Abstract
Despite the widespread use of Mycobacterium bovis bacillus Calmette-Guerin (BCG) childhood vaccine, tuberculosis (TB) remains a serious global health problem. A successful vaccine against TB that replaces or boosts BCG will include antigens that induce or recall appropriate T cell responses. Four Mycobacterium tuberculosis (Mtb) antigens, including members of the virulence factor families PE/PPE and EsX, or antigens associated with latency were produced as a single recombinant fusion protein. When administered with the adjuvant GLA-SE, a stable oil-in-water nanoemulsion, the fusion protein ID93 was immunogenic in mice, guinea pigs, and cynomolgus monkeys. In mice, ID93/GLA-SE combination induced polyfunctional CD4 TH1-cell responses characterized by antigen-specific IFN-gamma, tumor necrosis factor and interleukin-2, as well as a reduction in the number of bacteria in the lungs of animals subsequently infected with virulent or multidrug resistant Mtb strains. Furthermore, boosting BCG-vaccinated guinea pigs with ID93/GLA-SE resulted in reduced pathology and fewer bacilli, and prevented the death of animals challenged with virulent Mtb. Finally, ID93 elicited polyfunctional effector CD4 and CD8 T-cell responses in BCG-vaccinated or Mtb-exposed human peripheral blood mononuclear cells. This study establishes that the protein subunit vaccine ID93/GLA-SE protects against TB and MDR-TB in animals, and is a candidate for boosting the protective efficacy of the childhood BCG vaccine.
Keywords: vaccination, mycobacteria, drug-resistance
INTRODUCTION
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb). The World Health Organization has declared TB a global public health emergency and predicts that almost one billion people will be infected, with 35 million dying from the disease by 2020. TB persists as a global health concern in part because infected individuals are often non-compliant with the 6-month or longer drug treatment. This is particularly true in the developing world where more than 95% of cases occur. Treatment non-compliance has contributed to the current TB pandemic by increasing the probability of transmission and sustaining the development of multidrug (MDR) strains of Mtb. MDR strains are resistant to the two most powerful first-line drugs, rifampicin and isoniazid. Since the discovery of MDR-TB in the 1990s, the resistance pattern of TB has continued to evolve, and isolates resistant to both first- and second-line drugs have been identified (1). Therefore, development of a safe, efficacious, and affordable prophylactic vaccine that is also able to provide long-lasting protection in bacillus Calmette-Guerin (BCG)-immunized people is a critical step toward controlling TB.
High mortality and morbidity continue to be associated with Mtb infections despite the widespread use of BCG, a live, attenuated vaccine. BCG is the only TB vaccine currently licensed for use in humans and appears to be effective at preventing severe disseminated disease in newborns and young children, but fails to protect against pulmonary TB in adults (reviewed in (2)). Even though variable success is obtained with BCG vaccination in human trials, BCG is unlikely to be replaced in the near future and is the gold standard to which all other experimental vaccines are compared. A few countries with lower incidence of TB, including the United States of America, have not adopted or have withdrawn from routine BCG vaccination, preferring to screen for and treat TB with antibiotics. Therefore, the development of a TB vaccine that is safe and efficacious in BCG and non-BCG primed individuals alike would be advantageous.
Protective immunity to TB is conferred by TH1 CD4 and CD8 T cells (reviewed in (3)), and several groups have shown, in various TB animal models, that an effective vaccine requires the generation of a T cell-mediated immune response. We have recently identified Mtb antigens, recognized by human T cells, that are capable of eliciting dominant TH1 responses that are associated with reduced bacterial burden in a mouse model of TB (4). On the basis of this knowledge, we designed a recombinant subunit vaccine, called ID93, which combines four antigens belonging to families of Mtb proteins associated with virulence (Rv2608, Rv3619, Rv3620) or latency (Rv1813). In general, recombinant proteins are poorly immunogenic by themselves and require an adjuvant to elicit adaptive immune responses. We have developed a synthetic monophosphoryl lipid A and formulated it in a stable oil-in-water emulsion called GLA-SE (aka EM005) (5). This adjuvant system has been successfully combined with antigens for the induction of high antibody titers (6, 7) and of TH1 immune responses that have been associated with protection in TB and Leishmania challenge models (8, 9).
In the present study, we characterized immune responses induced by ID93/GLA-SE in mice, guinea pigs, and cynomolgus monkeys. We report on the prophylactic efficacy of this vaccine (i) in mice against Mtb H37Rv and MDR strain TN5904, and (ii) in guinea pigs against Mtb H37Rv using a BCG prime/boost strategy.
RESULTS
Cytokine recall responses of human PBMC to ID93
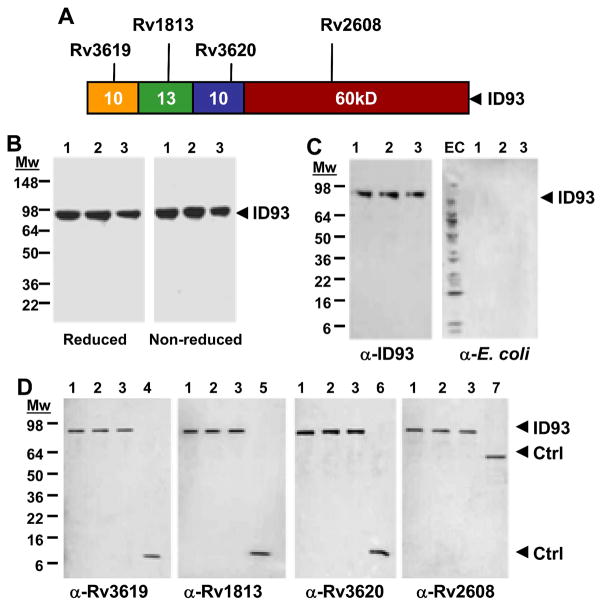
The ID93 recombinant protein is composed of the four Mtb antigens Rv3619, Rv1813, Rv3620, and Rv2608 linked in tandem (Fig. 1A). SDS-PAGE analysis of three purified scaled up fermentation lots of ID93 revealed a single major band at the expected 93kDa (Fig. 1B). A similar band profile was observed for samples in reducing and non-reducing conditions, demonstrating the absence of protein aggregates. The identity of the major band and relative absence of aggregates and large protein fragments was confirmed by immunoblotting with a mouse polyclonal serum raised against ID93 (Fig. 1C, α-ID93). Furthermore, ID93 was recognized by polyclonal sera raised against each of the single proteins, confirming the presence of the four antigens within the fusion protein (Fig. 1D, α-Rv3619, α-Rv1813, α-Rv3620, and α-Rv2608). Finally, the absence of bacterial byproducts in the purified ID93 product was confirmed by immunoblotting with polyclonal antibody to-Escherichia. coli (Fig. 1C, α-E.coli).
Fig. 1.
ID93 protein construct and characterization. (A) Schematic of the ID93 fusion protein. (B – D) SDS-PAGE and immunoblot of three lots of ID93. (B) 2 μg per lane of ID93 (lanes 1 – 3) were run in reducing and non-reducing conditions on a 4 – 20 % Tris glycine gel. (C) Immunoblot of ID93 with mouse antibody to ID93 and rabbit anti-sera to E. coli (50 ng and 1 μg of ID93, respectively). (D) Immunoblot of ID93 with mouse antibody to Rv3619, Rv1813, Rv3620, and Rv2608 (50 ng of ID93). EC, E. coli protein standards; ID93, lanes 1 – 3; lane 4, Rv3619; lane 5, Rv1813; lane 6, Rv3620; lane 7, Rv2608.
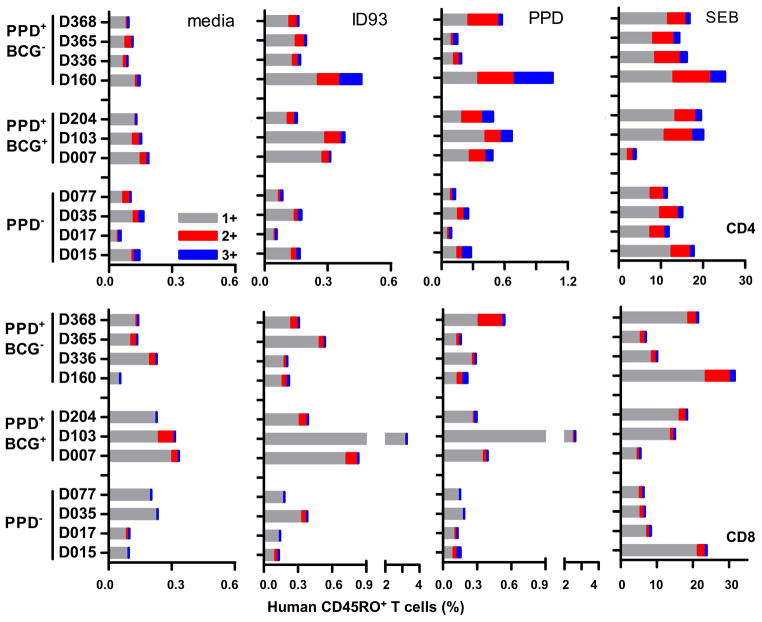
The antigenicity of ID93 was tested by ex vivo stimulation of human leukocytes. PBMC from 7 subjects reactive to Mtb purified protein derivatives (PPD+), either with (BCG+) or without (BCG−) a history of BCG vaccination, were used to characterize interferon-gamma (IFN-γ), tumor necrosis factor (TNF), and interleukin (IL)-2 cytokine responses to ID93 stimulation (Fig. 2). PBMC from PPD− subjects were used as negative controls. We determined the percent of CD4CD45RO+ and CD8CD45RO+ memory T cells expressing one (1+), any combination of two (2+), or the three cytokines (3+) by intracellular cytokine staining (ICS) and flow cytometry. ID93 stimulation of PPD+ PBMC resulted in a higher frequency of CD4 and CD8 T cells expressing one or more of these three cytokines compared to PPD− controls, indicating that the fusion protein was antigenic and retained an activity similar to that of the single antigens (4). Furthermore, T cells from BCG-vaccinated adults (PPD+BCG+) were as responsive to ID93 stimulation as T cells from Mtb-exposed subjects (PPD+BCG−), suggesting that ID93 might be efficacious in a BCG prime/protein boost vaccine regimen in previously vaccinated people.
Fig. 2.
Human PPD+ CD4 and CD8 T cells respond to ID93 antigen stimulation. PBMC from 7 healthy PPD+ subjects (3 with a history of BCG vaccination) with diverse HLA types were incubated for 8 h with medium, ID93 (20 μg/ml), PPD (10 μg/ml) or SEB (1 μg/ml). T cells were identified by intracellular cytokine staining based on CD3 expression, and further gated as CD4/CD45RO+ or CD8/CD45RO+ memory T cells. Percent of CD4 and CD8 T cells expressing IFN-γ, TNF, IL-2, or combinations of the three cytokines in response to antigen stimulation are shown.
The frequency hierarchy of 1+ T cells was IFN-γ (46 %) > TNF (22 %) > IL-2 (7 %), and IFN-γ/TNF double positive T cells were the most frequent among the 2+ (9 %). CD4 and CD8 T cells from all donors responded to SEB super antigen stimulation, while only PPD+ PBMC stained positive for cytokines in response to PPD antigen.
GLA-SE-adjuvanted ID93 vaccine generates TH1 immune responses that protects against TB and MDR-TB
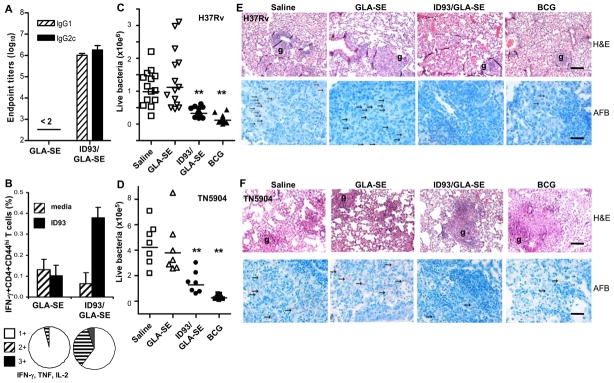
We conducted experiments to characterize the immunogenicity of ID93 adjuvanted with the synthetic monophosporyl lipid A adjuvant formulation GLA-SE in C57BL/6 mice. ID93-specific antibody and T cell responses were measured 1 wk and 3 wks, respectively, after the last injection. Vaccination with ID93/GLA-SE, but not with GLA-SE alone, induced measurable levels of antigen-specific IgG1 and IgG2c (Fig. 3A). Mean of reciprocal dilutions were 6.0 ± 0.1 log10 (IgG1) and 6.3 ± 0.2 log10 (IgG2c). The elevated titers of ID93-specific IgG2c, and a positive IgG2c:IgG1 log10 ratio are indicative of an IFN-γ-dependent switch in IgG subclasses. Characterization of cytokine expression after ID93 in-vitro recall of vaccine-induced T cells further confirmed that IFN-γ IL-2, and TNF were produced by CD4 TH1 phenotypic cells. Expression of IFN-γ, TNF, and/or IL-2 by splenic CD4/CD44high or CD8/CD44high T cells was determined at the single-cell level by ICS. There was a four-fold increase in the frequency of IFN-γ+CD4/CD44high T cells responding to ID93 stimulation in mice injected with ID93/GLA-SE, while cells from the GLA-SE only control did not respond to ID93 (Fig. 3B). The proportion of CD4/CD44high T cells expressing one, two or three cytokines was also determined. The small numbers of ID93-responsive CD4/CD44high T cells from mice injected with GLA-SE alone were mainly of the 1+ phenotype (96% of cells); the majority of these 1+ cells expressed TNF (45 %) or IL-2 (41 %). In contrast, CD4 T cells from animals that received the ID93/GLA-SE vaccine showed a significant increase in both the 2+ (34% of cells with 84% being IFN-γ/TNF double positives) and 3+ T cells (5%). Frequencies of IFN-γ, TNF and IL-2 producing ID93-specific CD8 T cells in the ID93/GLA-SE vaccine group were less than 0.1 %, suggesting that ID93/GLA-SE induced mainly a CD4 TH1 cell immune response.
Fig. 3.
Immunogenicity and efficacy of ID93/GLA-SE against M. tuberculosis H37Rv and MDR TN5904 strains. (A) ID93-specific IgG1 and IgG2c antibodies were determined on day 49 in sera from animals immunized 3x 3 wks apart with GLA-SE (20 μg TLR-4 agonist) or ID93 (8 μg) + GLA-SE. Mean of reciprocal dilution + SD and IgG2c:IgG1 ratio are shown. (B) T cell in vitro cytokine recall responses to ID93 were measured 3 wks after the last immunization by intracellular cytokine staining and flow cytometry. CD4 T cells were identified based on CD3 expression, and further gated as CD44hi. Bars show the percentage of cells expressing IFN-γ in response to ID93 stimulation (mean + SD, n = 3 mice), and pie charts show proportion of cells expressing one, two or the three cytokines IFN-γ, TNF, and IL-2. Data shown are representative of two experiments. (C and D) The number of viable bacteria in the lungs was determined 3 or 4 wks after challenge with aerosolized TN5904 (D) or H37Rv (C) strains, respectively. One way ANOVA followed by Dunnett’s Multiple Comparison Test was used for statistical analysis (vaccine groups were compared to saline control group); ** P < 0.01. (E and F) Histopathological evaluation of lung tissues post-challenge with H37Rv (E) or TN5904 (F). Granuloma (g) formation are shown in H & E-stained sections (scale 20 μm), and AFB ( → ) were evaluated (scale 5 μm). Data shown are representative of 4 mice/group in two independent experiments.
ID93/GLA-SE vaccine efficacy was subsequently tested in mice challenged with H37Rv or MDR TN5904 Mtb strains. Animals were vaccinated with GLA-SE, ID93/GLA-SE, BCG or saline (control), and challenged by aerosol administration of Mtb. ID93/GLA-SE significantly reduced the number of viable bacteria in lung (colony-forming unit, CFU) by 0.48 log10 (H37Rv) and 0.52 log10 (TN5904) compared to saline controls (P < 0.01), while immunization with GLA-SE alone was ineffective (−0.06 log10 and 0.05 log10, respectively; P > 0.05) (Fig. 3C and D, and Table 1). In comparison, one dose of BCG resulted in a decrease in lung CFU of 0.92 log10 (H37Rv) and 1.20 log10 (TN5904). A similar effect of ID93/GLA-SE was observed in the spleens of mice challenged with H37Rv, while no significant reduction was seen in this group after exposure to MDR TN5904 (Table 1). Hematoxylin and eosin (H&E) staining of formalin-fixed lung tissues showed little differences in granuloma size and lung cellularity between vaccine groups (Fig. 3E and F). However, numerous acid fast bacilli (AFB) could be detected throughout the lung sections of saline- and GLA-SE-immunized mice, while few AFB were seen in the lungs of mice vaccinated with ID93/GLA-SE or BCG. AFB results correlate well with the CFU observed in these animals (Table 1). Finally, similar pathology and AFB were observed in ID93/GLA-SE vaccinated mice after infection with both H37Rv and MDR TN5904 Mtb strains.
Table 1.
ID93/GLA-SE-induced protection against TB and MDR-TB.
| H37Rv | Lung | Spleen | ||
|---|---|---|---|---|
| Vaccinesa | CFU (log10)b | Reductionc | CFU (log10) | Reduction |
| Saline | 5.99 ± 0.25 | - | 5.46 ± 0.61 | - |
| GLA-SE | 6.05 ± 0.29 | −0.06 | 5.17 ± 0.57 | 0.29 |
| ID93/GLA-SE | 5.52 ± 0.18*d | 0.48 | 4.72 ± 0.35* | 0.74 |
|
| ||||
| BCG | 5.07 ± 0.44* | 0.92 | 4.02 ± 0.60* | 1.43 |
|
| ||||
| TN5904 | Lung | Spleen | ||
|
|
|
|||
| Vaccines | CFU (log10) | Reduction | CFU (log10) | Reduction |
|
| ||||
| Saline | 5.63 ± 0.17 | - | 5.17 ± 0.42 | - |
| GLA-SE | 5.58 ± 0.19 | 0.05 | 4.91 ± 0.73 | 0.26 |
| ID93/GLA-SE | 5.11 ± 0.26* | 0.52 | 4.90 ± 0.40 | 0.27 |
|
| ||||
| BCG | 4.42 ± 0.27* | 1.20 | 3.77 ± 0.25* | 1.40 |
Mice were immunized subcutaneoulsy three times, three wks apart with saline, GLA-SE (20 μg) adjuvant alone, or ID93 (8 μg) + adjuvant; or intradermally one time with 5x104 BCG.
Viable bacteria (CFU) expressed as log10 ± SD in the lungs and spleen of immunized animals (n = 7/group) 3 or 4 wks after a low dose aerosol challenge with Mtb H37Rv or MDR-TB TN5904 strain, respectively. Data shown are representative of 4 independent experiments with H37Rv and one experiment with TN5904.
Reduction of viable bacteria in the lungs and spleen of immunized animals compared to saline controls
Statistical significance between saline and vaccines groups were determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test.
P < 0.01.
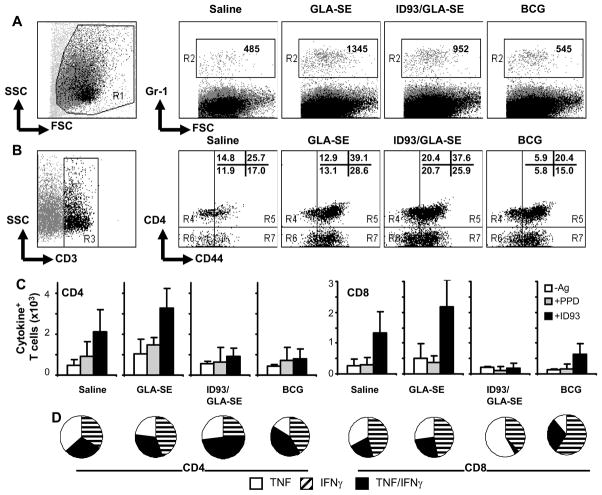
Analysis of cellular infiltrates to the lung after H37Rv challenge revealed an increase in granulocytes (Gr-1+, Fig. 4A) and T cells (Fig. 4B) in the GLA-SE and ID93/GLA-SE vaccine groups compared to saline or BCG groups. Unprotected animals (saline and GLA-SE groups) showed a higher frequency of CD44high CD4 and CD8 T cells responding to in vitro recall stimulation with PPD and ID93 (Fig. 4C), with most of the cells staining positive for TNF or IFN-γ (Fig. 4D). In contrast, the ID93/GLA-SE and BCG groups had fewer T cells responding to in vitro recall, but the majority of the responders in the lungs stained for both TNF and IFN-γ. The number of CD44high CD8 T cells positive for Granzyme B was also higher in saline (734±229) and GLA-SE (1255±277) groups compared to the BCG (372±200) and ID93/GLA-SE (571±225) groups.
Fig. 4.
Lung cellular infiltrates post-challenge. Mice were immunized 3x, 3 wks apart with saline, 20 μg GLA-SE, 8 μg ID93/GLA-SE, or once with 5×104 live BCG. Four weeks after the last boost, mice were challenged with an aerosol dose of 50 – 100 H37Rv bacilli. Lung cell phenotype and in vitro cytokine recall responses to ID93 were measured by intracellular cytokine staining and flow cytometry 4 wks after challenge. (A) Number of Gr-1+ cells in the lungs. (B) Number of T cells (x 103), identified based on CD3 expression, and further gated as CD4-CD44lo, CD4-CD44hi, CD4+CD44lo or CD4+CD44hi. (C) Number of CD44hiCD4+ and CD44hiCD8+ T cells expressing TNF and/or IFN-γ in response to media (“-Ag”), ID93 (10 μg/ml) or PPD (10 μg/ml) stimulation. Mean + SD (n = 2 pools of 3 mice each) are shown for a representative experiment. (D) Proportion of cells expressing IFN-γ, TNF, or TNF/IFN-γ effector cytokines in response to ID93 in vitro stimulation.
GLA-SE-adjuvanted ID93 vaccine boosts BCG and confers long-term protection in a guinea pig model of TB
Childhood vaccination with a single dose of the live attenuated BCG vaccine is standard practice in most of the world outside the U.S. and will not be quickly replaced. Its efficacy, however, wanes with time and additional injections during adolescence or adulthood are ineffective (reviewed in (2)). Therefore, developing a subunit vaccine that can be administered to BCG-immunized individuals and successfully boost their waning protective immune responses is of the utmost importance.
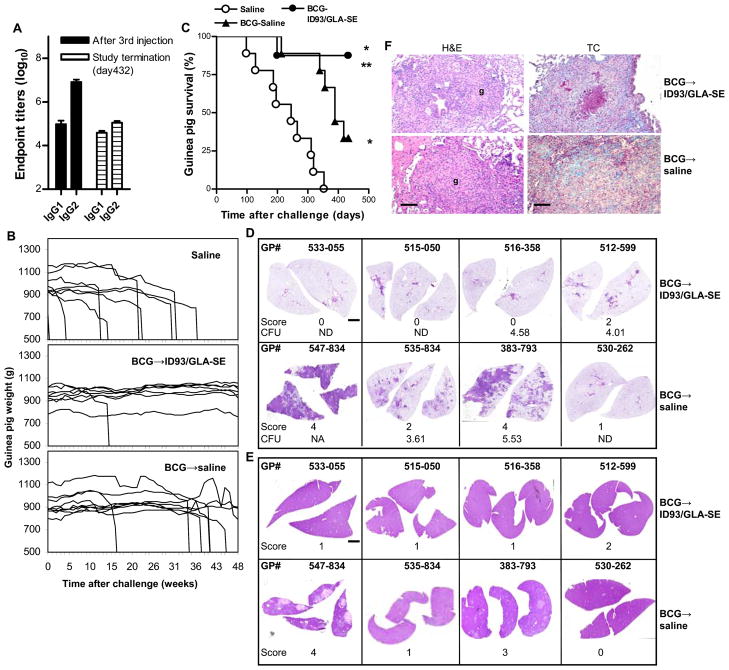
To demonstrate the efficacy of ID93/GLA-SE in a heterologous prime/boost strategy, guinea pigs were given a single dose of BCG or saline (control). Four months later, half of the animals in the BCG groups received the ID93/GLA-SE vaccine, while the other half was injected with saline. ID93-specific IgG1 and IgG2 antibodies were detected in sera of animals from the BCG → ID93/GLA-SE vaccine group (Fig. 5A). Mean of reciprocal dilutions were 5.0 ± 0.5 log10 (IgG1) and 6.9 ± 0.3 log10 (IgG2) one week after the last vaccine injection, and 4.6 ± 0.2 log10 (IgG1) and 5.0 ± 0.2 log10 (IgG2) more than one year later. Mean of reciprocal dilutions were less than 2.0 log10 in the BCG → saline or saline groups. Animals were challenged with Mtb and monitored for weight loss thereafter as an indicator of declining health and increased morbidity associated with TB (Fig. 5B). All animals in the saline control group (9/9) and two thirds of those in the BCG → saline vaccine group (6/9) exhibited weight loss greater than 20% (compared to their pre-infection body weight) and had to be euthanized or succumbed to the disease by day 432 after infection (Fig. 5C). On day 432, guinea pig 383–793 in the BCG → saline vaccine group showed signs of illness with 10.4 % body weight loss. In contrast, only 1 of 8 guinea pigs died in the BCG → ID93/GLA-SE vaccine group during the same period, and 0 of 7 exhibited symptoms (weight loss of ≥ 10%). The median survival was 245 and 389 days for the saline and BCG → saline groups, respectively (P < 0.001). The decreased mortality of the BCG → ID93/GLA-SE vaccine group was statistically different compared to the saline (P < 0.001) and BCG → saline (P < 0.05) groups.
Fig. 5.
ID93/GLA-SE vaccine confers long term protection against Mtb in guinea pigs in a BCG prime/protein boost regimen. Guinea pigs received either a single injection of saline (one of three groups) or of BCG (two groups). Four months later, BCG-primed animals received the ID93/GLA-SE vaccine or saline. (A) Serum ID93-specific IgG1 and IgG2 antibodies mean reciprocal dilution + SD. (B and C) Weekly weight monitoring after Mtb challenge for overt signs of disease and weight loss (B), resulting in euthanasia of the sick animals (C). Log Rank Test was used for statistical comparisons of median animal survival among the experiment groups; P < 0.01 compared to *saline or ** BCG → saline. (D and E) H&E-stained sections of lung and spleen, respectively, of each vaccinated animal on day 432 after challenge with H37Rv (scale: 0.2 mm). Guinea pig 547-834 in the BCG → saline group was euthanized on day 418 because of morbidity and excessive weight loss. (F). Granulomas (g) are shown in H&E-stained sections, and Masson’s trichrome (TC) stain was used to assess the amount of lung fibrosis in blue (scale: 20 μm). Data shown are representative of 4 animals/group.
Histopathological analyses were performed on formalin-fixed lung tissues (Fig. 5D and F) and liver (Fig. 5E) of 4 animals/group that were still alive at study termination on day 432, with the exception of guinea pig 547–834 in the BCG → saline group, whose organs were preserved at euthanasia on day 418. Three animals in the BCG → ID93/GLA-SE group showed no lesions in the lungs (score=0), while one animal exhibited a mild, multifocal granulomatous pneumonia (score=2). Minimal to mild cellular infiltrations (score=1–2) were observed in the livers. No AFB were found in any of the lung or liver sections analyzed in the BCG → ID93/GLA-SE group.
In the BCG → saline group, lung sections of animals 547–834 and 383–793 showed marked granulomatous bronchopneumonia (score=4). Lung granulomas had no evidence of central caseous necrosis or mineralization. We found scattered lymphocytes throughout the granuloma and a few infiltrating neutrophils. A few AFB in the sections of 547–834 and none in those from 383–793 were observed. Lung sections from 530–262 and 535–834 showed minimal to mild histological changes and no AFB (score=1 and 2, respectively). Liver sections from the animals in the BCG → saline group showed pathologies ranging from none or one lesion (530-262, 535-834, score=0 and 1, respectively) to marked, multiple coalescing foci of tuberculosis granulomatous hepatitis (383-793, 547-834, score=3 and 4, respectively). No AFB were detected in three out four animals in the BCG → saline group, and minimal AFB were present in liver sections from 547-834.
Two guinea pigs out of three (67 %) in the BCG → saline group had measurable CFU when we plated lung homogenates and tested for bacilli growth, while only 3 of 7 animals (43 %) were positive in the BCG → ID93/GLA-SE group (Fig. 5D). In spleens, the numbers of animals with detectable CFU were 2 out of 3 (67 %) with 4.65 and 4.30 log10 CFU and 1 out of 7 (14 %) with 3.55 log10 CFU, respectively.
GLA-SE-adjuvanted ID93 is safe and immunogenic in cynomolgus macaques
In order to demonstrate the safety and immunogenicity of ID93/GLA-SE in non-human primates, six male cynomolgus macaques received three doses of the vaccine. Injection-site reactions were minimal, with no more than barely perceptible erythema and edema (Draize scale range 0 – 1) observed for up to 3 days after immunization, and there were no significant changes in body weight and temperature observed (Fig.S1).
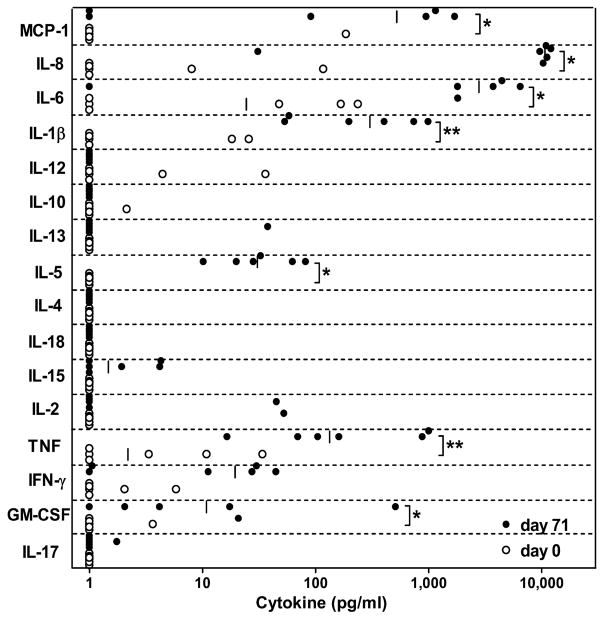
To characterize the cell-mediated immune response to the vaccine antigen by peripheral leukocytes, we performed a cytokine-profiling assay on blood collected from animals, before immunization and two weeks after the third dose. We determined the concentration of sixteen cytokines in whole blood supernatants in response to re-stimulation with ID93: IFN-γ, TNF, GM-CSF, MCP-1, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17 and IL-18. The results showed that in response to ID93, a subset of these cytokines representing pro-inflammatory, as well as TH1 and TH2 functional groups, was significantly up-regulated (Fig. 6). TNF, a soluble mediator of Mtb-specific immunity in infected individuals, was significantly up-regulated after vaccination with ID93/GLA-SE. The median concentration of TNF in ID93-stimulated blood from animals that had been vaccinated was approximately 60 times higher when compared to blood from the same animals prior to vaccination (133 pg/ml vs. 2 pg/ml, P=0.01). In addition, ID93-specific GM-CSF and IFN-γ responses were detected, but these differences were more modest and the latter were not statistically significantly different (11 pg/ml vs. <1.5 pg/ml, P=0.02 and 19 pg/ml vs. <1.5 pg/ml, P=0.06). No significant difference in the concentration of IL-2 was detected in this assay, and interestingly, although significant ID93-specific IL-5 responses were measured, there were no changes detected in the concentrations of IL-4 and IL-13, the other two TH2-type cytokines assayed. ID93-specific responses for all the other T-cell associated cytokines assayed were below the detection limit (smaller than 1.4 pg/ml)..The greatest differences measured were in IL-6 and IL-8, which had median concentrations from two and four logs higher in blood collected from ID93/GLA-SE-vaccinated animals than in blood collected from the same animals at study initiation (median 2776 vs. 24 pg/ml, P=0.05 and 10,671 vs <1.5 pg/ml (limit of detection), P=0.02, respectively). Two additional pro-inflammatory mediators, MCP-1 and IL-1β were also significantly up-regulated in blood from vaccinated animals after re-stimulation with ID93 when compared to blood from the same animals prior to vaccination (522 pg/ml vs. <1.5 pg/ml, P=0.05 and 302 pg/ml vs. <1.5 pg/ml, P=0.01, respectively). Overall, these results demonstrated that an ID93/GLA-SE formulation was well tolerated and immunogenic in cynomolgus macaques.
Fig. 6.
Cytokine responses to vaccine antigen in immunized macaques. Peripheral blood collected from ID93/GLA-SE-immunized animals was stimulated with ID93 or saline after which plasma was collected and assayed for cytokines by multiplexed bead-array assay. Background-subtracted antigen-specific cytokine responses are shown for blood collected pre-immunization (day 0, ○) and after third immunization (day 71, ●). * P ≤ 0.05, **P ≤ 0.01 by Wilcoxon Rank-Sum Test (n = 6).
DISCUSSION
Controlling the spread of TB and MDR-TB has become an urgent public health priority. This could be achieved by developing new TB vaccines or drugs, or by improving the long-term protection provided by BCG vaccine with prime/boost strategies. We designed the subunit vaccine, ID93/GLA-SE, to be used in both BCG-vaccinated and non-vaccinated individuals, and performed comprehensive pre-clinical analyses of its immunogenicity, safety, and efficacy against challenge with live virulent Mtb. ID93 consists of the fusion of four Mtb antigens that had been previously shown to confer partial protection in a mouse model of TB (4). ID93 was further shown to induce memory T-cell cytokine recall responses in BCG-vaccinated and in Mtb-exposed subjects. ID93/GLA-SE vaccination induced antigen-specific antibody and/or T-cell immune responses in mice, guinea pigs, and cynomolgus monkeys. Induction of predominantly TH1-type CD4 T-cell responses was associated with reduced TB and MDR-TB bacterial burdens in the lungs of vaccinated mice. Furthermore, we demonstrated that boosting with the ID93/GLA-SE vaccine enhanced BCG-induced immunity to Mtb in guinea pigs by significantly reducing TB-associated pathology and mortality when compared to BCG alone.
Combining multiple antigens in recombinant fusion proteins like Mtb72f, ID83, Ag85B-ESAT6, CSU-F36, and Ag85B-TB10 (8, 10-13) leads to increased vaccine efficacy against Mtb. The ID93 vaccine candidate is comprised of antigens from three different families of Mtb proteins: the EsX family of virulence factors (Rv3619, Rv3620), the PE/PPE (Rv2608) and the latency antigens (Rv1813). Because of their low intrinsic immunogenicity, protein-based vaccines need suitable adjuvant systems for the induction of strong in vivo immune responses. Three of the most advanced subunit vaccines against non-viral diseases currently in human clinical trials, RTS,S (malaria vaccine (14)), Mtb72F (TB vaccine (15)) and LEISH-F1 (leishmaniasis vaccine (16)), are based on liposomal (AS01) or oil-in-water (AS02, MPL®-SE) formulations containing the TLR-4 agonist MPL®. IDRI has developed a proprietary synthetic monophosphoryl lipid A molecule in an oil-in-water emulsion called GLA-SE (5). Our selection of GLA-SE was based on its ability to activate dendritic cells in a TLR-4 dependent fashion and potentiate TH1-type CD4 T-cell responses (8, 9), which are critical for controlling Mtb infection.
Three doses of the ID93/GLA-SE vaccine induced antigen-specific TH1 responses in mice, guinea pigs, and non-human primates. IgG2:IgG1 antibody ratios greater than 1 and/or increased production of IFN-γ and/or TNF were observed. In mice, the ability of ID93/GLA-SE to induce polyfunctional T cell responses was associated with a partial reduction in lung and spleen bacterial burden after aerosol exposure to Mtb, and the first adjuvanted protein-based vaccine with demonstrated efficacy against MDR-TB. Only one report to date has documented the efficacy of a TB vaccine against MDR-TB (17). In this study, Okada et al. used a DNA vaccine expressing Mtb heat-shock protein HSP65 and human IL-12 delivered by the hemagglutinating virus of Japan encapsulated in liposomes. However, it remains unclear wheather prophylactic DNA-based vaccines are a viable technology for human use because of safety concerns about the possibility of integration of foreign genetic material.
Most people in the world have been vaccinated with BCG; however, its efficacy wanes over time. Therefore, boosting the protective immunity provided by BCG might be the most realistic path toward a new TB vaccine. Homologous boosting with BCG was ineffective in humans (reviewed in (2)) and caused severe disseminated lesions in guinea pigs (18). As a result heterologous prime/boost strategies such as priming with BCG and boosting with adjuvanted (19, 20) or vectored (21) antigens found in BCG and/or Mtb have been pursued with promising results in mice. Given that all four Mtb antigens comprising ID93 are also found in BCG, ID93 may prove to be a superior vaccine candidate for boosting BCG-induced immunity. We demonstrated in the more susceptible TB guinea pig model that boosting BCG with ID93/GLA-SE reduced pathology and afforded long-term protection against TB. Protection with ID93/GLA-SE in guinea pigs was similar to that observed with Mtb72f/AS02A (22) and appeared greater than with the HyVac4/IC31 (23) or AdAg85A (24) vaccines, all three currently in clinical trials. The ID93-specific cytokine response in the blood of vaccinated cynomolgus macaques was a complex mixture of Th1- and Th2-type responses, a phenotype consistent with observed T cell memory responses in successful vaccine regimens, such as for yellow fever (25). Our finding are also consistent with previous work demonstrating that another subunit vaccine, Mtb72f/AS02A, was immunogenic in macaques and increased protection against Mtb in a prime/boost regimen (10). Interestingly a recent publication by van Dissel et al., suggests that humans may also react only slowly to adjuvanted protein vaccines, but generate a substantial response over time (26). Our most important finding is, the boosting potential of ID93 demonstrated with BCG-vaccinated guinea pigs and humans: When tested on PBMC isolated from healthy PPD+ BCG-vaccinated subjects, ID93 induced pre-existing CD4 and CD8 T-cell IFN-γ and TNF cytokine responses, suggesting that the ID93/GLA-SE vaccine may also boost BCG-induced protective responses. In conclusion, these results support the advancement of the ID93/GLA-SE TB vaccine and plans for a Phase I clinical trial are underway.
MATERIALS AND METHODS
Cloning and purification of ID93
ID93 was generated through a tandem fusion of the individual cloned and amplified genes of Rv3619, Rv1813, Rv3620, and Rv2608 using restriction site linkers. The recombinant pET28a plasmids (Novagen) containing the individual Rv3619, Rv1813, Rv3620, and Rv2608 genes were previously described (4). ID93 PCR primers were designed to incorporate specific restriction enzyme sites 5’ and 3’ of the gene of interest with primer sequences as follows: Rv1813-5’NdeI CAATTACATATGGGTA-CCCATCTCGCCAACGGTTCGATG; Rv1813-3’SacI CAATTAGAGCTCGTTGCA-CGCCCAGTTGACGAT; Rv3620-5’SacI CAATTAGAGCTCATGACCTCGCGTTTT-ATGACG; Rv3620-3’SalI CAATTAGTCGACGCTGCTGAGGATC-TGCTGGGA; Rv2608-5’SalI CAATTAGTCGACATGAATTTCGCCGTTTTGCCG; Rv2608-3’HindIII CAATTAAAGCTTTTAAGTACTGAAAAGTCGGGGTAGCGCCG; Rv3619-5’NdeI CAATTACATATGACCATCAACTATCAATTC and Rv3619-3’KpnI CAATTAGGTACCGGCCCAGCTGGAGCCGACGGC. The DNA sequences were amplified from plasmid DNA templates using Pfx DNA polymerase (Invitrogen, Carlsbad, CA) with 30 cycles at 94° C for 15 sec, 58° C for 30 sec and 68°C for 1h 30 min. The Rv1813 PCR product was digested with NdeI/SacI restriction enzymes then cloned into the pET28a vector. Rv3620 and the Rv1813pET construct were digested with SacI/SalI and ligated. Rv2608 was digested with SalI/HindIII and ligated into the Sa1I/HindIII-cut pET28a-Rv1813-3620 vector. Rv3619 was digested with NdeI / KpnI then ligated into the above vector. The resulting plasmid containing the fusion gene construct Rv3619-Rv1813-Rv3620-Rv2608 was DNA sequenced and named ID93 since it encodes a 93 kDa protein. ID93 was expressed in E. coli host BL-21plysS, purified under denaturing conditions by chromatography on DEAE and Q Sepharose columns, and analyzed by SDS-PAGE on a 4 – 20% Tris glycine gel (Invitrogen). Protein identity was confirmed by immunoblotting with mouse polyclonal sera raised against ID93 (1:1000), followed by goat anti-mouse IgG1 conjugated to horseradish peroxidase (1:1000, Southern Biotechnologies, Inc.). The presence of each of the four antigens within ID93 was confirmed by immunoblotting with mouse polyclonal sera raised against Rv3619, Rv1813, Rv3620 and Rv2608 (1:1000), followed by goat anti-mouse IgG1 conjugated to horseradish peroxidase (1:1000, Southern Biotechnologies, Inc.). The absence of E. coli byproducts was confirmed by immunoblotting with horseradish peroxidase-conjugated rabbit polyclonal anti-E. coli antibody (1:1000, ViroStat, Inc.). Residual LPS contamination was evaluated by the Limulus amoebocyte lysate assay (Cambrex Corp.) and determined to be less than 25 endotoxin units per mg of protein.
Immunization, Challenge, & CFU
All mice and guinea pigs were maintained in the Infectious Disease Research Institute animal care facility under specific pathogen-free conditions and were treated in accordance with the regulations and guidelines of IDRI Animal Care and Use Committee. The non-human primate study protocol and all experimental procedures were reviewed and approved by the SNBL USA Institutional Animal Care and Use Committee prior to study initiation.
Mice
Female C57BL/6 mice, 4 – 6 wk old, (Charles River) were immunized subcutaneously three times, 3 wks apart with ID93 (8 μg) + GLA-SE(20 μg). The BCG group received a single intradermal dose of 5 × 104 CFU (Pasteur strain, Sanofi Pasteur). Three to four wks after the last immunization, groups of seven mice were challenged by low dose aerosol exposure with Mtb strain H37Rv (American Type Culture Collection) or MDR strain TN5904 (provided by B. N. Kreiswirth, Public Health Research Institute TB Center, Newark, NJ) using a UW-Madison aerosol exposure chamber calibrated to deliver 50 – 100 bacteria into the lungs. TN5904 is a non-W Beijing family MDR clinical isolate with resistance to streptomycin, isoniazid, rifampin, and sodium para-aminosalicylate (27). Vaccine efficacy was determined 4 wks post-challenge by harvesting lungs and spleen from the infected mice and counting CFU as previously described (4).
Guinea pigs
Female out-bred Hartley guinea pigs, 400–450 g (Charles River) were injected intradermally with a single dose of 5 x 104 live BCG (prime), and 4 months later immunized three times (boosts), 3 wks apart with ID93 (20 μg) + of GLA-SE (20 μg). Four wks after the last immunization, groups of 8 - 9 guinea pigs were challenged by low dose aerosol exposure with Mtb H37Rv (20 – 50 bacteria in the lungs). Monitoring of weight loss and survival was done through Day 432 post-challenge at which time the experiment was terminated.
Non-human primates
Male cynomolgus macaques were selected from purpose-bred colonies housed at SNBL USA, Ltd (Everett, WA) and were maintained in accordance with guidelines set forth by the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animals were between 4-6.5 kg and 3-8 years old at study initiation. Six animals received three intramuscular injections of ID93 (10μg) + GLA-SE-H (a GLA-SE formulation suitable for use in humans; 20 μg) spaced 4 wks apart. Blood was obtained before the first injection (day 0) and 2 wks after the third dose (day 71) on restrained, conscious animals, and collected into Vacutainer tubes containing heparin as an anti-coagulant (BD Biosciences).
Determination of antibody titers
Sera from immunized mice and guinea pigs were tested for ID93-reactive IgG1 and IgG2c, respectively IgG1 and IgG2, as previously described (4). Anti-IgG1-HRP, anti-IgG2c-HRP, or anti-IgG2-HRP were used at 1:2000 in PBS Tween-20 0.05% BSA 0.1%. Reciprocal dilutions corresponding to endpoint titers were determined with GraphPad Prism 4 (GraphPad Software Inc.) with a cutoff of 0.1.
Flow cytometry
Mice
Splenocytes and lungs from immunized animals (1 – 2 × 106 cells) were stimulated with anti-CD28/CD49d (1 μg/ml each, eBioscience), PPD (10 μg/ml), or ID93 (20 μg/ml) for 8 h at 37°C in the presence of GolgiStop (eBioscience). The cells were then fixed, stained with fluorochrome-conjugated antibodies anti-CD3, CD8, CD44, IFN-γ, TNF, IL-2, Granzyme B (GrB, eBioscience) and CD4 (Invitrogen) as described elsewhere (4).
Human
PBMCs were purified from heparinized blood obtained from PPD- and PPD+ healthy subjects and stored frozen in liquid nitrogen. Informed consent was obtained from all the subjects and the study was approved by Western IRB, Seattle, WA. PBMC (1 – 2 × 106 cells) were stimulated with anti-CD28/CD49d (1 μg/ml each, eBioscience), and PPD (10 μg/ml), ID93 (20 μg/ml) or staphylococcal enterotoxin B (1 μg/ml) for 8 h at 37°C in the presence of GolgiStop (eBioscience). After stimulation, live cells were stained with Aqua Live/Dead fixable dye (Invitrogen) and then prepared for polychromatic flow cytometric analysis of CD3, CD45RO, CD4, CD8, IFN-γ and TNF (4).
Viable lymphocytes were gated by forward and side scatter, and 100,000 CD3+ events for each sample were acquired on a LSRII and analyzed with BD FACSDiva software v5.0.1 (BD Biosciences).
Peripheral blood cytokine profiling assay
Blood obtained from monkeys was assayed within 4 h of venipuncture. Under sterile conditions, 900 μl of blood was mixed with 100 μl of either phosphate-buffer saline, ID93 (20 μg/ml) or a mitogen positive control (phytohemaglutinin, 400 μg/ml), and allowed to incubate for 22 – 24 h at 37ºC, 5% CO2. Plasma was then collected by centrifugation, filtered and stored at −80ºC for subsequent cytokine analysis. Quantitation of cytokine levels was performed using a commercial multiplex bead-array assay (Millipore) specific for non-human primate cytokines per manufacturer’s instructions.
Histology
A single lobe of the lung and part of the liver were fixed for at least 7 days in 10% normal buffered formalin. After fixation, tissues were embedded in paraffin, cut and stained with H&E, Fite’s acid fast stain, or Trichrome stain (lung only) by the Benaroya Research Institute Histology Core facility (Seattle, WA) for mouse tissues, and BioGenetics Research Laboratories, Inc (Greenbank, WA) for guinea pig tissues. Between three and sixteen non-overlapping fields per tissue section were captured at a 1 – 600 x magnification. Qualitative analyses were performed by a veterinary pathologist and included comparing all fields within a group (4 animals per group, 2 – 3 sections examined per animal) for organization of cellular infiltrate, number of granulomas, visual quantification of AFB, and pulmonary fibrosis.
Lesion scoring
0 – normal tissue morphology with no lesion present; 1 – lesions involving <10% of the tissue and minimal infiltration of fibroblast, mononuclear or polymorphonuclear inflammatory cells; 2 – lesions affecting 10–20% of the tissue and mild cellular infiltration; 3 – lesions covering 21–40% of the tissue and moderate cellular infiltration; 4 – lesions covering 41–100% of the tissue and marked cellular infiltration.
Statistical analysis
Standard 1-way ANOVA followed by Dunnett’s Multiple Comparison Test were used for statistical analysis of CFU. Log Rank Test was used for statistical comparisons of median guinea pig survival among the experiment groups. Non-human primate cytokine data analysis was performed with MasterPlex QT (MiraiBio) and JMP software (SAS Institute). Test groups were compared with a non-parametric Wilcoxon Rank-Sum Test. P values of 0.05 or less were considered significant.
Supplementary Material
Acknowledgments
The authors thank A. Bhatia and R. Howard for reviewing this manuscript; D. Argilla, A. Bernard, K. Carper, T. Dutill, T. Evers, M. Henao Tamayo, E. Kristalinskaia, E. Laughlin, J. Laurance, G. Poshusta, V. Reese, C. Shanley, I. Tukacovic, W. Wicomb, I. Zharkikh, and J. Zheng for their technical expertise; L. Kunz for guinea pig tissue histology. We thank B. N. Kreiswirth, Public Health Research Institute TB Center, Newark, NJ for providing MDR strain TN5904. Funding: Supported in part by National Institutes of Health grants AI-044373 and AI-067251 to S.G.R., and contract AI-25479 and grant AI-078054 to R.N.C.
Footnotes
Author contributions: S.B., C.G.I., S.L.B., R.N.C. and S.G.R. designed the study, conducted the experiments, and analyzed the data; D.O. and I.M.O. conducted the MDR-TB experiments; T.S.V. provided GLA-SE-based adjuvant formulations; T.P. produced and analyzed ID93 lots; M.K. conducted the guinea pig experiment; H.P.W. analyzed the mouse histology; S.O.P. conducted the non-human primate study; S.B. drafted the paper and C.G.I., H.P.W., S.O.P., S.L.B., R.N.C. and S.G.R. contributed to the writing.
Competing interests: The authors have no competing interests.
REFERENCES AND NOTES
- 1.WHO. Fourth global report. World Health Organization; 2008. Anti-tuberculosis drug resistance in the world. [Google Scholar]
- 2.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–132. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, Mompoint F, Vedvick TS, Bertholet S, Coler RN, Reed SG. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–5963. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 7.Olugbile S, Kulangara C, Bang G, Bertholet S, Suzarte E, Villard V, Frank G, Audran R, Razaname A, Nebie I, Awobusuyi O, Spertini F, Kajava AV, Felger I, Druilhe P, Corradin G. Vaccine potentials of an intrinsically unstructured fragment derived from the blood stage-associated Plasmodium falciparum protein PFF0165c. Infect Immun. 2009;77:5701–5709. doi: 10.1128/IAI.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–3071. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, Coler RN, Vedvick TS, Reed SG. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–7045. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A. 2009;106:2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69:2773–2778. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Henao-Tamayo M, Harton M, Ordway D, Shanley C, Basaraba RJ, Orme IM. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. 2007;14:902–906. doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–6339. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 14.Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, Cummings JF, Kester KE, Ockenhouse CF, Stewart A, Ofori-Anyinam O, Ramboer I, Cahill CP, Lievens M, Dubois MC, Demoitie MA, Leach A, Cohen J, Ballou WR, Heppner DG., Jr Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One. 2009;4:e6465. doi: 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Eschen K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, Koutsoukos M, Moris P, Cain D, Dubois MC, Cohen J, Ballou WR. The candidate tuberculosis vaccine Mtb72F/AS02A: Tolerability and immunogenicity in humans. Hum Vaccin. 2009;5:475–482. doi: 10.4161/hv.8570. [DOI] [PubMed] [Google Scholar]
- 16.Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Piazza FM. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, Takao K, Kishigami C, Inoue Y, Matsumoto M, McMurray DN, Dela Cruz EC, Tan EV, Abalos RM, Burgos JA, Saunderson P, Sakatani M. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine. 2009;27:3267–3270. doi: 10.1016/j.vaccine.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Basaraba RJ, Izzo AA, Brandt L, Orme IM. Decreased survival of guinea pigs infected with Mycobacterium tuberculosis after multiple BCG vaccinations. Vaccine. 2006;24:280–286. doi: 10.1016/j.vaccine.2005.07.103. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich J, Billeskov R, Doherty TM, Andersen P. Synergistic effect of bacillus calmette guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J Immunol. 2007;178:3721–3730. doi: 10.4049/jimmunol.178.6.3721. [DOI] [PubMed] [Google Scholar]
- 20.Rouanet C, Debrie AS, Lecher S, Locht C. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect. 2009;11:995–1001. doi: 10.1016/j.micinf.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 22.Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, Basaraba RJ, Izzo AA, Lasco TM, Chapman PL, Reed SG, Orme IM. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72:6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skeiky YA, Dietrich J, Lasco TM, Stagliano K, Dheenadhayalan V, Goetz MA, Cantarero L, Basaraba RJ, Bang P, Kromann I, McMclain JB, Sadoff JC, Andersen P. Non-clinical efficacy and safety of HyVac4:IC31 vaccine administered in a BCG prime-boost regimen. Vaccine. 2010;28:1084–1093. doi: 10.1016/j.vaccine.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 24.Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One. 2009;4:e5856. doi: 10.1371/journal.pone.0005856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, 3rd, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff TH, Kromann I, Doherty TM, Andersen P. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 27.Small PM, Shafer RW, Hopewell PC, Singh SP, Murphy MJ, Desmond E, Sierra MF, Schoolnik GK. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med. 1993;328:1137–1144. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.