K, Welzl H. Effects of ketamine on tunnel maze and water maze performance in the rat. Behav Neural Biol. 1989;52:194–212. doi: 10.1016/s0163-1047(89)90313-0. [DOI] [PubMed] [Google Scholar]
- 35.Heale V, Harley C. MK801 and AP5 impair acquisition, but not retention, of the Morris milk maze. Pharmacol Biochem Behav. 1990;36:145–149. doi: 10.1016/0091-3057(90)90140-d. [DOI] [PubMed] [Google Scholar]
- 36.Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE. NMDA receptor antagonists can enhance or impair learning performance in animals. Exp Brain Res. 1989;75:449–456. doi: 10.1007/BF00249896. [DOI] [PubMed] [Google Scholar]
- 37.Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 38.Bashir Z, Alford S, Davies S, Randall A, Collingridge G. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- 39.Harris EW, Ganong A, Cotman CW. Long-term potentiation in the hippocampus involves activation in N-methyl-D-aspartate receptors. Brain Res. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- 40.Artola A, Singer W. NMDA receptors and developmental plasticity in visual neocortex. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. 2nd ed. Oxford: Oxford University Press; 1994. pp. 313–339. [Google Scholar]
- 41.Berlese DB, Sauzem PD, Carati MC, et al. Time-dependent modulation of inhibitory avoidance memory by spermidine in rats. Neurobiol Learn Mem. 2005;83:48–53. doi: 10.1016/j.nlm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Memory consolidation induces N-methyl-D-aspartic acid-receptor- and Ca2+/calmodulin-dependent protein kinase II-dependent modifications in alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor properties. Neuroscience. 2005;136:397–403. doi: 10.1016/j.neuroscience.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Roesler R, Vianna MR, Schroder N, Ferreira MB, Quevedo J. Aversive learning under different training conditions: effects of NMDA receptor blockade in area CA1 of the hippocampus. Neurochem Res. 2006;31:679–683. doi: 10.1007/s11064-006-9066-2. [DOI] [PubMed] [Google Scholar]
- 44.Carter AJ. Many agents that antagonize the NMDA receptor-channel complex in vivo also cause disturbances of motor coordination. J Pharmacol Exp Ther. 1994;269:573–580. [PubMed] [Google Scholar]
- 45.Kadotani H, Hirano T, Masugi M, et al. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintero GC, Erzurumlu RS, Vaccarino AL. Evaluation of morphine analgesia and motor coordination in mice following cortex-specific knockout of the N-methyl-D-aspartate NR1-subunit. Neurosci Lett. 2008;437:55–58. doi: 10.1016/j.neulet.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pittaluga A, Fedele E, Risiglione C, Raiteri M. Age-related decrease of the NMDA receptor-mediated noradrenaline release in rat hippocampus and partial restoration by D-cycloserine. Eur J Pharm. 1993;231:129–134. doi: 10.1016/0014-2999(93)90693-c. [DOI] [PubMed] [Google Scholar]
- 48.Gonzales RA, Brown LM, Jones TW, Trent RD, Westbrook SL, Leslie SW. N-methyl-D-aspartate mediated responses decrease with age in Fischer 344 rat brain. Neurobiol Aging. 1991;12:219–225. doi: 10.1016/0197-4580(91)90100-x. [DOI] [PubMed] [Google Scholar]
- 49.Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- 50.Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- 51.Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging. 1991;12:93–98. doi: 10.1016/0197-4580(91)90047-n. [DOI] [PubMed] [Google Scholar]
- 52.Tamaru M, Yoneda Y, Ogita K, Shimizu J, Nagata Y. Age-related decreases of the N-methyl-D-aspartate receptor complex in the rat cerebral cortex and hippocampus. Brain Res. 1991;542:83–90. doi: 10.1016/0006-8993(91)91001-h. [DOI] [PubMed] [Google Scholar]
- 53.Kito S, Miyoshi R, Nomoto T. Influence of age on NMDA receptor complex in rat brain studied in in vitro autoradiography. J Histochem Cytochem. 1990;38:1725–1731. doi: 10.1177/38.12.2147708. [DOI] [PubMed] [Google Scholar]
- 54.Pelleymounter MA, Beatty G, Gallagher M. Hippocampal 3H-CPP binding and spatial learning deficits in aged rats. Psychobiology. 1990;18:298–304. [Google Scholar]
- 55.Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magnusson KR, Scanga C, Wagner AE, Dunlop C. Changes in anesthetic sensitivity and glutamate receptors in the aging canine brain. J Gerontol A Biol Sci Med Sci. 2000;55:B448–454. doi: 10.1093/gerona/55.9.b448. [DOI] [PubMed] [Google Scholar]
- 57.Piggott MA, Perry EK, Perry RH, Court JA. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992;588:277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- 58.Magnusson KR. Aging of glutamate receptors: correlations between receptor binding and spatial memory performance in C57Bl mice. Mech Ageing Dev. 1998;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 59.Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00011. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meguro H, Mori H, Araki K, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 62.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 63.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 64.Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- 65.Magnusson KR, Bai L, Zhao X. The effects of aging on different C-terminal splice forms of the zeta1(NR1) subunit of the N-methyl-d-aspartate receptor in mice. Brain Res Mol Brain Res. 2005;135:141–149. doi: 10.1016/j.molbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8:43. doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coultrap SJ, Bickford PC, Browning MD. Blueberry-enriched diet ameliorates age-related declines in NMDA receptor-dependent LTP. Age. 2008;30:263–272. doi: 10.1007/s11357-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Mol Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 69.Newton IG, Forbes ME. Linville MC, et al. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging. 2007;29:1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi L, Adams MM, Linville MC, et al. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mesches MH, Gemma C, Veng LM, et al. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 72.Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci U S A. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das SR, Magnusson KR. Relationship between mRNA expression of splice forms of the zeta1 subunit of the N-methyl-D-aspartate receptor and spatial memory in aged mice. Brain Res. 2008;1207:142–154. doi: 10.1016/j.brainres.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magnusson KR, Kresge D, Supon J. Differential effects of aging on NMDA receptors in the intermediate versus the dorsal hippocampus. Neurobiol Aging. 2006;27:324–333. doi: 10.1016/j.neurobiolaging.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Ontl T, Xing Y, Bai L, et al. Development and aging of N-methyl-D-aspartate receptor expression in the prefrontal/frontal cortex of mice. Neuroscience. 2004;123:467–479. doi: 10.1016/j.neuroscience.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Adams MM, Smith TD, Moga D, et al. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- 77.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Sonntag WE, Bennett SA, Khan AS, et al. Age and insulin-like growth factor-1 modulate N-methyl-D-asparate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, Rosenke R, Kronemann D, et al. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–945. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magnusson KR. Influence of dietary restriction on ionotropic glutamate receptors during aging in C57Bl mice. Mech Ageing Dev. 1997;95:187–202. doi: 10.1016/s0047-6374(97)01884-8. [DOI] [PubMed] [Google Scholar]
- 81.Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol Aging. 2001;22:613–627. doi: 10.1016/s0197-4580(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 82.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Algeri S, Biagini L, Manfridi A, Pitsikas N. Age-related ability of rats kept on a life-long hypocaloric diet in a spatial memory test. Longitudinal observations. Neurobiol Aging. 1991;12:277–282. doi: 10.1016/0197-4580(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 84.Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi R, Komiya Y, Goto S. Effect of dietary restriction on learning and memory impairment and histologic alterations of brain stem in senescence-accelerated mouse (SAM) P8 strain. Ann N Y Acad Sci. 2006;1067:388–393. doi: 10.1196/annals.1354.055. [DOI] [PubMed] [Google Scholar]
- 86.Komatsu T, Chiba T, Yamaza H, et al. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol. 2008;43:339–346. doi: 10.1016/j.exger.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Masoro EJ. Dietary restriction and aging. J Am Geriatr Soc. 1993;41:994–999. doi: 10.1111/j.1532-5415.1993.tb06767.x. [DOI] [PubMed] [Google Scholar]
- 88.Bartke A, Coschigano K, Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci. 2001;56:B340–349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- 89.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80:589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 92.Magnusson KR. Influence of dietary restriction on ionotropic glutamate receptors during aging in C57B1 mice. Mech Ageing Dev. 1997;95:187–202. doi: 10.1016/s0047-6374(97)01884-8. [DOI] [PubMed] [Google Scholar]
- 93.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 94.Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- 95.Magnusson KR. Differential effects of aging on binding sites of the activated NMDA receptor complex in mice. Mech Ageing Dev. 1995;84:227–243. doi: 10.1016/0047-6374(95)01658-9. [DOI] [PubMed] [Google Scholar]
- 96.Magnusson KR, Cotman CW. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol Aging. 1993;14:197–206. doi: 10.1016/0197-4580(93)90001-r. [DOI] [PubMed] [Google Scholar]
- 97.Castorina M, Ambrosini AM, Pacifici L, Ramacci MT, Angelucci L. Age-dependent loss of NMDA receptors in hippocampus, striatum, and frontal cortex of the rat: prevention by acetyl-L-carnitine. Neurochem Res. 1994;19:795–798. doi: 10.1007/BF00967446. [DOI] [PubMed] [Google Scholar]
- 98.Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behav Neurosci. 1992;106:324–335. doi: 10.1037//0735-7044.106.2.324. [DOI] [PubMed] [Google Scholar]
- 99.Davis S, Markowska AL, Wenk GL, Barnes CA. Acetyl-L-carnitine: behavioral, electrophysiological, and neurochemical effects. Neurobiol Aging. 1993;14:107–115. doi: 10.1016/0197-4580(93)90030-f. [DOI] [PubMed] [Google Scholar]
- 100.Ingram DK, Garofalo P, Spangler EL, Mantione CR, Odano I, London ED. Reduced density of NMDA receptors and increased sensitivity to dizocilpine-induced learning impairment in aged rats. Brain Res. 1992;580:273–280. doi: 10.1016/0006-8993(92)90954-8. [DOI] [PubMed] [Google Scholar]
- 101.Ogawa N, Mizukawa K, Haba K, Asanuma M, Mori A. Effects of chronic bifemelane hydrochloride administration on receptors for N-methyl-D-aspartate in the aged-rat brain. Neurochem Res. 1992;17:687–691. doi: 10.1007/BF00968006. [DOI] [PubMed] [Google Scholar]
- 102.Bonhaus DW, Perry WB, McNamara JO. Decreased density, but not number, of N-methyl-D-aspartate, glycine and phencyclidine binding sites in hippocampus of senescent rats. Brain Res. 1990;532:82–86. doi: 10.1016/0006-8993(90)91745-3. [DOI] [PubMed] [Google Scholar]
- 103.Serra M, Ghiani CA, Foddi MC, Motzo C, Biggio G. NMDA receptor function is enhanced in the hippocampus of aged rats. Neurochem Res. 1994;19:483–487. doi: 10.1007/BF00967328. [DOI] [PubMed] [Google Scholar]
- 104.Fiore L, Rampello L. L-acetylcarnitine attenuates the age-dependent decrease of NMDA-sensitive glutamate receptors in rat hippocampus. Acta Neurol (Napoli) 1989;11:346–350. [PubMed] [Google Scholar]
- 105.Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 106.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nicolle MM, Bizon JL, Gallagher M. In vitro autoradiography of ionotropic glutamate receptors in hippocampus and striatum of aged Long-Evans rats: relationship to spatial learning. Neuroscience. 1996;74:741–756. doi: 10.1016/0306-4522(96)00147-9. [DOI] [PubMed] [Google Scholar]
- 108.Morris RGM. Synaptic plasticity and learning: selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 110.Moser M-B, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moser M-B, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lei Y, Yaroslavsky I, Tejani-Butt SM. Strain differences in the distribution of N-methyl-d-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain. Life Sci. 2009;85:794–799. doi: 10.1016/j.lfs.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finn R, Kovacs AD, Pearce DA. Altered sensitivity to excitotoxic cell death and glutamate receptor expression between two commonly studied mouse strains. J Neurosci Res. 2010;88:2648–2660. doi: 10.1002/jnr.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schauwecker PE. Differences in ionotropic glutamate receptor subunit expression are not responsible for strain-dependent susceptibility to excitotoxin-induced injury. Brain Res Mol Brain Res. 2003;112:70–81. doi: 10.1016/s0169-328x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 115.Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Greves M, Steensland P, Le Greves P, Nyberg F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-D-aspartate receptor subunits gene transcripts in male rats. Proc Natl Acad Sci U S A. 2002;99:7119–7123. doi: 10.1073/pnas.092135399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thorner MO. Statement by the growth hormone research society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64A:1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- 119.Ikeno Y, Lew CM, Cortez LA, Webb CR, Lee S, Hubbard GB. Do long-lived mutant and calorie-restricted mice share common anti-aging mechanisms?–a pathological point of view. Age (Dordr) 2006;28:163–171. doi: 10.1007/s11357-006-9007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004567. e4567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol. 2002;16:2657–2666. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- 125.Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giani JF, Bonkowski MS, Munoz MC, et al. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol A Biol Sci Med Sci. 2008;63:788–797. doi: 10.1093/gerona/63.8.788. [DOI] [PubMed] [Google Scholar]
- 127.Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: influence of genes and nutrition. Mech Ageing Dev. 2006;127:687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Christie JM, Wenthold RJ, Monaghan DT. Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J Neurochem. 1999;72:1523–1528. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- 129.Liu L, Brown JC, 3rd, Webster WW, Morrisett RA, Monaghan DT. Insulin potentiates N-methyl-D-aspartate receptor activity in Xenopus oocytes and rat hippocampus. Neurosci Lett. 1995;192:5–8. doi: 10.1016/0304-3940(95)11593-l. [DOI] [PubMed] [Google Scholar]
- 130.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 131.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 132.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. Faseb J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hempenstall S, Picchio L, Mitchell SE, Speakman JR, Selman C. The impact of acute caloric restriction on the metabolic phenotype in male C57BL/6 and DBA/2 mice. Mech Ageing Dev. 2010;131:111–118. doi: 10.1016/j.mad.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Le Couteur DG, Simpson SJ. Adaptive senectitude: the prolongevity effects of aging. J Gerontol A Biol Sci Med Sci. 2010;66:179–182. doi: 10.1093/gerona/glq171. [DOI] [PubMed] [Google Scholar]
- 136.Rowe WB, Blalock EM, Chen KC, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]