Abstract
Helicobacter pylori (H. pylori) infection is among the most common human infections and the major risk factor for peptic ulcer disease and gastric cancer. Within this work we present the implication of C-terminal region of H. pylori neutrophil activating protein in the stimulation of neutrophil activation as well as the evidence that the C-terminal region of H. pylori activating protein is indispensable for neutrophil adhesion to endothelial cells, a step necessary to H. pylori inflammation. In addition we show that arabino galactan proteins derived from chios mastic gum, the natural resin of the plant Pistacia lentiscus var. Chia inhibit neutrophil activation in vitro.
Keywords: Helicobacter pylori neutrophil activating protein, Helicobacter pylori, Peptic ulcer disease, Gastric cancer
INTRODUCTION
The Helicobacter pylori (H. pylori) neutrophil activating protein (HPNAP) is one of a number of virulence factors produced by the bacterium H. pylori[1]. This protein originally purified from aqueous extracts of H. pylori, was shown to induce neutrophil adhesion to endothelial cells in vitro[1] as well as in vivo[2], to increase the adhesion of neutrophils to endothelial cells[3], to induce migration and activation of human neutrophils and monocytes[4,5] and to be a potent stimulant of mast cells[6]. Its binding to neutrophil-glycosphingolipids[7] and mucin, a component of the stomach mucus layer[8], has also been reported. HPNAP induced reactive oxygen intermediates (ROI) production involves a cascade of intracellular activation events, including increase of cytosolic calcium ion concentration and phosphorylation of cytosolic proteins, leading to the assembly of the superoxide-forming nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase on the neutrophil plasma membrane[5,9,10].
Free radicals produced by neutrophils are a key component of the innate immune system and an effective antimicrobial agent against H. pylori as well as a factor that perpetuates mucosal damage and gastritis. Activation of neutrophils results in NADPH oxidase-mediated superoxide anion production which is highly destructive for the gastric mucosa, induces oxidative DNA damage and leads to substantial mucocal disruption. NADPH oxidase targeting is disrupted such that active enzyme complexes are present in patches at the cell surface but not on H. pylori phagosomes. Consequently, superoxide accumulates in the extracellular space but not near ingested bacteria. By this unusual mechanism, H. pylori evades oxidative killing and promotes tissue damage and ulceration. A possible blocking of reactive species production may lead to improvement of H. pylori induced chronic gastritis and reduction of signs of inflammation.
THE TWO ROLES OF HPNAP
Bacterial protection
HPNAP is a dodecameric protein consisting of 17 kDa monomers with a central cavity where iron can bind[11,12]. The observation that its synthesis is not affected by the iron content of the growth medium, led to the proposal that the primary role of HPNAP in vivo may not be to scavenge iron[13]. The primary sequence and overall structure of HPNAP[14] is similar to those of Dps family of iron-binding and DNA-protecting proteins[15]. Dps family proteins protect DNA from oxidative damage through direct interaction. Dps and DNA form a highly ordered and stable nucleoprotein complex called a biocrystal so that DNA is “sheltered” from the attack of the free oxidative radicals[16] by preventing the production of hydroxyl radicals produced by the Fenton reaction[17]. These proteins are present in many prokaryotes[18-22]. They bind ferrous ions and some of them lack the ability to bind DNA in vitro[12,19,23].
The role of HPNAP in protecting H. pylori from oxidative damage was first suggested by the observation that loss of alkyl hydroperoxide reductase (AhpC) leads to a concomitant increase in HPNAP expression[24]. Like other Dps proteins, HPNAP production is maximal in stationary-phase cells, and an H. pylori napA mutant survives less than the wild type strain upon exposure to oxidative stress conditions[25].
Our previous studies[26] revealed that HPNAP does not bind to DNA and therefore protection of the bacterial DNA by means of ferroxidase activity ensues by a mechanism similar to that suggested for other non DNA binding Dps. Molecular dynamics simulations (MDS) revealed that the ferroxidase site amino acids are indispensable for dimer formation and that ferrous ions contribute extensively to the stability of the dimers in solution.
HPNAP’s inflammatory role
The inflammatory role of HPNAP concerns the attraction and activation of neutrophils. In particular, the 150 kDa oligomeric protein isolated from H. pylori has been found to promote neutrophil adhesion to endothelial cells[1,11]. This protein was designated the HPNAP because of its ability to induce neutrophils to produce reactive oxygen radicals[5,11]. HPNAP is released in the medium, probably after cell lysis, and binds to the bacterial surface where it can act as an adhesin, mediating binding to mucin or to polymorphonuclear leukocyte sphingomyelin[7,8]. Purified recombinant HPNAP has been produced in Bacillus subtilis to avoid contamination by Escherichia coli lipopolysaccharide. This purified material was found to be chemotactic for human neutrophils and monocytes in vitro[5]. Moreover, using intravital microscopy, it has recently been demonstrated that in rats HPNAP is able to cross the endothelia efficiently and to promote rapid neutrophil adhesion in vivo[27]. HPNAP-induced adhesivity depends on the induction of expression and on the acquisition of a high-affinity state of β2-integrin on the plasma membrane of PMNs[5,27]. This conformational change requires a functional p38 mitogen-activated protein kinase (MAPK). Collectively, these observations suggest that HPNAP plays a central role in the accumulation of leukocytes at the site of infection[5,11,27]. HPNAP stimulates PMNs to synthesize and release several chemokines, including CXCL8 (interleukin-8), CCL3 (MIP-1α) and CCL4 (MIP-1β)[27]. Because neutrophils rapidly migrate in large numbers at infection sites, the fact that they also serve as a chemokine source may contribute to the generation of the conditions necessary for both the recruitment and activation not only of additional neutrophils, via CXCL8, but also of monocytes, dendritic cells, and lymphocytes through CCL3 and CCL4. After crossing epithelial monolayers, HPNAP is also able to activate the underlying mast cells to release tumor necrosis factor-α (TNF-α) and other pro-inflammatory molecules[6,13].
NEUTROPHIL ACTIVATION IN FOCUS
According to the literature HPNAP is the only protein of the DPs family capable of activating human leukocytes. Zanotti et al[14] investigated this unique property of HPNAP by analyzing not only its surface but also the surfaces of the structurally similar Flp, Dlp-1 and Dlp-2 which failed to activate human neutrophils. That was an attempt to identify regions located on the surface of these proteins whose different properties could account for such biological difference. They found that the surface of HPNAP was characterized by a large presence of positively charged residues, a property that was not shared by the other members of Dps family. The strong prevalence of positive charges of the electrostatic surface potential of HPNAP conferred a basic character on it. By taking into account the fact that positively charged residues of several proteins, including those of some chemokines which was believed to play a role in the activation of neutrophils[28,29] they suggested that the presence of the large number of basic residues on the HPNAP dodecamer surface was responsible for its neutrophil activating property.
However according to Kottakis et al[26] by replacing His25, His37, Asp52 and Lys134, that are located within the ferroxidase site, with Ala, a total loss of ferroxidase activity, dodecamer formation and DNA protection in environments rich in free radicals was observed.
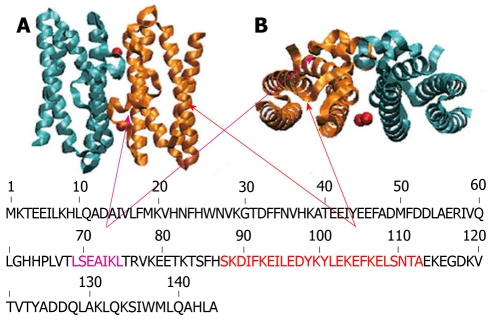
MDS revealed that dimer formation was highly unlikely following mutation of the above amino acids, since the ferrous ion is not attracted equally strongly by both subunits (Figure 1A and B). These findings indicate that iron plays an important role in the conformation of HPNAP by initiating the formation of stable dimers that are indispensable for the ensuing dodecamer structure. In addition, according to our experiments both HPNAP wild type as well as HPNAP mutant were able to activate neutrophils. In particular, by incubating neutrophils separately with the above proteins we observed a similar degree of activation for both cases[26]. Very surprisingly, neutrophil activation was stimulated by structural elements that are localized within the broad C-terminal region of both HPNAPmut and dodecamer HPNAPwild type. In particular, it was found that the dodecamer conformation was not necessary for activation and that helices H3 (Leu69-Leu75), H4 (Lys89-Leu114) or the linking coils (His63-Thr68 and Thr76-Ser88) were critical in stimulating neutrophil activation (Figures 2 and 3).
Figure 1.
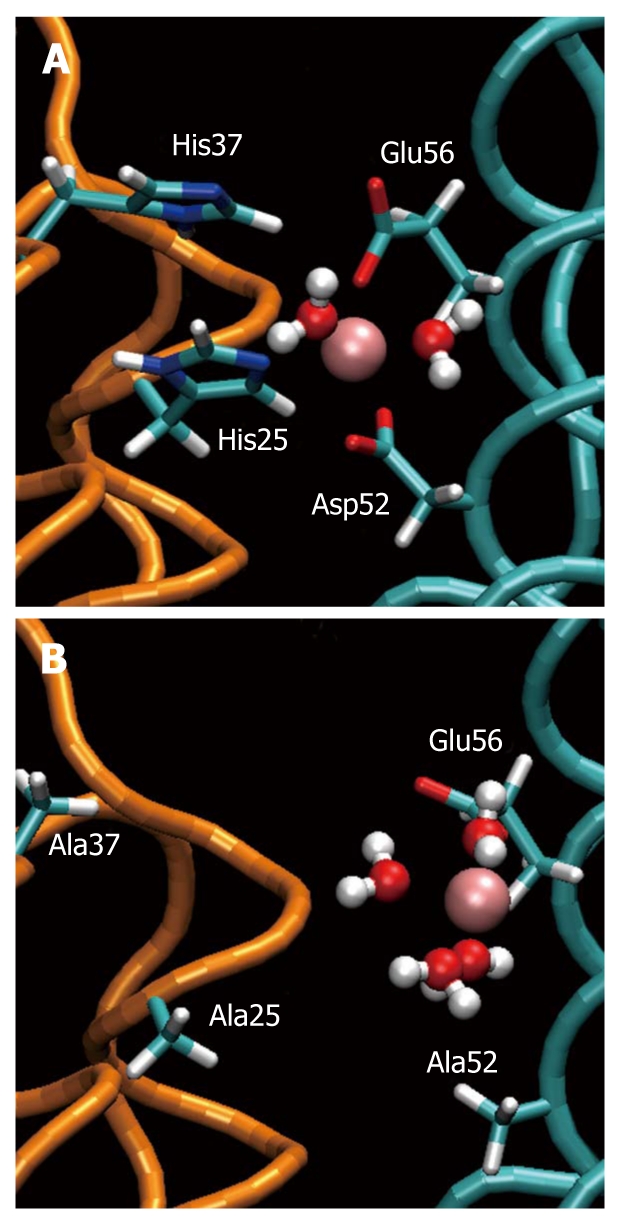
Ferroxidase site of Helicobacter pylori neutrophil activating protein. A: The “ferroxidase site” in the equilibrated wild type. The iron ion (pink) is kept in position by Asp52, Glu56, His25 and His37. Two water molecules are attracted by Fe(II); B: The same site in the equilibrated mutant. The ferrous ion is attracted one-sidedly by Glu56 and Asp53 (not shown) loosing its ability to stabilize the dimer. Four water molecules are attracted by Fe(II).
Figure 2.
Schematic representation of exposed helices of Helicobacter pylori neutrophil activating protein. Helicobacter pylori neutrophil activating protein dimer in stand up (A) and top view (B) with the exposed helices H3 and H4 (therefore possible candidates for interacting with the neutrophils) colored in violet and orange respectively.
Figure 3.
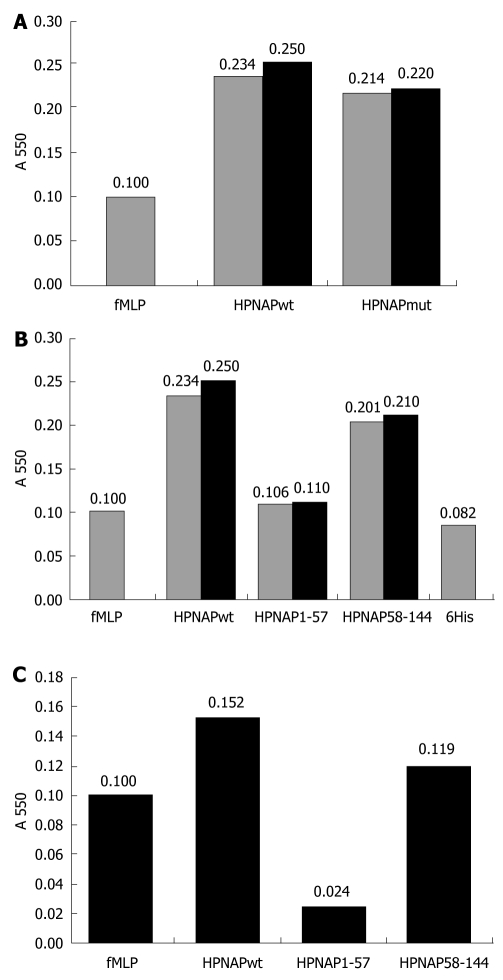
Neutrophil activation by Helicobacter pylori neutrophil activating protein. A: Neutrophil activation by Helicobacter pylori neutrophil activating protein (HPNAP)-wt and HPNAPmut. Bar 1, activation by formyl-met-leu-pro peptide (fMLP) (control), bar 2, activation by HPNAPwt, bar 3, activation by HPNAPmut; B: Neutrophil activation by HPNAPwt-hexa-histidine peptide (6xHis), HPNAP1-57-6His, HPNAP58-144-6His and 6His peptide. Bar 1, activation by fMLP (control), bar 2, activation by HPNAPwt-6His, bar 3, activation by HPNAP1-57-6His, bar 4, activation by HPNAP58-144-6His, bar 5, activation by 6His peptide; C: Values after subtraction of “6His” value from these of “HPNAPwt”, “HPNAP1-57” and “HPNAP58-144”.
It was recently reported that HPNAP promotes a Th1 immune response by inducing the expression of IL-12 and IL-23 in neutrophils and monocytes, and also elicits an antigen-specific Th1-polarized T cell response in gastric mucosa of H. pylori-infected patients in vivo[30]. It has been shown that HPNAP is able to shift antigen activated human T cells from a Th2 to a Th1 cytotoxic phenotype characterized by production of IFN-γ and TNF-α. Since HPNAP is a powerful stimulant for the production of ROS, mediating damage to DNA and enhancing cell turnover[19], it may be a risk factor for H. pylori-associated gastric cancer. Considering the upregulatory effects of HPNAP on the innate immune system, it could possibly be argued that the chronic inflammatory response mediated by HPNAP may be associated with an increased danger of occurrence of gastric cancer in view of the fact that H. pylori is classified as class 1 carcinogen. As a matter of fact, a recent impressive work[31] studied the serum positivity and mean absorbance value of HPNAP-specific antibodies in patients with gastric cancer in comparison to patients with chronic gastritis. Interestingly, HPNAP antibodies were significantly higher in the gastric cancer group indicating a possible pathogenetic role of HPNAP in gastric carcinogenesis.
ACTIVATED NEUTROPHILS ATTACH TO ENDOTHELIAL CELLS
Human neutrophils were separately incubated with HPNAP-6xHis, HPNAP1-57-6xHis (N-terminal region) and HPNAP58-144-6xHis (C-terminal region) on micro wells with pre attached endothelial cells and their attachment was quantified by using the myeloperoxidase (MPO) assay[32]. Besides the entire protein and its truncated forms neutrophils were also incubated by the same manner with the neutrophil stimulator formyl-Met-Leu-Pro peptide (fMLP) in order to control their “bioactivity”. In addition, a synthetic hexa-histidine peptide (6xHis) was also used for neutrophil activation in order to exclude the possibility that the obtained activation was attributed to the existence of the tailed histidines. Figure 4A shows that HPNAP-6xHis and the C-terminal region display almost the same ability to promote neutrophil adhesion to endothelial cells while the N-terminal region lacks this ability.
Figure 4.
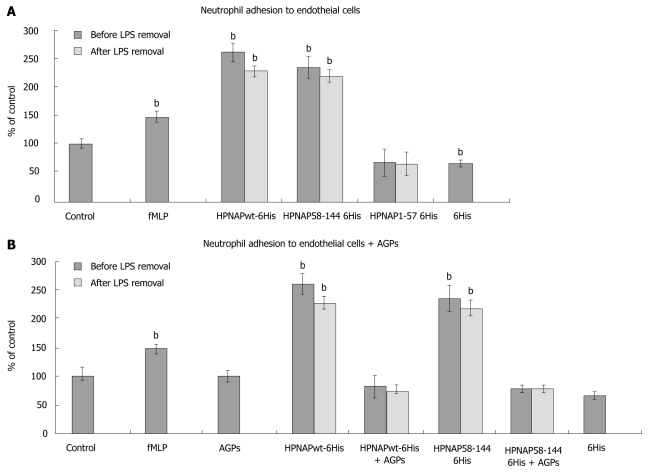
Neutrophil adhesion to endothelial cells. A: Neutrophil adhesion to endothelial cells. Black bars indicate neutrophil adhesion to endothelial cells prior to lipopolysaccharides (LPS) removal while grey bars indicate the adhesion after LPS removal. The data represent triplicates from at least three independent experiments. Error bars indicate standard deviation (SD). Statistical evaluation was performed by Mann-Whitney test. Significant differences with the control values are marked by (bP < 0.001) bar 1, control, bar 2, formyl-met-leu-pro peptide (fMLP), positive control of the procedure, bar 3, Helicobacter pylori neutrophil activating protein (HPNAP)wt-hexa-histidine peptide (6xHis) effect on neutrophil adhesion to endothelial cells before and after LPS removal, bar 4, HPNAP58-144-6xHis effect before and after LPS removal, bar 5, HPNAP1-57-6xHis effect before and after LPS removal, bar 6, 6xHis effect; B: Effect of arabino galactan proteins (AGPs) on neutrophil adhesion to endothelial cells, bar 1, control, bar 2, fMLP, positive control of the procedure, bar 3, effect of AGPs, bar 4, HPNAPwt-6xHis effect before and after LPS removal, bar 5, HPNAPwt-6xHis and AGPs co-effect, before and after LPS removal, bar 6, HPNAP58-144-6xHis effect before and after LPS removal, bar 7, HPNAP58-144-6xHis and mastic gum extract co-effect, before and after LPS removal, bar 8, 6xHis effect.
Considering the existence of lipopolysaccharides (LPS) and their involvement in the activation it is clearly shown that even after their removal the activation effects did not change significantly (Figure 4A). These results are consistent with previously published data[26], indicating that the 58-144 region of the HPNAP protein is the key component in neutrophil recruitment, activation and subsequent adhesion to endothelial cells, leading to oxidative burst and inflammation during H. pylori infection. Considering recently published data[33], on the safety and immunogenicity of an intramuscular vaccine comprising VacA, CagA and HPNAP, we suggest that the obtained neutrophil activation by the C-terminal region of HPNAP opens new ways for drug design dealing with H. pylori inflammation.
NEUTROPHIL ATTACHMENT INHIBITION BY ARABINO-GALACTAN-PROTEINS FROM CHIOS MASTIC GUM
Chios mastic gum (CMG) and its derivatives were largely used in traditional medicine to ease the discomfort in patients suffering from gastric pain. Its in vitro antibacterial properties against a great variety of bacteria are well established[34,35]. In this study we demonstrate that AGPs extracted from CMG as described in[34] inhibit the neutrophil attachment to endothelial cells caused by the HPNAP and its C-terminal region. In particular, human neutrophils were incubated with either HPNAP-6xHis or HPNAP58-144-6xHis both in the presence and in the absence of AGPs and their attachment to endothelial cells was investigated as above. Figure 4B shows the inhibition of neutrophil attachment to endothelial cells after co-incubation of entire HPNAP and its truncated forms (N-terminal and C-terminal) with the AGPs. In particular, bar 3 shows the absence of any influence of AGPs on neutrophil attachment to endothelial cells. The designation “control” on the figure represents the found attachment of isolated neutrophils to endothelial cells after incubation, without any other addition of proteins or AGPs. The marked percentages of all other combinations are calculated by taking into account the control values. Thus, comparison of the bars 4 (HPNAP entire) to 5 (HPNAP entire plus AGPs) and 6 (HPNAP58-144) to 7 (HPNAP58-144 plus AGPs) reveals that neutrophil activation and their subsequent attachment to endothelial cells is inhibited by the AGPs from CMG.
A recent study[36] focused on HPNAP mediated neutrophil activation before and after 2 mo of per os administration of CMG. According to this work, CMG induces a significant reduction in neutrophil activation when incubated with AGP plus HPNAP in H. pylori-infected patients and controls. CMG also induces a significant reduction in neutrophil activation when incubated with HPNAP in H. pylori-infected patients. These results indicate a substantial down-regulation of the innate cellular immune effectors, which - according to unpublished clinical data in the context of this study - are accompanied by a significant clinical improvement of the patients’ complaints (dyspepsia, epigastric discomfort, distention). However a demonstration of the histopathological improvement of the patients’ chronic gastritis would provide even more valuable evidence, concerning the potential anti-inflammatory and gastritis-suppressive effects of CMG.
Summarizing our results presented within this work we evidenced that the broad C-terminal region of HPNAP stimulates neutrophil adhesion and that the AGPs from CMG disrupt the process of neutrophil-endothelial cell attachment caused by HPNAP, an effect that should be further investigated and maybe exploited in a future anti-inflammatory therapy for H. pylori patients.
FAILURE OF ANTIBIOTIC THERAPY AND RECURRENCE OF H. PYLORI INFECTION - IS H. PYLORI AN INVINCIBLE ENEMY?
Triple as well as quadruple treatment regimens are so far a well established therapy of H. pylori infection. Eradication rates of over 80% (triple therapy) and 96% for the quadruple regimen with bismuth or ecabet sodium are documented[37,38]. However, a significant number of mutants is emerging-especially in developing countries - which confers resistance to standard antibiotics (amoxicillin, metronidazole, clarithromycin)[39-41] as well as to fluoroquinolones. Resistance rates of 35% for clarithromycin and 26% for fluoroquinolones are documented, a very worrying fact, which limits considerably the future perspectives of successful antibiotic treatment of H. pylori infection. Moreover, a high prevalence of recurrence of H. pylori infection in adults as well as in children could possibly render our efforts to eradicate this pathogen futile[42-45]. Indeed, an incidence of H. pylori annual recurrence of 2.67% and 13.00% in developed and developing countries respectively is documented in a recent study[46]. In view of these data, alternative methods of treatment as adjunct or main therapy regimens should be considered. HPNAP, a major stimulant of neutrophil recruitment and activation could be effectively targeted by natural agents that reduce inflammation (due to their activity as antioxidants) or suppress the production of inflammatory cytokines that attract neutrophils[47-50]. On the other side, the advent of the structure oriented drug design era would eventually provide us with valuable weapons to fight the H. pylori infection by the direct inhibition of HPNAP in vivo. The identification of structural elements at the C-terminal region of HPNAP monomer as a stimulus for neutrophil migration through endothelial cells and subsequent release of ROIs[26,51] renders this region eligible for drug mediated inhibition of its immunological effects upon neutrophils. Indeed, additional studies should be carried out in order to validate this assumption and open the way for new and alternative perspectives to fight this ubiquitous pathogen.
Footnotes
Supported by A Grant from the General Secretariat of Research and Technology, Ministry of Development of Greece, by the Program HERAKLITOS I as well as by Chios Gum Mastic Growers Association
Peer reviewer: Takashi Kawai, MD, PhD, Professor and Director of Endoscopy Center, Tokyo Medical University, 6-7-1, Nishishinjuku, Shinjuku-ku, Tokyo 160-0023, Japan
S- Editor Sun H L- Editor O’Neil M E- Editor Ma WH
References
- 1.Yoshida N, Granger DN, Evans DJ Jr, Evans DG, Graham DY, Anderson DC, Wolf RE, Kvietys PR. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 2.Kurose I, Granger DN, Evans DJ Jr, Evans DG, Graham DY, Miyasaka M, Anderson DC, Wolf RE, Cepinskas G, Kvietys PR. Helicobacter pylori-induced microvascular protein leakage in rats: role of neutrophils, mast cells, and platelets. Gastroenterology. 1994;107:70–79. doi: 10.1016/0016-5085(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 3.Evans DJ Jr, Evans DG, Lampert HC, Nakano H. Identification of four new prokaryotic bacterioferritins, from Helicobacter pylori, Anabaena variabilis, Bacillus subtilis and Treponema pallidum, by analysis of gene sequences. Gene. 1995;153:123–127. doi: 10.1016/0378-1119(94)00774-m. [DOI] [PubMed] [Google Scholar]
- 4.Montemurro P, Barbuti G, Dundon WG, Del Giudice G, Rappuoli R, Colucci M, De Rinaldis P, Montecucco C, Semeraro N, Papini E. Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells. J Infect Dis. 2001;183:1055–1062. doi: 10.1086/319280. [DOI] [PubMed] [Google Scholar]
- 5.Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671–676. doi: 10.1002/1521-4141(200203)32:3<671::aid-immu671>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Teneberg S, Miller-Podraza H, Lampert HC, Evans DJ Jr, Evans DG, Danielsson D, Karlsson KA. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J Biol Chem. 1997;272:19067–19071. doi: 10.1074/jbc.272.30.19067. [DOI] [PubMed] [Google Scholar]
- 8.Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun. 1998;66:444–447. doi: 10.1128/iai.66.2.444-447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecucco C, de Bernard M. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 2003;5:715–721. doi: 10.1016/s1286-4579(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 10.Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, Montecucco C. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol. 2003;33:840–849. doi: 10.1002/eji.200323726. [DOI] [PubMed] [Google Scholar]
- 11.Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G, Del Giudice G, Rappuoli R, Montecucco C. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol. 1999;34:238–246. doi: 10.1046/j.1365-2958.1999.01584.x. [DOI] [PubMed] [Google Scholar]
- 13.Dundon WG, Polenghi A, Del Guidice G, Rappuoli R, Montecucco C. Neutrophil-activating protein (HP-NAP) versus ferritin (Pfr): comparison of synthesis in Helicobacter pylori. FEMS Microbiol Lett. 2001;199:143–149. doi: 10.1111/j.1574-6968.2001.tb10665.x. [DOI] [PubMed] [Google Scholar]
- 14.Zanotti G, Papinutto E, Dundon W, Battistutta R, Seveso M, Giudice G, Rappuoli R, Montecucco C. Structure of the neutrophil-activating protein from Helicobacter pylori. J Mol Biol. 2002;323:125–130. doi: 10.1016/s0022-2836(02)00879-3. [DOI] [PubMed] [Google Scholar]
- 15.Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- 16.Wolf SG, Frenkiel D, Arad T, Finkel SE, Kolter R, Minsky A. DNA protection by stress-induced biocrystallization. Nature. 1999;400:83–85. doi: 10.1038/21918. [DOI] [PubMed] [Google Scholar]
- 17.Buda F, Ensing B, Gribnau MC, Baerends EJ. O2 evolution in the Fenton reaction. Chemistry. 2003;9:3436–3444. doi: 10.1002/chem.200204444. [DOI] [PubMed] [Google Scholar]
- 18.Bozzi M, Mignogna G, Stefanini S, Barra D, Longhi C, Valenti P, Chiancone E. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 19.Ceci P, Ilari A, Falvo E, Chiancone E. The Dps protein of Agrobacterium tumefaciens does not bind to DNA but protects it toward oxidative cleavage: x-ray crystal structure, iron binding, and hydroxyl-radical scavenging properties. J Biol Chem. 2003;278:20319–20326. doi: 10.1074/jbc.M302114200. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y, Wang G, Maier RJ. Helicobacter hepaticus Dps protein plays an important role in protecting DNA from oxidative damage. Free Radic Res. 2006;40:597–605. doi: 10.1080/10715760600618882. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Poole LB, Hantgan RR, Kamio Y. An iron-binding protein, Dpr, from Streptococcus mutans prevents iron-dependent hydroxyl radical formation in vitro. J Bacteriol. 2002;184:2931–2939. doi: 10.1128/JB.184.11.2931-2939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem. 2002;277:27689–27696. doi: 10.1074/jbc.M202094200. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa T, Mizunoe Y, Kawabata S, Takade A, Harada M, Wai SN, Yoshida S. The iron-binding protein Dps confers hydrogen peroxide stress resistance to Campylobacter jejuni. J Bacteriol. 2003;185:1010–1017. doi: 10.1128/JB.185.3.1010-1017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olczak AA, Olson JW, Maier RJ. Oxidative-stress resistance mutants of Helicobacter pylori. J Bacteriol. 2002;184:3186–3193. doi: 10.1128/JB.184.12.3186-3193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooksley C, Jenks PJ, Green A, Cockayne A, Logan RP, Hardie KR. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J Med Microbiol. 2003;52:461–469. doi: 10.1099/jmm.0.05070-0. [DOI] [PubMed] [Google Scholar]
- 26.Kottakis F, Papadopoulos G, Pappa EV, Cordopatis P, Pentas S, Choli-Papadopoulou T. Helicobacter pylori neutrophil-activating protein activates neutrophils by its C-terminal region even without dodecamer formation, which is a prerequisite for DNA protection--novel approaches against Helicobacter pylori inflammation. FEBS J. 2008;275:302–317. doi: 10.1111/j.1742-4658.2007.06201.x. [DOI] [PubMed] [Google Scholar]
- 27.Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N, Cassatella MA, Montecucco C, Tedesco F, de Bernard M. The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo. J Immunol. 2007;178:1312–1320. doi: 10.4049/jimmunol.178.3.1312. [DOI] [PubMed] [Google Scholar]
- 28.Laurence JS, Blanpain C, De Leener A, Parmentier M, LiWang PJ. Importance of basic residues and quaternary structure in the function of MIP-1 beta: CCR5 binding and cell surface sugar interactions. Biochemistry. 2001;40:4990–4999. doi: 10.1021/bi002593w. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Mayo KH, Daly TJ, Barry JK, La Rosa GJ. Subunit association and structural analysis of platelet basic protein and related proteins investigated by 1H NMR spectroscopy and circular dichroism. J Biol Chem. 1994;269:20110–20118. [PubMed] [Google Scholar]
- 30.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D’Elios MM, Del Prete G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long M, Luo J, Li Y, Zeng FY, Li M. Detection and evaluation of antibodies against neutrophil-activating protein of Helicobacter pylori in patients with gastric cancer. World J Gastroenterol. 2009;15:2381–2388. doi: 10.3748/wjg.15.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malfertheiner P, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, Norelli F, Contorni M, Peppoloni S, Berti D, et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135:787–795. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 34.Kottakis F, Lamari F, Matragkou Ch, Zachariadis G, Karamanos N, Choli-Papadopoulou T. Arabino-galactan proteins from Pistacia lentiscus var. chia: isolation, characterization and biological function. Amino Acids. 2008;34:413–420. doi: 10.1007/s00726-007-0554-8. [DOI] [PubMed] [Google Scholar]
- 35.Al-Habbal MJ, Al-Habbal Z, Huwez FU. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin Exp Pharmacol Physiol. 1984;11:541–544. doi: 10.1111/j.1440-1681.1984.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 36.Kottakis F, Kouzi-Koliakou K, Pendas S, Kountouras J, Choli-Papadopoulou T. Effects of mastic gum Pistacia lentiscus var. Chia on innate cellular immune effectors. Eur J Gastroenterol Hepatol. 2009;21:143–149. doi: 10.1097/MEG.0b013e32831c50c9. [DOI] [PubMed] [Google Scholar]
- 37.Costa F, D’Elios MM. Management of Helicobacter pylori infection. Expert Rev Anti Infect Ther. 2010;8:887–892. doi: 10.1586/eri.10.75. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi W, Tanabe S, Nakatani K, Ishido K, Nishimura K, Azuma M, Ae T, Sasaki T, Higuchi K, Katada C, et al. Quadruple therapy with ecabet sodium, omeprazole, amoxicillin and metronidazole is effective for eradication of Helicobacter pylori after failure of first-line therapy (KDOG0201 Study) J Clin Pharm Ther. 2010;35:303–307. doi: 10.1111/j.1365-2710.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- 39.Agudo S, Pérez-Pérez G, Alarcón T, López-Brea M. High prevalence of clarithromycin-resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J Clin Microbiol. 2010;48:3703–3707. doi: 10.1128/JCM.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Mansour K, Burucoa C, Zribi M, Masmoudi A, Karoui S, Kallel L, Chouaib S, Matri S, Fekih M, Zarrouk S, et al. Primary resistance to clarithromycin, metronidazole and amoxicillin of Helicobacter pylori isolated from Tunisian patients with peptic ulcers and gastritis: a prospective multicentre study. Ann Clin Microbiol Antimicrob. 2010;9:22. doi: 10.1186/1476-0711-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia GT, Aranda KR, Gonçalves ME, Cardoso SR, Iriya K, Silva NP, Scaletsky IC. High prevalence of clarithromycin resistance and cagA, vacA, iceA2, and babA2 genotypes of Helicobacter pylori in Brazilian children. J Clin Microbiol. 2010;48:4266–4268. doi: 10.1128/JCM.01034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leal YA, Gómez A, Madrazo-de la Garza A, Ramos I, Muñoz O, Torres J. A primary Helicobacter pylori infection does not protect against reinfection in children after eradication therapy. Rev Invest Clin. 2008;60:470–477. [PubMed] [Google Scholar]
- 43.Niv Y, Hazazi R, Waked A, Lederfein T, Achiel K. Helicobacter pylori recurrence and infection rate in Israeli adults. Dig Dis Sci. 2008;53:1211–1214. doi: 10.1007/s10620-007-0016-x. [DOI] [PubMed] [Google Scholar]
- 44.Ryu KH, Yi SY, Na YJ, Baik SJ, Yoon SJ, Jung HS, Song HJ. Reinfection rate and endoscopic changes after successful eradication of Helicobacter pylori. World J Gastroenterol. 2010;16:251–255. doi: 10.3748/wjg.v16.i2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva FM, Navarro-Rodriguez T, Barbuti RC, Mattar R, Hashimoto CL, Eisig JN. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter. 2010;15:46–52. doi: 10.1111/j.1523-5378.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 46.Niv Y. H pylori recurrence after successful eradication. World J Gastroenterol. 2008;14:1477–1478. doi: 10.3748/wjg.14.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Latif MM, Windle HJ, Homasany BS, Sabra K, Kelleher D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br J Pharmacol. 2005;146:1139–1147. doi: 10.1038/sj.bjp.0706421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee IO, Lee KH, Pyo JH, Kim JH, Choi YJ, Lee YC. Anti-inflammatory effect of capsaicin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2007;12:510–517. doi: 10.1111/j.1523-5378.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee KM, Yeo M, Choue JS, Jin JH, Park SJ, Cheong JY, Lee KJ, Kim JH, Hahm KB. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter. 2004;9:632–642. doi: 10.1111/j.1083-4389.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- 50.Toyoda T, Tsukamoto T, Takasu S, Shi L, Hirano N, Ban H, Kumagai T, Tatematsu M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer. 2009;125:1786–1795. doi: 10.1002/ijc.24586. [DOI] [PubMed] [Google Scholar]
- 51.Kottakis F, Befani C, Asiminas A, Kontou M, Koliakos G, Choli-Papadopoulou T. The C-terminal region of HPNAP activates neutrophils and promotes their adhesion to endothelial cells. Helicobacter. 2009;14:177–179. doi: 10.1111/j.1523-5378.2009.00678.x. [DOI] [PubMed] [Google Scholar]