Abstract
Objective
To examine the relationship between HIV-1 antigenic load (plasma RNA copies/ml) and broad HIV-1 neutralizing antibody activity.
Methods
Plasma from 120 HIV-1 infected patients, including HIV-1 Natural Viral Suppressors (similar to Elite Controllers), was tested for neutralization against 15 Tier 1/Tier 2 HIV-1 pseudoviruses. Broad HIV-1 neutralizing antibody activity was confirmed with IgG and heterlogous clade testing (18 pseudoviruses from Clades A, C, and CRF02_AG). Statistical analysis was performed to determine factors associated with broad HIV-1 neutralizing antibody activity.
Results
Ten individuals with broad HIV-1 neutralizing antibody activity were identified. These individuals had a median CD4 count of 589 cells/ul (range 202–927), 1,611 HIV-1 RNA copies/ml (range 110–8,964), and 13 years since HIV diagnosis (range 1–22). There was a significant correlation between the presence of broadly neutralizing antibodies in those with HIV-1 RNA between 100 and 10,000 copies/ml compared to those <100 or >10,000 copies/ml (p=.0003 and .0245, respectively). Individuals with HIV-1 RNA 100–10,000 copies/ml had a higher number of Tier 2 viruses neutralized compared to the <100or >10,000 copies/ml groups (p=< .0001 and p=.076, respectively). Male sex was associated with broad HIV-1 neutralizing antibody activity (p=.016).
Conclusion
These results indicate that low but persistent HIV antigen expression correlates with broad HIV-1 neutralizing antibody activity. At higher levels of plasma viremia, neutralization titers were diminished. Conversely, at lower levels, there appears to be insufficient antigen stimulation to maintain high neutralization titers. These findings may have important implications in furthering the understanding of the humoral response to HIV infection.
Keywords: HIV, broadly neutralizing antibody, neutralizing activity, HIV RNA, natural viral suppressor, elite controller
Introduction
The immune system requires the presence of sufficient quantities of antigen in order to elicit an immune response. Despite numerous studies in HIV-infected patients, the relationship between HIV-1 antigenic load and HIV-1 neutralization remains unclear. Several lines of evidence suggest that the antibody response to the HIV-1 envelope lacks durability.1,2 Because of this, we hypothesized that a persistent but low amount of circulating HIV-1 virus would be necessary to produce and maintain a robust humoral immune response. This study was undertaken to examine the relationship between HIV-1 antigenic load (as reflected by HIV-1 plasma RNA copies/ml) and broad HIV-1 neutralizing antibody activity.
Methods
Study Patients
Characterization of HIV-1 antigenic load and neutralizing activity was performed in 120 HIV-infected patients. These included HIV-1 infected patients with a wide range of viremia, who belonged to one of the following 4 cohorts: 1) HIV-1 Natural Viral Suppressors (NVS) defined as individuals with HIV-1 infection by both Western Blot and proviral DNA, and at least a 2-year period with <400 HIV-1 RNA copies/ml in the absence of highly active antiretroviral therapy (HAART);3,4 2) Low Viral Load (LVL) cohort consists of individuals with 500–20,000 HIV-1 RNA copies/ml in the absence of HAART; 3) Medium/High Viral Load (MHVL) cohort consists of individuals with >20,000 HIV-1 RNA copies/ml in the absence of HAART; and 4) HAART cohort which consists of individuals on their first HAART regimen with suppressed viral loads for at least one year. Demographic characteristics of these cohorts are given in Table 1. Forty eight NVS, 36 LVL, 18 MHVL, and 18 HAART patients were tested. This study has IRB approval, and all individuals provided informed consent.
Table 1.
Demographics of the 4 cohorts. HIV-1 Natural Viral Suppressors (NVS) are HIV-infected individuals with HIV-1 RNA <400 copies/ml in the absence of therapy (n=48).
| Cohort | NVS | LVL | MHVL | HAART |
|---|---|---|---|---|
| Age | 49 (range 29–60) | 43 (range 24–63) | 44 (range 22–58) | 45 (range 23–61) |
| Sex | 49% M 51% F | 59% M 41% F | 61% M 39% F | 78% M 22% F |
| Race | 100% AA | 97% AA 3% Caucasian |
89% AA 11% Caucasian |
95% AA 5% Caucasian |
| Risk factor for IIIV-1 infection | 59% IDU 43% S | 35% IDU 65% S | 52% IDU 48% S | 23% IDU 77% S |
| Median years with diagnosis of HIV-1 | 11 (range 2–21) | 6 (range 0–20) | 3 (range 1–21) | 6 (range 1–14) |
| Median of latest HIV-1 viral load (copies/ml) | <50 (range <40–537) | 1,785 (range 514–19,100) | 202,000 (range 28,721-> 1,000,000) | <50 |
| Median of latest CD4 count (cells/ul) | 817 (range 325–1929) | 632 (range 246–1313) | 77 (range 3–829) | 344 (range 134–644) |
Low Viral Load (LVL) are HIV-1 infected patients who are not treated by antiretrovirals whose HIV-1 RNA are 500–20,000 copies/ml (N=36). Medium-High Viral load (MHVL) are HIV-1 infected persons who are not on antiretroviral therapy whose HIV-1 RNA are >20,000 copies/ml (N=18). The HAART cohort (N=18) is comprised of individuals on their first antiretroviral regimen with suppressed HIV-1 viral loads for greater than one year. M=male, F=female, AA= African-American. IDU= injection drug use, S=sexual.
HIV-1 neutralization testing
HIV-1 neutralization testing was performed using a luciferase-based assay in TZM.bl cells as previously described.5 This assay measures the reduction in luciferase expression following a single round of virus infection. For plasma, starting at a 1:20 dilution, 3-fold serial dilutions of serum were performed in duplicate. 200 TCID50 of pseudovirus was added to each well (with plasma or IgG) and incubated for 1 hour at 37°C. TZM.bl cells were then added (1×104/well) in 10% D-MEM medium containing DEAE-Dextran (Sigma) at a final concentration of 11 μg/ml. Following 48 hours (37°C), 150ul of medium was added to 100ul of Bright-Glo luciferase reagent (Promega, Madison, WI), and luminescence measured using a Victor 3 luminometer (Perkin Elmer, Waltham, MA). Stocks of Env-pseudotyped viruses were prepared by transfection of 293T/17 cells.5 All patient plasma was tested against 3 Tier 1 Clade B pseudoviruses (SF162.LS, BaL.26, SS1196.1), 12 Tier 2 Clade B pseudoviruses (6535.3, QH0692.42, SC422661.8, PVO.4, TRO.11, AC10.0.29, RHPA4259.7, THRO4156.18, REJO4541.67, TRJO4551.58, WITO4160.33, CAAN5342.A2), and MuLV control.
The presence of broadly neutralizing antibodies in plasma was confirmed with purified IgG tested against the above Tier 1 and 2 viruses. IgG was purified by Protein A (GE Healthcare, Piscataway, NJ), and tested in neutralization assays starting at 333ug/ml or greater, with 3-fold serial dilutions. For patients with both plasma and IgG broadly neutralizing antibodies, heterologous clade testing was performed with the following 18 virus panel, depending on sample availability: Clade C (Du156.12, Du172.17, Du422.1, ZM53M.PB12, ZM135M.PL10a, ZM197M.PB7, ZM214M.PL15), Clade A (Q23.17, Q259.d2.17, Q461.e2, Q168.a2, 3415.v1.c1, 0439.v5.c1, 0260.v5.c1, 3365.v2.c20), CRF02_AG (257-31, 263–8, 211–9), and MuLV control.
Statistical analysis and definitions
Inhibitory dose 80 titer (ID80) was defined as the plasma dilution causing 80% reduction in relative luminescence units (RLU) compared to controls. Broadly neutralizing was defined as plasma with ID80 neutralization titers at least twice the MuLV control against 8 or more of the 12 Tier 2 viruses.6 Statistical analysis was performed with Graph Pad Prism (San Francisco, CA) for the Mann-Whitney and Fisher’s exact tests. Logistic regression was done with STATA statistical software (College Station, TX). P values <.05 were considered statistically significant.
Results
Identification of and characteristics of individuals with broadly neutralizing antibodies
Twelve of the 120 individuals demonstrated broadly neutralizing plasma, with the ability to neutralize at least 75% of the Tier 2 viruses. After testing of purified IgG against the 15 Tier 1 and Tier 2 viruses, 10 individuals had plasma neutralization profiles that closely matched their IgG neutralization profiles. Table 2 provides a summary of the plasma Tier 2 testing of the 10 patients with broadly neutralizing antibodies. The other 2 individuals had IgG that had no neutralizing activity against Tier 2 viruses, and in retrospect these 2 individuals had a positive MuLV control during ID50 plasma testing (but not ID80). One of these 2 individuals also had a contemporaneous serum sample tested, which did not show broadly neutralizing activity against HIV-1 (or activity against MuLV). Heterologous virus testing of IgG was done in 6 of 10 individuals with broadly neutralizing antibodies. Results demonstrated broad activity against Clade A, Clade C, and CR0F_AG viruses in all individuals tested. Table 3 provides a summary of the heterologous clade testing.
Table 2.
Plasma ID80 HIV neutralization assay results.
| Pt# | SF162 | BaL. 26 |
SS 1196.1 |
6535.3 | QH0 692.42 |
SC 422661.8 |
PVO.4 | TRO .11 |
AC10.0. 29 |
RHPA 4259.7 |
THRO 4156.18 |
REJO 4541.67 |
TRJO 4551.58 |
WITO 4160.33 |
CAAN 5342.A2 |
MuLV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (NVS) | 8,163 | 1585 | 437 | 1353 | 153 | 294 | 488 | 712 | 1670 | 932 | 347 | 623 | 269 | 33 | 1676 | <20 |
| 2 (NVS) | 1,539 | 515 | 88 | 875 | 38 | 74 | 57 | 211 | 265 | 227 | 28 | 214 | 34 | 20 | 229 | <20 |
| 3 (LVL) | 5,112 | 271 | 561 | 97 | 93 | 64 | 81 | 148 | 32 | 81 | 90 | 115 | <20 | 48 | 44 | <20 |
| 4 (LVL) | 1,652 | 344 | 86 | 124 | 47 | 45 | 24 | 59 | 46 | 54 | <20 | 67 | 24 | <20 | 96 | <20 |
| 5 (LVL) | 4,831 | 201 | 116 | 71 | 57 | 221 | 170 | 307 | 70 | 60 | 117 | 119 | 46 | 60 | 55 | <20 |
| 6 (LVL) | 3,120 | 394 | 245 | 140 | 80 | 131 | 217 | 514 | 177 | 358 | 42 | 128 | 72 | 620 | 72 | <20 |
| 7 (LVL) | 1,296 | 434 | 146 | 79 | 32 | 74 | 92 | 45 | 83 | 135 | <20 | 102 | 22 | <20 | 103 | <20 |
| 8 (LVL) | 4,275 | 812 | 279 | 147 | 110 | 318 | 174 | 429 | <20 | 261 | 45 | 833 | 181 | 272 | 66 | <20 |
| 9 (LVL) | 2,258 | 544 | 194 | 44 | 91 | 128 | 89 | 76 | <20 | 203 | <20 | 196 | 66 | <20 | 34 | <20 |
| 10 (LVL) | 7,771 | 499 | 146 | 149 | 44 | 79 | 114 | 120 | 54 | 57 | 25 | 125 | 23 | 68 | 93 | <20 |
| 11 (Control) | 2,905 | 120 | 51 | 57 | <20 | <20 | <20 | <20 | <20 | 33 | <20 | <20 | <20 | 93 | <20 | <20 |
| 12 (Control) | 521 | 43 | 23 | <20 | <20 | <20 | <20 | <20 | 29 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 13 (Control) | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 55 | <20 | <20 |
| 14 (Control) | 3,574 | 235 | 165 | 77 | 26 | 30 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 15 (Control) | 1,697 | 130 | 88 | 80 | 46 | 45 | 22 | 74 | <20 | 21 | 28 | 49 | <20 | <20 | <20 | <20 |
| 16 (Control) | 1,206 | 164 | 108 | 38 | 33 | 44 | <20 | 31 | <20 | <20 | 22 | 50 | <20 | 28 | <20 | <20 |
| 17 (Control) | 5,400 | 425 | 223 | 52 | 35 | 21 | <20 | 42 | <20 | 35 | <20 | 51 | <20 | <20 | <20 | <20 |
| 18 (Control) | 1,172 | 92 | 23 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 19 (Control) | 236 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 20 (Control) | 4,679 | 530 | 56 | 95 | 22 | <20 | <20 | <20 | <20 | <20 | 30 | 22 | <20 | <20 | <20 | <20 |
Results for 20 of the 120 individuals tested are shown (All 10 with broadly neutralizing antibodies, and 10 patients with non-broadly neutralizing plasma chosen sequentially). The first 3 columns of viruses (SF162, BaL.26, and SSI 196.1 are Tier 1 viruses), the remaining 12 viruses are Tier 2 viruses, with the MuLV control virus in the last column. 1D80 represents the highest concentration at which 80% of the infectious virus was neutralized. Broadly neutralizing plasma was defined as plasma demonstrating neutralization (ID80 titer at least twice the background shown in bold) in at least 8 of 12 Tier 2 HIV pseudoviruses.
Table 3.
Plasma ID80 heterologous HIV neutralization assay results.
| Pt# | Clade C |
Clade C |
Clade C |
Clade C |
Clade C |
Clade C |
Clade A |
Clade A |
Clade A |
Clade A |
Clade A |
Clade A |
CRFO2 _AG |
CRFO2 _AG |
CRFO2 _AG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Du156 .12 |
Du172 .17 |
Du422 .1 |
ZM53M .PB12 |
ZM135 M.PL10a |
ZM197 M.PB7 |
Q23 .17 |
Q259 .d2.17 |
Q461 .e2 |
Q168 .a2 |
3415 .vl.cl |
0439 .v5.cl |
257–31 | 263–8 | 211–9 | |
| 1 (NVS) | 335 | 27 | 213 | 1,022 | 60 | 199 | 1,005 | 775 | <20 | 420 | 344 | 185 | 25 | 136 | 23 |
| 2 (NVS) | 236 | 198 | 226 | 60 | <20 | <20 | 608 | 32 | <20 | 363 | 110 | 44 | <20 | 28 | <20 |
| 3 (LVL) | 26 | 27 | 44 | 36 | <20 | <20 | 88 | 143 | <20 | 68 | 97 | 36 | <20 | 82 | <20 |
| 4 (LVL) | 26 | <20 | 40 | 34 | <20 | 52 | 120 | 35 | <20 | 82 | 93 | 23 | <20 | 126 | <20 |
| 5 (LVL) | 144 | 36 | 32 | 117 | 28 | 26 | 152 | 78 | 34 | 150 | 203 | 67 | <20 | 91 | 50 |
| 6 (LVL) | 167 | <20 | 24 | 31 | 24 | 49 | 124 | 395 | 38 | 545 | 404 | 139 | <20 | 94 | 23 |
| 7 (Control) | <20 | <20 | <20 | <20 | <20 | <20 | 21 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 8 (Control) | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 38 | <20 | <20 | <20 | <20 |
Results for 8 individuals tested are shown (6 with broadly neutralizing plasma and 2 patients with non-broadly neutralizing plasma). Clade A, Clade C, and CRF02_AG viruses were tested. ID80 represents the highest concentration at which 80% of the infectious virus was neutralized. Values in bold show neutralization at least two-fold over the background. MuLV not shown but <20 for all individuals tested.
Overall, 2 of 48 NVS (4.2%), 8 of 34 LVL (22.2%), 0 of 18 MHVL (0%), and 0 of 18 HAART (0%) individuals had broadly neutralizing antibodies. These 10 carried a diagnosis of HIV-1 for a median of 13 years (range 1–22). Their median CD4 count was 589 cells/ul (range 202–927), and HIV-1 viral load of 1,611 copies/ml (range 110–8,964).
Six of these 10 individuals with broadly neutralizing plasma continue to maintain normal CD4 counts without therapy. However HAART was initiated in 4 individuals (three in the LVL group and one in the NVS group). One patient, 14 years after diagnosis, had an increase in HIV-1 RNA from a baseline of 5,000–10,000 copies/ml to close to 30,000 copies/ml with a CD4 loss from 507 to 288 cells/ul in 6 months. Two patients, while maintaining HIV-1 RNA <5,000 copies/ml, had slow but progressive CD4 loss to less than 300 cells/ul requiring HAART therapy. The final patient, although maintaining HIV-1 RNA <200 copies/ml over many years, likewise had a slow CD4 loss over and HAART therapy was initiated at a CD4 count of 489 (CD4% of 18) 19 years after diagnosis of HIV-1.
Correlation of the presence of broadly neutralizing antibodies with viral load
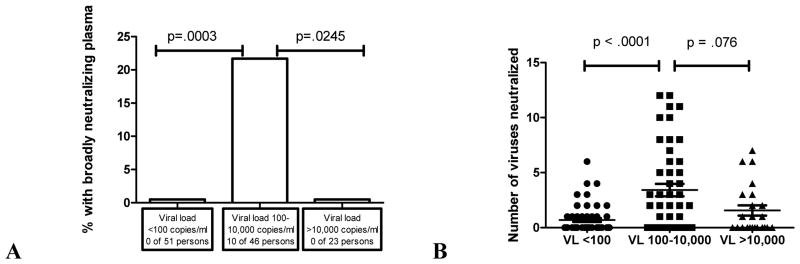
The presence of broadly neutralizing antibodies was analyzed according to cohort as well as HIV-1 viral load. Although all of the patients with broadly neutralizing antibodies were in the NVS and LVL groups, there was no correlation between presence of broadly neutralizing plasma and patient cohort (data not shown). However, there was a significant correlation between plasma viral load and the presence of broadly neutralizing antibodies in those with HIV-1 RNA between 102 and 104 copies/ml compared to those <102 and those with >104 HIV-1 RNA copies/ml (p=.0003 and .0245, respectively) with the two-tailed Fisher’s exact test. In addition, individuals with HIV-1 RNA between 102 and 104 copies/ml had a higher number of Tier 2 viruses neutralized compared to the <102 or >104 copies/ml groups. This reached significance when compared to the <102 group (p=< .0001) and there was a trend toward significance in the >104 group (p=.076) with the two-tailed Mann Whitney test. The correlation of broadly neutralizing antibodies with viral load, and the correlation between the total number of Tier 2 viruses neutralized for the 102–104 compared to the <102 group was significant whether or not the HAART-treated patients were included in the analyses (p=.0018 and p= .0002, respectively). Please refer to Figure 1 for a summary of the above.
Figure 1.
A. Correlation between plasma viral load and the presence of broadly neutralizing antibodies. There was a correlation between plasma viral load and the presence of broadly neutralizing antibodies in those with HIV-1 RNA between 102 and 104 copies/ml compared to those <102 or >104copies/ml (p=.0003 and .0245, respectively). B. Correlation between plasma viral load and number of Tier 2 viruses neutralized. Presence of HIV-1 RNA between 102 and 104 copies/ml correlated with number of Tier 2 viruses neutralized when compared with those <102 (p <.0001) and trended toward significance when compared with those with >104 copies/ml (p=.076). VL= viral load (HIV-1 RNA copies/ml). Broadly neutralizing was defined as plasma with ID80 neutralization activity against 8 or more of the 12 Tier 2 viruses tested. P value <.05 was considered significant (by two-tailed Fisher’s Exact test or two-tailed Mann Whiney test).
In addition, individuals with HIV-1 RNA between 102 and 104 copies/ml had a higher mean ID80 neutralization titer for each of the 15 viruses tested compared to the <102 or >104copies/ml groups. This reached significance for 46.7% of the individual viruses tested when analyzed by single patient neutralization using the two-tailed Mann Whitney test. The above is seen in Table 4.
Table 4.
Mean ID80 HIV-1 neutralization titers for Tier 1 and Tier 2 viruses.
| Patient Viral Load |
SF162 | BaL.26 | SS 1196.1 |
6535.3 | QH0 692.42 |
SC 422661.8 |
PVO.4 | TRO.11 | AC10.0.29 | RHPA 4259.7 |
THRO 4156.18 |
REJO 4541.67 |
TRJO 4551.58 |
W1TO 4160.33 |
CAAN 5342.A2 |
MuLV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 102–104 | 2330 | 270 | 104 | 99 | 37 | 45 | 51 | 81 | 72 | 74 | 33 | 81 | 37 | 51 | 70 | <20 |
| <102 | 950* | 109* | 44* | 29* | 26* | 25* | 26* | 26* | 25 | 28* | 25 | 35* | 23 | 36 | 30 | <20 |
| >104 | 1850 | 156* | 52* | 44 | 27* | 43 | 26 | 31 | 24 | 28 | 29 | 24* | 24 | 30 | 23 | <20 |
The first 3 columns of viruses (SF162, BaL.26, and SSI 196.1) are Tier 1 viruses), the remaining 12 viruses are Tier 2 viruses. Patients were analyzed according to viral load <102, 102–104, or >104 HIV-1 RNA copies/ml. Patients with HIV-1 RNA copies 102–104 had the highest mean titers for each of the 15 viruses tested. This was statistically significant for 46.7% (14 of 30) of the Tier 1 and Tier 2 viruses tested. There was no significant difference between the <102 and >104 groups.
p<.05 by the Mann Whitney test (results given in comparison to the 102–104 group only)
Inhibitory dose 80 titer (ID80) was defined as the plasma dilution causing 80% reduction in relative luminescence units (RLU) compared to controls.
Using logistic regression, there was no relation between presence of broadly neutralizing antibody and age, HIV risk factor, duration of HIV infection, HCV status, or CD4 count (data not shown). There was, however an association with male sex (p=.016), as 10 of 11 persons with broadly neutralizing plasma were male.
Discussion
The data suggests that in HIV-1 infected individuals, the ability to produce broadly neutralizing antibodies correlates with plasma viremia. The LVL group, which was defined as having viral loads between 500 and 20,000 copies/ml, had the most individuals with HIV-1 broadly neutralizing plasma. In addition, there were two individuals in the NVS cohort who had broadly neutralizing activity. Importantly, both of these NVS individuals had low level viremia detectable by routine HIV-1 RNA testing, as opposed to the majority of NVS who consistently have undetectable viremia and do not have broadly neutralizing antibodies. Because of this observation, the data was analyzed according to HIV-1 RNA copies, and the presence of broadly neutralizing antibodies correlated with a viral load of 102 and 104 copies/ml. We did not observe any persons with broadly neutralizing antibodies who had <102 or >104 HIV-1 RNA copies/ml. This suggests that an optimum amount of antigen production is needed for this response, which is less likely to be seen in patients with higher or lower HIV-1 RNA copies.
Patients with <102 HIV-1 RNA copies/ml, whether due to natural control or HAART, demonstrated diminished ID80 neutralization values, an observation made in prior studies.2,7 Likewise, there were no individuals with broadly neutralizing activity in this group. Overall, the low neutralization titers in the NVS and those on HAART suggests that a decrease in antigen load led to a decrease in antibody titer, a situation that has been described in patients after starting HAART.2 The lack of continual high titers in these situations may mirror the poor long-term immunogenicity of gp120 seen in the VaxGen vaccine trial.1 Importantly, a low plasma neutralization titer in an NVS patient does not necessarily mean that they never had a robust humoral response to HIV. As we have shown in the NVS, a patient can harbor a large number of memory B cells that can be stimulated to produce epitope-specific HIV antibodies that can not be found in circulation.8
Several previous studies in LTNPs noted increasing neutralization breadth and titers to heterologous viruses over time.9–11 Importantly, not all studies have shown this.12,13 LTNPs, which were defined by CD4 count only, have wide ranging viral loads, and the combined LVL and NVS groups in this study can be viewed as constituting a cohort similar to LTNPs (though not all would meet the CD4 or time requirements). More recent studies have focused on cohorts of patients, such as ours, that have a definition of HIV suppression based on viral load and not CD4 count.2,7,14,15 In this study 12% of the combined NVS/LVL groups had broadly neutralizing plasma, which is comparable to the 12% reported for a combined cohort of elite and viremic controllers (similar to our definition of NVS and LVL).14 The disparate results in this study between those NVS who have greater or less than 102 HIV-1 RNA copies/ml may account for the discrepancies in prior LTNP studies.
At the high levels of plasma viremia, a diminished humoral response was observed. The ID80 titers in those with >104 HIV-1 RNA copies/ml for the Tier 1 and Tier 2 viruses were lower than those with 102–104 HIV-1 RNA copies/ml but comparable to those with those with <102 HIV-1 RNA copies/ml. Also, there were no persons in this group that demonstrated broadly neutralizing plasma. This data is consistent with the observation of low antibody titers and neutralization breadth seen in the later stages of HIV,10,11,17 which could be due to progressive B cell dysfunction caused by HIV-1.
Recently, some groups have noted increasing neutralization breadth with higher viral loads.16,18–20 These studies have had fewer patients than this study, and most of them have tested fewer viruses than ours. However, it is likely that some of these patients do develop broadly neutralizing antibodies, which are lost as HIV disease progresses. It should be noted that 1 of the 10 patients with broadly neutralizing antibodies in this study (who also eventually received HAART) experienced an increase in HIV-1 viral load to close to 30,000 copies/ml. In this individual we retested the plasma from the time point just before starting HAART, and broadly neutralizing antibody response with a similar neutralization pattern to what was seen from the time point 18 months prior. Thus, the development of broadly neutralizing antibodies in this individual was related to the 13 years his immune system was exposed to HIV at lower viral loads (rather than the six months with higher viral loads), and that without HAART these antibodies could have disappeared with further HIV-mediated immune dysfunction and destruction.
Besides the role of the optimum amount of antigen associated with broadly neutralizing plasma highlighted in this study, duration of infection has also been noted to be important.9,10,16 In this study, the patients with broadly neutralizing antibodies also had been infected with HIV for a relatively long time (a median of 11 years since HIV diagnosis), but we were unable to show an association with time since diagnosis. Furthermore, this can not be considered the sole determinant, as the NVS and LVL had comparable length of HIV infection, but a difference in HIV-1 RNA copies and ability to neutralize various strains of HIV-1. We were unable to find other correlations with broadly neutralizing plasma other than the viral load and male sex. Regarding the later, 90% (9 of 10) of the patients with broadly neutralizing plasma were male; however, the significance of this association remains unknown.
The study’s strengths include the total number of patients tested (120 patients), the high number of Tier 1 and Tier 2 viruses tested (at least 15 viruses per patient). In addition, although we used a definition of broadly neutralizing that has been used previously,6 these were verified with both IgG and heterologous clade testing. Finally, this study was focused on individuals with low viral loads (82 NVS/LVL), where the majority of patients with broad neutralization were found. However, our findings need to be considered within the inherent limitations of a cross-sectional study. In terms of generalizability, it is important to note that the study subjects are predominantly African-American (>95%), Clade B infected, and have relatively high rates of IDU, thus the findings need to be confirmed in other cohorts of patients. In addition, although we have demonstrated a correlation between viral load and broadly neutralizing antibodies, this study was not designed to find causality or a putative mechanism (such as low antigen stimulation leading to low neutralization levels or high viral loads causing B cell dysfunction). Finally, correlations about the potential protective effects of neutralizing antibodies can not be done in this type of study. More prospective studies starting in acute HIV infection, such as the one by Piantadosi et al., should be able to answer this question.18
Conclusion
In conclusion, these results indicate that low but persistent HIV antigen expression correlates with high levels of broad HIV-1 neutralizing antibody activity. The presence of broadly neutralizing activity was associated with 102 and 104 copies of HIV-1 RNA. At higher levels of plasma viremia, neutralization levels were diminished. Conversely, at lower levels, either due to antiretrovirals or natural suppression by other mechanisms, there appears to be insufficient antigen stimulation to maintain high neutralization levels. These findings may have important implications in furthering the understanding of the humoral response to HIV infection.
Acknowledgments
We would like to thank members of the NVS Study and Becky Boyce, RN, the study coordinator.
Sources of funding: Research conducted as part of the Comprehensive Antibody - Vaccine Immune Monitoring Consortium (CA-VIMC) under the Collaboration for AIDS Vaccine Discovery (CAVD) with support from the Bill & Melinda Gates Foundation. M.M.S. supported by award number NIH K12RR023250 (PI: Alan R. Shuldiner) and NIH 1K23AI084580-01A1 (PI: Mohammad M. Sajadi).
Footnotes
Conflict of Interests: The authors (MMS, YG, ALD, MSS, MH, GKL, RRR) have no conflict of interests to report.
References
- 1.Pitisuttithum P, Berman PW, Phonrat B, et al. Phase I/II study of a candidate vaccine designed against the B and E subtypes of HIV-1. J Acquir Immune Defic Syndr. 2004 Sep 1;37(1):1160–5. doi: 10.1097/01.qai.0000136091.72955.4b. Erratum in: J Acquir Immune Defic Syndr 2004 Dec 15;37(5):1669. [DOI] [PubMed] [Google Scholar]
- 2.Bailey JR, Lassen KG, Yang HC, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006 May;80(10):4758–70. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sajadi MM, Heredia A, Le N, Constantine N, Redfield RR. HIV-1 Natural Viral Suppressors: Control of Viral Replication in the Absence of Therapy. AIDS. 2007 Feb 19;21(4):517–9. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 4.Sajadi MM, Constantine NT, Mann DL, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr. 2009 Apr 1;50(4):403–8. doi: 10.1097/QAI.0b013e3181945f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005 Aug;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory b cells in HIV-infected individuals. Nature. 2009 Apr 2;458(7238):636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 7.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008 Feb 15;197(4):563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 8.Guan Y, Sajadi MM, Kamin-Lewis R, et al. Humoral Immunity against Conserved Epitopes of the HIV-1 Envelope Protein Archived in Memory B cells in Natural Viral Suppressors: Discordance with Plasma Antibodies. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):3952–7. [Google Scholar]
- 9.Pilgrim AK, Pantaleo G, Cohen OJ, et al. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997 Oct;176(4):924–32. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 10.Cecilia D, Kleeberger C, Muñoz A, Giorgi JV, Zolla-Pazner S. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J Infect Dis. 1999 Jun;179(6):1365–74. doi: 10.1086/314773. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YJ, Fracasso C, Fiore JR, et al. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J Infect Dis. 1997 Nov;176(5):1180–7. doi: 10.1086/514111. [DOI] [PubMed] [Google Scholar]
- 12.Barker E, Mackewicz CE, Reyes-Terán G, et al. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood. 1998 Nov 1;92(9):3105–14. [PubMed] [Google Scholar]
- 13.Harrer T, Harrer E, Kalams SA, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996 May 1;12(7):585–92. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 14.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory b cells in HIV-infected individuals. Nature. 2009 Apr 2;458(7238):636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 15.Doria-Rose NA, Klein RM, Manion MM, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009 Jan;83(1):188–99. doi: 10.1128/JVI.01583-08. Epub 2008 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009 Jan;83(2):757–69. doi: 10.1128/JVI.02036-08. Epub 2008 Nov 5. Erratum in: J Virol. 2009 May;83(9):4713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong MT, Warren RQ, Anderson SA, et al. Longitudinal analysis of the humoral immune response to human immunodeficiency virus type 1 (HIV-1) gp160 epitopes in rapidly progressing and nonprogressing HIV-1-infected subjects. J Infect Dis. 1993 Dec;168(6):1523–7. doi: 10.1093/infdis/168.6.1523. [DOI] [PubMed] [Google Scholar]
- 18.Piantadosi A, Panteleeff D, Blish CA, et al. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009 Oct;83(19):10269–74. doi: 10.1128/JVI.01149-09. Epub 2009 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG, Schweighardt B, Wrin T, et al. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006 Jun;80(12):6155–64. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez SK, Sarr AD, MacNeil A, et al. Comparison of heterologous neutralizing antibody responses of human immunodeficiency virus type 1 (HIV-1)- and HIV-2-infected Senegalese patients: distinct patterns of breadth and magnitude distinguish HIV-1 and HIV-2 infections. J Virol. 2007 May;81(10):5331–8. doi: 10.1128/JVI.02789-06. Epub 2007 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]