Abstract
AIM: To evaluate the efficacy and safety of endoscopic removal and trimming of self-expandable metallic stents (SEMS).
METHODS: All SEMS had been placed for distal biliary strictures. Twenty-seven endoscopic procedures were performed in 19 patients in whom SEMS (one uncovered and 18 covered) removal had been attempted, and 8 patients in whom stent trimming using argon plasma coagulation (APC) had been attempted at Tokyo Medical University Hospital. The APC settings were: voltage 60-80 W and gas flow at 1.5 L/min.
RESULTS: The mean stent indwelling period for all patients in whom stent removal had been attempted was 113.7 ± 77.6 d (range, 8-280 d). Of the 19 patients in whom removal of the SEMS had been attempted, the procedure was successful in 14 (73.7%) without procedure-related adverse events. The indwelling period in the stent removable group was shorter than that in the unremovable group (94.9 ± 71.5 d vs 166.2 ± 76.2 d, P = 0.08). Stent trimming was successful for all patients with one minor adverse event consisting of self-limited hemorrhage. Trimming time ranged from 11 to 16 min.
CONCLUSION: Although further investigations on larger numbers of cases are necessary to accumulate evidence, the present data suggested that stent removal and stent trimming is feasible and effective for stent-related complications.
Keywords: Self-expandable metallic stent, Endoscopic biliary stenting, Endoscopic stent removal, Endoscopic stent trimming
INTRODUCTION
Placement of self-expandable metallic stents (SEMS) in the palliation of malignant biliary stricture has been increasingly employed in an attempt to prolong the patency period[1,2]. The recent improvement in multidisciplinary therapy that incorporates treatments such as chemotherapy and radiotherapy has resulted in an increased number of long-term survivors. This has however been accompanied by an increased chance of experiencing stent-related complications. The majority of stent-related complications involve stent occlusion, leading to cholangitis. Displacement of the stent in the duodenal wall, which is another complication, is not only associated with occlusion but also with the formation of erosions and ulcers from mechanical irritation, and serious bleeding and gastrointestinal tract perforation[3-5].
An intervention for stent occlusion is mechanical cleaning, using devices such as a balloon, but, the occlusion recurs in a comparatively short time in many cases[6]. For patients with repeated stent occlusion, the stent-in-stent procedure with plastic stents or SEMS is used. According to some recent reports[7-9], a procedure whereby SEMS are removed and replaced by new SEMS, has been performed as an alternative method for management of occlusion of SEMS. Furthermore, as an intervention in the displacement of a metallic stent, metallic stent trimming by argon plasma coagulation (APC) has been used in cases of stent migration to the duodenum[4,10-13]. In the present study, we retrospectively evaluated the efficacy and safety of endoscopic removal and trimming of SEMS.
MATERIALS AND METHODS
All SEMS had been placed for distal biliary strictures. Twenty-seven endoscopic procedures were performed in 19 patients in whom SEMS removal had been attempted and in 8 patients in whom APC trimming had been attempted at Tokyo Medical University Hospital between February 2004 and April 2009. The APC trimming group included 3 patients in whom stent removal had been switched to trimming because stent removal was impossible.
The ratio of males to females was 17:10 and the mean age was 68.1 ± 13.7 years (range, 43-91 years) (Table 1). Our policy for managing stent-related accidental symptoms is outlined below. When a stent was occluded, our policy was first to clean the occluded stent using a balloon catheter or basket catheter. If cholangitis due to stent occlusion recurred more than a twice, we attempted stent removal. However, even if the SEMS were positioned appropriately but removal was difficult, mechanical cleaning or a stent-in-stent maneuver was performed. Furthermore, if the end tip of the stent had migrated towards the duodenal wall, only stent trimming was performed. If the stent was occluded even after trimming, mechanical cleaning or a stent-in-stent procedure was performed.
Table 1.
Characteristics of patients
| Total | Stent removal | Stent trimming | |
| No. patients | 27 | 19 | 81 |
| Sex (male/female) | 17/10 | 13/6 | 4/41 |
| Mean age, yr, (range) | 68.1 (43-91) | 66.9 (43-91) | 70.9 (54-90) |
| Etiology of biliary strictures | |||
| Pancreatic carcinoma | 17 | 12 | 5 |
| Biliary ductal carcinoma | 3 | 2 | 1 |
| IPMC | 1 | 0 | 1 |
| Lymph node metastasis2 | 1 | 0 | 1 |
| Chronic pancreatitis | 2 | 2 | 0 |
| MFP | 2 | 2 | 0 |
| AIP | 1 | 1 | 0 |
| Type of SEMS | |||
| Uncovered Wallstent | 2 | 1 | 1 |
| Covered SEMS | 25 | 18 | 7 |
| Covered Wallstent (Partially covered type) | 7 | 4 | 3 |
| Niti-S Biliary ComVi Stent (Partially covered type) | 2 | 1 | 1 |
| Niti-S Biliary ComVi Stent (Fully covered type) | 14 | 11 | 3 |
| Niti-S Biliary Covered Stent with removal suture | 2 | 2 | 0 |
Including 3 cases with impossible stent removal;
Due to cancer of the uterine body. IPMC: Intraductal papillary mucinous carcinoma; MFP: Mass-forming pancreatitis; AIP: Autoimmune pancreatitis; SEMS: Self-expandable metallic stent.
Stent removal
The SEMS used for the 19 cases in whom stent removal was attempted are shown in Table 1. The stents used included one uncovered Wallstent (Boston Scientific Japan, Tokyo, Japan) and 18 covered SEMS. Therapeutic duodenoscope (TJF-240, TJF-260V and JF-260V, Olympus Medical Systems, Tokyo, Japan) were used for the removal of stents. Snare forceps (SD-5L-1, Olympus, Tokyo, Japan) were used to grip the end tip of the stent, which was then removed by pulling it towards the working channel. However, if removal through the working channel was difficult, the stent was removed by endoscopy while holding it. If a Niti-S Biliary covered stent (Taewoong Company, Seoul, Korea) was used with a removal suture, the removal cord was gripped using biopsy forceps and after pulling the stent a distance of about a third from the papilla, it was removed using snare forceps as previously mentioned.
Stent trimming
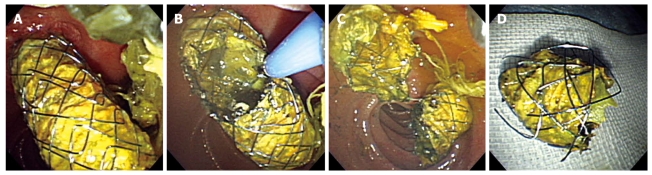
An APC system (ENDOPLASMA, PSD-60, Olympus Medial Systems, Tokyo, Japan) was used to perform stent trimming. The settings were: voltage at 60-80 W and gas flow at 1.5 L/min. The APC probe used for all the patients was the front emission elasticity APC probe (MAJ-1011, diameter 2.3 mm, Olympus Medical Systems, Tokyo, Japan). The stent amputation stump margin was set at 5-15 mm from the papilla. A rough distance was maintained so that the tip of the probe would be in minimal contact with the stent. The target line for cutting the stent was a circumferential incision in addition to ablation (Figure 1). In addition, to avoid gut distension due to the retention of excess gas and the accompanying pain, as well as the risk of perforations during the procedure, gas was suctioned periodically. Stent fragments were removed together with the endoscope using a catheter with a retrieval net or by pulling them out while being held by forceps.
Figure 1.
Stent trimming using argon plasma coagulation. A: Endoscopic imaging showing displacement of a Niti-S Biliary ComVi stent to the duodenum; B: Stent trimming was performed using argon plasma coagulation; C: Endoscopic imaging showing the cut down the stent; D: Eventually, the stent fragment was removed from the body.
RESULTS
Stent removal
The mean stent indwelling period of all SEMS was 113.7 ± 77.6 d (range, 8-280 d). There was no statistical difference between malignant and benign diseases (126.1 ± 80.0 and 78.8 ± 65.2 d, respectively, P = 0.25). Of the 19 patients in whom removal of the SEMS was attempted, the procedure was successful in 14 (73.7%) without complications (Table 2). Stent removal was impossible for one patient with an uncovered stent and 4 patients with a covered stent. The indwelling period in the stent removable group was shorter than that in the unremovable group (94.9 ± 71.5 d vs 166.2 ± 76.2 d, P = 0.08). Of the 14 patients who underwent successful stent removal, the indwelling period for the 10 patients who had a malignant disease was 111 ± 76.1 d (range, 15-232 d) and the period for the 4 patients who had a benign disease was 54.5 ± 41.6 d (range, 8-100 d). There was no statistical difference between the 2 groups (P = 0.1).
Table 2.
Outcome of removal of metallic stent
| No. of case | Disease | Type of SEMS | Duration of stent placement (d) | Outcome | Additional procedures |
| 1 | Pancreatic ca. | Uncovered Wallstent | 180 | Unsuccess | Trimming |
| 2 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Partially covered type) | 114 | Unsuccess | Trimming |
| 3 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 280 | Unsuccess | Trimming |
| 4 | Bile duct ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 81 | Unsuccess | Stent in stent |
| 5 | Bile duct ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 219 | Success | New SEMS |
| 6 | Pancreatic ca | Niti-S Biliary ComVi Stent (Fully covered type) | 232 | Success | New SEMS |
| 7 | Pancreatic ca. | Covered Wallstent (Partially covered type) | 15 | Success | New SEMS |
| 8 | Pancreatic ca. | Covered Wallstent (Partially covered type) | 78 | Success | New SEMS |
| 9 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 155 | Success | New SEMS |
| 10 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 32 | Success | New SEMS |
| 11 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 98 | Success | New SEMS |
| 12 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 50 | Success | New SEMS |
| 13 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 157 | Success | New SEMS |
| 14 | Pancreatic ca. | Niti-S Biliary ComVi Stent (Fully covered type) | 75 | Success | New SEMS |
| 15 | AIP | Niti-S Biliary ComVi Stent (Fully covered type) | 8 | Success | None |
| 16 | MFP | Covered Wallstent (Partially covered type) | 100 | Success | New SEMS |
| 17 | MFP | Covered Wallstent (Partially covered type) | 176 | Unsuccess | None |
| 18 | CP | Niti-S Biliary Covered Stent with removal suture | 33 | Success | New SEMS |
| 19 | CP | Niti-S Biliary Covered Stent with removal suture | 77 | Success | None |
ca.: Carcinoma; CP: Chronic pancreatitis; SEMS: Self-expandable metallic stent; MFP: Mass-forming pancreatitis; AIP: Autoimmune pancreatitis.
Of the 14 patients for whom stent removal was possible, covered SEMS were reinserted in 12, and the remaining 2 patients had a benign biliary stricture without additional stent placement because of successful dilation of the stricture.
In 5 patients for whom stent removal was impossible, a stent-in-stent procedure with a covered Wallstent was used for one patient, and trimming was performed for 3 patients as an alternative method (Table 2). For the one remaining patient, the progress was monitored because the stent was found to be open by imaging.
Stent trimming
Stent trimming was successful for all the patients (Table 3). Trimming time was 12 min for the single patient with the uncovered Wallstent, a mean of 14 min (range, 12-16 min) for 3 patients with the covered Wallstent, a mean of 13 min (range, 11-15 min) for 4 patients with the Niti-S Biliary ComVi Stent. Of the 2 patients for whom stent trimming was successful found to have bile duct stenosis and so a stent-in-stent procedure was performed using the covered Wallstent and Niti-S Biliary ComVi stent.
Table 3.
Outcome of stent trimming
| No. of case | Disease | SEMS | Procedural time (min) | Additional procedure after trimming |
| 1 | Pancreatic ca. | Uncovered Wallstent | 12 | None |
| 2 | Pancreatic ca. | Niti-S Biliary ComVi Stent | 11 | Stent in stent |
| 3 | Pancreatic ca. | Niti-S Biliary ComVi Stent | 14 | Stent in stent |
| 4 | Pancreatic ca. | Covered Wallstent | 14 | None |
| 5 | Pancreatic ca. | Covered Wallstent | 16 | None |
| 6 | Bile duct ca. | Niti-S Biliary ComVi Stent | 12 | None |
| 7 | IPMC | Covered Wallstent | 12 | None |
| 8 | Lymph node metastasis1 | Niti-S Biliary ComVi Stent | 15 | None |
Argon plasma coagulation setting: 60-80 W, Coagulation mode 1.5 L/min.
Due to cancer of the uterine body. ca.: Carcinoma; IPMC: Intraductal papillary mucinous carcinoma; SEMS: Self-expandable metallic stent.
There was self-limiting hemorrhage due to injury to the esophageal mucosa during removal of the stent fragments in one patient. The patient progressed well with conservative treatment.
DISCUSSION
Nowadays, placement of SEMS has become the gold standard for the treatment of malignant distal biliary stricture[1,2]. In addition, SEMS have recently been used for postoperative bile leaks, benign biliary strictures such as in chronic pancreatitis, or postoperative biliary strictures[14-17]. Nevertheless, stent occlusions do occur. Previously, when we encountered a SEMS occlusion, we used the stent-in-stent technique. Recently, stent replacement by stent removal, which is an alternative to the stent-in-stent technique, has been attempted[7-9]. In reports to date, the rate of complete removal of covered SEMS is 95.5% (169/177), which has been described as having a comparatively high rate of success (Table 4). However, an uncovered metallic stent is usually impossible to remove as a result of it being embedded in tissue, regardless of whether the disease is benign or malignant. There are also reports of a stent being removed by breaking it up with forceps[18-20], but this is time consuming and there is also the possibility of the channel being damaged by stent fragments. Therefore, this procedure is unlikely to become popular. In the present study, the rate of success for complete removal of the covered metallic stents was 77.8%,while that of the uncovered metallic stents was 0%, showing a comparatively good result for the rate of complete removal of the covered metallic stent. However, even with covered metallic stents, 22.2% were not removable, showing that in fact, there is a limitation to this technique’s total success rate. In reports to date, one of the reasons why it is difficult to remove covered metallic stents is said to be severe tissue growth over the uncovered part[8]. In addition, it has been reported that tumor growth causes duodenal stenosis, making it impossible to approach papillary areas[6,7].
Table 4.
Review of stent removal
| Author | Total No. of stent/patient | Type of SEMS | n | Disease | Mean duration of indwelling stent (range) | Success rate (%) |
| Kahaleh et al[7] | 18/18 | Uncovered Wallstent | 2 | BBS | (1-4 mo) | 2/2 (100) |
| Uncovered Wallstent | 2 | MBS | (10-11 mo) | 2/2 (100) | ||
| Covered Wallstent | 6 | BBS | 4.7 mo (1-10 mo) | 6/6 (100) | ||
| Covered Wallstent | 8 | MBS | 4.5 mo (1-16 mo) | 7/8 (87.5) | ||
| Familiari et al[8] | 39/29 | Covered Wallstent | 12 | 12/12 (100) | ||
| Shim-Hanarostent | 10 | 9/10 (90) | ||||
| Uncovered Wallstent | 10 | MBS (26) | 7.5 mo | 5/10 (50) | ||
| Niti-S Biliary Stent | 4 | BBS (3) | (5 d-16 mo) | 3/4 (75) | ||
| Sinus-Superflex Stent | 2 | 0/2 (0) | ||||
| Zilver Stent | 1 | 0/1 (0) | ||||
| Shin et al[9] | 30/30 | Uncovered Wallstent | 5 | MBS | Uncovered SEMSs | 0/5 (0) |
| Zilver Stent | 3 | MBS | 121.4 ± 45.4 d | 0/3 (0) | ||
| Covered Wallstent | 21 | MBS | Covered SEMSs | 18/21 (85.7) | ||
| Covered Wallstent | 1 | BBS | 129.7 ± 95.4 d | 1/1 (100) | ||
| Kahaleh et al[15] | 65/65 | Covered Wallstent | 65 | BBS | 4 mo (1-28 mo) | 65/65 (100) |
| Present study | 19/19 | Covered Wallstent | 2 | MBS | 46.5 d (15-78 d) | 2/2 (100) |
| Covered Wallstent | 2 | BBS | (100-176 d) | 1/2 (50) | ||
| Uncovered Wallstent | 1 | MBS | 180 d | 0/1 (0) | ||
| Niti-S Biliary ComVi Stent | 11 | MBS | 136 d (32-280 d) | 8/11 (72.7) | ||
| Niti-S Biliary ComVi Stent | 1 | BBS | 8 d | 1/1 (100) | ||
| Niti-S Biliary Covered Stent with removal suture | 2 | BBS | 55 d (33-77 d) | 2/2 (100) |
SEMS: Self-expandable metallic stent; BBS: Benign biliary strictures; MBS: Malignant biliary strictures.
In the present study, although there was no statistical significance, the mean indwelling period of removable SEMS was shorter than those of unremovable SEMS, suggesting that the length of the indwelling period may affect the successful removal of SEMS. Structural properties of the stent were also shown to affect the possibility of stent removal. For the covered Wallstent, the entire diameter was found to shrink and straighten when the stent was held and pulled towards the biliary axis. However, for the Niti-S Biliary ComVi stent, only the part that was held was found to shrink and even the straightening was inadequate, making it difficult to transmit force. Therefore, compared to the covered Wallstent, it was our experience that it was slightly difficult to remove. However, these possible correlations are unclear because of the small number of patients.
Another problem with the covered metallic stent is displacement of the stent towards the duodenum. Covered SEMS are easier to remove than uncovered SEMS. However, they migrate more easily than uncovered SEMS[21,22]. In future, the risk of stent displacement due to shrinkage of tumors in patients that respond to chemotherapy and radiotherapy is likely to increase. In cases where the prolapsing part is extremely long and cannot be held with a snare or holding forceps, it is sometimes effective to use recovery methods such as the wire loop technique[23]. Recently, stent trimming has occasionally been used. Basically, the purposes of trimming are to resolve any occlusion as a result of burial of the stent tip towards the duodenal wall, eliminate mechanical irritation of the duodenal wall, and ensure an accessible route to the bile duct. In particular, trimming using APC is known to be inexpensive and simple. Presently, the safety and usefulness of APC trimming have been confirmed in experimental[24] and clinical[4,10-13] studies. However, its indication, method of intervention, and settings have not yet been established because there are no uniform conditions for setting APC in trimming. From various studies, settings for the high frequency wave output ranged from 60 to 85 W, and gas flow from 0.8 L/min to 2.0 L/min, showing notable variation. The main reports to date are presented in Table 5[4,10-13]. In this study, gas flow was set at 1.5 L/min and the output was started at 60 W and gradually increased while observing the results of the trimming. However, at 60 W, the ablation effect was often inadequate; at 80 W, trimming could be done effectively and smoothly. In addition, even at 80 W, the trimming could be done adequately and safely without any major complications. Therefore, setting the output at 80 W initially contributed to a reduction of unnecessary testing time. With regard to the maximum output, there has been histological evaluation in animal models[24] but its status in the human body is unknown. Standardization of a safe and effective setting of the output would be ideal.
Table 5.
Review of biliary stent trimming
| Auther | Total No. of cases | SEMS | No. of case | Setting of APC system | Procedural time | Success rate |
| Demarquay et al[4] | 3 | Uncovered Wallstent | 3 | 85 W, coag. 0.8 L/min | NA | 100% (3/3) |
| Vanbiervliet et al[10] | 24 | Uncovered Wallstent | 23 | 70-80 W, coag. 0.8 L/min | < 30 min | 95.7% (22/23) |
| Covered Wallstent | 1 | 70-80 W, coag. 0.8 L/min | < 30 min | 0% (0/1) | ||
| Guda et al[11] | 2 | Uncovered Wallstent | 1 | 80 W, coag. 2.0 L/min | NA | 100% (1/1) |
| Uncovered Wallstent | 1 | 60 W, coag. 2.0 L/min | NA | 100% (1/1) | ||
| Rerknimitr et al[12] | 2 | Covered Wallstent | 1 | 60 W, coag. 0.8 L/min | 20 min | 100% (1/1) |
| Uncovered Wallstent | 1 | 70 W, coag. 0.8 L/min | NA | 100% (1/1) | ||
| Christiaens et al[13] | 2 | Uncovered SEMS (nitinol) | 1 | 60 W, coag. 1.8 L/min | NA | 100% (1/1) |
| Uncovered SEMS (nitinol) | 1 | 60 W, coag. 1.8 L/min | NA | 100% (1/1) | ||
| Present study | 8 | Covered Wallstent | 3 | 60-80 W, coag. 1.5 L/min | < 20 min | 100% (3/3) |
| Uncovered Wallstent | 1 | 60-80 W, coag. 1.5 L/min | 12 min | 100% (1/1) | ||
| Niti-S Biliary ComVi Stent | 4 | 60-80 W, coag. 1.5 L/min | < 20 min | 100% (4/4) |
NA: Not available; SEMS: Self-expandable metallic stent; APC: Argon plasma coagulation; coag.: Coagulation mode.
There are various types of SEMS in use and these include Elgiloy, which is a cobalt alloy as in the Wallstent (Boston Scientific Co., Tokyo, Japan), other stainless steel stents, and recently, Nitinol, which is made up of a shape-memory alloy as in the Niti-S stent (TaeWoong Medical Co., Seoul, Korea). To date, there are reports on APC trimming with the uncovered metallic stents, Elgiloy and Nitinol, stating that trimming can be done with no major complications[4,10-13]. In the present study, the trimming time was about 15 min (range, 11-16 min) for all the patients, with no significant difference in time. With regard to the differences between the uncovered and covered metallic stents, Vanbiervliet et al[10] and Christiaens et al[13] were unsuccessful at trimming the covered metallic stents, and the reason given was poor generation of the heat required for trimming due to hindrance of the flow of electricity to the stent by the coated polyurethane resin membrane. However, Chen et al[24] reported there was no difference in technique or time required for trimming covered versus uncovered stents. This result was different from the results in clinical reports. Presently, no conclusion can be reached regarding the differences. In addition, food residue that attaches to the periphery of stents can also be considered a hindrance to the discharge of electricity[12]. As a pre-trimming intervention, adequate removal of food residue may shorten the time of the procedure.
In conclusion, although further investigations on larger numbers of cases are necessary to accumulate evidence, the present data suggest that stent removal and stent trimming is feasible and effective for stent-related complications.
COMMENTS
Background
Recently, self-expandable metallic stents (SEMS) removal has been performed as an alternative management method of occlusion of SEMS. Furthermore, metallic stent trimming by argon plasma coagulation (APC) has been used in cases of stent displacement or migration to the duodenum.
Research frontiers
In reports to date, the rate of complete removal of covered SEMS was 95.5% (169/177), which has been described as having a comparatively high rate of success. However, an uncovered metallic stent is usually impossible to remove. In the present study, of the 19 patients in whom removal of the SEMS had been attempted, the procedure was successful in 14 (73.7%) without complications. The rate of success for complete removal of the covered metallic stents was 77.8% (14/18), while that of the uncovered metallic stents was 0% (0/1). In addition, stent trimming was successful in all patients. Trimming time ranged from 11 to 16 min. The present data suggested that stent removal and trimming is feasible and effective for stent-related complications.
Innovations and breakthroughs
The present study showed a comparatively good result for the rate of complete removal of the covered metallic stent. However, even with covered metallic stents, 22.2% (4/18) were unremovable, showing that there is a limitation to this technique’s total success rate. This report suggests that the indwelling period length and structural properties of the stent may also affect the successful removal of SEMS. There are few reports about stent trimming of biliary covered SEMS. In addition, several investigators have reported no success in trimming the covered metallic stent. Meanwhile, in the present study, stent trimming was successful for all covered SEMS.
Applications
Stent removal and trimming is feasible and effective for stent-related complications.
Terminology
Covered SEMSs can be removed theoretically, because the membrane of SEMS prevents embedding caused by tumor ingrowth or tissue hyperplasia.
Peer review
This is a well written paper on removal and trimming of self expandable metal biliary stents with good results. The technique and setting used are useful for the readers.
Acknowledgments
The authors are indebted to Professor J Patrick Barron of the Department of International Medical Communications Center at Tokyo Medical University for his review of this manuscript.
Footnotes
Peer reviewers: Basil Ammori, Department of Surgery, Salford Royal Hospital, Stott Lane, Salford, Greater Manchester, M6 8HD, United Kingdom; Michael A Fink, MBBS, FRACS, Department of Surgery, The University of Melbourne, Austin Hospital, Melbourne, Victoria 3084, Australia
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
References
- 1.Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 2.Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–734. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roebuck DJ, Stanley P, Katz MD, Parry RL, Haight MA. Gastrointestinal hemorrhage due to duodenal erosion by a biliary wallstent. Cardiovasc Intervent Radiol. 1998;21:63–65. doi: 10.1007/s002709900213. [DOI] [PubMed] [Google Scholar]
- 4.Demarquay JF, Dumas R, Peten EP, Rampal P. Argon plasma endoscopic section of biliary metallic prostheses. Endoscopy. 2001;33:289–290. doi: 10.1055/s-2001-12813. [DOI] [PubMed] [Google Scholar]
- 5.Yarze JC, Poulos AM, Fritz HP, Herlihy KJ. Treatment of metallic biliary stent-induced duodenal ulceration using endoscopic laser therapy. Dig Dis Sci. 1997;42:6–9. doi: 10.1023/a:1018812416496. [DOI] [PubMed] [Google Scholar]
- 6.Bueno JT, Gerdes H, Kurtz RC. Endoscopic management of occluded biliary Wallstents: a cancer center experience. Gastrointest Endosc. 2003;58:879–884. doi: 10.1016/s0016-5107(03)02309-5. [DOI] [PubMed] [Google Scholar]
- 7.Kahaleh M, Tokar J, Le T, Yeaton P. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004;60:640–644. doi: 10.1016/s0016-5107(04)01959-5. [DOI] [PubMed] [Google Scholar]
- 8.Familiari P, Bulajic M, Mutignani M, Lee LS, Spera G, Spada C, Tringali A, Costamagna G. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005;62:903–910. doi: 10.1016/j.gie.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Shin HP, Kim MH, Jung SW, Kim JC, Choi EK, Han J, Lee SS, Seo DW, Lee SK. Endoscopic removal of biliary self-expandable metallic stents: a prospective study. Endoscopy. 2006;38:1250–1255. doi: 10.1055/s-2006-944969. [DOI] [PubMed] [Google Scholar]
- 10.Vanbiervliet G, Piche T, Caroli-Bosc FX, Dumas R, Peten EP, Huet PM, Tran A, Demarquay JF. Endoscopic argon plasma trimming of biliary and gastrointestinal metallic stents. Endoscopy. 2005;37:434–438. doi: 10.1055/s-2005-860989. [DOI] [PubMed] [Google Scholar]
- 11.Guda NM, Freeman ML. Endoscopic transection of distally migrated biliary self-expanding metallic stents by using argon plasma coagulation: a report of 2 cases (with video) Gastrointest Endosc. 2006;63:512–514. doi: 10.1016/j.gie.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Rerknimitr R, Naprasert P, Kongkam P, Kullavanijaya P. Trimming a metallic biliary stent using an argon plasma coagulator. Cardiovasc Intervent Radiol. 2007;30:534–536. doi: 10.1007/s00270-006-0013-z. [DOI] [PubMed] [Google Scholar]
- 13.Christiaens P, Decock S, Buchel O, Bulté K, Moons V, D’Haens G, Van Olmen G. Endoscopic trimming of metallic stents with the use of argon plasma. Gastrointest Endosc. 2008;67:369–371. doi: 10.1016/j.gie.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Baron TH, Poterucha JJ. Insertion and removal of covered expandable metal stents for closure of complex biliary leaks. Clin Gastroenterol Hepatol. 2006;4:381–386. doi: 10.1016/j.cgh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, Sundaram V, Tokar J, Adams RB, Yeaton P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video) Gastrointest Endosc. 2008;67:446–454. doi: 10.1016/j.gie.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697–700. doi: 10.1055/s-2008-1077353. [DOI] [PubMed] [Google Scholar]
- 17.Mahajan A, Ho H, Sauer B, Phillips MS, Shami VM, Ellen K, Rehan M, Schmitt TM, Kahaleh M. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video) Gastrointest Endosc. 2009;70:303–309. doi: 10.1016/j.gie.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A, Keeffe EB, Imperial JC. A novel technique for endoscopic removal of expandable biliary Wallstent. Gastrointest Endosc. 1999;50:279–281. doi: 10.1016/s0016-5107(99)70242-7. [DOI] [PubMed] [Google Scholar]
- 19.Itoi T, Nakamura K, Sofuni A, Itokawa F, Shinohara Y, Takeda K, Tsuchida A, Aoki T, Moriyasu F. Extraction of uncovered biliary metallic stent using percutaneous transhepatic cholangioscopy. Digestive Endoscopy. 2002;14:175–177. [Google Scholar]
- 20.Baron TH, Blackard WG, Morgan DE. Endoscopic removal of a “floating” biliary Gianturco Z stent five years after placement for a benign anastomotic stricture in a liver transplant patient. Gastrointest Endosc. 1997;46:80–82. doi: 10.1016/s0016-5107(97)70217-7. [DOI] [PubMed] [Google Scholar]
- 21.Wamsteker EJ, Elta GH. Migration of covered biliary self-expanding metallic stents in two patients with malignant biliary obstruction. Gastrointest Endosc. 2003;58:792–793. doi: 10.1016/s0016-5107(03)02024-8. [DOI] [PubMed] [Google Scholar]
- 22.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986–995. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 23.Itoi T, Nakamura K, Sofuni A, Itokawa F, Moriyasu F, Tsuchida A. Endoscopic removal of a dislocated covered wallstent using a wire-loop technique. Digestive Endoscopy. 2003;15:312–315. [Google Scholar]
- 24.Chen YK, Jakribettuu V, Springer EW, Shah RJ, Penberthy J, Nash SR. Safety and efficacy of argon plasma coagulation trimming of malpositioned and migrated biliary metal stents: a controlled study in the porcine model. Am J Gastroenterol. 2006;101:2025–2030. doi: 10.1111/j.1572-0241.2006.00744.x. [DOI] [PubMed] [Google Scholar]