Abstract
AIM: To determine the association between serum levels of growth-related gene product β (GROβ) and clinical parameters in esophageal squamous cell carcinoma (ESCC).
METHODS: Using enzyme-linked immunosorbent assay, serum GROβ levels were measured in ESCC patients (n = 72) and healthy volunteers (n = 83). The association between serum levels of GROβ and clinical parameters of ESCC was analyzed statistically.
RESULTS: The serum GROβ levels were much higher in ESCC patients than in healthy controls (median: 645 ng/L vs 269 ng/L, P < 0.05). Serum GROβ levels were correlated positively with tumor size, lymph node metastasis, and tumor-node-metastasis (TNM) staging, but not with gender or the histological grade of tumors in ESCC patients. The sensitivity and specificity of the assay for serum GROβ were 73.61% and 56.63%, respectively.
CONCLUSION: GROβ may function as an oncogene product and contribute to tumorigenesis and metastasis of ESCC.
Keywords: GROβ, Esophageal squamous cell carcinoma, Metastasis, Cytokine, Tumor markers
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive malignancies in China, South Africa, and some other developing countries[1,2]. In China, patients with ESCC have a poor prognosis, with an average 5-year survival rate of less than 30%[3-5]. It is thus imperative to find new markers, especially serum protein markers, to facilitate the early detection and diagnosis of ESCC.
CXC chemokine receptors are integral membrane proteins that specifically bind and are activated by cytokines of the CXC chemokine family, a group of small cytokines[6] involved in the response to injury[7] and inflammatory reactions[8] as well tumorigenesis, cancer progression, and metastasis[9,10]. CXCL16 expression is increased in multiple tumor tissues and cell lines, and upregulation is associated with tumor progression and metastasis[11]. The plasma levels of CXCL4 and CXCL6 are much higher in patients with osteosarcoma than in healthy controls, and elevated plasma CXCL4 and CXCL6 levels are associated with poor prognosis[10]. Growth-related gene product (GRO) is a member of the CXC chemokine family, which is composed of GROα, GROβ and GROγ[12,13]. GROα is highly expressed in a variety of tumors and plays an important role in tumor proliferation[14], angiogenesis[15], and metastasis[16]. Compared with GROα, the roles of GROβ in tumors are poorly understood.
Recently, authors from South Africa reported that GROβ is highly expressed in ESCC tissues and cell lines[17]. Although this suggests that GROβ may be secreted from tumor tissues into the extracellular milieu, no report has yet investigated serum GROβ expression levels in human ESCC. In this study, we measured the serum levels of GROβ in ESCC patients and healthy controls by enzyme-linked immunosorbent assay (ELISA), and analyzed the association between clinical parameters of ESCC and serum GROβ levels.
MATERIALS AND METHODS
Clinical specimen collection and preparation
Serum samples were collected from 72 ESCC patients [52 males and 20 females; mean age, 61 ± 7.7 years (SD); range 41-76 years] and 83 healthy controls [45 males and 38 females; mean age, 57 ± 8.4 years (SD); range 41-70 years] after the informed consent was obtained. Pathological diagnosis was independently conducted by two senior pathologists. The healthy individuals were negative for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus. Abdominal ultrasound examination, routine blood tests, and biochemistry tests were performed for the healthy controls, and the results were within normal ranges. Sample preparation was referred to the reference[18]. The clinical characteristics of the serum samples are shown in Table 1.
Table 1.
Clinical parameters of esophageal squamous cell carcinoma patients and healthy controls n (%)
| ESCC patients (n = 72) | Healthy controls (n = 83) | |
| Age (yr) | ||
| < 50 | 5 (7) | 16 (19) |
| ≥ 50 | 67 (93) | 67 (81) |
| Gender | ||
| Male | 52 (72) | 45 (54) |
| Female | 20 (28) | 38 (46) |
| Histological grade | ||
| Well | 16 (22) | - |
| Well/moderate | 2 (3) | - |
| Moderate | 33 (46) | - |
| Moderate/poor | 3 (4) | - |
| Poor | 15 (21) | - |
| Unknown | 3 (4) | - |
| Tumor size (cm) | ||
| < 5 | 32 (44) | - |
| ≥ 5 | 37 (51) | - |
| Unknown | 3 (5) | - |
| TNM stage | ||
| I | 14 (19) | - |
| II | 21 (29) | - |
| III | 31 (43) | - |
| Unknown | 6 (8) | - |
| Lymph node metastasis | ||
| Yes | 34 (47) | - |
| No | 31 (43) | - |
| Unknown | 7 (10) | - |
ESCC: Esophageal squamous cell carcinoma.
ELISA for GROβ
A human GROβ ELISA development kit (PeproTech, Rocky Hill, NJ, USA) was used to measure GROβ in human serum. Briefly, 96-well ELISA microplates were coated overnight with 100 μL GROβ antibody (PeproTech, Rocky Hill, NJ, USA) at a final concentration of 0.25 mg/L in PBS. After washing with PBS/0.05% (w/v) Tween-20 (PBST, pH 7.4), the wells were blocked with blocking buffer at room temperature for 1 h. Then, 100 μL diluted serum samples (at 1:5 dilution) were added and incubated at room temperature for 2 h. Similarly, 100 μL PBST lacking antibody was used as a negative control. Following three washes with PBST, 100 μL antibody diluted to a concentration of 0.25 mg/L was added. After incubation at room temperature for 2 h, 100 μL avidin-horseradish peroxidase-conjugated secondary antibody (at 1:2000 dilution) was added, and plates were incubated at room temperature for 30 min. The excess conjugate was removed by washing the plates three times with PBST. The amount of bound conjugate was determined by adding ABTS liquid substrate solution to each well, and plates were incubated at room temperature for color development. The absorbance was measured at 405 nm using a Model 680 microplate reader (Bio-Rad Lab. Inc., Hercules, CA, USA). All analyses were performed in triplicate. The coefficient of variation was lower than 15% between analyses.
Statistical analysis
Statistical significance was determined with the nonparametric Mann-Whitney U-test (differences between two groups) and the Kruskal-Wallis nonparametric test (differences among more than two groups) with SPSS 16.0 software (Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Serum GROβ levels in ESCC patients and healthy controls
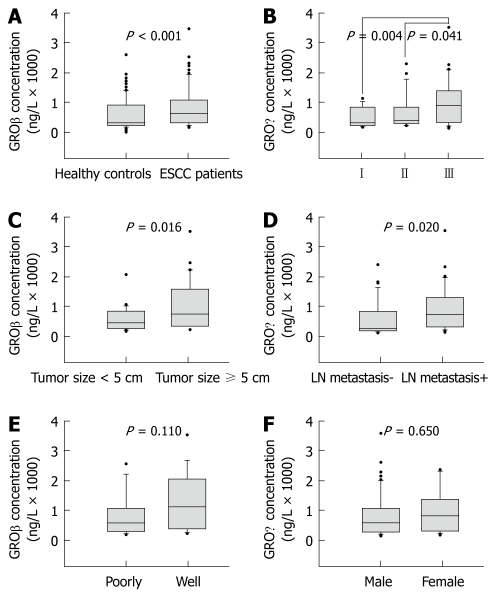
A total of 155 participants were enrolled in this study. The serum levels of GROβ in ESCC patients (n = 72) and healthy controls (n = 83) were assessed by ELISA. Serum concentrations of GROβ ranged from 146 ng/L to 3721 ng/L (median = 645 ng/L) in ESCC patients, and from 0 ng/L to 2781 ng/L (median = 269 ng/L) in healthy controls. GROβ levels were significantly higher in ESCC patients than in healthy controls (Mann-Whitney U-test, P < 0.001, Figure 1A). The sensitivity and specificity of serum GROβ were 73.61% and 56.63%, respectively.
Figure 1.
Correlations between serum growth-related gene product β level and clinicopathological parameters in esophageal squamous cell carcinoma. Serum growth-related gene product β (GROβ) levels were measured in esophageal squamous cell carcinoma (ESCC) patients (n = 72) and healthy volunteers (n = 83) using enzyme-linked immunosorbent assay. Serum levels of GROβ were significantly increased in ESCC patients (A). Serum levels of GROβ were correlated positively with tumor-node-metastasis stage (B), tumor size (C), and lymph node (LN) metastasis (D), but not with histological grade (E) or gender (F) in ESCC patients. The boxes represent the distribution of the 25th and 75th percentile data (the line in the box is the median, and the dots are the outlying points).
Association between serum concentrations of GROβ and clinical parameters in ESCC
We assessed the potential correlations between serum levels of GROβ and clinical parameters, including tumor-node-metastasis (TNM) stage, tumor size, lymph node metastasis, histological grade, and gender. The median levels of GROβ in the ESCC patients at different stages were: stage I, 284 ng/L (range, 146-1141 ng/L); stage II, 360 ng/L (range, 211-2453 ng/L); and stage III, 966 ng/L (range, 165-3721 ng/L)”. The serum GROβ levels were significantly different (Kruskal-Wallis nonparametric test, P = 0.006) among the three TNM stages, and significantly higher in patients at stage III than in those at stage II (Mann-Whitney U-test, P = 0.041, Figure 1B) and stage I (Mann-Whitney U-test, P = 0.004, Figure 1B). However, there was no significant difference between stage I and stage II (Mann-Whitney U-test, P = 0.135). Furthermore, patients with tumor diameters ≥ 5 cm had higher serum levels of GROβ (median = 786 ng/L; range, 191-3721 ng/L) than those with smaller tumors (median = 456 ng/L; range, 146-2008 ng/L; Mann-Whitney U-test, P = 0.016; Figure 1C). Serum levels of GROβ in patients with lymph node metastasis (median = 819 ng/L; range, 165-3721 ng/L) were significantly higher than those without lymph node metastasis (median = 330 ng/L; range, 146-2453 ng/L; Mann-Whitney U-test, P = 0.020; Figure 1D). However, there was no significant difference between serum levels of GROβ in patients with poorly differentiated tumors (median = 607 ng/L; range, 191-2705 ng/L) and well-differentiated tumors (median = 1178 ng/L; range, 239-3721 ng/L; Mann-Whitney U-test, P = 0.110, Figure 1E). Serum levels of GROβ were not gender dependent (Mann-Whitney U-test, P = 0.650, Figure 1F).
DISCUSSION
Identification of targets for early detection of ESCC is important to improve the prognosis of the patients with this pernicious disease. Currently, carcinoembryonic antigen, cytokeratin 19 fragments, and squamous cell carcinoma-associated antigen are routinely used as serum markers for detection of ESCC. Due to the low sensitivity and specificity of detection of these markers[19], additional serum markers must be established for early detection and diagnosis of ESCC.
GROβ, also known as the chemokine CXCL2, is a member of the CXC chemokine family and shares the same receptor with interleukin (IL)-8, interleukinIL-8RB/CXCR2. GROβ is widely expressed in the central nervous system[20] and possesses important functions, such as attracting neutrophils to sites of inflammation, mobilizing stem cells[21-25], and modulating neurotransmitter release[26]. The roles of GROβ in tumor formation and development have been investigated[27], and a recent report described significant elevation of GROβ mRNA in colon cancer tissues[28]. Upregulation of GROβ promotes colony formation by melanocytes in soft agar and tumor formation in nude mice[13,29]. Conversely, decreased expression of GROβ inhibits the proliferation and colonization capacity of esophageal cancer cells[30]. Through binding to its receptor CXCR2, GROβ forms an autocrine loop that activates the Ras-Erk1/2 signaling pathway, which is important for cell proliferation[17,25,26,31]. This pathway in turn activates the transcription and expression of the early growth response 1 gene (egr1), a transcription factor that regulates the expression of downstream factors related to cell growth and cell cycle regulation (p65, p27), thereby promoting tumor growth[32-34].
Using cDNA microarray analysis, authors from South Africa showed that GROβ was highly expressed in ESCC tissues and cultured cells[17]. As a chemokine, GROβ may be secreted into the extracellular matrix. However, no report has investigated the clinical significance of serum GROβ levels in ESCC. To compare the levels of serum GROβ in patients with ESCC and healthy controls, we measured serum GROβ concentrations with ELISA. The level of serum GROβ was much higher in patients with ESCC than in healthy controls, which supported the hypothesis that GROβ may function as an oncogene in ESCC.
We assessed the potential correlations between serum levels of GROβ and several clinical parameters of ESCC, including tumor size, TNM stage, lymph node metastasis, histological grade and gender. Serum levels of GROβ were correlated significantly with tumor size and the extent of metastasis. Patients with larger tumors (≥ 5 cm in diameter) had higher levels of serum GROβ than those with smaller tumors (P < 0.05). Likewise, serum GROβ levels in patients with lymph node metastasis were significantly higher than those without lymph node metastasis (P < 0.05). The elevation of serum GROβ in ESCC patients may therefore reflect the enhanced potential of tumor cells to proliferate and metastasize, which is consistent with TNM staging[35]. Indeed, GROβ serum levels were significantly increased in stage III patients compared with stage II and stage I patients (P < 0.05). The histological grade of tumors is also an important indicator associated with the development and prognosis of cancer[36]. However, no significant differences were found in the serum levels of GROβ in ESCC patients with different histological tumor grades (P > 0.05) in our study. Because our study involved a relatively small number of ESCC patients and the sensitivity and specificity of our assay to measure serum GROβ were not sufficiently high, further confirmation in a larger sample size is warranted.
In conclusion, we found that serum GROβ levels were increased in ESCC patients and correlated positively with tumor size, TNM stage and lymph node metastasis, but not with gender or the histological grade of ESCC. GROβ may function as an oncogene product and play a role in tumor formation, progression and metastasis. Examining and monitoring serum GROβ levels may be useful in estimating the prognosis of ESCC.
COMMENTS
Background
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive malignancies in China, South Africa and some other developing countries. In China, prognosis of the patients with ESCC is poor, with a 5-year survival rate of less than 30%. It is important to find new markers, especially serum protein markers to facilitate the early detection and diagnosis of ESCC.
Research frontiers
Growth-related gene product β (GROβ) was reported to be highly expressed in ESCC tissues and cell lines. As a chemokine, GROβ could be secreted from cells to extracellular matrix. But no report has yet investigated serum GROβ expression levels in human ESCC. In the present study, serum level of GROβ between patients with ESCC and healthy controls were firstly measured, and the association between clinical parameters of ESCC and serum GROβ levels were also analyzed.
Innovations and breakthroughs
The serum level of GROβ was increased in ESCC patients and was positively correlated with tumor size, tumor-node-metastasis staging and lymph node metastasis of the patients. GROβ might serve as an oncogene and contribute to tumorigenesis and metastasis of ESCC.
Applications
Examining and monitoring serum GROβ levels are useful in estimating the prognosis of ESCC patients.
Terminology
GROβ, also known as CXCL2 chemokine, is a member of the CXC chemokine family. It is widespread in the central nervous system and involved in many important functions, such as attracting neutrophils to inflammation sites, mobilization of stem cells and neurotransmitter release modulation.
Peer review
GRO, a member of the CXC chemokine subfamily, plays a major role in inflammation and wound healing. CXC chemokines have been found to be associated with tumorigenesis, angiogenesis and metastasis. The roles of GROβ in tumor formation and development were unclear. The papers from tumor cell lines, animal and human studies on this field showed controversial results.
Acknowledgments
We thank Drs. Yu-Lin Sun, You-Sheng Mao, Fang Liu and Lan-Ping Zhou for critical reading of the manuscript and sample preparations. Additional correspondence authorship: Zhao X, Center of Basic Medical Sciences, Navy General Hospital, Beijing 100048, China.
Footnotes
Supported by The Grants from International Science & Technology Cooperation and Exchange Programs, No. 2008DFA31130; Joint China/South Africa Science and Technology Agreement; National Natural Science Foundation of China, No. 81021061, No. 0772507 and No. 30700992; State Key Projects for Basic Research of China, No. 2011CB910703
Peer reviewer: Yu-Yuan Li, Professor, Department of Gastroenterology, First Municipal People’s Hospital of Guangzhou, 1 Panfu Road, Guangzhou 510180, Guangdong Province, China
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 2.Hendricks D, Parker MI. Oesophageal cancer in Africa. IUBMB Life. 2002;53:263–268. doi: 10.1080/15216540212643. [DOI] [PubMed] [Google Scholar]
- 3.Lu CL, Lang HC, Luo JC, Liu CC, Lin HC, Chang FY, Lee SD. Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma, in Taiwan. Cancer Causes Control. 2010;21:269–274. doi: 10.1007/s10552-009-9458-0. [DOI] [PubMed] [Google Scholar]
- 4.Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Lu F, Zhang S. [Analyses of variation trend and short-term detection of Chinese malignant tumor mortality during twenty years] Zhonghua Zhongliu Zazhi. 1997;19:3–9. [PubMed] [Google Scholar]
- 6.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 7.Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M. CXC chemokines play a critical role in liver injury, recovery, and regeneration. Am J Surg. 2009;198:415–419. doi: 10.1016/j.amjsurg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y, Zhao L, Yan F. Chemokines as novel therapeutic targets in autoimmune thyroiditis. Recent Pat DNA Gene Seq. 2010;4:52–57. doi: 10.2174/187221510790410787. [DOI] [PubMed] [Google Scholar]
- 9.Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Flores R, Yu A, Okcu MF, Murray J, Chintagumpala M, Hicks J, Lau CC, Man TK. Elevated expression of CXC chemokines in pediatric osteosarcoma patients. Cancer. 2011;117:207–217. doi: 10.1002/cncr.25563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng L, Chen N, Li Y, Zheng H, Lei Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim Biophys Acta. 2010;1806:42–49. doi: 10.1016/j.bbcan.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Modi WS, Yoshimura T. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol Biol Evol. 1999;16:180–193. doi: 10.1093/oxfordjournals.molbev.a026101. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, Tsubakimoto K, Endo T, Richmond A. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 15.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 16.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Mi W, Cai J, Ying W, Liu F, Lu H, Qiao Y, Jia W, Bi X, Lu N, et al. Quantitative proteomic signature of liver cancer cells: tissue transglutaminase 2 could be a novel protein candidate of human hepatocellular carcinoma. J Proteome Res. 2008;7:3847–3859. doi: 10.1021/pr800153s. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi H, Ohno S, Miyazaki M, Hashimoto K, Egashira A, Saeki H, Watanabe M, Sugimachi K. CYFRA 21-1 determination in patients with esophageal squamous cell carcinoma: clinical utility for detection of recurrences. Cancer. 2000;89:1413–1417. doi: 10.1002/1097-0142(20001001)89:7<1413::aid-cncr1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol. 1999;5:13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- 21.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 22.Rossi EM, Pylkkänen L, Koivisto AJ, Vippola M, Jensen KA, Miettinen M, Sirola K, Nykäsenoja H, Karisola P, Stjernvall T, et al. Airway exposure to silica-coated TiO2 nanoparticles induces pulmonary neutrophilia in mice. Toxicol Sci. 2010;113:422–433. doi: 10.1093/toxsci/kfp254. [DOI] [PubMed] [Google Scholar]
- 23.Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008;15:285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorburn E, Kolesar L, Brabcova E, Petrickova K, Petricek M, Jaresova M, Slavcev A, Striz I. CXC and CC chemokines induced in human renal epithelial cells by inflammatory cytokines. APMIS. 2009;117:477–487. doi: 10.1111/j.1600-0463.2009.02446.x. [DOI] [PubMed] [Google Scholar]
- 25.Ragozzino D, Giovannelli A, Mileo AM, Limatola C, Santoni A, Eusebi F. Modulation of the neurotransmitter release in rat cerebellar neurons by GRO beta. Neuroreport. 1998;9:3601–3606. doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- 26.Limatola C, Mileo AM, Giovannelli A, Vacca F, Ciotti MT, Mercanti D, Santoni A, Eusebi F. The growth-related gene product beta induces sphingomyelin hydrolysis and activation of c-Jun N-terminal kinase in rat cerebellar granule neurones. J Biol Chem. 1999;274:36537–36543. doi: 10.1074/jbc.274.51.36537. [DOI] [PubMed] [Google Scholar]
- 27.Daller B, Müsch W, Röhrl J, Tumanov AV, Nedospasov SA, Männel DN, Schneider-Brachert W, Hehlgans T. Lymphotoxin-β receptor activation by lymphotoxin-α(1)β(2) and LIGHT promotes tumor growth in an NFκB-dependent manner. Int J Cancer. 2011;128:1363–1370. doi: 10.1002/ijc.25456. [DOI] [PubMed] [Google Scholar]
- 28.Doll D, Keller L, Maak M, Boulesteix AL, Siewert JR, Holzmann B, Janssen KP. Differential expression of the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and their impact on metastatic disease and survival. Int J Colorectal Dis. 2010;25:573–581. doi: 10.1007/s00384-010-0901-1. [DOI] [PubMed] [Google Scholar]
- 29.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Bruyère C, Lonez C, Duray A, Cludts S, Ruysschaert JM, Saussez S, Yeaton P, Kiss R, Mijatovic T. Considering temozolomide as a novel potential treatment for esophageal cancer. Cancer. 2010:Epub ahead of print. doi: 10.1002/cncr.25687. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Khachigian LM, Esau L, Birrer MJ, Zhao X, Parker MI, Hendricks DT. A key role for early growth response-1 and nuclear factor-kappaB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer. Mol Cancer Res. 2009;7:755–764. doi: 10.1158/1541-7786.MCR-08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redmond KL, Crawford NT, Farmer H, D’Costa ZC, O’Brien GJ, Buckley NE, Kennedy RD, Johnston PG, Harkin DP, Mullan PB. T-box 2 represses NDRG1 through an EGR1-dependent mechanism to drive the proliferation of breast cancer cells. Oncogene. 2010;29:3252–3262. doi: 10.1038/onc.2010.84. [DOI] [PubMed] [Google Scholar]
- 34.Dong Q, Zhang J, Hendricks DT, Zhao X. GROβ and its downstream effector EGR1 regulate cisplatin-induced apoptosis in WHCO1 cells. Oncol Rep. 2011;25:1031–1037. doi: 10.3892/or.2011.1163. [DOI] [PubMed] [Google Scholar]
- 35.Ito Y, Ichihara K, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, Higashiyama T, Takamura Y, Kobayashi K, et al. Establishment of an intraoperative staging system (iStage) by improving UICC TNM classification system for papillary thyroid carcinoma. World J Surg. 2010;34:2570–2580. doi: 10.1007/s00268-010-0710-2. [DOI] [PubMed] [Google Scholar]
- 36.Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol. 2010;49:57–62. doi: 10.3109/02841860903334411. [DOI] [PubMed] [Google Scholar]