Introduction
Movement disorders may result from autoimmune damage of the central nervous system. Examples include Sydenham´s chorea and paraneoplastic encephalitis. Recently Dalmau et al identified a new type of encephalitis, mostly in young women and children, presenting with dyskinesias, psychiatric disturbances, autonomic instability and seizures1, 2. There is increasing evidence that this disorder is mediated by circulating antibodies against NR1/NR2 heteromers of the N-methyl-D-aspartate-glutamic-receptors(NMDAR)2,3. Exceptionally, patients with NMDAR encephalitis may not develop the full spectrum of symptoms. Here we report a patient with isolated subacute hemidystonia associated with NMDAR antibodies.
Case report
A 19 year old woman was admitted in October 2008 because of headache and abnormal movements. She had longstanding migraine without aura and a remote history of childhood recurrent pharyngeal infections requiring tonsillectomy. Four days before admission she developed severe occipital throbbing headache followed by left hand and foot abnormal postures and stiffness that varied spontaneously in intensity over periods of several hours. She had no previous febrile illness. A few days earlier she took a low dose of cocaine, which she used occasionally. On admission she had dystonic posturing of the left side of the face, hand, and foot (VIDEO). The intensity of symptoms fluctuated; they were precipitated by stress and anxiety and mildly ameliorated with voluntary movements. There were no sensory tricks or mirror movements. MRI of the brain showed no abnormalities. An EEG obtained during her dystonic episodes was normal. The following investigations were normal or negative: full blood count, cupremia/cupruria, serum ceruloplasmin, thyroid hormones, anti-nuclear-antibodies, anti-DNA-antibodies, anti-thyroid-antibodies, anti-phospholipid-antibodies, rheumatoid factor, blood smear for acanthocytes. Anti-streptolysin-O-antibodies (ASLO) titres were 650 UI/ml (normal<200 UI/ml). Pharyngeal smear culture failed to demonstrate current streptococcal infection. CSF analysis was normal, without oligoclonal bands and negative microbiological studies (culture, PCR for HSV, EBV, CMV). Onconeuronal-antibodies(anti-Hu, Ri, Yo, Ma2, Tr, amphiphysin, CV2) were negative. Using reported techniques2, antibodies to NR1/NR2 heteromers of the NMDA receptor expressed on HEK293 cells were identified in serum (1/20) and CSF (1/40). In addition, in vitro incubation of cerebellar granular neurons (where NMDAR are highly expressed)4 with the CSF of the patient caused suppression of the NMDAR dependent calcium influx into the cells (Figure 1, for methodology see reference 4). Thoraco-abdomino-pelvic CT and abdomino-pelvic ultrasound were normal. She was treated with intravenous methylprednisolone (1 gram/daily for 5 days) with rapid resolution of her symptoms. In addition she also received a single dose of 1,2-million units of penicillin G benzathine. Three months after discharge, the patient consulted again due to reappearance of hemidystonia. ASLO titres were 421 UI/ml. Treatment with intravenous immunoglobulins (0.4 grams/kg for 5 days) was started, but on the fourth day it was discontinued due to the development of severe headache, malaise, and unexpected worsening of patient’s dystonia. She was then treated with high dose intravenous corticosteroids, resulting again in rapid symptom improvement. Because symptoms relapsed 8 weeks later, she was started on prednisone (1 mg/kg) and mycophenolate (2 gr/day), with steady improvement. One year later she is asymptomatic, with mycophenolate and a tapering dose of prednisone.
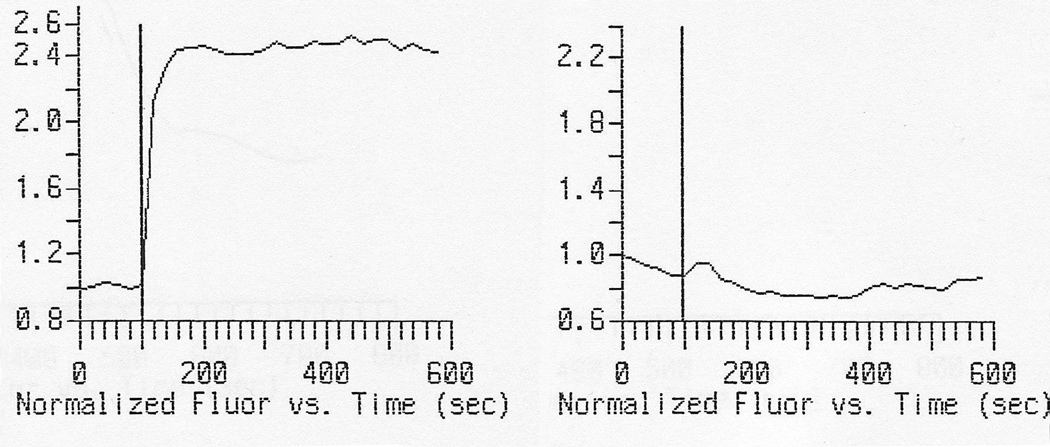
Figure 1.
Left panel: Normalized fluorometric measured intracellular calcium in time (seconds) before and after instillation of NMDA into the media (at 100 sec) in primary cultures of neurons. Right panel: After preincubation during 1 hour with 1:10 CSF of the patient3.
Discussion
Patients with anti-NMDAR encephalitis often develop complex abnormal movements including orofacial dyskinesias, dystonic posturing of the extremities, chorea, oculogyric crises, myoclonus, and opisthotonos2,5,6. Dystonic posturing might be sustained, with a variable distribution and response to sensory stimulation, including unilateral symptoms5,6. Abnormal movements may be the presenting symptom5 or as in our patient, the sole manifestation. NMDAR antibodies targeting conformal extracellular epitopes of the receptor are thought to be pathogenic. In vitro studies have demonstrated that antibodies induce downregulation of postsynaptic NMDAR clusters in cultured rat hippocampal neurons. Moreover, similar effects have been observed in the hippocampus of rats infused with patients’ antibodies3. The CSF of our patient altered NMDA triggered calcium influx into living neurons, suggesting a direct functional effect of the antibody. These results, however, need replication in other patients.
Parainfectious aetiology is often considered in the differential diagnosis of patients with anti-NMDAR encephalitis. Studies are often negative, but a few paediatric patients have been reported with positive mycoplasma serology5,7. Whether previous streptococcal infection played a role in triggering NMDAR autoimmune response in our patient remains speculative. We conclude that anti-NMDAR encephalitis should be considered in patients with new onset unexplained dystonia.
Supplementary Material
Videotaped examination of the patient showing dystonic posturing of the left face, hand and foot and improvement after treatment.
Acknowledgements
Supported in part with FIS PI06/0804
We thank Mr. Pablo Presencia-Ortí for technical assistance.
Dr. Dalmau receives research support from EUROIMMUN and the NIH/NCI [RO1CA107192 (PI) and RO1CA89054-06A2 (PI)].
Footnotes
Author roles:
1. Research project: A. Conception. B. Organization. C. Execution.
2. Manuscript: A. Writing of the first draft. B. Review and critique
Ignacio Rubio-Agustí 1 A, 1 B, 1C, 2 A
Josep Dalmau 1 C, 2 B
Teresa Sevilla 1 B
María Burgal 1 C
Eduardo Beltrán 1C
Luis Bataller 1 A, 1 B, 1C, 2 B
Full Financial disclosure:
Dr. Rubio-Agustí, Dr Bataller, Dr Sevilla, Dra Burgal and E. Beltrán report no disclosures. Dr. Dalmau has received honoraria for lectures not funded by industry; has received license fee payments from EUROIMMUN for an NMDA receptor autoantibody test (patent pending PCT/US07/18092, filed: August 15, 2007); and has received royalty payments and may accrue revenue for US Patent 6,387,639, issued: May 14, 2002: Patent for Ma2 autoantibody test.
References
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes BA, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms underlying anti-NMDA receptor encephalitis. Ann Neurol. 2009;66 Suppl 13:S63–S64. [Google Scholar]
- 4.Marcaida G, Miñana MD, Burgal M, et al. Ammonia prevents activation of NMDA receptors by glutamate in rat cerebellar neuronal cultures. Eur J Neurosci. 1995;7:2389–2396. doi: 10.1111/j.1460-9568.1995.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 5.Florance NR, Davis RL, Lam C, et al. Anti-NMDA-receptor encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinig TJ, Thompson PD, Matar W, et al. The distinctive movement disorder of Ovarian teratoma-associated encephalitis. Mov Dis. 2008;23:1256–1261. doi: 10.1002/mds.22073. [DOI] [PubMed] [Google Scholar]
- 7.Gable MS, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of 10 cases and comparison with viral encephalitis. Europ J Clin Microbiol Infect Dis. 2009;28:1421–1429. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videotaped examination of the patient showing dystonic posturing of the left face, hand and foot and improvement after treatment.