Abstract
Phthalate esters are a class of compounds utilized extensively in widely-distributed consumer goods, and have been associated with various adverse health outcomes in previous epidemiologic research. Some of these health outcomes may be the result of phthalate-induced increases in oxidative stress or inflammation, which has been demonstrated in animal studies. The aim of this study was to explore the relationship between urinary phthalate metabolite concentrations and serum markers of inflammation and oxidative stress (C-reactive protein (CRP) and gamma glutamyltransferase (GGT), respectively). Subjects were participants in the National Health and Nutrition Examination Survey (NHANES) between the years 1999 and 2006. In multivariable linear regression models, we observed significant positive associations between CRP and mono-benzyl phthalate (MBzP) and mono-isobutyl phthalate (MiBP). There were CRP elevations of 6.0% (95% confidence interval (CI) 1.7% to 10.8%) and 8.3% (95% CI 2.9% to 14.0%) in relation to interquartile range (IQR) increases in urinary MBzP and MiBP, respectively. GGT was positively associated with mono(2-ethylhexyl) phthalate (MEHP) and an MEHP% variable calculated from the proportion of MEHP in comparison to other di(2-ethylhexyl) phthalate (DEHP) metabolites. IQR increases in MEHP and MEHP% were associated with 2.5% (95%CI 0.2% to 4.8%) and 3.7% (95%CI 1.7% to 5.7%) increases in GGT, respectively. CRP and GGT were also inversely related to several phthalate metabolites, primarily oxidized metabolites. In conclusion, several phthalate monoester metabolites that are detected in a high proportion of urine samples from the US general population are associated with increased serum markers of inflammation and oxidative stress. On the other hand, several oxidized phthalate metabolites were inversely associated with these markers. These relationships deserve further exploration in both experimental and observational studies.
Keywords: Biomarkers, Exposure, Epidemiology, Population, Risk
INTRODUCTION
Phthalate esters are a class of compounds utilized extensively in widely-distributed consumer goods. High molecular weight phthalates such as di(2-ethylhexyl) phthalate (DEHP) and benzylbutyl phthalate (BzBP) are used to add flexibility to plastics such as those found in polyvinyl chloride (PVC) flooring, medical tubing, and food packaging materials (ATSDR 2002; CDC 2010). Low molecular weight phthalates such as diethyl phthalate (DEP) and dibutyl phthalate (DBP) may also be used as plasticizers and are widely used as solvents in cosmetics, insecticides, and other products (ATSDR 1996; 2001). Previous human epidemiologic research has reported associations between exposure to phthalates and various adverse health outcomes, including altered male reproductive function and development (Hauser et al. 2007, Hauser et al. 2006, Main et al. 2006; Swan 2008; Zhang et al. 2006), altered thyroid function (Meeker et al. 2007; Huang et al. 2007; Boas et al. 2010), increased waist circumference and insulin resistance (Stahlhut et al. 2007; Hatch et al. 2008), decreased gestational age or increased risk of premature birth (Latini et al. 2003, Meeker et al. 2009, Whyatt et al. 2009), and respiratory symptoms and asthma (Bornehag et al. 2010; 2004; Kolarik et al. 2008; Hoppin et al. 2004).
Several of the phthalates elicit adverse reproductive effects through endocrine disruption mechanisms. Perhaps best described is the role of testosterone synthesis suppression in reproductive tract development of male laboratory animals by DEHP, DBP, and BzBP (Noriega et al. 2009; Clark and Cochrum 2007; Higuchi et al. 2003; Nagao et al. 2000). In addition to endocrine disruption, some phthalates stimulate oxidative stress, a cellular condition in which damage to cellular macromolecules occurs as a result of excessive amounts of reactive oxygen species. Laboratory experiments implicate a role for oxidative stress in phthalate-stimulated liver tumorigenesis (Ito et al. 2007), male reproductive toxicity (Zhou et al. 2010; Wellejus et al. 2002), and developmental toxicity (Seo et al. 2004; Kasahara et al. 2002). Moreover, oxidative stress has been suggested as the etiologic link for recently reported relationships between urinary phthalate metabolites of DEHP and DEP with increased DNA damage in human sperm (Hauser et al. 2007; Duty et al. 2003). Recent epidemiologic studies have reported relationships between biomarkers of phthalate exposure and increased levels of the oxidative stress markers malondialdehyde (MDA) and 8-hydroxydeoxyguanosine (8-OHdG) (Hong et al. 2009; Ji et al. 2010).
Oxidative stress and inflammation are known to be interrelated, and oxidative stress-stimulated inflammation has been proposed to be part of the etiologic pathway for DEHP-induced tumorigenesis (Ito et al. 2007). In addition, increased release of the proinflammatory cytokines tumor necrosis factor- α (TNF-α) and Fas ligand (FasL) in the male reproductive tract is linked to decreased sperm production (Yao et al. 2007). Phthalate metabolites stimulate release of the pro-inflammatory cytokine IL-6 from human lung epithelial cells (Jepsen et al. 2004). Moreover, consistent with inflammatory activity, DEHP or its metabolite MEHP increase integrin (CD11b) expression in neutrophils (Gourlay et al. 2003) and activate MAPK pathways in liver cells (Pauley et al. 2002), respectively. Bornehag and Nanberg (2010) postulated that asthma and allergic effects of phthalates may be due to their ability to act as adjuvants and cause inflammation. Likewise, inflammation was hypothesized to be a mechanism involved in the reported relationships between phthalates and decreased gestational age or preterm birth (Latini et al. 2003; Meeker et al. 2009).
In the present study we analyzed the National Health and Nutrition Examination Survey (NHANES) datasets from 1999 to 2006 to explore associations between urinary phthalate metabolite concentrations and levels of gamma glutamyltransferase (GGT) and C-reactive protein (CRP) measured in serum. Serum CRP is a commonly used measure of inflammation (Everett et al. 2010; Pearson et al. 2003), and increased serum GGT has been demonstrated to be a sensitive marker of oxidative stress in population studies (Lee and Jacobs 2009a; 2009b).
METHODS
NHANES is an ongoing cross-sectional study designed to collect nationally representative data on dietary intake and disease. Methods for demographic and survey data collection are described in detail elsewhere (NCHS 2010a). Briefly, participants across the country are interviewed in their homes and subsequent laboratory tests, conducted on subsets of participants, are performed in mobile examination centers (MECs). Minority groups are oversampled, which, when analyzed with proper weightings, enables more accurate distributions of population characteristics. The present analysis included measurements from eight years of NHANES data, 1999-2006, merged according to the NCHS tutorial (NCHS 2010b). There were 10,476 subjects from these years who had data for one or more of the phthalate metabolites, urine creatinine levels, and either serum GGT or serum CRP. Pregnant subjects (n=445) were excluded from the primary data analysis. This resulted in 10,031 total subjects available for analysis, though the numbers for each model varied by phthalate metabolite and/or serum GGT and CRP availability.
Urinary phthalate metabolites
Upon MEC collection of urine, samples were stored at 4 degrees C or frozen at −20 degrees C and shipped to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis. Measurements of urine phthalate metabolite levels were made using HPLC separation followed by either electrospray ionization (ESI) or atomic pressure chemical ionization (APCI) and tandem mass spectrometry (NCHS 2009, NCHS 2010c), described in detail elsewhere (Silva et al. 2004; 2003). Values below the limit of detection (LOD) were replaced with a value of the LOD divided by the square root of two (Hornung and Reed 1990). Of all the phthalate metabolites measured, those with greater than 75% of samples below the LOD were not considered further in the present analysis as reported previously by Stahlhut et al. (2007). This excluded mono-cyclohexyl phthalate (MCHP), mono-isononyl phthalate (MiNP), mono-n-octyl phthalate (MnOP), and mono-n-methyl phthalate (MnMP). In addition to examination of the remaining metabolites, we also created a variable to represent a combination of the metabolites of DBP, which was measured as represented by the MnBP variable in years 1999-2000 and calculated as the sum of mono-isobutyl phthalate (MiBP) and mono-n-butyl phthalate (MnBP) in 2001-2006.
For DEHP, we hypothesized that subjects with higher levels of the biologically active monoester metabolite, mono-(2-ethyl)-hexyl phthalate (MEHP), in relation to other potentially less toxic oxidized DEHP metabolites, mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), may have a heightened metabolic susceptibility to MEHP exposure (Hauser 2008; Meeker et al. 2007). To test this hypothesis we created a variable to represent the percentage of the sum of DEHP metabolites that was measured as MEHP (MEHP%). This was done by converting the DEHP metabolites MEHP, MEHHP, and MEOHP into nanomoles (nmol) using their respective molecular weights (278, 294, and 292 g/mol) and dividing the molar mass of MEHP by the mass of the sum of all three metabolites and then multiplying by 100. Though levels of urinary mono-2-ethyl-5-carboxypentyl phthalate (MECPP), another oxidized metabolite of DEHP, were available in this dataset, we did not include them in the MEHP% variable because this analyte was measured on a much smaller number of participants (N = 4903). In addition, MECPP was highly correlated with both MEHHP (R = 0.95, p < 0.0001) and MEOHP (R = 0.96, p < 0.0001). Thus, its inclusion in the MEHP% variable would not be expected to impact results.
Because all metabolite concentrations as well as the summed and percent measures were right-skewed, the data were log-transformed prior to analysis. Also, metabolite measurements were creatinine standardized for presentation of descriptive statistics and calculation of simple correlations by dividing metabolite concentrations by urinary creatinine (presented as ug/g creatinine). For regression analysis unadjusted metabolite levels were used and urinary creatinine was included as a covariate in all models as recommended by Barr et al. (2005).
Serum CRP and GGT
GGT and CRP levels were measured in serum collected at the same time as urine samples, and analyzed as described by the National Center for Health Statistics (NCHS) (2010a). Briefly, GGT was measured in serum from participants aged 13 years and older with an enzymatic reaction in which the compound transfers gamma-glutamyl groups from a colorless substrate to glycylglycine with the production of the colored byproduct, p-nitroanaline. The absorbance of the reaction is measured at 410 nm over a fixed period of time by a Beckman Synchron LX20 chemistry analyzer. CRP was analyzed by latex-enhanced nephelometry in which the serum sample is mixed with a latex-bound mouse anti-CRP antibody which is then measured with a Behring Nephelometer. Both distributions were right-skewed and log-transformed for analysis.
Covariates
Demographic data were collected in an in-home survey component of NHANES. From these data, we examined age, sex, poverty income ratio (PIR), and race and ethnicity as potential confounding variables. We also considered alcohol use and education level, but did not include these variables in final models in order to maximize the sample size of our study (N missing for alcohol use = 4,674, N missing for education = 4,293) since there was a lack of association between these variables and urinary phthalate metabolites in the subset of participants with these data (p > 0.05). From examination and laboratory data we included body mass index (BMI) as well as (log-transformed) serum cotinine as a measure of exposure to tobacco smoke. As mentioned previously, creatinine was included as a covariate in all models to adjust for urine sample dilution (Barr et al. 2005). Finally, NHANES dataset (i.e. 2-year survey increments) was also considered as a categorical covariate but was not retained in the final models.
Statistical analysis
Data analysis was performed using SAS 9.2 (SAS Institute, Cary, NC). NHANES data are collected using a complex, multistage study design. Ensuring accurate population representation entails oversampling of some groups based on age, sex, and race and ethnicity. In order to create a sample that, when analyzed properly, is representative of national demographic and health characteristics, sample weights are created for use in univariate and bivariate modeling. For our analysis, an 8 year weight for individual probabilities was created from phthalate metabolite weights according to the NCHS web tutorial (NCHS 2010b) to account for the sampling method. In addition, stratum and cluster weights were included in regression models to correct for the study design. We also constructed models without including the sample weights for comparison.
In descriptive analyses, distributions of phthalate metabolites, GGT and CRP were assessed with appropriate weights by age group, sex, ethnicity, and dataset by calculating the sample size, geometric mean and standard error, and selected percentiles for each group. Correlation analyses between log-transformed and creatinine-standardized phthalate metabolites and log-transformed GGT and CRP were performed to examine crude relationships between the predictors and outcomes. For these relationships weights were not used. Phthalate metabolite and GGT/CRP levels were also compared between categorical variables using appropriate parametric and non-parametric tests. We then constructed full multivariable linear regression models with log-transformed GGT or CRP as the dependent variable and one log-transformed phthalate metabolite as a predictor along with age (continuous variable), sex (dichotomous), race and ethnicity (categorical), log-transformed cotinine, PIR (continuous), BMI (continuous), and log-transformed creatinine as covariates. All models included the same covariates for consistency. Analyses were performed both with and without including the sample weights to examine the effects of weighting. Secondary analyses were conducted by repeating all models when stratifying by gender, age (6-12, 13-19, 20-35, 36-50, 51-65, 66-80, >81 years), BMI (<18.5, 18.5-24.9, 25-29.9, 30-34.9, >34.9 kg/m2), race and ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, other Hispanic, other race/multi-racial), or study year (NHANES 1999-2000, 2001-02, 2003-04, 2005-06) groups. Secondary analyses were also conducted to explore non-linear relationships by assessing associations between phthalate metabolite quintiles and GGT or CRP. In order to test our hypothesis that individuals with a high concentration of MEHP in relation to other DEHP metabolites may be more susceptible to DEHP-related impacts on health, in another secondary analysis we created a multivariate linear regression model with the aforementioned covariates as well as MEHP, MEHP%, and an MEHP*MEHP% interaction term. In a final exploratory analysis, we performed similar multivariate regression analyses among pregnant women.
RESULTS
Study population characteristics are presented in Table 1, and distributions of urinary phthalate metabolites are displayed in Table 2. As mentioned previously, MCHP, MiNP, MnOP, and MnMP were not considered in subsequent analyses due to a high proportion of samples below the LOD (> 75%). The number of observations for each metabolite differs due to the number of years in which that metabolite was measured. In the combined datasets several phthalate metabolites were associated with age, race, and gender as previously described for NHANES 1999-2000 (Silva et al. 2004). For GGT and CRP, sample-weighted geometric mean (median) values were 20.1 U/L (18.4 U/L) and 0.14 mg/dL (0.15 mg/dL), respectively. Males had higher GGT and lower CRP compared to females (p<0.05), and there were increasing trends in both GGT and CRP with increasing age, BMI, and serum cotinine (p<0.05) (results not shown).
Table 1.
Study population characteristics.
| Category | N | % Unweighteda | % Weightedb | |
|---|---|---|---|---|
| Gender | Male | 5106 | 50.9 | 49.64 |
| Female | 4925 | 49.1 | 50.36 | |
| Age (years) | 6-12 | 1401 | 14.23 | 9.62 |
| 13-19 | 2866 | 29.16 | 12.88 | |
| 20-35 | 1528 | 15.55 | 23.27 | |
| 36-50 | 1509 | 15.36 | 25.29 | |
| 51-65 | 1286 | 13.09 | 17.65 | |
| 66-80 | 1032 | 10.5 | 9.85 | |
| >81 | 205 | 2.09 | 1.43 | |
| Race/ethnicity | Non-Hispanic White | 4022 | 40.1 | 69.47 |
| Mexican American | 2641 | 26.33 | 8.27 | |
| Non-Hispanic Black | 404 | 4.03 | 5.48 | |
| Other Hispanic | 2578 | 25.7 | 11.84 | |
| Other race/multi-racial | 386 | 3.85 | 4.93 | |
| BMI (kg/m2) | Underweight (<18.5) | 1325 | 13.41 | 9.56 |
| Normal weight (18.5-24.9) | 3666 | 37.11 | 34.85 | |
| Pre-obese (25-29.9) | 2580 | 26.11 | 28.36 | |
| Obese class I (30-34.9) | 1411 | 14.28 | 16.31 | |
| Obese class II and III (>34.9) |
898 | 9.09 | 10.92 | |
| Study year | 1999-2000 | 2431 | 24.23 | 22.74 |
| 2001-2002 | 2670 | 26.62 | 26.57 | |
| 2003-2004 | 2513 | 25.05 | 25.17 | |
| 2005-2006 | 2417 | 24.1 | 25.53 |
unweighted represents actual sample percentages
weighted represents population percentages
Table 2.
Sample-weighted, creatinine-corrected urinary phthalate metabolite concentrations, NHANES 1999-2006 (μg/g creatinine).
| Metabolite | Parent compound | Years measured |
N | % > LOD |
Geometric mean a |
25th | 50th | 75th | 90th | 95th | Maximum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono(2-ethylhexyl) Phthalate (MEHP) |
DEHP (Di(2-ethylhexyl) phthalate) |
1999-2006 | 10,031 | 80.6 | 2.99 | 1.31 | 2.76 | 5.91 | 13.5 | 26.3 | 726 |
| Mono-ethyl Phthalate (MEP) |
DEP (Diethyl phthalate) | 1999-2006 | 10,026 | 99.9 | 167 | 64.3 | 145 | 383 | 986 | 1,879 | 100,900 |
| Mono-benzyl Phthalate (MBzP) |
BzBP (Benzylbutyl phthalate) |
1999-2006 | 10,031 | 98.6 | 13.0 | 6.83 | 12.6 | 24.4 | 48.1 | 74.0 | 13,332 |
| Mono-isononyl Phthalate (MiNP) |
DiNP (Di-isononyl phthalate) |
1999-2006 | 10,031 | 8.1 | NC | <LOD | <LOD | <LOD | <LOD | 3.16 | 133 |
| Mono-n-octyl Phthalate (MnOP) |
DOP (Di-n-octyl phthalate) | 1999-2006 | 10,031 | 4.8 | NC | <LOD | <LOD | <LOD | <LOD | 2.99 | 53.7 |
| Mono-cyclohexyl Phthalate (MCHP) |
DCHP (Dicyclohexyl phthalate) |
1999-2006 | 10,031 | 9.3 | NC | <LOD | <LOD | <LOD | <LOD | 1.27 | 51.3 |
| Mono-n-butyl Phthalate (MnBP) |
DBP (Di-butyl phthalate) |
2001-2006 | 7,600 | 99.1 | 18.9 | 10.9 | 18.2 | 31.6 | 54.1 | 83.3 | 6,426 |
| Mono-(2-ethyl-5-hydroxyhexyl) Phthalate (MEHHP) |
DEHP (Di(2-ethylhexyl) phthalate) |
2001-2006 | 7,600 | 99.2 | 21.2 | 10.2 | 18.3 | 37.8 | 92.7 | 189 | 2,676 |
| Mono-(2-ethyl-5-oxohexyl) Phthalate (MEOHP) |
DEHP (Di(2-ethylhexyl) phthalate) |
2001-2006 | 7,600 | 98.3 | 13.9 | 6.71 | 12.1 | 24.6 | 59.8 | 118 | 1,706 |
| Mono-isobutyl Phthalate (MiBP) |
DiBP (Di-butyl phthalate) |
2001-2006 | 7,600 | 90.7 | 3.57 | 1.95 | 3.57 | 6.61 | 11.5 | 16.2 | 8,452 |
| Mono-n-methyl Phthalate (MnMP) |
DMP (Dimethyl phthalate) | 2001-2006 | 7,600 | 60.8 | 1.39 | <LOD | 1.37 | 2.92 | 6.41 | 11.5 | 880 |
| Mono-(3-carboxypropyl) Phthalate (MCPP) |
DOP (Di-n-octyl phthalate) | 2001-2006 | 7,600 | 96.5 | 2.43 | 1.40 | 2.34 | 4.04 | 7.39 | 10.7 | 426 |
| Mono-2-ethyl-5-carboxypentyl Phthalate (MECPP) |
DEHP (Di(2-ethylhexyl) phthalate) |
2003-2006 | 4,930 | 100 | 35.0 | 16.7 | 30.0 | 60.6 | 157 | 278 | 3,157 |
| Mono-(carboxynonyl) Phthalate (MCNP) |
DiDP (Di-isodecyl phthalate) |
2005-2006 | 2,417 | 91.5 | 2.67 | 1.47 | 2.48 | 4.50 | 8.67 | 13.4 | 702 |
| Mono-(carboxyoctyl) Phthalate (MCOP) |
DiNP (Di-isononyl phthalate) |
2005-2006 | 2,417 | 96.3 | 5.29 | 2.61 | 4.56 | 9.26 | 23.9 | 40.2 | 3,876 |
LOD = limit of detection; NC = not calculated due to high percentage of samples <LOD.
Multivariable linear regression results are presented in Table 3. Results from unweighted models are presented, as they were consistent with the results that incorporated sample weights though more precise. Since there is a wide range of weights in the dataset the weighted method can result in an inefficient analysis, and it has been suggested that inclusion of covariates used in the creation of weights—such as age, sex, ethnicity and PIR—may serve as a suitable substitute (Korn and Graubard 1991). We found significant positive associations between GGT and MEHP (β = 0.012, p = 0.03) and MEHP% (β = 0.036, p = 0.0002). These effect estimates correspond with 2.5% (95%CI 0.2% to 4.8%) and 3.7% (95%CI 1.7% to 5.7%) increases in GGT in relation to an interquartile range (IQR) increase in MEHP or MEHP%, respectively. On the other hand, GGT was inversely associated with MCPP (β = −0.026, p = 0.003) and MCNP (β = −0.031, p = 0.035). For CRP, there were significant positive associations with the metabolites MBzP (β = 0.035, p = 0.006) and MiBP (β = 0.048, p = 0.003), and a suggestive positive association with the sum of the DBP metabolites MnBP and MiBP (β = 0.026, p = 0.10). These effect estimates correspond to increases in CRP of 6.0% (95%CI 1.7% to 10.8%) and 8.3% (95% CI 2.9% to 14.0%) for IQR increases in urinary MBzP and MiBP, respectively. Conversely, CRP was inversely associated with MEHHP (β = −0.032, p = 0.02) and MEOHP (β = −0.027, p = 0.05).
Table 3.
Adjusteda regression coefficients (95% confidence intervals) for a change in ln-transformed CRP or GGT in relation to a unit increase in ln-transformed urinary phthalate metabolite concentration.
| GGT | CRP | |||||
|---|---|---|---|---|---|---|
| Phthalate Metabolite | N | β (95% CI) | p-value | N | β (95% CI) | p-value |
| MEHP | 7185 | 0.012 (0.001, 0.023) | 0.03 | 8346 | −0.014 (−0.036, 0.008) | 0.21 |
| MEHHP | 5528 | 0.002 (−0.011, 0.015) | 0.79 | 6443 | −0.032 (−0.058, −0.005) | 0.02 |
| MEOHP | 5528 | −0.019 (−0.033, −0.006) | 0.006 | 6443 | −0.027 (−0.054, 0.0004) | 0.05 |
| MECPP | 3616 | −0.008 (−0.026, 0.009) | 0.37 | 4207 | −0.032 (−0.067, 0.003) | 0.07 |
| MEHP%b | 5528 | 0.036 (0.017, 0.055) | 0.0002 | 6443 | 0.014 (−0.024, 0.051) | 0.47 |
| MnBP | 5528 | 0.001 (−0.016, 0.018) | 0.91 | 6443 | 0.028 (−0.006, 0.062) | 0.1 |
| MiBP | 5528 | −0.004 (−0.012, 0.012) | 0.62 | 6443 | 0.048 (0.017, 0.079) | 0.003 |
| DBPCOMc | 7185 | −0.008 (−0.024, 0.007) | 0.3 | 8346 | 0.026 (−0.005, 0.056) | 0.1 |
| MEP | 7181 | 0.008 (−0.002, 0.018) | 0.11 | 8342 | −0.020 (−0.040, 0.0003) | 0.05 |
| MBzP | 7185 | 0.009 (−0.004, 0.023) | 0.16 | 8346 | 0.035 (0.010, 0.061) | 0.006 |
| MCNP | 1777 | −0.031 (−0.059, −0.002) | 0.03 | 2070 | 0.018 (−0.038, 0.075) | 0.52 |
| MCOP | 1777 | 0.016 (−0.041, 0.009) | 0.21 | 2070 | −0.002 (−0.053, 0.048) | 0.93 |
| MCPP | 5528 | −0.026 (−0.043, −0.009) | 0.003 | 6443 | −0.006 (−0.039, 0.027) | 0.73 |
Models adjusted for age, sex, race and ethnicity, serum cotinine, PIR, BMI, and urinary creatinine
Proportion of MEHP compared to sum of MEHP, MEHHP and MEOPH.
Sum of MnBP and MiBP.
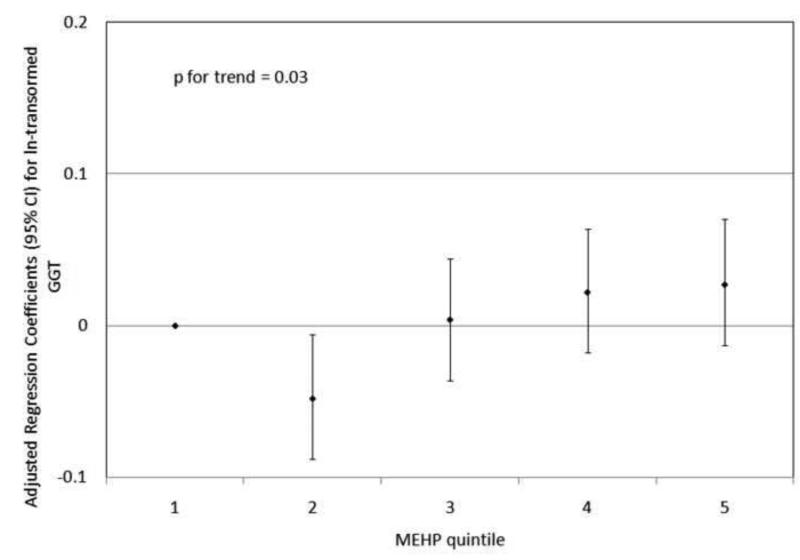
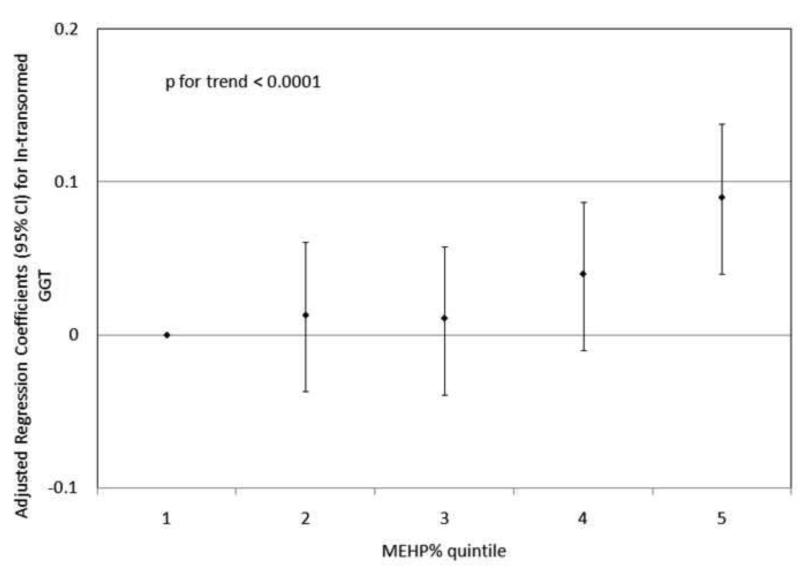
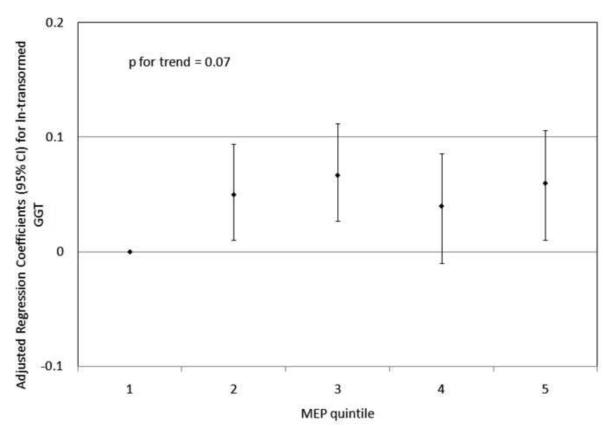
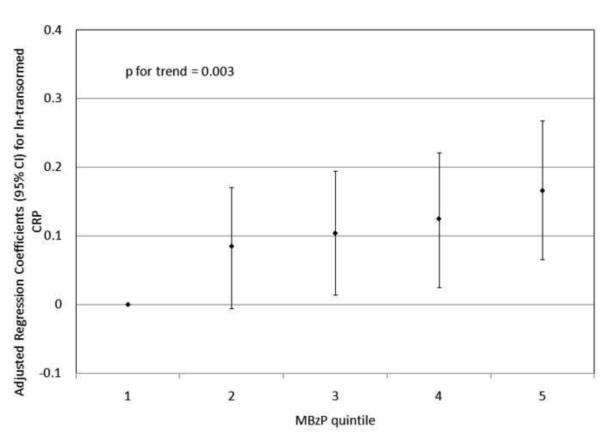
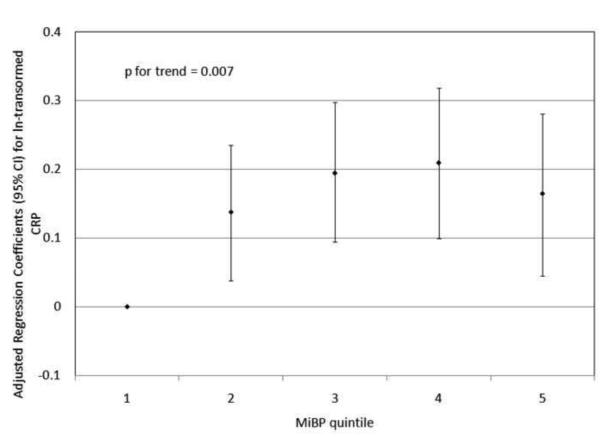
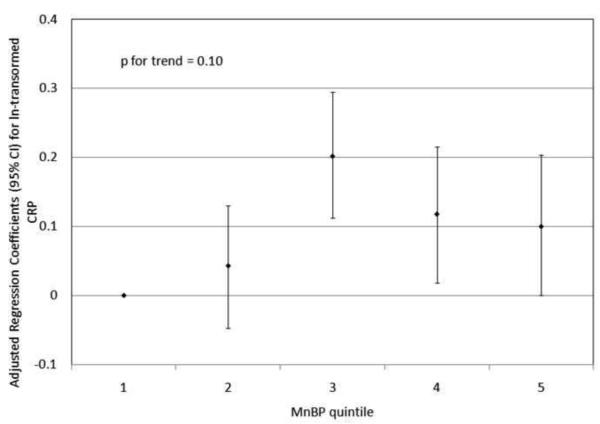
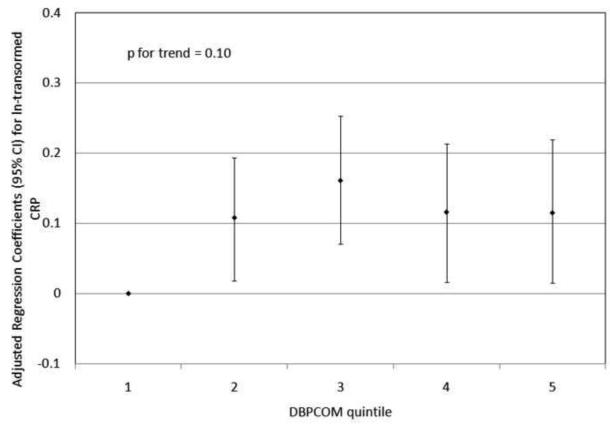
To investigate the possibility of non-linear relationships, we also regressed phthalate metabolite quintiles on ln-transformed GGT and CRP. For GGT, there were non-linear but statistically significant trends with increasing quintiles for MEHP (Figure 1a) and MEHP% (Figure 1b). MEP quintiles 2, 3, and 5 had significantly elevated adjusted regression coefficients compared to quintile 1, though the trend was not statistically significant (p=0.07; Figure 1c). For CRP, a statistically significant monotonic trend was observed for MBzP (Figure 2a). Adjusted regression coefficients for quintiles 2 through 5 for MiBP, MnBP, and their sum were all greater than quintile 1 (reference), but the trends with increasing quintiles did not appear to be monotonic (Figures 2b – 2d).
Figure 1.
Adjusted regression coefficients (95% confidence intervals) for change in ln-transformed GGT in relation to phthalate metabolite quintiles for a) MEHP; b) MEHP%; and c) MEP. adjusted for age, sex, race and ethnicity, serum cotinine, PIR, BMI, and urinary creatinine.
Figure 2.
Adjusted regression coefficients (95% confidence intervals) for change in ln-transformed CRP in relation to phthalate metabolite quintiles for a) MBzP; b) MiBP; c) MnBP; and d) sum of DBP metabolites MiBP and MnBP. adjusted for age, sex, race and ethnicity, serum cotinine, PIR, BMI, and urinary creatinine.
In secondary analyses stratified by gender, age, BMI, race, and study year, adjusted effect estimates were overall consistent between strata with regard to direction and magnitude of the effect estimates, with no evidence of effect modification of the relationship between phthalate metabolites and GGT or CRP by these variables (results not shown). In further secondary analyses where we constructed full models examining the interaction of MEHP and MEHP%, there was a significant positive association between the MEHP*MEHP% interaction term and both GGT (β = 0.018, p = 0.009) and CRP (β = 0.034, p = 0.02). Finally, in an analysis of pregnant women included in the NHANES dataset (N=445; results not shown), which also included trimester at time of sampling as a covariate, most adjusted effect estimates were consistent with results from the full dataset with regard to direction and magnitude. However, associations were not statistically significant, likely due to the smaller sample size. For example, we observed suggestive positive relationships between CRP and MnBP (β = 0.104; p = 0.14) and sum of DBP metabolites (β = 0.107; p = 0.14) where adjusted effect estimates were greater than those observed in the full dataset (β = 0.028 and 0.026, respectively). Also, when stratifying by study year, these relationships were statistically significant among pregnant women in the 2003-2004 dataset (both p-values <0.05).
DISCUSSION
In the present study of up to eight years of NHANES data, we report statistically significant relationships between several urinary phthalate metabolites and serum levels of GGT or CRP. Because urinary concentrations of phthalate metabolites are considered reliable indices of exposure (Silva et al. 2004), our results suggest that exposures to phthalates may modulate oxidative stress and inflammation pathways as reflected by GGT and CRP, respectively. While numerous statistical comparisons were made and thus we cannot rule out chance findings, previous studies have shown that several phthalates are associated with oxidative stress and inflammation which may be relevant to a range of health effects potentially linked to phthalate exposure.
Serum GGT has been used in recent studies as a biomarker of oxidative stress (Whitfield 2001; 2007). Gamma glutamyltransferase has the critical function of breaking down extracellular glutathione into amino acids that subsequently can be taken up by the cell. Because glutathione is important in protection against oxidative stress, yet must be synthesized intracellularly from constitutive amino acids, upregulation of GGT is considered part of the cellular antioxidant response pathway. Although it is not well understood how oxidative stress could increase serum concentrations of GGT, one plausible explanation is that oxidative stress or glutathione conjugation of reactive metabolites deplete intracellular GSH, followed by compensatory increase of GGT which is then released into the serum (Whitfield 2007).
In addition, serum GGT is used clinically as a liver function test, which could be explained by oxidative stress effects of alcohol on the liver (Whitfield 2007). Interestingly, several of the phthalates, most notably DEHP, cause changes in liver function in laboratory rodents (Rusyn et al. 2006). Our findings of an association between urinary MEHP and serum GGT are consistent with these laboratory animal studies. Serum GGT has also been used as a biomarker of excessive or harmful consumption of alcohol (Whitfield 2007). Due to missing data we did not include alcohol consumption in our final models in order to maximize the sample size of our study. Our decision to not include alcohol consumption in the final models was further supported by the lack of an association between alcohol use and urinary phthalate metabolites among participants who contributed data on alcohol use, suggesting alcohol consumption would not act as a confounder in our analysis.
MEHP, a monoester metabolite of DEHP, was positively associated with GGT, as were elevated quintiles of MEP, a monoester metabolite of DEP. Interestingly, these same two urinary metabolites were also associated with significant elevations in sperm DNA damage in 379 men recruited through a US fertility clinic (Hauser et al. 2007), and oxidative stress is likely to be the primary cause of sperm DNA damage (Aitken and Deluliis 2010; Aitken et al. 2008). Our findings are also consistent with experimental studies reporting MEHP induction of oxidative stress in rat testis, measured as increased lipid peroxidation (Santhosh et al. 1998; Rusyn et al. 2006), increased MDA and 8-OHdG levels (Seo et al. 2004), and increased generation of reactive oxygen species with simultaneous declines in testis concentrations of the antioxidants glutathione GSH and ascorbic acid (Kasahara et al. 2002). Likewise, our finding with MEP, the monoester metabolite of DEP, is consistent with a study of fish that found increased oxidative stress as measured by increased hepatic lipid peroxide and compensatory changes in the oxidative stress defense enzymes (Kang et al. 2010).
MEHP is metabolized further to the oxidized metabolites MEHHP, MEOHP, and MECPP. In contrast to MEHP, the latter three metabolites of DEHP were inversely associated with GGT to varying degrees. While this is not consistent with a recent study of 960 Korean adults that reported positive associations between MEHHP and MEOHP with urinary markers of oxidative stress (MDA and 8-OHdG)(Hong et al. 2009), it is consistent with a study of men from an infertility clinic that reported inverse associations between MEHHP and MEOHP and sperm DNA damage (Hauser et al. 2007). Because MEHP is likely the most bioactive form of DEHP (Erkekoglu et al. 2010; Chu et al. 1978), it has been suggested that increased urine concentrations of MEHHP, MEOHP and other oxidized DEHP metabolites may reflect an individual's increased ability to convert MEHP to less toxic metabolites that are more easily excreted in urine (Hauser 2008; Meeker et al. 2007). As such, the positive association of MEHP with GGT and inverse associations of oxidized MEHP metabolites with GGT would be consistent with MEHP being the more toxic DEHP metabolite.
Interindividual differences in the proportion of MEHP in relation to other less toxic metabolites may serve as a marker for individual metabolic susceptibility to DEHP exposure (Hauser 2008; Meeker et al. 2007). This may be further supported by our secondary analysis of the MEHP% variable. We found that increased MEHP%, indicating higher levels of MEHP in relation to the oxidized DEHP metabolites (MEHHP and MEOHP), was associated with a significant increase in GGT. In addition, we found that a MEHP*MEHP% interaction term was positive and significant for both GGT and CRP. These findings indicate that an increase in the proportion of MEHP in comparison to other DEHP metabolites may result in an increased effect in relation to the same amount of MEHP, which further supports the hypothesis that the relative concentration of MEHHP and MEOHP may represent individuals who are more likely to efficiently oxidize MEHP to less toxic metabolites and thus may be less susceptible to effects related to DEHP exposure compared to individuals with a high MEHP%. Another factor that likely contributes to the variability in MEHP% between individuals relates to sample timing if human exposure to DEHP is episodic. Since the oxidized DEHP metabolites have a longer biological half-life (10-15 hours) compared to MEHP (5 hours) (Lorber et al. 2010), a higher MEHP% for an individual may also reflect that the person experienced a more recent DEHP exposure event compared to an individual with a low MEHP%. However, high reliability in urinary MEHP% within individuals over time was recently reported (Adibi et al. 2008) which may provide support for underlying inter-individual differences in DEHP metabolism.
Some of our findings appear to be inconsistent with the literature, which could be due to chance findings, differences in study design or populations, outcome measures being assessed, phthalate exposure levels, methods used to measure phthalate exposure, statistical methods and covariates that were considered, or other reasons. MBzP and various DBP metabolites have been reported to be associated with oxidative stress in previous studies (Hong et al. 2009; Seo et al. 2004), but neither were associated with GGT in the present analysis. We also observed inverse relationships between GGT and MCNP (an oxidized metabolite of diisodecyl phthalate) and MCPP (an oxidized metabolized of both DBP and di-n-octyl phthalate). Although these latter phthalates are less studied than the other phthalates we evaluated, our findings with these phthalates may provide further indication for an inverse relationship between oxidative metabolism of phthalates and serum GGT. Further research on the metabolism and mechanisms of action for MCNP and MCPP and their parent compounds will be needed to better interpret the inverse associations of GGT with these metabolites.
Increased serum concentration of CRP is widely used as a biomarker of inflammation associated with various diseases (Marnell et al. 2005) and, more recently, with environmental toxicant exposure (Everett et al. 2010). Named for its ability to precipitate the so-called “C” polysaccharide of Streptococcus pneumonia, CRP binds to phosphocholine of microorganism cell walls and damaged host cell membranes, as well as nuclear antigens (Marnell et al. 2005). As an acute phase serum protein, CRP concentrations in serum increase markedly in response to cell injury or infection. Although CRP is made in various tissues, increased serum levels of CRP are mainly the result of increased CRP production in the liver in response to the pro-inflammatory cytokine interleukin (IL)-6 (Bottazzi et al. 2010).
Serum CRP was positively associated with MBzP, MiBP, and the sum of DBP metabolites in urine. These associations are consistent with previous experimental studies. MBzP and MiBP stimulate increased PPARα expression in rodent cell lines, indicating increased peroxisome proliferation and inflammation (Hurst and Waxman 2003, Bility et al. 2004). Similarly, MnBP and MBzP increased release of the inflammatory cytokines IL-6 and IL-8 from human epithelial cells in vitro (Jepsen et al. 2004). Although MEHP stimulated pro-inflammatory cytokine release from cultured human epithelial cells (Jepsen et al. 2004; Rael et al. 2009), we did not observe an association between urinary MEHP and serum CRP in the present study. However, CRP was inversely associated with the oxidized DEHP metabolite MEOHP. Furthermore, consistent with our hypothesis surrounding the importance of interindividual variability in DEHP/MEHP metabolism, CRP was positively associated with a MEHP*MEHP% interaction term.
A secondary analysis of pregnant women revealed a suggestive positive relationship between MnBP and the sum of DBP metabolites with CRP despite a much smaller sample size. Interestingly, inflammation is associated with preterm birth (Romero et al. 2007) and MnBP was the phthalate metabolite most strongly associated with preterm birth in our recent exploratory nested case-control study that measured third trimester urinary phthalate metabolites in 60 pregnant women (Meeker et al. 2009).
There were several limitations to the present analysis. First, due to the cross-sectional study design we are unable to make any conclusions regarding causation in the relationships between phthalate exposure and inflammation or oxidative stress. Also, since the data were cross-sectional only one urine sample per subject was analyzed for phthalate metabolite levels, and it has been suggested that one data point may not be representative of the subject's average body burden (Fromme et al. 2007). However, several studies have demonstrated that single spot urine sample phthalate levels may be representative of long-term averages though temporal reliability likely varies by metabolite (Hauser et al. 2004, Teitelbaum et al. 2008; Suzuki et al. 2009). The dataset was also limited to single serum measures of CRP and GGT which may also vary over time (Meier-Ewert et al. 2001; Gu et al. 2009; Lazo et al. 2008). In addition, there are markers other than CRP and GGT (e.g. cytokines, MDA, 8-OHdG, and many others) that may be potentially more sensitive for examining inflammation and oxidative stress responses to environmental contaminants that were not available in the NHANES datasets. Future studies of oxidative stress and inflammatory outcomes in relation to phthalate exposure should examine multiple markers for comparison, and, where possible, measure these biomarkers in fluids or tissues most relevant to the particular downstream health outcomes of interest. Finally, our secondary analysis of pregnant women, who may be particularly susceptible to inflammation- and oxidative stress-inducing xenobiotics, suffered from a much smaller sample size and lacked information on other important pregnancy factors (e.g., preeclampsia, gestational diabetes, and other complications).
Despite these limitations our study had several strengths that warrant further research on this topic. First, the study utilized state-of-the-art methods to measure exposure biomarkers of phthalate metabolites in urine and serum markers of inflammation and oxidative stress. Second, the use of the NHANES dataset allowed for a large sample size and excellent statistical power. Our primary findings were also robust to multiple statistical modeling approaches and various sensitivity analyses that we conducted in our secondary analyses. Finally, in our primary analysis of non-pregnant participants, our results are likely representative of the US population and thus have good generalizability.
In conclusion, we found that several phthalate monoester metabolites that are detected in a high proportion of urine samples from the US general population are associated with increased serum markers of inflammation and oxidative stress. Though causation cannot be determined, these results suggest that the relationships between phthalates and inflammation, oxidative stress, and related adverse health effects deserve further exploration in more detailed molecular epidemiology studies, especially among potentially sensitive subgroups. In addition, future investigations should be aimed at explaining the potential inverse (i.e., protective) relationships between oxidized phthalate metabolite concentrations in urine and serum markers of inflammation and oxidative stress.
Acknowledgments
Funding: Work supported by grants R01ES018872, P42ES017198, and P20ES018171 from the National Institute of Environmental Health Sciences (NIEHS), and RD83480001 from the US Environmental Protection Agency (USEPA).
Abbreviations
- CRP
C-reactive protein
- GGT
Gamma glutamyltransferase
- DEHP
Di-2-ethylhexyl phthalate
- DEP
Diethyl phthalate
- BzBP
Benzylbutyl phthalate
- DiNP
Di-isononyl phthalate
- DOP
Di-noctyl phthalate
- DCHP
Dicyclohexyl phthalate
- DBP
Di-butyl phthalate
- DMP
Dimethyl phthalate
- DiDP
Di-isodecyl phthalate
- MEHP
Mono-(2-ethyl)-hexyl phthalate
- MEP
Mono-ethyl phthalate
- MBzP
Mono-benzyl phthalate
- MiNP
Mono-isononyl phthalate
- MCHP
Mono-cyclohexyl phthalate
- MnBP
Mono-n-butyl phthalate
- MEHHP
Mono-(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
Mono-(2-ethyl-5-oxohexyl) phthalate
- MiBP
Mono-isobutyl phthalate
- MnMP
Mono-n-methyl phthalate
- MCPP
Mono(3-carboxypropyl) phthalate
- MECPP
Mono-2-ethyl-5-carboxypentyl phthalate
- MCNP
Mono(carboxynonyl) phthalate
- MCOP
Mono-(carboxyoctyl) phthalate
- PTGS2
Prostaglandin-endoperoxide synthase 2
- MDA
Malondialdehyde
- 8-OhdG
8-hydroxydeoxyguanosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Di(2-ethylhexyl) phthalate (DEHP): ToxFAQs. 2002 Available from: http://www.atsdr.cdc.gov/toxfaqs/TF.asp?id=377&tid=65 [Accessed 8 July 2010] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for di-n-butyl phthalate. 2001 Available from: http://www.atsdr.cdc.gov/toxprofiles/tp135.pdf [Accessed 27 July 2010] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR) Diethyl phthalate: ToxFAQs. 1996 Available from: http://www.atsdr.cdc.gov/toxfaqs/TF.asp?id=602&tid=112 [Accessed 8 July 2010] [PubMed]
- Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, Peters JM. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol. Sci. 2004;82(1):170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Boas M, Federiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedüs L, Hilsted L, Juul A, Main KM. Childhood exposure to phthalates – associations with thyroid function, insulin-like growth factor I (IGF-I) and growth. Environ. Health Perspect. 2010;118(10):1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int. J. Androl. 2010;33(2):333–345. doi: 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, Hägerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ. Health Perspect. 2004;112(14):1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–83. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Chemical information for benzylbutyl phthalate. 2010 Available from: http://www.cdc.gov/exposurereport/data_tables/BzBP_ChemicalInformation.html [Accessed 27 July 2010]
- Chu I, Villeneuve DC, Secours V, Franklin C, Rock G, Viau A. Metabolism and tissue distribution of mono-2-ethylhexyl phthalate in the rat. Drug Metab. Dispos. 1978;6(2):146–149. [PubMed] [Google Scholar]
- Clark BJ, Cochrum RK. The steroidogenic acute regulatory protein as a target of endocrine disruption in male reproduction. Drug Metab Rev. 2007;39:353–70. doi: 10.1080/03602530701519151. [DOI] [PubMed] [Google Scholar]
- Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, Herrick RH, Christiani DC, Hauser R. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ. Health Perspect. 2003;111(9):1164–1169. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, Hincal F. Evaluation of cytotoxicity and oxidative DNA damaging effects of Di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol. and Appl. Pharmacol. 2010;248(1):52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Everett CJ, King DE, Player MS, Matheson EM, Post RE, Mainous AG., 3rd Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ. Res. 2010;110(1):79–82. doi: 10.1016/j.envres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Fromme H, Gruber L, Schlummer M, Wolz G, Böhmer S, Angerer J, Mayer R, Liebl B, Bolte G. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ. Int. 2007;33(8):1012–1020. doi: 10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory response of rat and human neutrophils exposed to di-(2-ethyl-hexyl)-phthalate-plasticized polyvinyl chloride. Artif. Organs. 2003;27(3):256–260. doi: 10.1046/j.1525-1594.2003.07107.x. [DOI] [PubMed] [Google Scholar]
- Gu Y, Zeleniuch-Jacquotte A, Linkov F, Koenig KL, Liu M, Vlikokhatnaya L, Shore RE, Marrangoni A, Toniolo P, Lokshin AE, Arsian AA. Reproducibility of serum cytokines and growth factors. Cytokine. 2009;45(1):44–49. doi: 10.1016/j.cyto.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ. Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum. Reprod. 2007;22(3):688–695. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int. J. Androl. 2008;31(2):112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- Higuchi TT, Palmer JS, Gray LE, Jr., Veeramachaneni DN. Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol Sci. 2003;72:301–13. doi: 10.1093/toxsci/kfg036. [DOI] [PubMed] [Google Scholar]
- Hong YC, Park EY, Park MS, Ko JA, Oh SY, Kim H, Lee KH, Leem JH, Ha EH. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett. 2009;184(2):139–144. doi: 10.1016/j.toxlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Ulmer R, London SJ. Phthalate exposure and pulmonary function. Environ. Health Perspect. 2004;112(5):571–574. doi: 10.1289/ehp.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007;22(10):2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol. Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, Nakajima T. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J Occup Health. 2007;49:172–82. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol. In Vitro. 2004;18(3):265–269. doi: 10.1016/j.tiv.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ji K, Kho YL, Park Y, Choi K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: a pilot study with “Temple Stay” participants. Environ. Res. 2010;110(4):375–382. doi: 10.1016/j.envres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem. J. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JC, Jee JH, Koo JG, Keum YH, Jo SG, Park KH. Anti-oxidative status and hepatic enzymes following acute administration of diethyl phthalate in olive flounder Paralichthys olivaceus, a marine culture fish. Ecotoxicol Environ Saf. 2010;73:1449–55. doi: 10.1016/j.ecoenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Kolarik B, Naydenov K, Larsson M, Bornehag CG, Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ. Health Perpect. 2008;116(1):98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn EL, Graubard BI. Epidemilogic studies utilizing surveys: accounting for the sampling design. Am. J. Public Health. 1991;81(9):1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 2003;111(14):1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann. Intern. Med. 2008;148(5):348–352. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR., Jr. Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants? Free Radic. Res. 2009a;43(6):533–537. doi: 10.1080/10715760902893324. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR., Jr. Serum gamma-glutamyltransferase: new insights about an old enzyme. J. Epidemiol. Community Health. 2009b;63(11):884–886. doi: 10.1136/jech.2008.083592. [DOI] [PubMed] [Google Scholar]
- Lorber M, Koch HM, Angerer J. A critical evaluation of the creatinine correction approach: can it underestimate intakes of phthalates? A case study with di-2-ethylhexyl phthalate. J. Expo. Sci. Environ. Epidemiol. 2010 doi: 10.1038/jes.2010.43. [Epub ahead of print] doi:10.1038/jes.2010.43. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006;114(2):270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–11. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ. Health Perspect. 2007;115(7):1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ. Health Perspect. 2009;117(10):1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin. Chem. 2001;47:426–430. [PubMed] [Google Scholar]
- Nagao T, Ohta R, Marumo H, Shindo T, Yoshimura S, Ono H. Effect of butyl benzyl phthalate in Sprague-Dawley rats after gavage administration: a two-generation reproductive study. Reprod Toxicol. 2000;14:513–32. doi: 10.1016/s0890-6238(00)00105-2. [DOI] [PubMed] [Google Scholar]
- NCHS (National Center for Health and Statistics) Documentation, codebook, and frequencies for urinary phthalates, phytoestrogens and PAHs 2001 to 2002. 2009 Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/phpypa_b.pdf [Accessed 12 July 2010]
- NCHS (National Center for Health and Statistics) National Health and Nutrition Examination Survey. 2010a Available: http://www.cdc.gov/nchs/nhanes.htm [Accessed 30 June 2010]
- NCHS (National Center for Health and Statistics) Continuous NHANES web tutorial. 2010b Available: http://www.cdc.gov/nchs/tutorials/Nhanes/index_current.htm [Accessed 30 June 2010]
- NCHS (National Center for Health and Statistics) 2005-2006 Data documentation, codebook, and frequencies for urinary phthalates. 2010c Available: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/PHTHTE_D.htm [Accessed 12 July 2010]
- Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE., Jr. Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci. 2009;111:163–78. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- Pauley CJ, Ledwith BJ, Kaplanski C. Peroxisome proliferators activate growth regulatory pathways largely via peroxisome proliferator-activated receptor alpha-independent mechanisms. Cell Signal. 2002;14:351–358. doi: 10.1016/s0898-6568(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention, American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Hearth Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, Bar-Or D. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid. Med. Cell Longev. 2009;2(3):166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006;36(5):459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh A, Nair KG, Arun P, Deepadevi KV, Manojkumar V, Lakshmi LR, Kurup PA. Effect of DEHP [di-(2-ethyl hexyl) phthalate] on lipid peroxidation in liver in rats and in primary cultures of rat hepatocytes. Indian J. Med. Res. 1998;108:17–23. [PubMed] [Google Scholar]
- Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem. Toxicol. 2004;42(1):107–114. doi: 10.1016/j.fct.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2003;789(2):393–404. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007;115(6):876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Niwa M, Yoshinaga J, Watanabe C, Mizumoto Y, Serizawa S, Shiraishi H. Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ. Health Prev. Med. 2009;14(3):180–187. doi: 10.1007/s12199-009-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Wellejus A, Dalgaard M, Loft S. Oxidative DNA damage in male Wistar rats exposed to di-n-butyl phthalate. J. Toxicol. Environ. Health A. 2002;65:813–824. doi: 10.1080/00984100290071126. [DOI] [PubMed] [Google Scholar]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- Whitfield JB. Serum gamma-glutamyltransferase and risk of disease. Clin Chem. 2007;53:1–2. doi: 10.1373/clinchem.2006.080911. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, Perera FP, Andrews H, Just AC, Hoepner L, Tang D, Hauser R. Prenatal di(2-ethylhexyl) phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124(6):e1213–e1220. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. J Biol Chem. 2007;282:5420–31. doi: 10.1074/jbc.M609068200. [DOI] [PubMed] [Google Scholar]
- Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23(2):118–124. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Zheng LX, Chen BH. Phthalate exposure and human semen quality in Shanghai: a cross-sectional study. Biomed. Environ. Sci. 2006;19(3):205–209. [PubMed] [Google Scholar]
- Zhou D, Wang H, Zhang J. Di-n-butyl phthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol Ind Health. 2010 doi: 10.1177/0748233710381895. [DOI] [PubMed] [Google Scholar]