Abstract
The molecular identification of adult hepatic stem/progenitor cells has been hampered by the lack of truly specific markers. To isolate putative adult liver progenitor cells, we used cell surface-marking antibodies, including MIC1-1C3, to isolate subpopulations of liver cells from normal adult mice or those undergoing an oval cell response and tested their capacity to form bilineage colonies in vitro. Robust clonogenic activity was found to be restricted to a subset of biliary duct cells antigenically defined as CD45−/CD11b−/CD31−/MIC1-1C3+/CD133+/CD26−, at a frequency of one of 34 or one of 25 in normal or oval cell injury livers, respectively. Gene expression analyses revealed that Sox9 was expressed exclusively in this subpopulation of normal liver cells and was highly enriched relative to other cell fractions in injured livers. In vivo lineage tracing using Sox9creERT2-R26RYFP mice revealed that the cells that proliferate during progenitor-driven liver regeneration are progeny of Sox9-expressing precursors. A comprehensive array-based comparison of gene expression in progenitor-enriched and progenitor-depleted cells from both normal and DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine or diethyl1,4-dihydro-2,4,6-trimethyl-3,5-pyridinedicarboxylate)-treated livers revealed new potential regulators of liver progenitors.
Keywords: liver progenitor, FACS, microarray, Sox9
The liver has an impressive capacity for regeneration, and this process is thought to be driven mostly by the proliferation of mature hepatocytes and duct cells in a stem/progenitor-independent fashion after many common forms of injury (Duncan et al. 2009). However, in instances of severe injury that limit hepatocyte/cholangiocyte proliferation, progenitors are activated, resulting in a ductular reaction known as the “oval cell response.” This reaction is characterized by the appearance of a large number of small proliferating cells with a high nuclear:cytoplasm ratio near the portal triad that can proliferate and migrate into the lobule to become mature hepatocytes; these are regarded as the classical adult facultative liver progenitors (for review, see Oertel and Shafritz 2008). Oval cell-inducing injury models include 2-acetylaminofluorene/partial hepatectomy (Solt et al. 1977), choline deficiency coupled with ethionine administration (CD+E) (Wilson and Leduc 1958), or treatment with porphyria-inducing DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine or diethyl1,4-dihydro-2,4,6-trimethyl-3,5-pyridinedicarboxylate) (Preisegger et al. 1999).
There is a large and growing unmet demand for livers suitable for therapeutic transplantation. Although it is unclear whether adult hepatic progenitor cells will be a useful substitute for transplantation, understanding their regulation might permit the stimulation of residual in situ progenitor function in patients or guide the directed differentiation of induced pluripotent stem (iPS)/embryonic stem (ES) cells. In addition, a substantial body of literature suggests that hepatic progenitors may be the cell of origin in some liver cancers, particularly cholangiocarcinomas. Recently, the availability of tools for isolating subpopulations of mouse liver cells has improved, thanks to the discovery that some hematopoietic lineage-marking antibodies also label hepatic subsets (e.g., CD133 and Sca-1) and the development of new liver-specific cell surface or genetic markers (Dorrell et al. 2008; Sackett et al. 2009a,b). The purification of clonogenic liver progenitors has been described previously in fetal and newborn mice (Suzuki et al. 2002), as well as adult mice undergoing oval cell activation (Suzuki et al. 2008). However, the conditions used to identify progenitors in young animals or adults with oval cell injury do not apply to the isolation of progenitors from normal mature adult mice. Using cell surface markers against blood cell antigens, enrichment of progenitors from adult normal livers has been described recently (Kamiya et al. 2009). However, the relationship of the clonogenic progenitor from normal livers and the cells emerging during the oval cell response was unknown, and the gene expression profile of progenitors was not described.
Here, the use of novel cell surface markers developed for cells emerging during this type of liver injury has permitted substantial enrichment of this clonogenic population (Dorrell et al. 2008). We describe the phenotypic definition and isolation of a clonal progenitor present at a similar frequency in both the normal and regenerating adult mouse capable of in vitro bilineage colony formation. The gene expression profile of this population was studied in both normal adult and injured livers, and was compared with that of the progenitor-depleted fractions. These surveys revealed that factors such as Sox9 and FoxJ1 are enriched in both progenitor populations in a pattern consistent with the regulation of progenitor function. Comparison of the microarray analysis data from an accompanying study in this issue of Genes & Development (Shin et al. 2011) indicate that FoxL1-positive clonogenic liver progenitors represent a highly similar but not completely identical population of cells that shares most molecular characteristics. When lineage tracing was performed to mark the progeny of Sox9-expressing cells, bilineage marking was observed in both normal and DDC-treated livers. The gene expression profiles of the progenitor-enriched populations characterized here reveal new information regarding the nature of adult hepatic progenitors and should guide future efforts to enhance their activity in situ or specify this fate during ES/iPS differentiation.
Results
Identification and isolation of duct cell subpopulations in the adult mouse liver
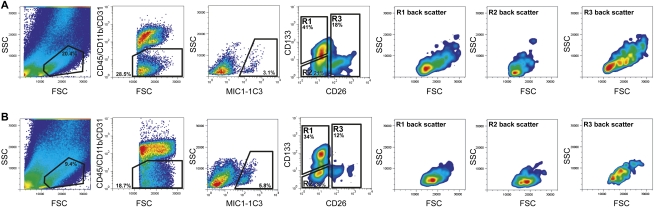
Although the existence of stem/progenitor cells in the adult liver is generally accepted, their phenotypic identity and the circumstances in which they are detectable remain controversial. To isolate and study hepatic progenitors in adult mouse liver tissue, we labeled cell populations obtained by sequential enzymatic dispersal with combinations of antibodies recognizing cell surface antigens and then isolated them by fluorescence-activated cell sorting (FACS). Figure 1 illustrates the sequential gating strategy used to define subpopulations of nonparenchymal cells (NPCs) from normal (Fig. 1A) or DDC-treated oval cell-activated (Fig. 1B) liver cell preparations. These gates allowed the exclusion of hepatocytes (high FSC/SSC), erythrocytes (low FSC/SSC), leukocytes, Kupffer cells (CD45+/CD11b+) cells, and endothelial cells (CD31+). Approximately 3% of the remaining hepatic NPCs were labeled by the duct cell surface marker MIC1-1C3 (Dorrell et al. 2008) in normal livers; DDC-treated mouse livers yielded such cells at a frequency of ∼6%. Subfractionation of the ductal MIC1-1C3+ population on the basis of CD133 and CD26 expression yielded three populations with distinct characteristics. As illustrated by FSC/SSC backscattering, the CD45−/11b−/31−/MIC1-1C3+/133+/26− fraction (R1) contained cells of intermediate size and low granularity, CD45−/11b−/31−/MIC1-1C3+/133−/26− (R2) cells were smaller (or of mixed size, when isolated from DDC-treated tissue), and CD45−/11b−/31−/MIC1-1C3+/26+ (R3) cells were noticeably more granular than those found in R1 or R2.
Figure 1.
FACS-based isolation of mouse liver NPC subsets. Cells isolated from normal (A) or DDC-injured (B) livers were enzymatically dispersed, antibody-labeled, and sorted by FACS. Successive gating showed sequential selection of cell-sized events (FSC vs. SSC), nonhematopoietic/endothelial events (CD45/CD11b/CD31 vs. FSC), and duct cells (MIC1-1C3 vs. FSC). Dead cells and debris were excluded using propidium iodide labeling and size exclusion, respectively. MIC1-1C3+ cells were divided into three subfractions (R1–R3) based on CD26 and CD133 expression; the size/granularity characteristics of the cells in R1–R3 are shown by backscattering on FSC/SSC.
Assessment of progenitor activity by ex vivo colony formation
In order to survey liver cell subsets for the presence of progenitors, an ex vivo culture assay was developed. To limit the growth of mature cell types such as hepatocytes and facilitate the study of individual factors in promoting progenitor growth/survival, fully defined serum-free conditions were used. When CD45−/11b−/31−/MIC1-1C3+/26−cells from normal mouse livers were cultured in this assay, regions of epithelial cell growth emerged (examples of which are shown in Fig. 2A–D). Initiating cells were noticeably motile; they often migrated several millimeters in the 24 h post-plating before attaching, spreading, and initiating colonies when observed by time-lapse photography (data not shown). To confirm that this growth was indeed clonal rather than the result of cell aggregation, in three experiments, NPCs were prepared in parallel from β actin-EGFP transgenic and wild-type mice and mixed 50:50 prior to ex vivo culture. When the resulting colonies were evaluated by fluorescent microscopy, 100% (600 of 600) of the colonies were homogenously EGFP+ or EGFP−, indicating that all growth was clonal (data not shown).
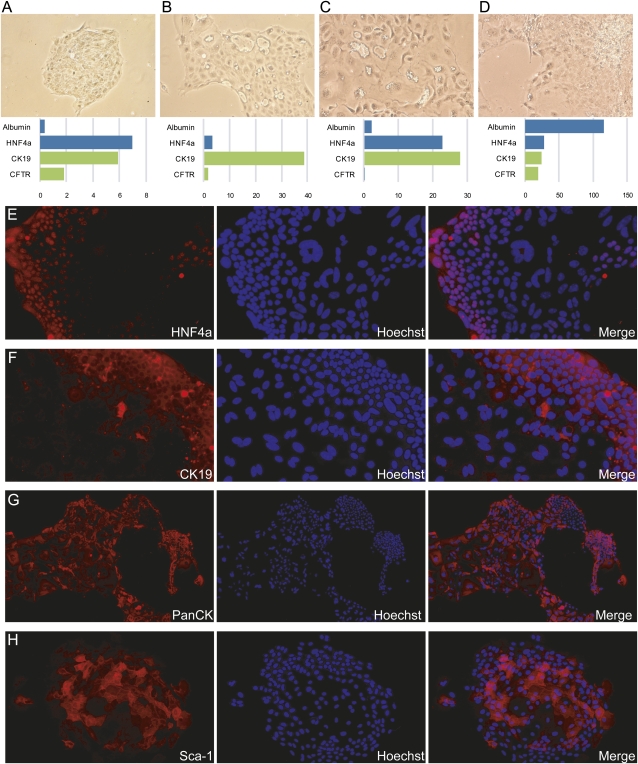
Figure 2.
Mouse liver cell colonies exhibit markers of both hepatocytic and ductal cell types. (A–D) Four representative individual colonies produced by FACS-sorted CD45−/11b−/31−/MIC1-1C3+/26− cells are shown; the gene expression measurements for each colony are indicated below it. Expression data are presented as qRT–PCR-measured linearized ΔCt values relative to the mean of GAPDH and β-actin housekeeping genes. Albumin and HNF4a are markers of hepatocytic differentiation, whereas CK19 and CFTR are duct-associated. Phase contrast images are shown at 100×. (E–H) Additional colonies were grown on chamber slides and labeled with lineage-marking antibodies. Antibody labeling was visualized by Cy3 fluorescence and nuclei were labeled with Hoechst 33342. Magnification, 200×.
The gene expression profiles of individual colonies revealed significant variation with respect to duct and hepatocytic lineage markers. Nevertheless, nearly all colonies contained cells of both the hepatocyte and bile duct epithelial lineages. Immunofluorescent detection of the hepatocytic marker HNF4a (Fig. 2E) revealed that positive cells tended to be smaller and located toward the edges of the colony. The same patterns were observed in colonies after labeling with bile duct marker cytokeratin 19 (CK19) labeling (Fig. 2F), suggesting that many cells coexpress hepatocytic and duct lineage markers. Pan-cytokeratin labeling, which marks ducts more strongly than hepatocytes in normal tissue, was patchy on colonies; most cells were clearly positive, but regions of dim/negative cells were observed (Fig. 2G). Sca-1, a marker of hematopoietic progenitors that may also indicate primitiveness in epithelial cells (Clayton and Forbes 2009), was observed on cells toward the center of colonies (Fig. 2H) in a pattern opposite to that of HNF4a or CK19.
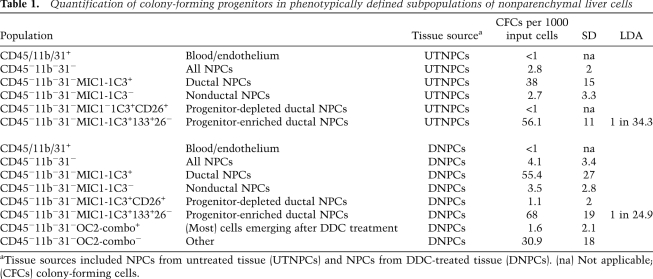
The frequencies of colony-forming progenitor cells detected in populations of normal and DDC-treated nonparenchymal liver cells are listed in Table 1. In order to capture the most robustly clonogenic cells, only colonies containing 50 or more cells were counted, but a substantial number of smaller clones were also observed. Hepatocytes did not initiate clones in these cultures. Of the three subfractions marked by the MIC1-1C3 antibody (M+), the M+133+26− fraction contained by far the highest frequency of colony-forming cells. CD26 depletion was important; CD26+ duct cells do not form colonies. The combination of CD133 and MIC1-1C3 enriched for colony formation about another twofold compared with either marker alone. Colony counts from wells seeded with 1000 cells and frequencies calculated by limiting dilution both indicated a slightly higher frequency of progenitors in the DDC-treated fractions compared with their untreated counterparts. During the oval cell response, a variety of peri-ductal cells emerge or increase in frequency, and several antibodies are available that label these NPCs (Dorrell et al. 2008). To determine whether any of these DDC-induced populations contained clonogenic progenitors, a combination of six different antibodies (“OC2-combo,” containing OC2-1C6, OC2-2A6, OC2-3C5, OC2-3C7, OC2-4E8, and OC2-6E10) was used. As indicated in Table 1, cells labeled by any of these markers were dramatically depleted for colony-forming activity. Therefore, most of the cells emerging during the oval cell response are not clonogenic.
Table 1.
Quantification of colony-forming progenitors in phenotypically defined subpopulations of nonparenchymal liver cells
The self-renewal capacity of colony-forming cells was tested by secondary colony formation assays. Individual colonies were harvested on day 12 by cloning ring isolation and trypsinization, seeded in new wells in fresh medium, and assessed 12 d later. Primary colonies gave rise to 0–54 secondary colonies (mean, 9.8) and tertiary colonies were generated at a frequency of 0–53 tertiary colonies (mean, 7.3) per secondary colony when cultures were supplemented with Wnt3A (Barker et al. 2010). Thus, progenitor self-renewal was achieved for at least three passages.
Transcriptional analysis of hepatic cell subpopulations
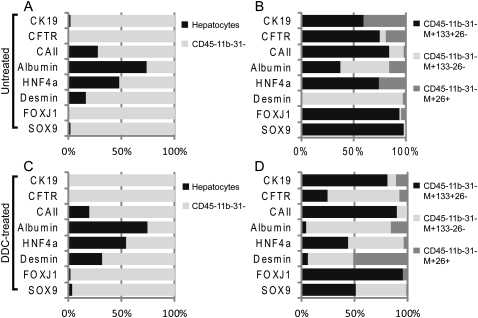
Gene expression analysis was performed by quantitative RT–PCR (qRT–PCR) on RNA from cell populations FACS-sorted directly into Trizol. As expected, the expression of genes associated with bile duct, stellate, or progenitor cells were associated with the CD45−/11b−/31− NPC population rather than hepatocytes, whether isolated from untreated (Fig. 3A) or DDC-injured (Fig. 3C) liver. The subdivision of NPCs from untreated livers into subpopulations of MIC1-1C3+ cells showed distinct patterns of gene expression (Fig. 3B). Substantial CK19 and cystic fibrosis transmembrane protein (CFTR) expression was detected in both the M+133+26− and M+26+ populations, indicating a bile duct identity for both. These two populations differed with respect to carbonic anhydrase II (CAII) expression, however; only the M+133+26− duct population contained measurable CAII. Another noteworthy distinction was the selective expression of the Sox9 liver progenitor-associated transcription factor (Furuyama et al. 2011; Kopp et al. 2011) in only the M+133+26− cell fraction. FoxJ1, another transcription factor associated with epithelial cell differentiation (Rawlins et al. 2007), exhibited the same pattern. The expected presence of CD26/DPPIV mRNA in the M+26+ fraction and its absence from the M+133+26− fraction were also confirmed by qRT–PCR (data not shown). The third subpopulation, M+133−26−, contained a very large proportion of desmin mRNA, suggesting the presence of stellate cells. When these same three antigenically defined NPC subpopulations were isolated from DDC-treated liver, certain differences were observed. Figure 3D shows that the DDC-treated M+133+26− and M+26+ fractions had profiles similar to their normal counterparts, but that the M+133−26− fraction contained CK19 mRNA and a large proportion of the CFTR mRNA; i.e., ducts. In addition, this population contained proportionally less desmin and approximately half of the SOX9 mRNA. These observations suggested that the M+133−26− fraction is quite heterogeneous after DDC treatment and contained duct cells as well as stellate cells.
Figure 3.
Mouse liver cell subset analysis reveals a duct subpopulation with progenitor gene expression. qRT–PCR results obtained from FACS-isolated hepatocyte and NPC populations were calculated as ΔCt values relative to the mean of GAPDH and β-actin; the relative expression in each cell subpopulation is indicated. The proportion of the total signal detected for a given gene in the populations being compared is indicated on the Y axis. (A,C) The expression levels of duct-, hepatocyte-, and progenitor-associated genes in hepatocytes and NPCs from normal (A) or DDC-injured (C) livers are compared. (B,D) The expression levels of duct-, hepatocyte-, and progenitor-associated genes in three subpopulations of MIC1-1C3+ NPCs from normal (B) or DDC-injured (D) livers are compared. Sox9 and FoxJ1 expression are restricted to the MIC1-1C3+/CD133+/CD26− duct subpopulation. Data from two replicate qRT–PCR analyses on material obtained from three separate cell isolation experiments were used.
The transcriptomes of progenitor-containing populations
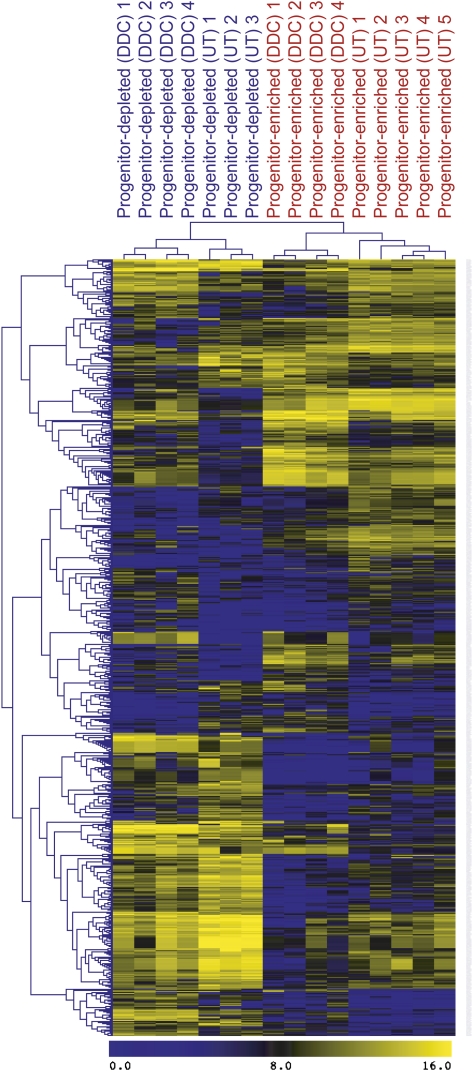
The identification of a clonogenic bipotential progenitor in normal livers afforded us the opportunity to determine which genes were up-regulated or down-regulated in this population during oval cell activation. mRNA profiles of the progenitor-enriched and progenitor-depleted nonparenchymal fractions of both normal and DDC-treated livers were obtained using Agilent Whole Mouse Genome Arrays. Global expression profiles were assembled by analysis of four biological replicates per DDC-treated population; for the untreated populations, five progenitor-enriched and three progenitor-depleted samples were used. This data set is available through ArrayExpress (AE accession: E-MTAB-459). We performed a cluster analysis of the global gene expression profiles obtained for the four sorted cell populations, focusing on 726 probes that were differentially expressed with a fold change of at least three between any pair of cell types. As shown in Figure 4, each of the four groups contained large blocks of genes with distinctive expression. An overview of the gene ontology (GO)-based distributions is shown as GO and Kegg analysis in Supplemental Figure S2.
Figure 4.
Hierarchical clustering of microarray-assessed gene expression in mouse NPC subpopulations. Analysis of global gene expression profiles was performed using the 726 probes that were differentially expressed with a fold change of at least three between any cell type pair. (UT) Untreated; (DDC) oval cell induction.
Lists of gene categories with specific differential expression patterns in these cell populations corresponding to potential functional groups were derived. Supplemental Table S2 lists categories with the most dramatic differential regulation between the M+133+26− progenitor fraction and M−133−26− nonprogenitor fractions of untreated tissue (dormant progenitors), and Supplemental Table S3 lists the corresponding information for DDC-treated tissue (injury-activated). The gene expression profile of activated bipotential clonogenic liver cells (BCLCs) was highly similar to the FoxL1-positive progenitors described in the accompanying study (Shin et al. 2011) and featured differential expression of Prom1(CD133), Epcam, Cd44, Sox9, and Trop2, which have been associated with progenitor function in the liver. Similarly, Cldn3, Ncam1, and Onecut2 are markers for both the FoxL1 and M+133+26− progenitor populations. The presence of these same factors in BCLCs from uninjured livers indicates that they pre-exist in clonogenic progenitors prior to injury. Therefore, it is likely that FoxL1 is an early marker of activated progenitors. When compared with nonclonogenic NPCs, both classes of progenitors feature a down-regulation of categories associated with cancer and tight junctions and an up-regulation of hepatocytic genes. This is compatible with the notion that the progenitors are bipotential and capable of differentiation into the hepatocytic lineage, although they are part of the biliary tree anatomically. When the activated progenitor fraction of DDC-treated tissue was compared with the dormant progenitor fraction of untreated livers (Supplemental Table S4), it was observed that the activated progenitor fraction features higher expression of cell cycle-associated genes and lower expression of zinc finger domain-containing genes. An example of the latter is Zscan12/Zfp449, a putative transcription factor with 45-fold lower expression in the M+133+26− fraction of DDC-treated cells than in untreated cells. These down-regulated zinc finger transcription factors are therefore candidates for controlling the activation of progenitors during liver injury.
In vivo potential of clonogenic progenitors
Intrasplenic transplantation NPC subpopulations (including the CD45−/11b−/31−/MIC1-1C3+/133+/26− progenitor fraction) to Fah−/− mice, in which transplanted cells undergo selection for hepatocyte function (Overturf et al. 1996), yielded evidence of liver engraftment in two of 20 transplanted mice. Supplemental Figure S1 illustrates FAH (Supplemental Fig S1A) and H&E (Supplemental Fig S1B) staining of the liver of an Fah−/− recipient transplanted with 1 × 104 CD45−/11b−/31−/MIC1-1C3+/26− cells and subjected to two rounds of NTBC withdrawal. This region, showing a region of FAH+ hepatocytes in a FAH− background, demonstrates that it is possible to derive hepatocytes in vivo after transplantation of progenitor cells. The low engraftment frequency is consistent with the observation that progenitor-derived colonies were comprised of bilineage cells rather than fully hepatocyte-like cells; additional differentiation stimuli are likely required before the progenitors or their immediate progeny can become fully hepatocytic.
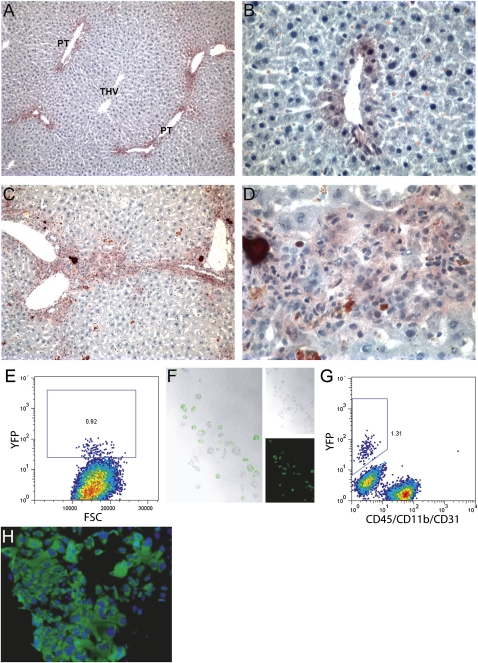
To further demonstrate the in vivo differentiation potential of the progenitors, we took advantage of the fact that Sox9-expressing cells reside within the clonogenic progenitor population defined here. This suggested that Sox9 expression may be a marker for the adult liver progenitor in vivo as well. To determine the extent to which Sox9-expressing progenitors give rise to hepatocytes and duct cells under normal conditions and during liver regeneration, Sox9creERT2-R26RYFP mice (Kopp et al. 2011) were treated with tamoxifen to induce marking of Sox9+ cells and their progeny with YFP. Examples of immunohistochemistry-visualized YFP expression in untreated tissue 14 d after recombinase activation are shown in Figure 5, A and B. Small numbers of marked peri-portal hepatocytes and duct cells were detected, indicating that Sox9-expressing cells contributed to both cell lineages even in the absence of a major regenerative stimulus. However, 14 d of DDC treatment produced a more dramatic response (Fig. 5C,D); most of the cells emerging as a result of DDC-induced ductal proliferation and proximal hepatocyte-like cells are YFP+ and are therefore the product of a Sox9-expressing precursor. To further study the identity and properties of these marked cells, hepatocytes and NPCs were isolated by perfusion and assessed for YFP expression. Approximately 1% of hepatocytes (selected by density selection and gated by size/granularity during flow cytometric analysis) were YFP+ (Fig. 5E). After FACS isolation and overnight culture, hepatocyte identity and YFP positivity were confirmed by visual inspection (Fig. 5F). YFP+ NPCs were also recovered (Fig. 5G) and cultured in a progenitor assay. Approximately one-third (26 of 74) of the resulting colonies were YFP+ (e.g., Fig. 5H), indicating that the Sox9-expressing cells and clonogenic progenitors identified using cell surface markers are the same population. The presence of YFP− colonies may indicate that some progenitors are derived from precursors that never expressed Sox9 or that Cre recombinase activity was incomplete. Collectively, these observations suggest that the Sox9-expressing cell population encompasses progenitor cells in the adult liver.
Figure 5.
Cells emerging from a DDC-induced ductular reaction are the progeny of a Sox9-expressing precursor. YFP expression was visualized in Sox9creERT2; R26RYFP mice 9 wk after tamoxifen injection. (A,B) Liver sections from control mice that received a normal diet during this interval. (C,D) Liver sections from mice that were DDC-treated to induce oval cell activation for the final 2 wk before harvest. (PT) Portal triad. Flow cytometric detection of YFP+ hepatocytes (E) and duct cells (G) recovered from the perfused liver of a tamoxifen-treated Sox9creERT2; R26RYFP mouse are shown. (F) Phase contrast and fluorescent imaging of sorted hepatocytes after overnight culture to confirm their identity and YFP fluorescence. (H) YFP+ colonies were initiated by clonogenic progenitors from these animals, indicating that the populations defined by Sox9 expression or surface marker labeling have the same identity. Magnifications: A,C, 100×; F,H,I, 400×; B,D, 500×.
Discussion
The existence of stem/progenitor cells in the adult liver and their roles in injury and carcinogenesis have been debated for many years. Histopathological studies have indicated that a bipotential precursor to both hepatocytes and bile duct epithelial cells may reside with the Canals of Hering. However, some have challenged the notion that there is a progenitor in the adult liver and have proposed that the cells that emerge during the oval cell response may be the product of transdifferentiation between the bile duct and hepatocyte lineages (Michalopoulos 2011). Specifically, it has been suggested that hepatocytes can give rise to ductal lineages both in vitro and in vivo. Although experiments in chimeric mice have shown conclusively that the majority of NPCs that are generated during DDC-induced oval cell activation are not derived from hepatocytes (Wang et al. 2003), the origin of the progenitor remained unknown. Recently, genetic lineage tracing using FoxL1Cre; RosaYFP mice indicated that FoxL1 expression, activated in response to liver injury, marks progenitor cells that give rise to both hepatocytes and cholangiocytes in vivo (Sackett et al. 2009b).
In this study, we used FACS to prospectively isolate a clonogenic epithelial population from normal adult livers. These M+133+26− progenitor cells generate colonies that express markers of both the hepatocyte and bile duct lineages, indicating that they are bipotential at the clonal level. Furthermore, the oval cell injury-activated progenitor population is similar to that reported in the accompanying study (Shin et al. 2011) describing FoxL1Cre; RosaYFP marked cells. The high colony-forming frequency was achieved by using novel surface markers not used previously for this purpose, most notably MIC1-1C3 (Dorrell et al. 2008).
In most cases, fully defined medium was used to generate the colonies characterized here, obviating the need for conditioned medium as reported by previous studies. Thus, the assay can be used to study the growth factors/matrix conditions required for clonogenic growth and differentiation of this population. In preliminary experiments, we observed that the addition of IL-6 shifts colony gene expression toward the hepatocytic lineage, showing that the assay can be used to test fate-altering exogenous agents. We have not yet demonstrated that the in vitro self-renewal capacity of the clonogenic population is unlimited, and therefore we chose to not use the term “stem cell” to describe these cells. We believe that the term BCLCs is appropriate based on these results. However, when medium conditioned by embryonic day 14.5 (E14.5) liver cells is used, as shown in the accompanying study (Shin et al. 2011), the progenitors can be serially passaged at least 15 times. The factor(s) within the conditioned medium that permit much more extensive self-renewal remain to be defined.
Microarray analysis and qRT–PCR indicated that these clonogenic progenitors express Sox9, whereas other nonhepatocyte cell populations in the normal liver do not. Sox9 is known to contribute to the regulation of developing reproductive tissues, neural crest stem cells, and chondrocytes (Thomsen et al. 2008). Within the developing mouse pancreas, Sox9 expression marks a progenitor population but is restricted to a subset of duct cells in the adult mouse (Seymour et al. 2007, 2008). Given the common developmental origin of the liver and pancreas in the foregut, it is unsurprising that progenitor cells in these tissues may have similar regulatory features. Although our data show that the BCLCs express Sox9, they cannot be interpreted to indicate the reverse; i.e., that every Sox9-positive cell is a progenitor. In this context, it is interesting to note that Shin et al. (2011) found two populations of Sox9+ cells in the injured liver distinguishable by the presence or absence of FoxL1 expression. Since only one of 25 M+133+26− cells forms a colony in vitro, it is possible that this population remains heterogeneous and that further enrichment is possible. Nonetheless, it is clear that the bulk of cells that emerge de novo after DDC injury, including hepatocytes, derive from a Sox9-positive precursor. A recent study (Furuyama et al. 2011) describing lineage tracing of Sox9 cells in the adult mouse is consistent with our own data and indicates that these cells function as progenitors during a wide variety of liver injury regimens, including carbon tetrachloride administration or oval cell activation by a choline-deficient/ethionine-supplemented diet.
The delineation of adult hepatic progenitors has important biomedical implications. It now becomes possible to understand how this population awakens from dormancy during the oval cell response. This mechanism is likely to be used during some forms of chronic liver injury in humans, especially primary biliary cirrhosis (Roskams et al. 2003). In addition, the ability to prospectively isolate these cells affords the possibility of studying genetic and epigenetic changes during liver carcinogenesis. Finally, the identification of transcriptional regulators strongly enriched in the hepatic progenitor population should facilitate future studies of the regulation of progenitor self-renewal and differentiation. It is likely that this population remains heterogenous and that further fractionation of the M+133+26− fraction will yield cell subtypes with distinct potentials.
Materials and methods
Tissue sources and liver cell isolation
Animal care and immunization procedures were performed in accordance with protocol IS00000788 of the institutional review committee at Oregon Health and Science University and protocol S08215 of the University of California at Irvine and University of California at San Diego Institutional Animal Care and Use Committees. c129/S3 mice, obtained from the Jackson Laboratory, were fed a Purina 5015 diet with or without 0.1% (w/w) DDC (Sigma-Aldrich and Harlan Teklad) for 2 wk. For transplantation studies, Fah−/− c129/S3 recipient animals were used (Grompe et al. 1993). Lineage analysis studies were performed with Sox9creERT2; R26RYFP mice (Kopp et al. 2011) maintained on 70% CD1 (Charles River) and 30% C57BL/6 (Charles River). To induce recombination of the YFP reporter allele in Sox9+ cells, 24-d-old Sox9creERT2; R26RYFP mice were injected with three doses of tamoxifen subcutaneously (5 mg/40 g body weight) on consecutive days. Nine weeks later, these mice were fed a Purina 5015 diet with or without 0.1% (w/w) DCC for 2 wk to induce oval cell induction in the liver.
Hepatocytes and NPCs were isolated as described previously (Dorrell et al. 2008) with the following modifications. Accutase (Innovative Cell Technologies) was substituted for the published combination of 10 mg/mL Collagenase D and 10 mg/mL Pronase; although Pronase did not reduce cell viability, it selectively destroyed the MIC1-1C3 antigen and was therefore unsuitable for duct cell preparation. Erythrocytes present in the first NPC fraction were lysed by cell pellet resuspension in 0.2% NaCl for 1 min. Finally, once all three NPC fractions were collected and combined, these were resuspended in 35% Percoll (GE Healthcare) and centrifuged at 900g for 10 min to exclude debris (which reduces the efficiency of FACS sorting).
Immunofluorescent imaging
Mouse liver cryosections (5 μm) were prepared using a Reichert 2800 Frigocut (Reichert Scientific Instruments) and fixed in acetone for 10 min at −20°C. Nonspecific labeling was blocked by incubation in 2% goat serum (Hyclone) for 10 min. Lineage-marking antibodies used included anti-HNF4a (Santa Cruz Biotechnology), anti-CK19 (a gift from X. Wang), anti-panCK (Abcam), and Sca-1 (BD Biosciences). Primary labeling used hybridoma supernatants diluted 1:20 in DPBS for 30 min and secondary labeling with 1:200 dilutions of DyLight488- or Cy3-conjugated anti-rat Ig preadsorbed against mouse serum proteins (Jackson ImmunoResearch) for 20 min. Nuclei were visualized with Hoechst 33342 (Molecular Probes). A Zeiss Axioskop 2 plus microscope (Carl Zeiss) was used for imaging.
Flow cytometry and FACS
Dissociated cells were resuspended at 1 × 106 cells per milliliter in DMEM + 2% FBS prior to the addition of MIC1-1C3 hybridoma supernatant at a 1:20 dilution, or a 1:200 dilution of purified MIC1-1C3 antibody (Novus Biologicals) and incubation for 30 min at 4°C. After a wash with cold DPBS, cells were resuspended in DMEM + 2% FBS containing a 1:200 dilution of APC-conjugated goat anti-rat secondary antibody adsorbed against mouse serum proteins (Jackson Immunoresearch). After another wash, cells were resuspended in DMEM + 5% rat serum (Serotec) and held for 10 min on ice to block the secondary antibody. A final incubation with FITC-conjugated anti-CD26 (BD Biosciences), PE-conjugated anti-CD133 (eBioscience), and PE-Cy7-conjugated anti-CD45 (BD Biosciences) + anti-CD11b/Mac1 (BD Biosciences) + anti-CD31 (Abcam) facilitated cell subfractionation and exclusion gating of hematopoietic (CD45+ and CD11b+) and endothelial (CD31+) cells. Propidium iodide staining was used to label dead cells for exclusion. Cells were analyzed and sorted with a Cytopeia inFluxV-GS (Becton-Dickenson); for FSC, pulse-width gating was used to exclude cell doublets from analysis and collection.
Colony formation assay
Cells were seeded at a density generally 102–104 per square centimeter (dependent on the anticipated colony formation frequency) on Primaria (BD Falcon) tissue culture plasticware precoated rat tail collagen. The coating was made from a stock of 1 mg/mL rat tail collagen in 0.1% glacial acetic acid, diluted 1:10 in DMEM, applied to the plate surface for 10 min, and then aspirated before the addition of cells + medium. Serum-free medium, including defined concentrations of free fatty acids, was prepared as described (Macdonald et al. 2002) and supplemented with 50 ng/mL murine epidermal growth factor, 25 ng/mL murine hepatocyte growth factor (Chemicon), and 10 μM Insolution Y-27632 (EMD Chemical). In certain experiments, medium was supplemented with Wnt3A (100 ng/mL; Stem Cell Technologies). Colonies, defined as organized roughly circular clusters of at least 10 cells, were scored on day 12. Because of the challenge associated with identifying these (extremely flat) colonies by phase contrast alone, staining with Methylene Blue was used unless colonies were to be analyzed by immunofluorescent labeling or collected for RNA analysis or replating.
Transplantation assay
The injection of sorted cell populations to the spleen and the withdrawal of NTBC to induce hepatocyte selection were performed as described previously (Overturf et al. 1996). Drug withdrawal was done in periods of 3 wk, followed by readministration until normal weight was restored in the recipient animals.
Limiting dilution analysis
For limiting dilution assays of ex vivo colony formation, Poisson statistics for the single-hit model were applied. Cells were seeded at densities ranging from five to 100 per well and colony counts were performed at day 12.
Microarray analyses
Amplified cDNA was prepared from RNA from five samples for each sorted cell population sample using the WT-Ovation Pico Amplification system (NuGEN Technologies). Amplified cDNA (2 μg) was directly labeled using the BioPrime Array CGH Genomic Labeling system (Invitrogen) with Cy3-labeled nucleotides (GE Amersham Biosciences). Labeled samples were hybridized overnight to the Agilent 4X44 Whole Mouse Genome Array. Arrays were washed and then scanned with the model G2565B Agilent DNA microarray scanner (Agilent Technologies). Median intensities of each element on the array were captured with Agilent Feature Extraction version 10.5.1.1 (Agilent Technologies). Quality control diagnostic plots were prepared for each array, and those failing to exhibit high-quality hybridizations were excluded from further analysis, resulting in the final data sets.
The data were normalized by the quantile normalization method using the “normalizeBetweenArrays” function in the LIMMA (linear models for microarray data) package in R as described (Gentleman 2005). For statistical analysis, genes were called differentially expressed using the Significance Analysis of Microarray (SAM) one-class response package with a false discovery rate of 10% and a minimum fold change of 1.5×. Genes marked as absent (i.e., with expression levels near background) were omitted. All expression data were deposited in Gene Expression Omnibus (GEO accession GSE29121).
The heat map in Figure 5 was produced using the TIGR Multiexperiment Viewer (Saeed et al. 2003).
Functional enrichment of GO and KEGG pathways were determined using the DAVID/EASE Web site (Dennis et al. 2003; Huang et al. 2009). Reported statistical significances were corrected for multiple testing using Benjamini-Hochberg and FDR values were calculated by the Web site.
RNA isolation and qRT–PCR
Cell populations were sorted directly into Trizol LS (Invitrogen). First strand cDNA synthesis used MMLV reverse transcriptase and random oligonucleotide primers (both Invitrogen). RNA levels were assessed by qRT–PCR using a Bio-Rad iCycler and IQ5 detection system. All reactions were performed using 45 cycles of 15 sec at 95°C, 20 sec at 68°C, and 25 sec at 72°C. Reaction mixtures included Platinum Taq DNA polymerase (Invitrogen), 2.5 mM MgCl2, 10 μM 5′ and 3′ primers, 10 mM dNTPs, and 0.5× SYBR green. Primer sequences are listed in Supplemental Table S1. Gene expression levels were reported as the difference between baseline-corrected, curve-fitted cycle thresholds for the gene of interest minus the average cycle thresholds of two housekeeping genes (GAPDH and β-actin). Curve-fitting of qRT–PCR cycle threshold results was generated by IQ5 software (Bio-Rad), and statistical mean and standard deviation data were obtained with Microsoft Excel.
Acknowledgments
This work was supported by National Institutes of Health grants DK051592 (M.G.), P01 DK049210 (K.H.K.), and DK078803 to M.S., and an NIH/NRSA post-doctoral fellowship to J.L.K. OHSU has commercially licensed part of the technology disclosed herein (MIC1-1C3/MPdi1); C.D. and M.G. are inventors of this reagent. This potential conflict of interest is reviewed and managed by OHSU.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.2029411.
Supplemental material is available for this article.
References
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Clayton E, Forbes SJ 2009. The isolation and in vitro expansion of hepatic Sca-1 progenitor cells. Biochem Biophys Res Commun 381: 549–553 [DOI] [PubMed] [Google Scholar]
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4: 3 doi: 10.1186/gb-2003-4-5-p3 [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M 2008. Surface markers for the murine oval cell response. Hepatology 48: 1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Dorrell C, Grompe M 2009. Stem cells and liver regeneration. Gastroenterology 137: 466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. 2011. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41 [DOI] [PubMed] [Google Scholar]
- Gentleman R 2005. Bioinformatics and computational biology solutions using R and Bioconductor. Springer, New York [Google Scholar]
- Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P 1993. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 7: 2298–2307 [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H 2009. Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice. Gastroenterology 137: 1114–1126e.14 doi: 10.1053/j.gastro.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M 2011. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138: 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JM, Xu ASL, Kubota H, LeCluyse E, Hamilton G, Liu H, Rong YW, Moss N, Lodestro C, Luntz T, et al. 2002. Protocols for isolation and ex vivo maintenance of hepatic stem cells and their normal and transformed derivatives. In Methods of tissue engineering (ed. Lanza W et al. ), pp. 151–201 Academic Press, San Diego, CA [Google Scholar]
- Michalopoulos G.K 2011. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol 221: 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Shafritz DA. 2008.. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta 1782: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M 1996. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 12: 266–273 [DOI] [PubMed] [Google Scholar]
- Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS 1999. Atypical ductular proliferation and its inhibition by transforming growth factor β1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest 79: 103–109 [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL 2007. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci 104: 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams TA, Libbrecht L, Desmet VJ 2003. Progenitor cells in diseased human liver. Semin Liver Dis 23: 385–396 [DOI] [PubMed] [Google Scholar]
- Sackett SD, Gao Y, Shin S, Esterson YB, Tsingalia A, Hurtt RS, Brondell K, Kaestner KH, Greenbaum LE 2009a. Foxl1 promotes liver repair following cholestatic injury in mice. Lab Invest 89: 1387–1396 [DOI] [PubMed] [Google Scholar]
- Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, Greenbaum LE 2009b. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 49: 920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M 2007. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci 104: 1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M 2008. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol 323: 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, et al. 2011. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev (this issue) doi: 10.1101/gad.2027811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt DB, Medline A, Farber E 1977. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol 88: 595–618 [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Zheng Yw YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H 2002. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol 156: 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H 2008. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology 48: 1964–1978 [DOI] [PubMed] [Google Scholar]
- Thomsen MK, Francis JC, Swain A 2008. The role of Sox9 in prostate development. Differentiation 76: 728–735 [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M 2003. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci 100: 11881–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW, Leduc EH 1958. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol 76: 441–449 [DOI] [PubMed] [Google Scholar]