Abstract
AMPA receptors (AMPARs) are the primary mediators of excitatory synaptic transmission in the brain. Alterations in AMPAR localization and turnover have been considered critical mechanisms underpinning synaptic plasticity and higher brain functions, but the molecular processes that control AMPAR trafficking and stability are still not fully understood. Here, we report that mammalian AMPARs are subject to ubiquitination in neurons and in transfected heterologous cells. Ubiquitination facilitates AMPAR endocytosis, leading to a reduction in AMPAR cell-surface localization and total receptor abundance. Mutation of lysine residues to arginine residues at the GluA1 C-terminus dramatically reduces GluA1 ubiquitination and abolishes ubiquitin-dependent GluA1 internalization and degradation, indicating that the lysine residues, particularly K868, are sites of ubiquitination. We also find that the E3 ligase Nedd4 is enriched in synaptosomes and co-localizes and associates with AMPARs in neurons. Nedd4 expression leads to AMPAR ubiquitination, leading to reduced AMPAR surface expression and suppressed excitatory synaptic transmission. Conversely, knockdown of Nedd4 by specific siRNAs abolishes AMPAR ubiquitination. These data indicate that Nedd4 is the E3 ubiquitin ligase responsible for AMPAR ubiquitination, a modification that regulates multiple aspects of AMPAR molecular biology including trafficking, localization and stability.
Keywords: glutamate receptors, AMPA receptors, Nedd4, E3 ligase, ubiquitination, trafficking
Introduction
AMPA receptors (AMPARs) are heterotetrameric glutamate-gated ion channels that mediate most of the synaptic transmission in the brain. Alteration in AMPAR synaptic expression has been considered the most important molecular mechanism in the formation of both Hebbian-type and homeostatic synaptic plasticity (Malinow and Malenka, 2002; Collingridge et al., 2004; Turrigiano, 2008). Since AMPARs traffic rapidly between the plasma membrane and cytosolic compartments via vesicle-mediated membrane insertion, internalization and recycling (Malinow and Malenka, 2002; Song and Huganir, 2002; Newpher and Ehlers, 2008), levels of surface AMPAR accumulation can be efficiently regulated by altering receptor dynamics. Ultimately, the total abundance of AMPARs is determined through a balance between receptor synthesis and degradation. However, exactly how neurons regulate AMPAR trafficking and turnover, a question critical to our understanding of synaptic plasticity and higher brain functions, remains less well understood.
Ubiquitin is a small 76 amino acid protein ubiquitously expressed in all eukaryotes. Ubiquitin can be covalently conjugated to other proteins (ubiquitination) through a series of reactions catalyzed by three enzymes: E1–E3. The ubiquitin-activating enzyme E1 activates ubiquitin in an ATP-dependent manner, while E3 is the ligase that links ubiquitin to its substrate at lysine residues and determines substrate specificity. Once a single ubiquitin is conjugated to the target protein (monoubiquitination), an internal lysine in ubiquitin itself can be linked to a second ubiquitin and so on to form a ubiquitin chain (polyubiquitination). Ubiquitination of membrane proteins functions as a tag that can be readily recognized by endocytotic machinery, leading to receptor internalization. Polyubiquitinated proteins are often sorted to the proteasome or lysosome for degradation (Nandi et al., 2006; Schmitt, 2006). Of particular interest is the ubiquitin-proteasome system (UPS), which is present in synapses (Bingol et al., 2010; Bingol and Schuman, 2006) and plays an important role in synaptic function, including synapse development and maturation (DiAntonio et al., 2001; Ding and Shen, 2008), synaptic plasticity (Hegde, 2004), presynaptic vesicle release (Willeumier et al., 2006) and postsynaptic reorganization through proteolysis of postsynaptic proteins including PSD-95 and GRIP (Colledge et al., 2003; Ehlers, 2003; Bingol and Schuman, 2004; Guo and Wang, 2007). Furthermore, ubiquitination has been implicated in glutamate receptor trafficking and turnover, including NMDA receptors (NMDARs) (Kato et al., 2005) and AMPARs (Patrick et al., 2003; Bingol and Schuman, 2004). In C. elegans ubiquitination of AMPARs regulates GluR synaptic accumulation (Burbea et al., 2002). Consistently, in Drosophila, inhibition of the proteasome by subunit mutation increases GluRIIB expression and enhances synaptic transmission at the neuromuscular junction (Haas et al., 2007). However, in the mammalian system, direct evidence for AMPAR ubiquitination and the identity of the participating E3 ligase(s) remain to be established.
Here, we have examined the existence and functions of AMPAR ubiquitination in a mammalian system. We find that AMPARs in rat neurons are subject to direct ubiquitination. Conjugation of multiple ubiquitin molecules to lysine residues at the intracellular C-terminals of GluA1 subunits facilitates AMPAR internalization and reduces receptor cell-surface expression. Importantly, we identify Nedd4 as the E3 ligase involved in AMPAR ubiquitination. Nedd4 is enriched in synapses, co-distributes and physically associates with AMPAR subunits. Nedd4 expression induces GluA1 ubiquitination, resulting in a reduction in AMPAR surface expression. Consistently, Nedd4 knockdown suppresses ubiquitin-induced GluA1 ubiquitination. These results strongly indicate an important role for Nedd4-mediated ubiquitination in AMPAR trafficking.
Materials and methods
Neuronal and HEK cell culture and transfection
Primary cultured cortical neurons were prepared from embryonic day 18 rat embryos as previously described (Man et al. 2007; Hou et al. 2008a). Briefly, dissociated neurons from rat hippocampus or cortex were seeded onto poly-L-lysine-coated coverslips at approximately 0.3×106 cells per 60mm dish, each containing five coverslips, and maintained for 2 wks until experiments. Transfections on cultured neruons and human embryonic kidney (HEK) 293A cells were performed with Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. More details can be found in Supplemental Materials and Methods.
Immunoprecipitation, western blotting and analysis
Cells were rinsed with cold PBS and resuspended in 100–200 μl modified RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP40, 1% SDOC and 0.1% SDS) containing mini cOmplete protease inhibitor (Roche) and 5 μM ubiquitin aldehyde (Sigma) to inhibit deubiquitination. Lysates were further solubilized by sonication and 10 minutes incubation on ice followed by centrifugation for 10 minutes at 13,000 × g to remove insolubilities. Supernatant volumes were adjusted to 500 μl with NP40 buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 5 mM EDTA and 1% NP40 plus mini cOmplete and 5 μM ubiquitin aldehyde) and incubated overnight for 8–12 hours on rotation at 4°C with protein A-Sepharose beads (Santa Cruz Biotechnology) and antibodies against either GFP or GluA1. More details can be found in Supplemental Materials and Methods.
AMPAR internalization assays
AMPAR internalization was measured as described previously (Man et al. 2000; Man et al. 2007). Briefly, 2 week-old cortical neurons were transfected at 11 DIV with wildtype or 4KR mutant GFP-GluA1, together with either pcDNA as a vector control or with HA-ubiquitin. 1 day after transfection, neurons were incubated with (1:500) anti-GFP antibodies for 10 min on ice to label surface GluA1. Cells were then washed and transferred to the incubator with 50 μM glutamate for 15 min to allow receptor endocytosis. Following fixation, the remaining surface-associated antibodies were blocked with non-conjugated secondary antibodies under non-permeant conditions (1:300) and internalized antibody-bound AMPARs were visualized following incubation with fluorescent secondary antibodies under permeant conditions (1:700). For internalization assays on endogenous AMPARs, the same procedures were followed, except that the surface receptors were labeled by anti-GluA1Nt antibodies (1:100).
mEPSC whole-cell patch clamp recording
Hippocampal neurons were transfected around 10 DIV with either EGFP alone, or together with Nedd4. Total cDNA amounts were balanced by adding empty vectors in control transfections. 2 days following transfection, a coverslip of neurons was transferred to a recording chamber with the extracellular solution containing (in mM) 140 NaCl, 3 KCl, 1.5 MgCl2, 2.5 CaCl2, 11 glucose and 10 HEPES, pH 7.4, which was supplemented with TTX (1 μM) to block action potentials, APV (50 μM) to block NMDARs and bicuculline (20 μM) to block GABAA receptor-mediated inhibitory synaptic currents. Whole-cell voltage-clamp recordings were made with patch pipettes filled with an intracellular solution containing (in mM) 100 Cs-methanesulfonate, 10 CsCl, 10 HEPES, 0.2 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 5 QX-314 and 10 Na-phosphocreatine, pH 7.4, with the membrane potential clamped at −70 mV. Recordings were performed in turns on the same day, on control and Nedd4 coverslips that were from the same batch of cells and transfected at the same time.
Data analysis
All values were reported as mean ± SEM. Statistical analysis was performed using the two-population student’s t test or two-way ANOVA as indicated. N indicates the number of independent experiments in westerns, or the number of cells in immunostaining assays (where the number of independent assays was also specified).
Other methods can be found in Supplemental Materials and Methods (including Plasmids and mutagenesis; Immunoprecipitation of surface AMPARs; Immunocytochemistry on AMPAR surface expression and ubiquitin; Synaptosome preparation; Virus preparation; and Image collection).
Results
AMPAR Subunits are subject to ubiquitination
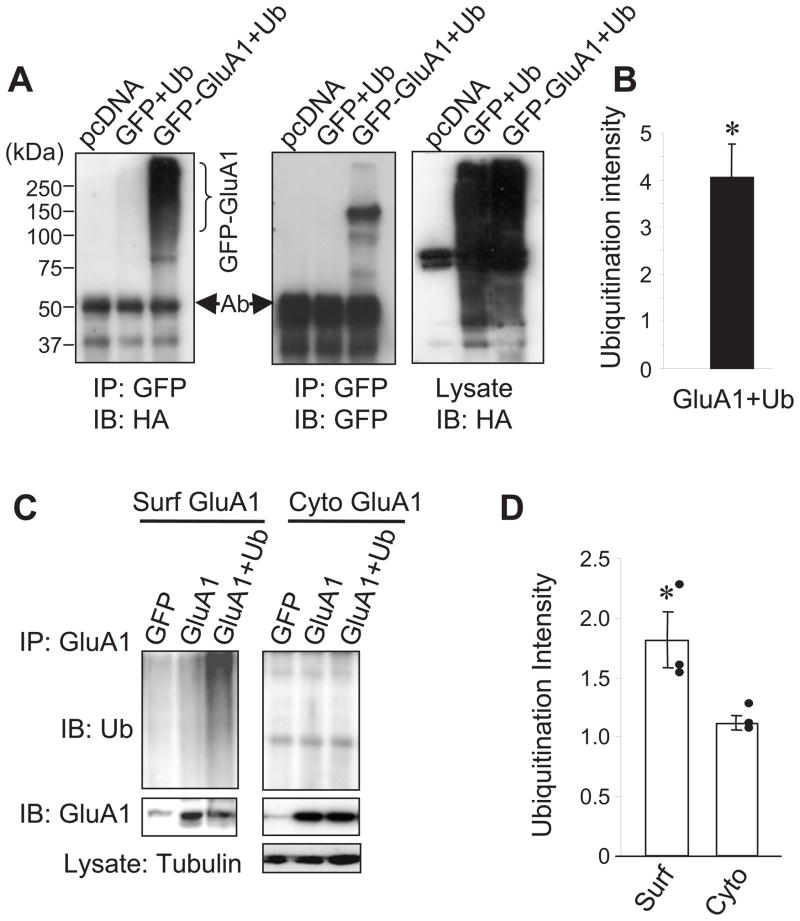
To examine whether AMPARs are capable of being modified by ubiquitination, we co-expressed HA-tagged ubiquitin (Ub) together with GFP-tagged GluA1 subunits (GluA1) in HEK 293A cells. 2 days after transfection, GluA1 subunits were immunoprecipitated with anti-GFP antibodies and anti-HA antibodies were used to confirm the presence of ubiquitination. In support of AMPAR ubiquitination, a strong ubiquitin smear, which is a typical biochemical signature for protein ubiquitination resulting from conjugation with a varied number of ubiquitin moieties, was reliably observed in isolated GluA1 (Fig. 1A left and 1B). In contrast, despite similar levels of intense total ubiquitination species in lysates (Fig. 1A, right), control cells expressing free GFP and HA-ubiquitin showed no ubiquitin signals in GFP immunoprecipitates (Fig. 1A, left). All the cell lysates used for ubiquitination assays in this study were prepared under denaturing conditions to avoid conventional protein-protein association. Therefore, the ubiquitination signals represent a bona fide conjugation of ubiquitin to GluA1 subunits, rather than a conventional protein-protein association. The high molecular weight of the ubiquitin smear indicates the conjugation of multiple ubiquitin molecules with GluA1 subunits. To examine whether AMPAR ubiquitination occurs at the plasma membrane, we incubated GluA1-expressing HEK cells with anti-GFP antibodies to immunoprecipitate surface GluA1. Following a first round of immunoprecipitation to isolate surface GluA1, the remaining supernatants were incubated with anti-GluA1 antibodies to isolate intracellular GluA1. Western analysis revealed intense ubiquitination of surface GluA1 (Fig. 1C left and 1D). However, although more GluA1 was isolated from the cytosolic compartment, no significant ubiquitination signals were detected (Fig. 1C right and 1D). These results indicate that ubiquitination occurs mainly on surface GluA1 subunits. The minimal ubiquitination of intracellular receptors may indicate that the ubiquitinated intracellular receptors are degraded with high efficiency.
Figure 1.
AMPAR GluA1 subunits are subject to ubiquitination. (A) HEK cells were co-transfected with GFP-GluA1 or GFP as a control, together with HA-ubiquitin (Ub). pcDNA vector was also transfected as a control. Cell lysates were prepared in a denaturing lysis buffer and GluA1 or GFP proteins were immunoprecipitated with anti-GFP antibodies and probed with anti-HA for ubiquitin. The same western blot was reprobed to confirm pull-down of GluA1 (middle) and general ubiquitination levels in ubiquitin transfected cell lysates (right). Note an antibody band around 50 kD (Ab) and a non-specific band in the pcDNA lane. (B) Measurement of ubiquitination intensity (n=8 independent experiments). (C) HEK 293A cells transfected with GluA1 and ubiquitin were incubated with anti-GFP antibodies to isolate surface GluA1 following immunoprecipitation. The remaining supernatants were then incubated with anti-GluA1Ct antibodies to isolate intracellular GluA1. Strong ubiquitination signals were detected in isolated cell-surface GluA1 (Surf, left panel), but not in cytosolic GluA1 of intracellular compartments (Cyto, right panel). GluA1 was reprobed in immunoprecipitates. Lysate tubulin was probed as a loading control (bottom panel). (D) Pooled data of average levels of GluA1 ubiquitination (n=3 independent experiments). Bar graph data represent means ± SEM, * P<0.05, t test.
Ubiquitination facilitates GluA1 degradation
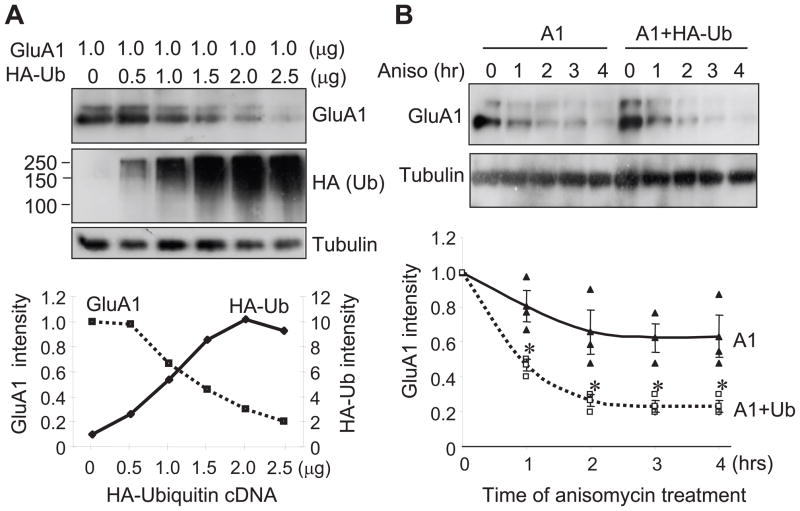
A physiological consequence of protein ubiquitination, especially polyubiquitination, is to direct the protein to degradation pathways. To examine the effect of ubiquitination on AMPAR turnover, we co-expressed GFP-GluA1 in HEK 293A cells with varied amounts of ubiquitin. We found that the abundance of GluA1 showed linear downregulation along with higher levels of ubiquitin expression (Fig. 2A). To directly investigate GluA1 degradation, we treated cells with 5 mM anisomycin to inhibit protein synthesis and time chased the remaining GluA1 abundance up to 4 hrs. GluA1 levels showed a time-dependent decrease, with about 40% decrease (0.63 ± 0.12, n=3) after 4 hrs of anisomycin incubation, indicating constitutive receptor degradation. In contrast, in cells transfected with HA-ubiquitin, GluA1 abundance showed a facilitated rate of reduction. At 4 hrs of anisomycin treatment, GluA1 was reduced by approximately 80% (0.23 ± 0.03, n=3) (Fig. 2B), strongly indicating that ubiquitination enhances AMPAR turnover.
Figure 2.
Ubiquitination causes a reduction in GluA1 protein abundance. (A) GluA1 was transfected in HEK 293A cells with varied amounts of HA-ubiquitin. GluA1 abundance (top) showed an inverse relationship to the level of ubiquitination (middle). Tubulin was probed as a control for equal loading (n=1). (B) GluA1 degradation time course in the presence of anisomycin (Aniso, 5 μM). Co-transfection of ubiquitin facilitated GluA1 degradation rate (n=3 independent experiments). Data represent means ± SEM, * P<0.05, two-way ANOVA test.
Lysine residues at the GluA1 C-terminal are targets of ubiquitination and responsible for degradation
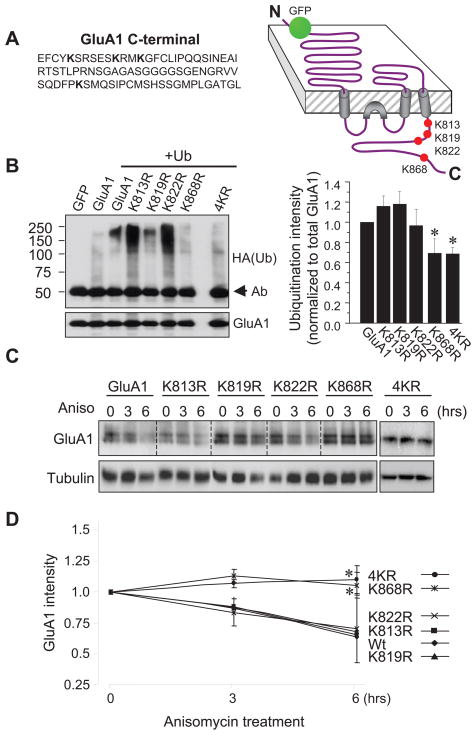
During ubiquitination, a ubiquitin molecule is covalently conjugated to a lysine residue of the target protein. When the intracellular domain of the GluA1 subunit was examined, we found four lysine residues all localized on the C-terminus. To identify the site(s) of ubiquitination, we replaced each lysine with arginine at each individual site (K813R, K819R, K822R and K868R) or all four lysines together (4KR) (Fig. 3A) in an N-terminal GFP-tagged GluA1 construct. Mutation sites were confirmed by sequencing. In HEK 293A cells co-transfected with the KR mutants and HA-ubiquitin, GFP-tagged GluA1 mutants were immunoprecipitated with anti-GFP antibodies and ubiquitinated species in the immunoprecipitates were probed with anti-HA antibodies. We found that typical ubiquitination smears were still detected in K813R, K819R and, to a lesser extent, K822R. In contrast, the intensity of the ubiquitin conjugates in K868R was markedly reduced. Consistently, GluA1 ubiquitination intensity was also significantly reduced when all four lysines were mutated (4KR) (Fig. 3B). Since all the KR mutants showed higher protein levels compared to the wildtype control, ubiquitination signals were normalized to GluA1 protein abundance. A clear reduction in ubiquitination intensity on K868R and 4KR was revealed following normalization (Fig. 3B). Results from these experiments indicate lysine 868 as the principal residue for GluA1 ubiquitination.
Figure 3.
Lysine residues at GluA1 C-terminus are sites of ubiquitination. (A) Illustration of the lysine residues at the C-terminus of GluA1 and various forms of KR mutants. (B) Ubiquitination assays on HEK 293A cells overexpressing GFP-GluA1 wildtype or KR mutants, together with HA-ubiquitin. Bar graphs show normalized ubiquitination intensity to total GluA1 protein levels. Ubiquitination intensity in K813R, K819R and K822R mutants was comparable to that of wildtype GluA1, whereas K868R and 4KR showed significant reductions in ubiquitination intensity (n=4 independent experiments). (C) HEK 293A cells transfected with GluA1 or KR mutants were incubated with anisomycin for 3 or 6 hrs to block protein synthesis, and the remaining GluA1 amount was examined to indicate protein degradation. Tubulin was reprobed as a loading control. (D) In the presence of anisomycin, GluA1 wildtype (wt) and K813R, K819R and K822R mutants showed a similar time-dependent reduction. In contrast, GluA1 degradation was completely abolished in K868R and 4KR mutants (n=2–3 independent experiments). Data represent means ± SEM, * P<0.05, t test.
We found that elevated protein ubiquitination was accompanied with enhanced GluA1 degradation (Fig. 2). However, this degradation could be a consequence of ubiquitination of other proteins, which in turn modulates GluA1 proteolysis. We reasoned that if this ubiquitin-dependent receptor degradation is due to direct ubiquitination on GluA1 subunits, the KR mutants should become resistant to the degradation process. To test this, we expressed GluA1WT and the GluA1 KR mutants in HEK cells to compare their degradation rates. Following incubation with 5 mM anisomycin to inhibit protein synthesis for 0, 3 and 6 hrs, transfected cells were lysed and probed for total GluA1 abundance. We found that protein levels of K813R, K819R and K822R decreased at a rate comparable to that of the wild type GluA1. In contrast, the amount of K868R and 4KR showed little change over a course of 6 hrs (Fig. 3C and 3D). The reduced degradation rate in K868R and 4KR mutants is consistent with the important role of K868 in ubiquitination.
Ubiquitination and proteasomal degradation of AMPARs in neurons
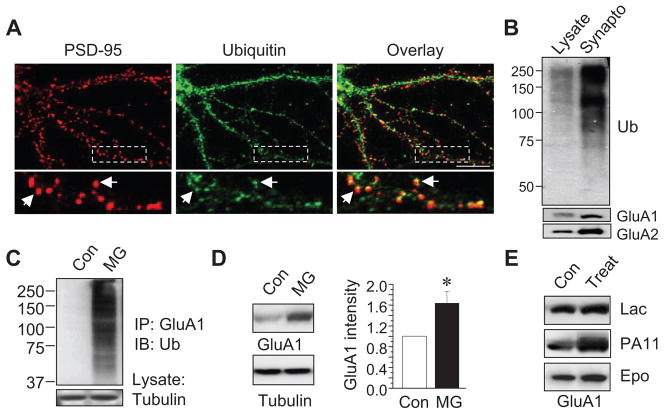
We then explored the occurrence of AMPAR ubiquitination in neurons. Because AMPARs are enriched at the postsynaptic domain on dendritic spines, we first examined whether ubiquitin is localized at the vicinity of synapses. In cultured cortical neurons, ubiquitin and a synaptic marker protein PSD-95 were immunostained. Ubiquitin signals were detected throughout the neuron. In dendrites, ubiquitin formed intense clusters, most of which co-distributed with the postsynaptic scaffolding molecule PSD-95 (Fig. 4A), indicating a concentrated localization of ubiquitin in spines. Consistently, western blotting demonstrated strong ubiquitin immunosignals in brain synaptosomal preparations compared to the lysate (Fig. 4B), indicating the occurrence of ubiquitination modification on synaptic proteins.
Figure 4.
Ubiquitination and proteasome-mediated degradation of AMPARs in neurons. (A) Double staining of ubiquitin (green) and the postsynaptic marker protein PSD-95 (red) in 2 wk old cultured cortical neurons. The boxed area was zoomed in for clarity (bottom). Arrows indicate co-localized puncta. Two independent immunostainings showed similar distribution pattern. (B) Western blotting showed high levels of ubiquitin (Ub) immunosignals in a synaptosome fraction prepared from rat brain cortical tissue. Protein assays were performed in lysates prior to westerns to ensure that equal amount of total protein was loaded. (C) Cultured cortical neurons were incubated with MG132 (5 μM) for 24 hrs, followed by receptor ubiquitination assays. A strong ubiquitin smear was detected in MG treated neurons, indicating endogenous AMPAR ubiquitination. (D and E) Cultured cortical neurons were treated for 3 hours with different proteasomal inhibitors including MG-132 (5 μM) (n=4 independent experiments), lactacystin (Lac, 10 μM), PR11 (0.5 μM) and epoxomicin (Epo, 10 μM). Proteasome inhibition induced a significant increase in GluA1 amount. Bar graph data represent means ± SEM, * P<0.05, t test. Scale bar, 10 μm.
Polyubiquitinated proteins are normally directed to the proteasome for degradation. Many membrane receptors can be degraded by the proteasome, including the IL-2 receptor, growth hormone receptor, opioid receptor and the inhibitory GABAA receptors (Hegde 2004). Degradation of synaptic scaffolding proteins such as PSD-95 is also proteasome dependent (Colledge et al. 2003). In cultured cortical neurons at basal conditions, we failed to observe obvious AMPAR ubiquitination. This might be due to either a minimal level of ubiquitination or rapid degradation and removal of ubiquitinated receptors by the proteasome. To accumulate ubiquitinated receptors for better detection, we pre-incubated neurons with the proteasome inhibitor MG-132 (5 μM) for 24 hours. Under this condition, a strong ubiquitination smear was detected in immunoprecipitated GluA1 subunits (Fig. 4C), indicating the presence of receptor ubiquitination in neurons. In agreement with proteasome-mediated degradation, we found a rapid elevation of AMPAR amount when proteasomal activity was inhibited. Treatment of cultured cortical neurons with MG-132 (5 μM) for 3 hrs significantly increased GluA1 levels (Fig. 4D). To confirm that the effect was indeed proteasome dependent, we found a similar increase in GluA1 by other proteasome specific inhibitors including lactacystin (10 μM) and epoxomicin (10 μM), as well as PR11 (0.5 μM) (Fig. 4E), indicating proteasome-mediated AMPAR degradation at basal conditions.
Ubiquitination regulates AMPAR internalization and cell-surface expression
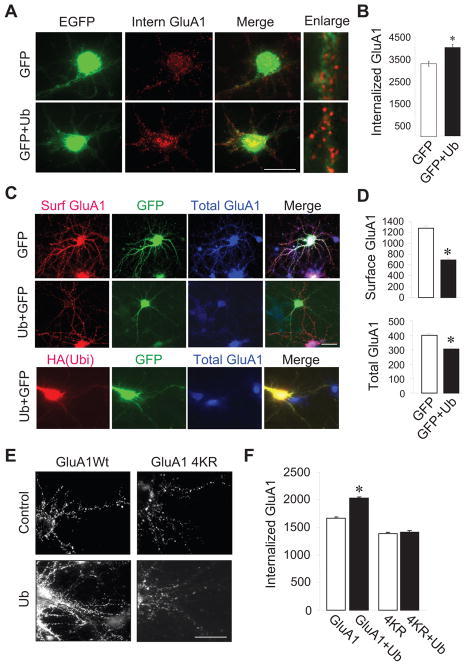
A major function of ubiquitination is to sort membrane proteins to the endocytotic pathway (d’Azzo et al. 2005). To directly assess effects of ubiquitination on AMPAR trafficking, we performed internalization assays (Man et al. 2007; Hou et al. 2008b). Cortical neurons were transfected with GFP alone or together with HA-ubiquitin. 2 days after transfection, neurons were incubated with anti-GluA1 N-terminal antibodies at 4°C to label receptors on the cell surface. Cells were then treated with glutamate (50 μM) at 37°C for 15 minutes to induce receptor internalization. Following blockade of the remaining surface receptors with non-conjugated secondary antibodies, the internalized receptors were then specifically labeled under permeant conditions by fluorescent secondary antibodies. We found that GluA1 internalization was markedly enhanced in cells overexpressing ubiquitin compared to those transfected with GluA1 alone (internalized GluA1 intensity: 3301 ± 111, n=15 cells in GFP alone control; 3997 ± 140, n=17 cells in GFP+ubiquitin) (Fig. 5A and 5B).
Figure 5.
Ubiquitin regulates AMPAR internalization and surface expression. (A and B) Cultured cortical neurons were transfected with GFP or GFP plus HA-ubiquitin. Surface AMPARs were labeled by anti-GluA1Nt antibodies, followed by glutamate (50 μM, 15 min) treatment to induce receptor internalization. Ubiquitin expression increased GluA1 internalization. (red, Intern GluA1, n=15–17 cells from two independent assays). (C) Cultured cortical neurons were transfected with HA-ubiquitin together with GFP or GFP alone as a control. Surface (Surf GluA1, red) and total (Total GluA1, blue) GluA1 were selectively labeled (top panels). To confirm the co-expression of HA-ubiquitin and GFP, some cells were double stained using antibodies against HA (red) and GluA1C (blue) (bottom panel). (D) Measurement of surface and total GluA1 immunointensity. (E and F) Cortical neurons were transfected with wildtype and 4KR GFP-GluA1, together with or without HA-ubiquitin. Neurons were incubated with anti-GFP antibodies to label surface receptors and then treated with glutamate (50 μM, 15 min) to induce receptor internalization. The internalized receptors were specifically labeled (n=20–23 cells from two independent assays). Bar graph data represent means ± SEM, * P<0.05, t test. Scale bar, 20 μm.
A direct consequence of receptor endocytosis is to down regulate surface receptor expression. In addition, the internalized AMPARs can be recycled back to the plasma membrane, while a fraction of those can be sorted for degradation (Ehlers 2000), resulting in a loss of total receptor amount. To examine the effect of ubiquitination on AMPAR surface localization and stability, we transfected cortical neurons with HA-ubiquitin plus GFP, or GFP alone as a control. We then immunolabeled the surface and total endogenous GluA1 subunits in transfected neurons under nonpermeant and permeant conditions with antibodies against GluA1Nt and GluA1Ct, respectively. We found that in neurons overexpressing ubiquitin, GluA1 surface expression was reduced by 45% (control, 1272 ± 11, n=19 cells; ubiquitin, 691 ± 13, n=16 cells) (Fig. 5C and 5D). Meanwhile, ubiquitin expression also reduced the total amount of GluA1 by 25% (control, 399 ± 8, n=19 cells; ubiquitin, 302 ± 10, n=16 cells) (Fig. 5C and 5D), consistent with ubiquitination-dependent AMPAR internalization and degradation in neurons. To ensure that the GFP signals could reliably indicate ubiquitin over-expression, we immunostained HA in neurons co-transfected with HA-ubiquitin and GFP. We found that all of the green cells contained red (50 green cells were all red), and most of the red cells contained green (in 50 red cells, 45 showed green) (Fig. 5C, bottom), indicating reliable co-expression of double transfected constructs.
To further confirm that ubiquitination on GluA1 is necessary for receptor internalization, we evaluated the effects of the KR mutations on AMPAR trafficking. Cortical neurons were transfected with GFP-GluA1 or GFP-4KR with or without HA-ubiquitin. 24 hours later, cells were incubated with anti-GFP antibodies at 4°C to label surface GluA1 and then treated with glutamate (50 μM) at 37°C for 15 minutes to induce receptor endocytosis. Immunofluorescence intensity acquired following a blockade of the remaining surface receptors was used to indicate receptor internalization. Internalized GluA1 immunofluorescence of 4KR was significantly reduced compared with wildtype GFP-GluA1 (Fig. 5E and 5F), indicating a role for GluA1 ubiquitination in basal receptor trafficking. More importantly, we found that whereas overexpression of HA-ubiquitin markedly enhanced internalization of wildtype GFP-GluA1, co-transfection with ubiquitin failed to facilitate 4KR internalization (1669 ± 125, n=23 cells in GluA1 alone; 2032 ± 119, n=23 cells in GluA1+ubiquitin; 1385 ± 138, n=20 cells in 4KR; 1411 ± 119, n=20 cells in 4KR+ubiquitin) (Fig. 5E and 5F). These results strongly indicate that ubiquitin-induced receptor internalization results from direct ubiquitination of lysine residues at the GluA1 C-terminus.
The E3 ligase Nedd4 induces AMPAR ubiquitination in heterologous cells
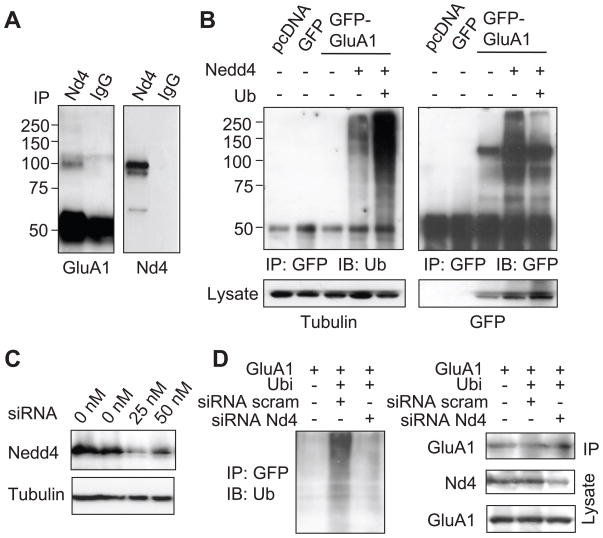
During ubiquitination, an E3 ligase interacts with a particular substrate protein and conjugates ubiquitin molecules to a lysine residue, thus achieving specificity in protein ubiquitination. In searching for the E3 ligase responsible for AMPAR modification, our recent study demonstrated that intracellular accumulation of sodium triggers proteasome-mediated AMPAR degradation, suggesting the presence of sodium-related machinery in AMPAR ubiquitination. We therefore chose to examine the role of Nedd4 (neural precursor cell expressed, developmentally down-regulated 4) in AMPAR ubiquitination. Indeed, in our earlier work we have identified Nedd4 as an AMPAR E3 ligase (Zhang et al., 2009). Nedd4 is a HECT domain-containing single molecule E3 ligase (Ingham et al., 2004), which is highly expressed in neurons and has been shown to be regulated by sodium (Harvey et al., 1999; Dinudom et al., 2001; Kabra et al., 2008). Nedd4 ligases have been implicated in regulating the trafficking and turnover of many membrane proteins including sodium channels (Staub et al., 2000; Snyder, 2005; Kabra et al., 2008). To examine the interaction of Nedd4 with its substrate AMPARs, we transfected HEK cells with GFP-GluA1, and performed co-immunoprecipitation assays with antibodies against endogenous Nedd4. GluA1 was positively detected in immunoprecipitated Nedd4 complexes, but not in the IgG immunoprecipitation control (Fig. 6A). In order to evaluate the role of Nedd4 in AMPAR ubiquitination, we transfected HEK cells with GFP-GluA1 alone, or together with Nedd4, or Nedd4 plus HA-ubiquitin. GluA1 was immunoprecipitated with anti-GFP and probed with anti-ubiquitin antibodies. As shown previously (Fig. 1C), little ubiquitination of GluA1 from HEK lysate was detected under basal conditions (Fig. 6B), indicating inefficient ubiquitination in heterologous cells. However, co-transfection of GFP-GluA1 with Nedd4 produced a significant amount of ubiquitination species in GFP-GluA1 immunoprecipitates, which was further enhanced by co-expressing ubiquitin (Fig. 6B), indicating GluA1 as a substrate of the Nedd4 ligase.
Figure 6.
Nedd4 mediates GluA1 ubiquitination. (A) Lysate of HEK cells expressing GFP-GluA1 was incubated with anti-Nedd4 antibodies to isolate endogenous Nedd4. GluA1 was positively detected in Nedd4 complexes, indicating an association. IgG was used as a control. (B) In transfected HEK cells, GluA1 was immunoprecipitated and probed for ubiquitin. Co-expression of GluA1 with Nedd4 markedly increased receptor ubiquitination (left panel). The membrane was reprobed to confirm the pull-down of GluA1 (right panel). (C) siRNA dramatically reduced Nedd4 abundance in HEK cells. (D) HEK cells were transfected with GFP-GluA1, HA-ubiquitin, together with Nedd4 siRNA, or scrambled siRNA as a control. GFP-GluA1 was immunoprecipitated for ubiquitination assays. The blot was reprobed for GluA1, and cell lysate was probed for Nedd4 (Nd4) and GluA1.
To further confirm the involvement of endogenous Nedd4, we introduced an siRNA specifically targeting Nedd4. The effect of the siRNA was confirmed by a dramatic reduction in Nedd4 levels in HEK cells transfected with Nedd4 siRNA (Fig. 6C). We then expressed GFP-GluA1 and HA-ubiquitin, together with Nedd4 siRNA or a scrambled siRNA control. As shown earlier in this study Fig. 1A), expression of ubiquitin induces strong ubiquitination of GluA1 (Fig. 6D). However, in the presence of Nedd4 siRNA, which reduced total Nedd4 in the lysate, ubiquitin overexpression failed to induce GluA1 ubiquitination as compared to cells expressing scrambled siRNA control (Fig. 6D). These results strongly indicate that endogenous Nedd4 mediates the ubiquitination effect and functions as the ubiquitin ligase for AMPAR ubiquitination.
Nedd4 co-localizes and associates with AMPAR in neurons
If Nedd4 functions as a ligase for AMPAR ubiquitination, it should be localized in close proximity to its target so as to promote efficient ubiquitin ligation. Towards this end, we double stained Nedd4 with AMPAR GluA2 subunits, and the synaptic protein Shank in cultured cortical neurons. The Nedd4 immunofluorescence signal showed a punctate pattern in dendrites, and co-distributed with both AMPARs (Supplemental Figure, SFig. A) and Shank (SFig. B), indicating an enrichment of Nedd4 at synaptic sites. Furthermore, rat brain lysates and synaptosome preparations of equal total protein amounts were analyzed by western blotting. The synaptosome sample showed markedly higher amounts of AMPAR subunits and Nedd4 compared to brain lysate, confirming synaptic distribution of the Nedd4 ligase (SFig. C). Similar to the results observed from transfected HEK cells (Fig. 6A), we found that GluA1 co-immunoprecipitated with Nedd4 using lysates from cultured cortical neurons (SFig. D), suggesting a possible role for Nedd4 as an AMPAR ubiquitin ligase in neurons.
Nedd4 ubiquitinates and regulates AMPARs surface stability in neurons
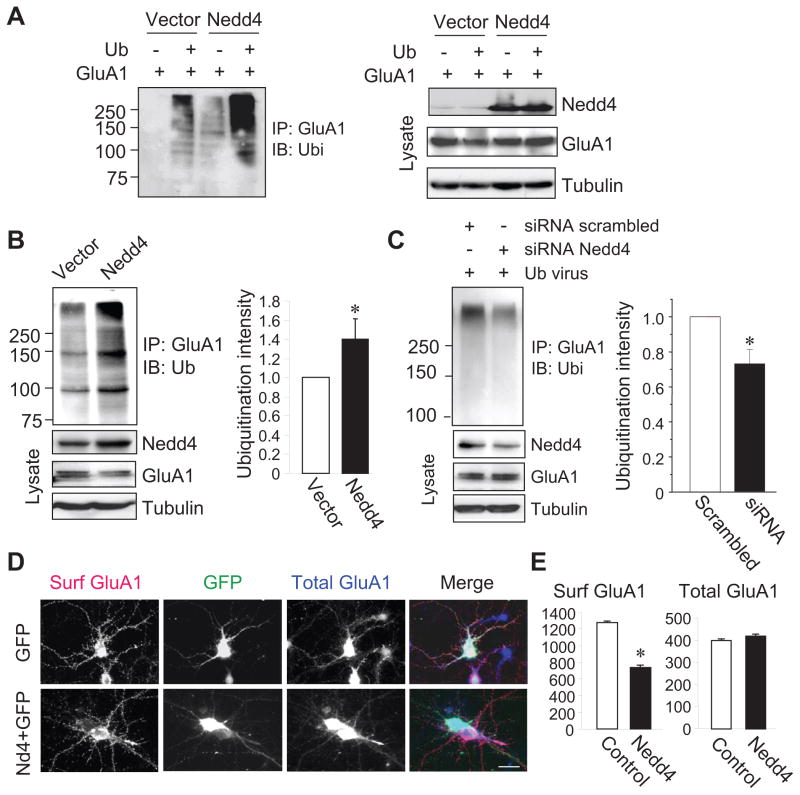
In order to confirm the involvement of Nedd4 in AMPAR ubiquitination in neurons, we cloned Nedd4 into a lentiviral vector so that biochemical analysis could be performed. To test the Nedd4 lentiviral constructs, HEK cells were transfected with HA-ubiquitin and GFP-GluA1 followed by viral Nedd4 infection. A marked increase in Nedd4 amount was observed in viral Nedd4-infected cells (Fig. 7A right). Correspondingly, Nedd4 infection increased GluA1 ubiquitination levels, which was further enhanced by co-expression of HA-ubiquitin (Fig. 7A left). Consistently, incubation of viral Nedd4 with cultured neurons also increased Nedd4 expression and levels of GluA1 ubiquitination (Nedd4, 1.40 ± 0.22, n=3) (Fig. 7B). To investigate the role of endogenous Nedd4 in AMPAR ubiquitination in neurons, cultured cortical neurons were transfected with Nedd4-specific siRNA followed by infection with an ubiquitin adenovirus to elevate basal ubiquitination levels. We found that siRNA knockdown reduced Nedd4 abundance in neurons, and significantly reduced GluA1 ubiquitination as compared to the scrambled siRNA control (siRNA, 0.73 ± 0.08, n=3) (Fig. 7C), strongly indicating that Nedd4 functions as an endogenous AMPAR E3 ubiquitin ligase in neurons. If so, Nedd4 activity should have a similar effect on AMPAR surface localization as that from ubiquitin overexpression. We thus examined surface and total GluA1 levels under nonpermeant and permeant conditions, respectively, in cortical neurons. 2 days after Nedd4 transfection, a marked reduction in surface-localized GluA1 was observed compared to the GFP transfection control (control, 1246 ± 15, n=19 cells; Nedd4, 736 ± 7, n=17 cells) (Fig. 7D and 8E). Interestingly, unlike the effect of ubiquitin overexpression, Nedd4 showed no significant effect on total AMPAR protein amount (control, 412 ± 9, n=19 cells; Nedd4, 417 ± 12, n=17 cells) (Fig. 7D and 8E). These data strongly indicate that endogenous Nedd4 in neurons catalyzes AMPAR ubiquitination and regulates receptor cell-surface localization.
Figure 7.
Nedd4 ubiquitinates AMPARs and suppresses receptor surface expression in neurons. (A) HEK cells were first transfected with GluA1 and HA-ubiquitin cDNA plasmids and then incubated with lentiviral Nedd4 and a viral vector control. GluA1 immunoprecipitates were probed with anti-ubiquitin antibodies (left panel). Nedd4, GluA1 and tubulin were probed in cell lysates (right panel). (B) Neurons were infected with viral Nedd4 and ubiquitination of endogenous GluA1 was examined (left panel). Pooled data are shown in the bar graph (right panel, n=3). (C) Neurons were transfected with Nedd4 siRNA or scrambled control siRNA, with a subsequent incubation with the viral ubiquitin construct. Nedd4 siRNA suppressed Nedd4 expression and reduced GluA1 ubiquitination (n=3). (D and E) Neurons were transfected with GFP plus Nedd4, or GFP alone as a control. Surface (Surf) and total GluA1 were detected sequentially (n=19 cells in control and n=17 in Nedd4, from two independent assays). Bar graph data represent means ± SEM, * P<0.05, t test. Scale bar, 20 μm.
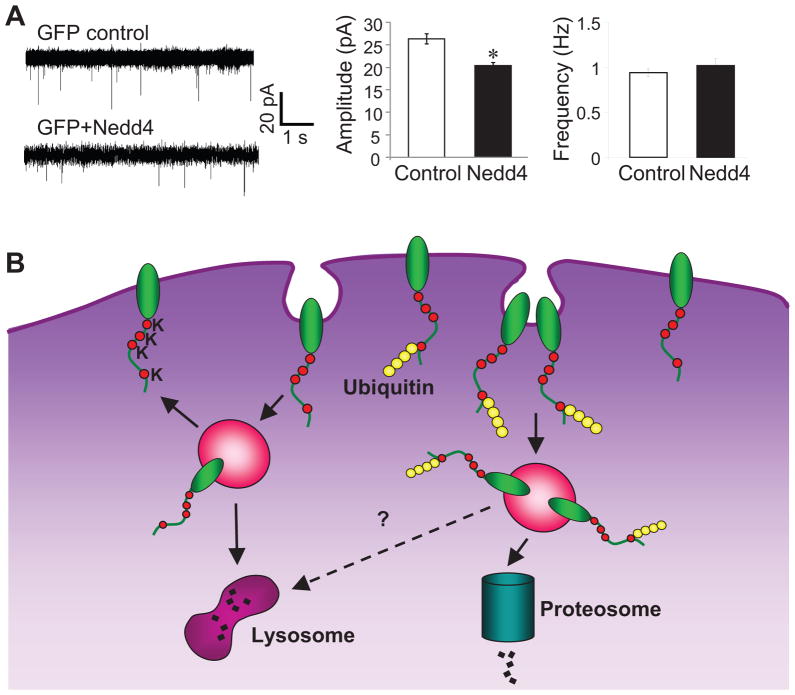
Figure 8.
Nedd4 suppresses AMPAR-mediated excitatory synaptic transmission. (A) Whole-cell recording of mEPSCs in neurons expression EGFP or EGFP and Nedd4. Typical mEPSCs (left) and pooled data (right) show reduced mEPSC amplitude in Nedd4-expressing neurons (n=5 cells for control, n=4 cells for Nedd4; from 3 independent experiments). (B) An illustration of ubiquitination-dependent AMPAR internalization and degradation. The non-modified native AMPARs are subject to constitutive internalization, of which most will be recycled back to the plasma membrane for reuse and a small portion will be sorted to lysosome for degradation. E3 ligase Nedd4 conjugates ubiquitin units to the last lysine in GluA1 C-terminal, and the ubiquitinated receptors are targeted for internalization and sorted for degradation in proteasome.
If Nedd4-dependent ubiquitination regulates AMPAR surface localization, we wanted to know whether Nedd4 also altered AMPAR-mediated synaptic transmission. We therefore examined miniature excitatory postsynaptic currents (mEPSCs) by whole cell patch clamp recording on transfected hippocampal neurons (Fig. 8A). Compared to control neurons that expressed EGFP alone, neurons co-expressing EGFP and Nedd4 showed a significant reduction in mEPSCs amplitude (control 26.4 ± 1.1 pA, n=5 cells; Nedd4 20.3 ± 0.8 pA, n=4 cells), whereas the miniature frequency showed no change (control 0.94 ± 0.04 Hz, n=5 cells; Nedd4 1.02 ± 0.08 Hz, n=4 cells). This result is consistent with the Nedd4-induced reduction in AMPAR surface expression and indicates a removal of receptors from synaptic sites by locally localized Nedd4.
Discussion
We show that mammalian AMPARs are subject to direct modulation by ubiquitination. Covalent conjugation with ubiquitin molecules shifts the molecular weight (MW) of GluA1 from 100 kD to as high as approximately 300 kD, indicating the addition of a large number (more than 20) of ubiquitin moieties. Given the spectrum of the ubiquitin smear in isolated GluA1, AMPARs are likely modified mainly by polyubiquitination, but could be multi- or monoubiquitination as well. This is consistent with recent studies showing high MW ubiquitinated receptors including glutamate receptors (Rezvani et al., 2007) and GABAA receptors (Saliba et al., 2007). In C. elegans only five ubiquitin moieties or less are conjugated with GluA1 (Burbea et al., 2002), suggesting a more intense ubiquitination process in mammalian AMPARs. At basal conditions, only a limited amount of ubiquitin conjugation can be detected on AMPARs, similar to previous work on C. elegans AMPARs (Burbea et al., 2002) and mammalian GABAA receptors (Saliba et al., 2007) This might result from an inactive ubiquitination process at basal neuronal activity levels, or short-lived ubiquitinated receptors. In neurons, AMPARs are normally assembled into heterotetrameric complexes with the most typical combinations being either GluA1 and GluA2 or GluA2 and GluR3 subunits (Lu et al., 2009). In heterologous cells, transfected GluA1 subunits are known to form homotetrameric channels which, like endogenous receptors, can be targeted onto the plasma membrane and respond to agonist activation. Nevertheless, transfected GluA1 might be processed differently; for instance, proteins may be misfolded, leading to an ER-associated degradation (ERAD) response, where defective proteins are selectively ubiquitinated prior to proteasome-mediated degradation (Plemper and Wolf, 1999). If this is the case, ubiquitination should occur on GluA1 localized within intracellular compartments, but not at the plasma membrane. Contrary to this possibility, our results from surface pulldown assays reveal that ubiquitin conjugation occurs mainly on cell-surface, rather than intracellular AMPARs, which argues against the involvement of ERAD. The preference of ubiquitination on surface AMPARs may be due to plasma membrane-limited localization of participating ubiquitin ligases such as Nedd4 (Dunn et al., 2004; Ingham et al., 2004). There are four lysine residues residing at the intracellular C-terminus of GluA1. Mutation of K868 or all four lysines to arginines dramatically suppressed the intensity of GluA1 ubiquitination, indicating that the last lysine residue at GluA1 C-terminus K868 is the key site for ubiquitin conjugation, consistent with previous work showing the involvement of C-terminal lysines in C. elegans AMPAR ubiquitination. Reasons for the incomplete knockdown of ubiquitination in the lysine mutant remain unclear, but it might be attributable to ubiquitination at non-lysine residues (Ikeda et al., 2002).
Our results demonstrate that overexpression of ubiquitin increases receptor internalization and reduces GluA1 surface expression, indicating the role of the UPS in AMPAR internalization. Similar results have been observed in a recent study published during the revision of this paper (Schwarz, et al., 2010). Interestingly, Schwarz et al. show that AMPAR ubiquitination is facilitated by AMPA treatment, indicating the existence of activity-dependent receptor autoregulation. The observed effects might potentially be a result of ubiquitination of other synaptic proteins, such as PSD-95, which subsequently destabilizes surface AMPARs (Colledge et al., 2003; Bingol and Schuman, 2004). However, our data show that mutation of the lysine residues at the GluA1 intracellular terminus suppresses both constitutive and glutamate-induced receptor internalization, supporting a role for the direct involvement of GluA1 ubiquitination in AMPAR internalization. The molecular basis underlying ubiquitination-mediated endocytosis remains unclear. It has been established that AMPARs internalize via the clathrin-coated pit pathway following the binding of the clathrin adaptor protein AP2 to the GluR C-terminus (Man et al., 2000; Lee et al., 2002). Given that AP2 binding is not directly regulated by ubiquitination, it is intriguing to postulate that a distinct, ubiquitination-sensitive clathrin adaptor, such as EPS15 (d’Azzo et al., 2005; Piper and Luzio, 2007), might be implicated in ubiquitination-dependent AMPAR trafficking.
In line with the role of ubiquitination in directing proteins to the degradation pathway, we observed that overexpressing ubiquitin reduces total receptor abundance in neurons. Similarly, in C. elegans GluR ubiquitination also leads to a reduction in GluR synaptic accumulation (Burbea et al., 2002). In Drosophila, inhibition of the proteasome by subunit mutation increases GluRIIB expression and enhances synaptic transmission at the neuromuscular junction (Haas et al., 2007). These findings strongly indicate the involvement of the UPS in AMPAR turnover. Because AMPARs can also be degraded by the lysosome (Ehlers, 2000), it would be interesting to know whether the distinct machineries involved in AMPAR degradation are exclusively utilized under different conditions, or are employed in a sequentially coordinated manner so as to accomplish complete proteolysis (Geetha and Wooten, 2008).
The E3 ligase is a key component in the molecular machinery of AMPAR ubiquitination. Although activity of several E3 ligases such as the anaphase-promoting complex (Juo and Kaplan, 2004) as well as the Skp1/Cullin/F Box component including LIN-23 (Dreier et al., 2005) and KEL-8 (Schaefer and Rongo, 2006) have been implicated in AMPAR turnover, they do not seem to directly target AMPARs for ubiquitination. We find that Nedd4 is preferentially localized in synapses and associates with AMPARs in neurons. Overexpression of Nedd4 causes GluA1 ubiquitination, which is accompanied with suppression of AMPAR cell-surface expression and excitatory synaptic currents. Consistently, knockdown of endogenous Nedd4 reduces AMPAR ubiquitination. All of these results strongly indicate a role for Nedd4 as an AMPAR E3 ligase. For protein association, Nedd4 typically binds to a PPXY domain in its substrates such as epithelial sodium channels via its WW domain (Staub et al., 1996; Snyder, 2005). However, no such domain exists at the GluA1 C-terminus, suggesting the involvement of an unconventional interacting motif or an unidentified intermediate protein. Indeed, many substrates of Nedd4-like E3 ligases such as EPS15, Notch and TGF-β receptor 1 do not contain the PPXY domain (Chen and Matesic, 2007). In addition, the WW domain has been shown to be able to interact with a motif containing a phosphorylated serine or threnonine (Lu et al., 1999). Interestingly, unlike the effect of ubiquitin, Nedd4 overexpression in neurons only decreases surface AMPAR expression without changing the total receptor amount. This could be due to a mild effect of Nedd4 on AMPAR ubiquitination compared to a stronger ubiquitin-induced effect. Alternatively, Nedd4 might participate in other cellular functions such as the facilitation of AMPAR gene transcription or translation, which in turn would counterbalance ubiquitination-dependent AMPAR degradation. Indeed, Nedd4 has been shown to be able to translocate into the nucleus and regulate nuclear targets (Hamilton et al., 2001). An important feature of Nedd4 is that it contains a C2 domain, through which Nedd4 associates with plasma membrane phospholipids in a calcium-dependent manner (Wang et al., 2010; Ingham et al., 2004). It is intriguing to postulate that calcium-induced membrane translocation of Nedd4 may be important in linking neuronal activity to surface receptor ubiquitination, endocytosis and degradation. In addition, whether Nedd4 is the sole E3 ligase for AMPAR ubiquitination in vivo remains to be addressed with more thorough investigation.
Supplementary Material
Acknowledgments
We thank Dr. Peter Snyder (University of Iowa) for providing us with HA-ubiquitin and Nedd4 cDNA construct, and Dr. Xianmin Yu (Florida State University) for technical assistance. This work was supported in part by US National Institutes of Health grant MH079407 (H. Y. M.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid
- AMPAR
AMPA receptor
- GluA1
glutamate receptor subunit 1
- PBS
phosphate-buffered saline
- ACSF
artificial cerebrospinal fluid
- RIPA buffer
radioimmunoprecipitation assay buffer
- NP40 buffer
Nonidet P40 buffer
- PVDF
polyvinylidene difluoride
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- SDOC
sodium deoxycholate
- HEK
human embryonic kidney
- UPS
ubiquitin proteasome system
References
- Bingol B, Schuman EM. A proteasome-sensitive connection between PSD-95 and GluA1 endocytosis. Neuropharmacology. 2004;47:755–763. doi: 10.1016/j.neuropharm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Chen C, Matesic LE. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev. 2007;26:587–604. doi: 10.1007/s10555-007-9091-x. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Ding M, Shen K. The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. Bioessays. 2008;30:1075–1083. doi: 10.1002/bies.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A, Harvey KF, Komwatana P, Jolliffe CN, Young JA, Kumar S, Cook DI. Roles of the C termini of alpha -, beta -, and gamma -subunits of epithelial Na+ channels (ENaC) in regulating ENaC and mediating its inhibition by cytosolic Na+ J Biol Chem. 2001;276:13744–13749. doi: 10.1074/jbc.M011273200. [DOI] [PubMed] [Google Scholar]
- Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Dunn R, Klos DA, Adler AS, Hicke L. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J Cell Biol. 2004;165:135–144. doi: 10.1083/jcb.200309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Geetha T, Wooten MW. TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic. 2008;9:1146–1156. doi: 10.1111/j.1600-0854.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Wang Y. Glutamate stimulates glutamate receptor interacting protein 1 degradation by ubiquitin-proteasome system to regulate surface expression of GluA2. Neuroscience. 2007;145:100–109. doi: 10.1016/j.neuroscience.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Haas KF, Miller SL, Friedman DB, Broadie K. The ubiquitin-proteasome system postsynaptically regulates glutamatergic synaptic function. Mol Cell Neurosci. 2007;35:64–75. doi: 10.1016/j.mcn.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MH, Tcherepanova I, Huibregtse JM, McDonnell DP. Nuclear import/export of hRPF1/Nedd4 regulates the ubiquitin-dependent degradation of its nuclear substrates. J Biol Chem. 2001;276:26324–26331. doi: 10.1074/jbc.M101205200. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Dinudom A, Komwatana P, Jolliffe CN, Day ML, Parasivam G, Cook DI, Kumar S. All three WW domains of murine Nedd4 are involved in the regulation of epithelial sodium channels by intracellular Na+ J Biol Chem. 1999;274:12525–12530. doi: 10.1074/jbc.274.18.12525. [DOI] [PubMed] [Google Scholar]
- Hegde AN. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog Neurobiol. 2004;73:311–357. doi: 10.1016/j.pneurobio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008a;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Regulation of AMPA receptor localization in lipid rafts. Mol Cell Neurosci. 2008b;38:213–223. doi: 10.1016/j.mcn.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ikeda A, Longnecker R. Lysine-independent ubiquitination of Epstein-Barr virus LMP2A. Virology. 2002;300:153–159. doi: 10.1006/viro.2002.1562. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem. 2008;283:6033–6039. doi: 10.1074/jbc.M708555200. [DOI] [PubMed] [Google Scholar]
- Kato A, Rouach N, Nicoll RA, Bredt DS. Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc Natl Acad Sci U S A. 2005;102:5600–5605. doi: 10.1073/pnas.0501769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluA2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci. 2006;31:137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Bingol B, Weld HA, Schuman EM. Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr Biol. 2003;13:2073–2081. doi: 10.1016/j.cub.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007;19:459–465. doi: 10.1016/j.ceb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24:266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H, Rongo C. KEL-8 is a substrate receptor for CUL3-dependent ubiquitin ligase that regulates synaptic glutamate receptor turnover. Mol Biol Cell. 2006;17:1250–1260. doi: 10.1091/mbc.E05-08-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt HP. Protein ubiquitination, degradation and the proteasome in neuro-degenerative disorders: no clear evidence for a significant pathogenetic role of proteasome failure in Alzheimer disease and related disorders. Med Hypotheses. 2006;67:311–317. doi: 10.1016/j.mehy.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30 (49):18–29. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. Embo J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 2000;57:809–815. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem. 2010;285:12279–12288. doi: 10.1074/jbc.M109.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeumier K, Pulst SM, Schweizer FE. Proteasome inhibition triggers activity-dependent increase in the size of the recycling vesicle pool in cultured hippocampal neurons. J Neurosci. 2006;26:11333–11341. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29:4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.