Abstract
Background.
This study asked whether older adults were more likely than younger adults to err in the initial direction of their anticipatory postural adjustment (APA) prior to a step (indicating a motor program error), whether initial motor program errors accounted for reaction time differences for step initiation, and whether initial motor program errors were linked to inhibitory failure.
Methods.
In a stepping task with choice reaction time and simple reaction time conditions, we measured forces under the feet to quantify APA onset and step latency and we used body kinematics to quantify forward movement of center of mass and length of first step.
Results.
Trials with APA errors were almost three times as common for older adults as for younger adults, and they were nine times more likely in choice reaction time trials than in simple reaction time trials. In trials with APA errors, step latency was delayed, correlation between APA onset and step latency was diminished, and forward motion of the center of mass prior to the step was increased. Participants with more APA errors tended to have worse Stroop interference scores, regardless of age.
Conclusions.
The results support the hypothesis that findings of slow choice reaction time step initiation in older adults are attributable to inclusion of trials with incorrect initial motor preparation and that these errors are caused by deficits in response inhibition. By extension, the results also suggest that mixing of trials with correct and incorrect initial motor preparation might explain apparent choice reaction time slowing with age in upper limb tasks.
Keywords: Aging, CRT, Step initiation, Inhibition, Anticipatory postural adjustment
AGING is accompanied by increased reaction time (RT), particularly for choice reaction time (CRT) tasks, in which the central nervous system must inhibit incorrect responses while activating the correct response for a particular stimulus. Age-related slowing of CRT occurs across a variety of response modalities, including verbal, button pressing, arm raising, and stepping (1–3). Evidence for slowing of CRTs can also be seen in the brain with slowed readiness potentials (4,5). Age-related RT deficits increase as tasks become more complex (6). For instance, some studies have found age-related RT deficits only in dual-task situations (7). Other studies have found age-related differences in CRT tasks but not in simple reaction time (SRT) tasks (5). Still others have found that CRT performance deficits begin at a younger age than SRT deficits (8,9). The cognitive deficit responsible for greater slowing in CRT tasks than in SRT tasks in older adults is unclear.
Age-related slowing may be connected to the well-known decline that aging brings in inhibitory control (2). CRT tasks require the inhibition of the non-selected response along with the activation of the selected response (10–12). It is possible that findings of age-related slowing are due to mixing of trials in which the correct initial movement is programmed and trials in which the non-selected response is not inhibited, and therefore, the incorrect initial movement is programmed. Thus, age-related impairments of motor inhibition may underlie findings of slow CRT performance in elderly populations.
To evaluate the hypothesis that increased CRT in older adults is due to impaired inhibition of incorrect preparatory responses, we needed a task in which the preparatory control is specific to the forthcoming focal movement, so that an incorrect initial program will lead to an incorrect preparatory movement, which must be corrected before the primary movement can be executed. One example of this sort of preparatory movement is the “anticipatory postural adjustment” (APA). APAs are defined as changes in postural control associated with voluntary movements; they occur prior to the onset of the disturbance of posture and equilibrium resulting from the movement (13). Although, in a well-coordinated movement, the APA and the primary movement combine seamlessly, neurophysiological evidence suggests that they have separate neural origins (14). One activity known to reliably produce an APA is the initiation of stepping (15–18). To prepare for a step, body weight is shifted laterally, onto the stepping foot, in preparation for shifting weight onto the support leg (19). If the weight is shifted in the wrong direction, it must be corrected before the step can safely take place. APAs associated with step initiation have durations of several hundred milliseconds and are easily measured with force plates under the feet (17,20,21).
In the experiment that follows, we tested the hypothesis that overall CRT differences between younger and older adults are due to individual trials with errors in direction of initial postural preparation. Participants stepped as soon as possible after the presentation of a light cue, with the foot that was on the same side as the cue. In one block (the SRT condition), participants knew in advance that the light would always be on the same side as their dominant foot. In the other block (CRT condition), the light was equally likely to be on either the right or left side. If age-related differences in CRT performance are due to occasional errors in the initial APA direction, then age-related speed differences between older and younger adults should depend on the trials with incorrect initial APAs. Also, if impaired motor inhibition is responsible for slowed CRT in older adults, the latency of CRT should be related to response inhibition impairment, as measured by the Stroop color-word task (22).
METHODS
Participants
Two groups of participants participated in the study: 12 young healthy participants (ages 26–30, M 28) and 12 healthy older participants (ages 50–78, M 67). None of the participants had orthopedic problems, balance trouble, neurological disorders, or history of stroke. All participants gave informed consent and were paid for their participation. The study was approved by the Institutional Review Board of Oregon Health & Science University.
Task and Design
The task was to initiate walking as quickly as possible in response to a light cue, stepping off with the foot that was on the same side as the light and taking three steps before stopping. In the SRT condition, participants were informed that the light would always appear on the same side as their dominant stepping foot. In the CRT condition, participants were informed that the light was equally likely to be on the left or right side. The two conditions were blocked and counterbalanced, with every participant completing 20 trials in each condition. Between blocks, participants were invited to sit and rest for about 5 minutes.
Setup and Protocol
Participants stood with their feet on separate force platforms, at a self-determined comfortable stance width for walking. Experimenters marked participants’ foot placement with tape so that every trial would begin from the same position. Two green laser pointers were mounted on the ceiling and pointed at a low wall approximately 3 m in front of the participants, in front of the right and left foot. A line of tape was placed vertically on the wall halfway between the locations of the target lights to enhance participants’ ability to distinguish which side the light was on. An eight-camera system (Motion Analysis System, Santa Rosa, CA) gathered position data from 24 passive reflective markers on each participant's trunk, head, and limbs, sampled at 60 Hz. Before each trial, participants were instructed to stand with their weight evenly balanced and to look straight ahead. The experimenter monitored the force on each force plate on a computer screen and, when necessary, instructed the participant to shift to the left or right to achieve approximately balanced weight (with no more than 51% of weight on either foot) before starting the trial. The light cue appeared 2,200 ms after the start of the trial and remained on for 600 ms. Data collection lasted 4,000 ms.
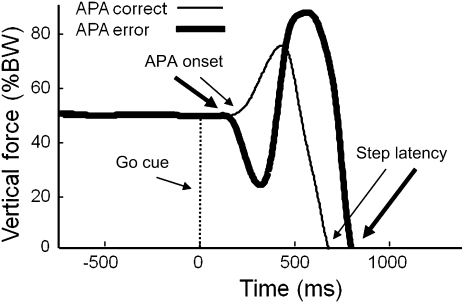
Figure 1 shows the vertical force under the stepping foot (as a percentage of body weight) for two trials with correct and incorrect initial APAs. The thin line represents a trial in which the initial APA was in the correct direction (ie, increased force under the foot to be lifted). The thick line represents a trial in which the initial APA was in the wrong direction (ie, increased force under the initial stance leg), and this delayed the step.
Figure 1.
Postural preparation prior to stepping. Vertical force as a percentage of body weight, for two example trials. Heavy line: trial with initial anticipatory postural adjustment (APA) error. Thin line: trial with correct initial APA.
To determine whole body center of mass (COM), we computed the weighted sum of the COM position of each body segment (23) based on measurements of the length, width, and circumference of 26 body segments (including the head, limbs, and trunk) and self-reported heights and weights for each participant (24).
Definitions
Step latency: the time between the appearance of the light cue and the first moment when vertical force under either foot decreased to zero. Step errors: trials in which the first step was not with the foot on the same side as the light. APA onset: the first time when the difference in vertical force under the two feet increased by 5% of body weight. APA errors: trials in which participants executed an APA in the direction associated with a step error, then corrected that APA, and stepped with the correct foot. APA amplitude: the amount of force under the more-heavily–weighted foot at the peak of the APA, expressed as a percent of body weight. Step length: the forward distance traveled by the marker on the stepping foot from when it began moving to when it came to rest on the floor. COM displacement: forward distance traveled by the COM during the time corresponding to the step latency.
Stroop Task
For additional insight into the relation between inhibitory control and CRT stepping, we asked participants to perform a Stroop color-word task (22). The Stroop task, which measures how well participants can inhibit a habitual response to a stimulus in order to respond to a less salient aspect of the stimulus, includes three conditions: reading, color naming, and conflict. For each condition, participants were given a page with 100 stimuli on it (four columns of 25 stimuli each). Words were written in 20-point font, and the page was placed on a table in front of the participant at a comfortable reading distance. The experimenter asked the participants to respond verbally to all items in order as quickly as possible, pointing to each item as they went, so the experimenter could monitor performance for errors. Stimuli for the reading condition comprised the words (black, red, green, purple, blue) written in black ink and arranged in random order. The instructions were to read the words. The stimuli for the color naming condition comprised color blocks from the set (black, red, green, purple, blue) arranged in random order. The instructions were to name the ink colors. The stimuli for the conflict condition comprised the same words as those on the first page, written in the same colors as those on the second page. Both the word order and the color order were randomized and different from the other two conditions. The instructions were to ignore the words and name the ink colors. Before every condition, participants performed a few practice trials to demonstrate that they understood the task and were able to correctly read the words and identify the colors.
Statistical Analysis
Before analyzing the data, we removed trials in which participants (a) drifted laterally so that one side had more than 52.5% of the weight during the baseline period, (b) initiated an APA sooner than 100 ms after the light cue, (c) failed to step within 2 seconds after the light cue, and (d) stepped with the wrong foot. This left 962 trials (an average of 37 trials per participant). Because many of our analyses depended on dividing trials according to the presence or absence of an APA error, we had different numbers of trials in each cell. We therefore used a linear mixed model approach to determine the effects of age group, condition, and presence or absence of APA error on step characteristics. By entering data from each trial individually into each model and using participant as a random effect, we built a model that could account for the different numbers of trials in different cells. Signal processing was performed Matlab (The Mathworks, Inc., Natick, MA). and statistics were computed with R (A language and environment for statistical computing; R Development Core Team, R Foundation for Statistical Computing; Vienna, Austria). Alpha was set at .05 for all tests.
The primary analysis, to determine the effects of age and condition on step latency, APA onset, and APA errors, used a linear mixed model, with age group (younger vs older) and condition (SRT vs CRT) as fixed factors and participant as a random factor. APA errors occurred in 83 trials (24 among 12 of the 13 young participants and 59 among 12 of the 13 older participant) for the combined CRT and SRT conditions.
The secondary analysis determined how errors in APA selection affected APA onset, step latency, step length, and COM displacement, also using a linear mixed model with age group and APA error (present or absent) as fixed factors and participant as random factor.
We did not include condition in these secondary models because there were only eight trials with APA errors in the SRT condition. To determine the effect of APA errors on APA amplitude, we applied a linear mixed model analysis with age group and APA error as fixed factors and participant as a random factor. We used this approach to compare amplitudes of APAs in trials with no error to amplitudes of first (erroneous) and last (correct) APAs in trials with APA errors.
To determine the effect of mixing trials with and without APA errors on the apparent coupling between the APA and the step, we computed within-subject correlations between APA onset and step latency as our dependent variable for all data, and we computed it again for data without APA errors.
RESULTS
Influence of Group and RT Condition on Response Times and Errors
There were 20 trials for which no step was recorded during the four-second recording period, and 10 trials in which participants stepped with the wrong foot. These trials were not analyzed. There were also 48 trials with weight shifting greater than 5% of body weight during the baseline period. Because an early weight shift (possibly indicating that participants anticipated the cue) could affect the size of the APA, these trials were removed before proceeding with data processing, leaving 962 trials for most of the analyses. Markers going out of sight of the camera led to the loss of eight additional trials from the COM data and an additional 65 trials from the step length data.
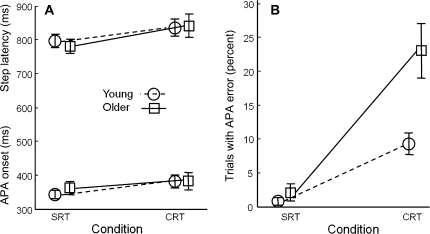
Figure 2 shows the mean step latency, APA onset, and APA errors, for younger and older participants in SRT and CRT conditions. Inferential statistics are reported in Table 1. As can be seen in Figure 2A, response times were shorter in the SRT condition than in the CRT condition for both step latency, F(1,934) = 45.5, p < .0001, and APA onset, F(1,934) = 27.2, p < .0001. Age group had no effect on either of these variables nor did it interact with condition. APA errors (initial weight shifts in the wrong direction) are shown in Figure 2B. There were almost no APA errors in the SRT condition in either age group, but in the CRT condition, older adults made almost three times as many APA errors (23%) as did younger adults (8%). The effect of age group was significant, F(1,24) = 21.9, p < .001, as was the effect of condition, F(1,934) = 66.7, p < .001, and the interaction, F(1, 934) = 17.1, p < .0001.
Figure 2.
Effect of trial condition and group on step preparation. Circles connected with dashed lines: mean values from 13 young adults. Squares connected with solid lines: mean values from 13 older adults. (A) Step latency (upper lines) and anticipatory postural adjustment (APA) onset (lower lines). (B) Trials with initial APA errors. Error bars represent standard errors across participant means.
Table 1.
Effects of Condition and Age Group
| Condition |
Age Group |
Interaction |
|||||||
| df | F | p | df | F | p | df | F | p | |
| Step latency | 1, 934 | 45.5 | <.0001** | 1, 24 | <0.1 | .88 | 1, 934 | 0.7 | .39 |
| APA onset | 1, 934 | 27.2 | <.0001** | 1, 24 | 0.2 | .67 | 1, 934 | 3.3 | .07 |
| APA errors | 1, 934 | 66.7 | <.0001** | 1, 24 | 21.9 | <.001** | 1, 934 | 17.1 | <.0001** |
Notes: Degrees of freedom, F statistic, and p value for step latency, APA onset, and APA errors, respectively, as a function of condition (simple reaction time or choice reaction time) and age group (young vs older). A linear mixed model was applied, with condition and age group as fixed factors and participant as random factor. Thirteen participants in each group were included. APA = anticipatory postural adjustment.
**Indicates p < .001.
Influence of APA Errors on Step Latency
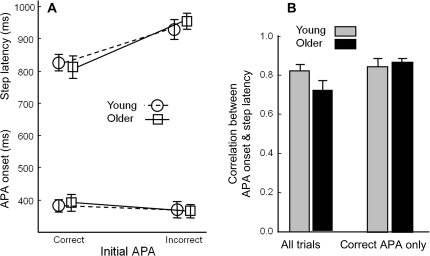
We next considered the influence of APA errors on the timing of stepping. In Table 2 and Figure 3, trials are divided according to whether an APA error occurred or not. In each group, 12 of the 13 participants had at least one trial with an APA error. Figure 3A shows the timing of APA onset and step latency. Neither group nor APA error had a significant effect on the APA onset. However, step latency was delayed in trials with APA errors relative to trials with correct initial APAs, F(1,934) = 125.3, p < .0001.
Table 2.
Effects of APA Errors and Age Group
| APA Errors |
Age Group |
Interaction |
|||||||
| df | F | p | df | F | p | df | F | p | |
| Step latency | 1, 934 | 125.3 | <.0001** | 1, 24 | <0.1 | .87 | 1, 934 | 0.1 | .90 |
| APA onset | 1, 934 | 0.2 | .75 | 1, 24 | 0.2 | .66 | 1, 934 | 2.3 | .13 |
| APA–step correlation | 1, 22 | 16.4 | <.001** | 1, 24 | 0.6 | .45 | 1, 22 | 7.3 | .01 * |
| Step length | 1, 861 | 0.3 | .60 | 1, 24 | 5.1 | .03* | 1, 861 | <0.1 | .95 |
| ΔCOM/height | 1, 926 | 73.0 | <.0001** | 1, 24 | 9.0 | .006* | 1, 926 | <0.1 | .85 |
| ΔCOM/step length | 1, 861 | 85.8 | <.0001** | 1, 24 | <0.1 | 0.9 | 1, 858 | 1.6 | .21 |
Notes: Degrees of freedom, F statistic, and p value for step latency, APA onset, APA-step correlation, step length, and center of mass displacement, respectively, as a function of APA error (present or absent) and age group (young vs older). A linear mixed model was applied, with APA error and age group as fixed factors and participant as random factor. Thirteen participants in each group were included, with 12 participants in each group contributing trials with APA errors. APA = anticipatory postural adjustment; COM = center of mass.
*Indicates statistical significance (p < .05).
**Indicates p < .005.
Figure 3.
Effect of anticipatory postural adjustment (APA) errors on timing of step initiation. (A) Step latency (upper lines) and APA onset (lower lines). Circles connected with dashed lines: mean values from 13 young adults’ trials with correct APAs and 12 of the same 13 adults’ trials with APA errors. Squares connected with solid lines: mean values from 13 older adults’ trials with correct APAs and 12 of the same 13 adults’ trials with APA errors. (B) Within-subject correlations between APA onset and step latency. Error bars represent standard errors across participant means.
The within-subject correlation between APA onset and step latency is a measure of the consistency of the APA–step coupling. Figure 3B shows the influence of APA errors on these correlations. When all trials were included, the average correlation between APA onset and step latency was .82 for the younger adults but only.73 for older adults. However, when only trials with correct initial APAs were included in the analysis, the correlations were greater and more similar (means of .85 and .86, respectively). The effect of including or excluding trials with multiple APAs in the correlation was significant, F(1,22) = 16.4, p < .001. There was no main effect of age, but there was an interaction, such that the inclusion of trials with APA errors made a larger difference for the older adults than for the younger adults, F(1,22) = 7.3, p = .01.
Influence of APA Errors on Movement
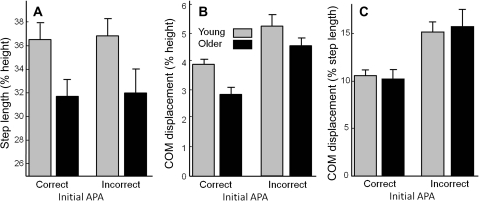
Figure 4 illustrates the effect of age and initial APAs on step length and COM movement. Figure 4A shows the step length, as a percentage of participant height. Younger adults took larger initial steps than older adults did, F(1,24) = 5.1, p = .03. Initial APA errors did not affect step length.
Figure 4.
Effect of anticipatory postural adjustment (APA) error on step length and forward displacement of center of mass (COM) prior to step. (A) Step length, normalized by participant height. (B) COM displacement, normalized by participant height. (C) COM displacement, normalized by step length. Data plotted are mean values from trials with correct APAs from 13 participants in each group and mean values from trials with APA errors from 12 of the same 13 participants in each group. Error bars represent standard errors across participant means.
Figure 4B shows the forward distance traveled by the COM between the go cue and the step latency, as a percentage of participant height. Young adults leaned forward farther than older adults, F(1,24) = 9.0, p = .006. All participants leaned forward farther during trials with APA errors than in trials with correct initial APA, F(1,926) = 72.9, p < .0001.
Figure 4C shows the forward distance traveled by the COM between the go cue and the step latency, normalized by the length of the resulting step. The COM traveled farther forward before the step in trials with APA errors than in trials with correct initial APAs, F(1,861) = 85.8, p < .0001.
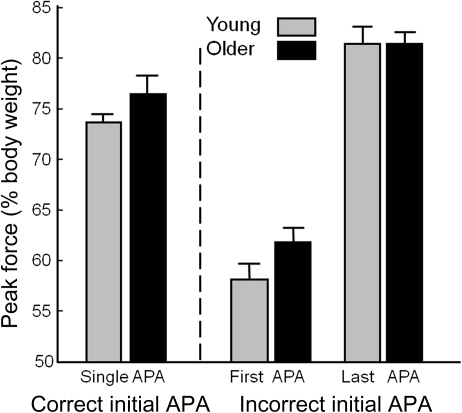
Figure 5 shows the effect of APA errors on APA amplitude. The leftmost pair of bars shows the peak vertical force under the stepping foot in trials with a correct APA. The middle pair shows the force generated in the first APA when it would later be corrected, and the rightmost pair shows the force generated in the final APA after a correction. In trials with an initial APA error, the first APA was about 50% smaller than in single-APA trials, F(1,934) = 861.9, p < .0001, and the last weight shift was slightly larger than in single-APA trials, F(1,934) = 117.2, p < .0001. There was no APA amplitude difference between older and younger adults. There was, however, an interaction, indicating that the first APA in trials with APA errors (i.e., the APA in the wrong direction) was smaller in young adults than in older adults, F(1,934) = 12.5, p < .001.
Figure 5.
Effect of anticipatory postural adjustment (APA) error on APA amplitude. First two bars: peak vertical force associated with a correct initial APA. Middle two bars: peak vertical force associated with an APA error. Last two bars: peak vertical force associated with a correction to an APA error. Data plotted are mean values from trials with correct APAs from 13 participants in each group and mean values from trials with APA errors from 12 of the same 13 participants in each group. Error bars represent standard errors across participant means.
Relation Between Cognition and Stepping
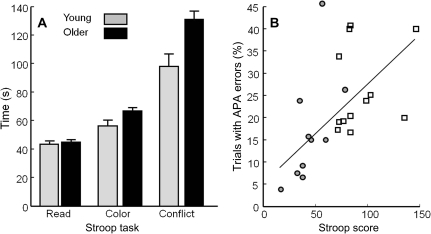
Figure 6 and Table 3 quantify the relations between performance on the Stroop task and our stepping measures. Figure 6A shows the mean scores for the three components of the Stroop task. There were no differences between young and older adults in reading time. However, older adults were 18% slower than young adults in color naming, t(22) = 2.6, p = .01, and—of greatest interest for our hypothesis—33% slower in the conflict task, t(22)=4.7, p = .0001. On average, participants made 1.5 errors. Numerically, older adults made more errors than young adults, but this difference was not significant, t(22) = 1.7; p = .11.
Figure 6.
Stroop task performance and relation to anticipatory postural adjustment (APA) errors. (A) Raw scores on each of the three component tests. Error bars represent standard errors across participant means. (B) Relation of APA errors to interference score. Circles: younger adults. Squares: older adults.
Table 3.
Correlations Between Stroop Score and APA/Step Measurements
| Age | APA Error | APA Onset | Step Latency | Step Length | |
| Stroop (raw) | .77* | .50* | −.07 | .17 | −.38* |
| Stroop (age corrected) | — | .45* | −.34 | .09 | −.18 |
Notes: Spearman's rank order correlations (rho) between Stroop conflict score and APA and step measurements, without and with age adjustment. APA = anticipatory postural adjustment.
*Indicates statistical significance (p < .05).
The measure that correlated most strongly with Stroop interference score (time + errors in conflict condition − time in reading condition) was number of APA errors (ρ = .50; first row of Table 3). Figure 6B shows individual values for this relation. In addition, there was a strong correlation between Stroop interference and age (ρ = .77). Therefore, we recalculated the correlations between Stroop interference and step preparation, controlling for the effect of age (second row of Table 3). When age was controlled, a significant relation remained between Stroop interference and APA errors (ρ = .45, p = .02). In addition, the (negative) relation between Stroop interference and APA onset approached significance (ρ = −.34, p = .06), suggesting that people who were better at inhibiting a habitual verbal response (reading) were also better at inhibiting step preparation until they had processed the directional information.
DISCUSSION
Initiation of the Wrong Motor Program Delays CRT Stepping
This study tested the hypothesis that age differences in CRT stepping are the result of occasional errors in initial movement program. We tested young and older adults in SRT and CRT stepping conditions, and we predicted that performance deficits in older adults would depend significantly on the inclusion of trials with errors in the initial movement program, as manifested by errors in the initial APA. This hypothesis was supported. In the CRT condition, older adults made almost three times as many APA errors as younger adults, and step latencies were much slower in trials with APA errors than in trials without APA errors. Overall, step latencies were slower in this experiment than in previously published work (9,25–27). We propose two explanations for this difference. First, It is well known that the strength of a stimulus is related to the speed of response (28). The light cue used here was small (less than 1 cm) and distant (3 m from the participant). Second, unpublished data from our laboratory suggest that pre-leaning can have a large effect on RT. Because we wanted to be able to detect even small APA errors, our threshold for eliminating trials with pre-leaning was very strict.
Data from previous studies are also consistent with our hypothesis. In a study in which participants pointed rapidly to the left or right in response to a cue, researchers found that long times between APA onset and arm movement were associated with invalid precues (29). The authors of this study suggested that APA errors might account for their results, but they did not measure or report APA errors. Supportive evidence also comes from two studies in which participants were required to rapidly push or pull on a handle in response to a cue; in both these studies, the average time between the APA onset and the arm movement was longer for older adults than for young adults (1,30). Although neither of these studies reported APA errors, the results are consistent with the hypothesis that APA errors caused the delay, and one of the studies mentions this possibility (30). Similar results have been seen in stepping tasks. One study showed that the average time between the APA onset and the step was longer for older adults than for young adults, whereas the APA onset was not affected by age (16). Another study demonstrated that in a CRT stepping task, older adults stepped over obstacles more slowly than young adults, and the age difference was exacerbated by the inclusion of trials in which participants first picked up the wrong foot and then put it down and picked up the correct foot. (17). Thus, a plausible explanation for slower CRT stepping in older adults is that they more often initially program the wrong movement and correct it only after a weight shift in the wrong direction has been launched.
Inhibitory Deficits Are Associated With Motor Program Selection Errors
We propose that motor program errors were more common in older adults than in younger adults because older adults had more failures in response inhibition. A large body of literature indicates that older adults have deficits in inhibitory control (31,32). Evidence in our study connecting inhibition failures with motor program errors comes from the age-adjusted correlation between APA errors and Stroop interference. This suggests that processes required for successful performance on the Stroop task overlap with processes required for initiation of the correct APA for stepping. The Stroop task involves multiple executive functions, including conflict resolution, perceptual inhibition, and task set maintenance as well as response inhibition. To confirm that response inhibition failure is the executive function responsible for APA errors, future studies should include tests such as the flankers task (33) or the stop-signal task (34,35) that isolate more specific executive functions.
The idea that selection of desired responses is dependent on inhibition of undesired responses is supported by ample behavioral and physiological evidence (36). In one recent study, researchers recording from neurons in the supplementary motor area of monkeys performing a go/no-go task discovered that neurons associated with the “go” cue and neurons associated with the “no-go” cue were intermingled (11). It appears that when a stimulus is first detected, “go” neurons for all possible conditions become active, along with “no-go” neurons for the undesired conditions. Because the “no-go” neurons normally become active first, the correct alternative is usually selected. Thus, these authors conclude that abnormal circuitry in the pre-SMA leads to inhibitory deficits, which cause errors in CRT tasks (11,12).
The scenario just described is similar in spirit to the independent horse race model proposed to predict outcomes of the stop-signal paradigm (34). The stop-signal task is an RT experiment in which a stop cue is occasionally presented. If the stop cue is presented with or soon after the go cue, stopping is easy. If the stop cue is presented very late, stopping is impossible. Stop-signal performance is often described as a race between a go process and a stop process, each triggered by their respective cues. An important implication of race models is that there is no early decision process that selects one course of action and deselects another, leaving the motor system with a single clear course of action, as other researchers have proposed (37,38). Instead, race models propose that activation and inhibition are in a race all the way to the end. Support for the race model comes from electromyographic evidence of partial responses in focal muscles prior to successful inhibition of errors in a stop-signal task (39). The data presented here represent the first use of postural activity as a measure of partial responses.
APA Errors Could Increase Fall Risk
The APA errors reported here could have important functional significance for aging and fall risk. In trials with APA errors, the COM traveled about 50% further forward (relative to step length) than in trials with correct initial APAs, for both older and younger adults. This could destabilize gait and contribute to falls. Cognitive decline and slow performance in CRT stepping are strong predictors of falls in elderly populations (40,41). The results of this study suggest that the initiation of an incorrect motor program along with forward motion of the COM may increase the likelihood of falling in the elderly. Thus, cognitive training that specifically targets response inhibition could be investigated as an intervention for fall reduction.
Coupling Between APA and Step Can Be Intermittently Disrupted
Prior evidence suggests that the APA and the step are programmed separately and then joined together (with a greater or lesser degree of success) into one motor plan (13,42,43). Successful coupling between the APA and the step should lead to strong within-subject correlations between APA onset and step latency. When, in the present study, all trials were included in this analysis, correlations were stronger for young adults than for older adults, suggesting that the APA/step coupling is tighter for young adults than for older adults. However, when only trials with correct initial APAs were included, this difference vanished. Thus, older adults do not have a general deficit in coupling between APA and step. Instead, APA/step coupling is occasionally disrupted by the initiation of the wrong motor program, which subsequently needs to be corrected before the step can proceed. This disruption occurs more often in older adults than in young adults and more often in CRT tasks than in SRT tasks, suggesting that there is a cognitive element to the process (44). Preliminary evidence suggests that this disruption occurs even more frequently in patients with Parkinson's disease and may contribute to freezing of gait (45).
CONCLUSIONS
Errors in the direction of the initial APA account for the difference in performance between young and older adults in CRT stepping. This suggests that slower CRT stepping in older adults is caused by mixing of trials in which the correct response is originally programmed and trials in which the incorrect response is programmed first and then corrected before the step can occur. The correlation between APA errors and performance on the Stroop task suggests that APA errors may be caused by a failure to inhibit the lateral weight shift until the directional information was processed. Thus, failures of response inhibition could cause APA errors, which in turn lead to response delays. It is possible that the slower CRT performance demonstrated by older adults in upper limb tasks might also rely on a mixing of trials with correct and incorrect initial impulses and that electromyographic of postural muscles activated before arm movement would reveal a pattern analogous to the one seen here. These results suggest that deficits in response inhibition, rather than general slower processing time, might account for findings of slowed CRT in older adults.
FUNDING
This research was supported by the National Institutes of Health (AG 006457-23, T32 NS045553, and T32 AT002688).
Acknowledgments
We thank Triana Nagel and Amanda Chao for help with data collection. We thank Arash Salarian for help with statistical analysis and presentation.
References
- 1.Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. J Gerontol. 1988;43:M105–M113. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- 3.Patla AE, Frank JS, Winter DA. Balance control in the elderly: implications for clinical assessment and rehabilitation. Can J Public Health. 1992;83(suppl 2):S29–S33. [PubMed] [Google Scholar]
- 4.Roggeveen AB, Prime DJ, Ward LM. Lateralized readiness potentials reveal motor slowing in the aging brain. J Gerontol Psychol Sci. 2007;62:P78–P84. doi: 10.1093/geronb/62.2.p78. [DOI] [PubMed] [Google Scholar]
- 5.Yordanova J, Kolev V, Hohnsbein J, Falkenstein M. Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes: evidence from high-resolution event-related potentials. Brain. 2004;127:351–362. doi: 10.1093/brain/awh042. [DOI] [PubMed] [Google Scholar]
- 6.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 7.Redfern MS, Muller ML, Jennings JR, Furman JM. Attentional dynamics in postural control during perturbations in young and older adults. J Gerontol A Biol Sci Med Sci. 2002;57:B298–B303. doi: 10.1093/gerona/57.8.b298. [DOI] [PubMed] [Google Scholar]
- 8.Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychol Aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Luchies CW, Schiffman J, Richards LG, Thompson MR, Bazuin D, DeYoung AJ. Effects of age, step direction, and reaction condition on the ability to step quickly. J Gerontol Med Sci. 2002;57:M246–M249. doi: 10.1093/gerona/57.4.m246. [DOI] [PubMed] [Google Scholar]
- 10.Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 12.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 13.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 14.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- 15.Breniere Y, Do MC, Bouisset S. Are dynamic phenomena prior to stepping essential to walking? J Mot Behav. 1987;19:62–76. doi: 10.1080/00222895.1987.10735400. [DOI] [PubMed] [Google Scholar]
- 16.Mercer VS, Sahrmann SA. Postural synergies associated with a stepping task. Phys Ther. 1999;79:1142–1152. [PubMed] [Google Scholar]
- 17.St George RJ, Fitzpatrick RC, Rogers MW, Lord SR. Choice stepping response and transfer times: effects of age, fall risk, and secondary tasks. J Gerontol A Biol Sci Med Sci. 2007;62:537–542. doi: 10.1093/gerona/62.5.537. [DOI] [PubMed] [Google Scholar]
- 18.Zettel JL, McIlroy WE, Maki BE. Effect of competing attentional demands on perturbation-evoked stepping reactions and associated gaze behavior in young and older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1370–1379. doi: 10.1093/gerona/63.12.1370. [DOI] [PubMed] [Google Scholar]
- 19.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 20.Mancini M, Zampieri C, Carlson-Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur J Neurol. 2009;16:1028–1034. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melzer I, Liebermann DG, Krasovsky T, Oddsson LI. Cognitive load affects lower limb force-time relations during voluntary rapid stepping in healthy old and young adults. J Gerontol A Biol Sci Med Sci. 2010;65:400–406. doi: 10.1093/gerona/glp185. [DOI] [PubMed] [Google Scholar]
- 22.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 23.Vaughan C, Davis B, Jeremy C. Dynamics of Human Gait. Champaign, IL: Human Kinetics Publishers; 1992. [Google Scholar]
- 24.Chandler R, Clauser C, McConville J, Reynolds H, Young J. Investigation of Inertial Properties of the Human Body. US Dept of Transportation, National Highway Traffic Safety Administration, Washington, DC publication AMRL-TR-74-137. Springfield, VA: National Technical Information Service; 1975. [Google Scholar]
- 25.Kukulka CG, Hajela N, Olson E, Peters A, Podratz K, Quade C. Visual and cutaneous triggering of rapid step initiation. Exp Brain Res. 2009;192:167–173. doi: 10.1007/s00221-008-1566-7. [DOI] [PubMed] [Google Scholar]
- 26.Melzer I, Oddsson LI. The effect of a cognitive task on voluntary step execution in healthy elderly and young individuals. J Am Geriatr Soc. 2004;52:1255–1262. doi: 10.1111/j.1532-5415.2004.52353.x. [DOI] [PubMed] [Google Scholar]
- 27.Rogers MW, Kukulka CG, Brunt D, Cain TD, Hanke TA. The influence of stimulus cue on the initiation of stepping in young and older adults. Arch Phys Med Rehabil. 2001;82:619–624. doi: 10.1053/apmr.2001.20833. [DOI] [PubMed] [Google Scholar]
- 28.Palmer J, Huk A, Shadlen M. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis. 2005;5:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- 29.Brown JE, Frank JS. Influence of event anticipation on postural actions accompanying voluntary movement. Exp Brain Res. 1987;67:645–650. doi: 10.1007/BF00247295. [DOI] [PubMed] [Google Scholar]
- 30.Stelmach GE, Populin L, Muller F. Postural muscle onset and voluntary movement in the elderly. Neurosci Lett. 1990;117:188–193. doi: 10.1016/0304-3940(90)90142-v. [DOI] [PubMed] [Google Scholar]
- 31.Olincy A, Ross RG, Youngd DA, Freedman R. Age diminishes performance on an antisaccade eye movement task. Neurobiol Aging. 1997;18:483–489. doi: 10.1016/s0197-4580(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 32.Potter LM, Grealy MA. Aging and inhibitory errors on a motor shift of set task. Exp Brain Res. 2006;171:56–66. doi: 10.1007/s00221-005-0244-2. [DOI] [PubMed] [Google Scholar]
- 33.Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 34.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 35.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houghton G, Tipper SP. Inhibitory mechanisms of neural and cognitive control: applications to selective attention and sequential action. Brain Cogn. 1996;30:20–43. doi: 10.1006/brcg.1996.0003. [DOI] [PubMed] [Google Scholar]
- 37.Gazzaniga M, Ivry R, Mangun G. Cognitive Neuroscience: The Biology of the Mind. New York: W. W. Norton; 1998. [Google Scholar]
- 38.Ratcliff R, Gomez P, McKoon G. A diffusion model account of the lexical decision task. Psychol Rev. 2004;111:159–182. doi: 10.1037/0033-295X.111.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- 40.Alexander NB, Hausdorff JM. Guest editorial: linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2008;63:1325–1328. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]
- 41.Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol Med Sci. 2001;56:M627–M632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- 42.Belenkiy V, Gurfinkel V. On elements of control of voluntary movements. Biofizica. 1967;12:135–141. [PubMed] [Google Scholar]
- 43.Jacobs JV, Horak FB. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res. 2007;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen R, Nutt J, Horak F. Failure to Synchronize Postural Preparation and Stepping May Trigger Freezing of Gait in Parkinson's Disease. Chicago, IL: Society for Neuroscience; 2009. [Google Scholar]