Abstract
c-Jun N-terminal kinase 2 (JNK2) isoforms are transcribed from the jnk2 gene and are highly homologous with jnk1 and jnk3 transcriptional products. JNK proteins mediate cell proliferation, stress response, and migration when activated by a variety of stimuli, including receptor tyrosine kinases (RTKs), but their ability to influence tumor metastasis is ill defined. To evaluate JNK2 in this manner, we used the highly metastatic 4T1.2 mammary tumor cells. Short hairpin RNA expression directed toward JNK2 (shJNK2) decreases tumor cell invasion. In vivo, shJNK2 expression slows tumor growth and inhibits lung metastasis. Subsequent analysis of tumors showed that shJNK2 tumors express lower GRB2-associated binding protein 2 (GAB2). In vitro, knockdown of JNK2 or GAB2 inhibits Akt activation by hepatocyte growth factor (HGF), insulin, and heregulin-1, while phosphorylation of ERK is constitutive and Src dependent. Knockdown of GAB2 phenocopies knockdown of JNK2 in vivo by reducing tumor growth and metastasis, supporting that JNK2 mediates tumor progression by regulating GAB2. The influence of jnk2 in the host or microenvironment was also evaluated using syngeneic jnk2–/– and jnk2+/+ mice. Jnk2–/– mice experience longer survival and less bone and lung metastasis compared to jnk2+/+ mice after intracardiac injection of 4T1.2 cells. GAB2 has previously been shown to mediate osteoclast differentiation, and osteoclasts are critical mediators of tumor-related osteolysis. Thus, studies focusing on the role of JNK2 on osteoclast differentiation were undertaken. ShJNK2 expression impairs osteoclast differentiation, independently of GAB2. Further, shJNK2 4T1.2 cells express less RANKL, a stimulant of osteoclast differentiation. Together, our data support that JNK2 conveys Src/phosphotidylinositol 3-kinase (PI3K) signals important for tumor growth and metastasis by enhancing GAB2 expression. In osteoclast progenitor cells, JNK2 promotes differentiation, which may contribute to the progression of bone metastasis. These studies identify JNK2 as a tumor and host target to inhibit breast cancer growth and metastasis.
Keywords: JNK2, metastasis, GAB2, receptor tyrosine kinases, osteoclast
Introduction
RTKs, such as cMet, IGF-IR (insulin-like growth factor-I receptor), and ErbB1-3, play pivotal roles in breast cancer progression by enhancing tumor cell proliferation, survival, migration, invasion, and metastasis. RTK inhibitors have proven to be highly effective in treating breast cancer, but drug resistance and side effects limit their usefulness. Metastasis is a major contributor to breast cancer–related mortality. A better understanding of RTK signaling in metastasis should open new avenues to enhance the efficacy of or reduce resistance to RTK-targeting anticancer agents.
Ligand activation of RTKs promotes their association with a wide array of proteins to activate downstream signaling pathways. Among these proteins, RTKs bind and tyrosine phosphorylate adaptor proteins, including GAB1-2. Adaptor proteins interact with other signaling proteins to amplify and diversify cellular signaling. GAB proteins are up-regulated in a variety of cancers and impart properties associated with tumor cell proliferation, migration, and metastasis.1-3 Several studies have implicated GAB2 in mammary tumorigenesis and metastasis,4 and it is frequently overexpressed in human breast carcinomas and cell lines.5-7 In the MMTV-Neu model, deletion of gab2 potently inhibits cell migration and lung metastasis.4 A recent report showed that the nonreceptor tyrosine kinase Src constitutively binds to and phosphorylates GAB2,8 augmenting its binding to PI3K and Shp2 to enhance Akt and ERK activity.9,10 In nontumorigenic MCF-10A breast epithelial cells, co-overexpression of Src enhances the oncogenic properties of GAB2 overexpression,11 illustrating the importance of collaboration between Src and GAB2 in tumor progression. Together, these signaling proteins transmit cell survival, proliferative, and invasive properties, among others.
Further downstream, RTKs activate JNK in a Src- and/or PI3K-dependent fashion. Both epidermal growth factor receptor (EGFR) and Kit induce JNK phosphorylation via GAB1 and GAB2.8,12,13 JNKs are also activated downstream of IR and IGF-IR.14,15 Once stimulated, JNKs phosphorylate transcription factors and other proteins, like IRS-1, p53, and paxillin. Despite the high homology among the family of proteins, several models have demonstrated key isoform-specific functions in responses to RTK or oncogenic signaling. For example, jnk1 is required for BCR-ABL–mediated transformation of B lymphoblasts in vitro. Absence of jnk1 also resulted in less leukemic cell infiltration of peripheral organs and delayed mortality.17 Moreover, Hirosumi et al. reported that loss of jnk1, but not jnk2, resulted in less adiposity and weight gain, improved insulin sensitivity, and enhanced insulin receptor signaling in two mouse obesity models.18 In contrast, JNK2 appears to be the primary target for other RTK-mediated responses. JNK2 is phosphorylated in response to EGF (epidermal growth factor) and is inactivated by gefitinib, an EGFR inhibitor.19 Constitutive JNK2 activity is present in glial tumors and correlates with EGFR overexpression.20,21 Specifically, JNK2α2 promotes tumorigenicity of human glioblastoma cells.22 JNK2’s role in cancer is further evidenced by the observation that jnk2–/– mice experience a lower frequency of papillomas after topical application of TPA (12-O-tetradecanoylphorbol-13-acetate).23 These data support JNK2 as a candidate for inhibition to slow tumor progression.
We have recently shown that jnk2 deletion or a reduction of JNK2 expression leads to a decrease in migration of various mammary cancer cells (Mitra et al., submitted). Our studies herein further investigate the role of JNK2 in invasion and metastasis using an aggressive 4T1.2 mammary cancer metastasis model. The 410.4 cell line was isolated from a spontaneous murine mammary tumor and was further modified to produce a thioguanine-resistant variant referred to as the 4T1 subcell line, which itself was further propagated.24 Intracardiac injection of the 4T1.2 cells results in bone and lung metastasis and elevated serum PTHrP (parathyroid hormone–related protein), along with elevated plasma calcium, indicative of osteolytic disease.25 Using these cells, we show that JNK2 is activated by RTKs in a Src- and PI3K-dependent fashion. Reduction of JNK2 expression slows tumor growth and strongly inhibits metastases to the lung and bone. Our data support that JNK2 conveys these effects in response to a variety of RTKs expressed by breast cancer cells by regulating GAB2 expression and its downstream signaling. Moreover, when 4T1.2 cells were injected into the left ventricle, mice with systemic knockout of jnk2 experienced slower tumor progression compared to jnk2 wild-type mice. Subsequent in vitro studies showed that reduction of JNK2 expression diminishes osteoclast differentiation by inhibiting receptor activator of NF-κB ligand (RANKL) expression in tumor cells and decreasing expression of RANK, tartrate-resistant acid phosphatase (TRAP), and cathepsin K expression in osteoclast precursor cells, which may slow the vicious cycle of bone metastasis.
Results
Inhibition or knockdown of JNK2 expression in mammary cancer cells reduces tumor cell invasion
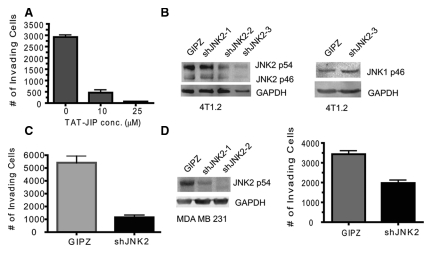
Cancer cell invasion allows tumor cells to escape the microenvironment and is a critical step in the metastatic process. Invasive cells digest and move through extracellular matrix (ECM) in response to chemoattractants. The Boyden chamber assay emulates in vivo invasion by requiring cells to traverse through extracellular matrices in response to growth factors. Given that JNK2 is stimulated in response to RTK activity and because RTKs are critical for tumor invasion, we hypothesized that JNK2 facilitates tumor cell invasion. We used the cell-permeable TAT-JIP (JNK interacting protein-1) fusion peptide to inhibit all JNK isoforms.26 4T1.2 cells were pretreated with TAT-JIP (10 and 25 µM) and then subjected to an in vitro Boyden chamber invasion assay. Figure 1A shows that TAT-JIP significantly reduced the number of invading cells by over 80% in a concentration-dependent fashion (P < 0.0001).
Figure 1.
Inhibition or knockdown of JNK2 in mammary cancer cells reduces cell invasion. (A) 4T1.2 cells were pretreated with TAT-JIP at the indicated concentrations for 45 minutes and then plated into Transwell (BD Falcon, Bedford, MA) inserts containing Matrigel (BD Biosciences). Twenty-four hours later, noninvading cells were swiped from the upper chamber, and cells attached to the lower surface were stained with crystal violet and counted (ANOVA, P < 0.001). (B) 4T1.2 cells were transduced with shJNK2 lentivirus or GIPZ nonsilencing virus and treated with puromycin. The surviving cell population was then lysed and JNK2 and JNK1 expression measured using Western blot analysis. Clone shJNK2-3 was used for further studies. (C) shJNK2 and GIPZ were subjected to invasion assays in the same fashion described in A (2-tailed Student t test, P = 0.0016). (D) Expression of GIPZ and shJNK2 in MDA MB 231 cells was obtained as in B. Cells were then subjected to an invasion assay as described above (2-tailed Student t test, P = 0.0034).
To address the role of JNK2 isoforms in growth factor–mediated invasion, 4T1.2 cells were transduced with GFP-tagged JNK2 shRNA or GIPZ (nonsilencing control) containing lentivirus. ShJNK2 expression led to a >70% decrease in JNK2 p54 and p46 in clone shJNK2-3 (with no significant change in JNK1 expression) compared to GIPZ expressing cells (Fig. 1B). Other researchers recently reported that the 4T1 cells respond to HGF, implicating the expression of cMet in these cells.27 Given HGF’s role in metastasis, we tested if JNK2 mediates HGF-induced tumor cell invasion. Knockdown of JNK2 significantly impaired cell invasion by approximately 80% (P = 0.0016) (Fig. 1C). A similar response was observed when fetal bovine serum (FBS) was used as the chemoattractant (data not shown). Matrix metalloproteases (MMP) 2 and 9 (among others) are well-established extracellular proteases that mediate breast cancer invasion. Hence, we evaluated if MMP 2 or 9 activities are affected by JNK2 expression. However, JNK2 did not alter MMP2 and MMP9 function, as no difference was observed between the cell lines using a gelatin zymography assay (data not shown).
To further extend our findings, knockdown of JNK2 was performed in the same fashion using the human MDA MB 231 breast cancer cells. The invasive potential of cells with >70% knockdown (clone shJNK2-2) was then compared to GIPZ expressing cells. ShJNK2 expression inhibited MDA MB 231 cell invasion through Matrigel (BD Biosciences, Franklin Lakes, NJ) by 42% using HGF as the chemoattractant (P = 0.0034) (Fig. 1D). These data support a role for JNK2 in inducing tumor cell invasion in response to HGF and FBS (data not shown).
ShJNK2 expression inhibits mammary tumor growth rate and decreases lung metastasis
Considering the strong inhibition of cell invasion observed, we then questioned if shJNK2 expression would reduce tumor growth or metastasis. Cells expressing shJNK2 or GIPZ were injected into the mammary fat pad of Balb/c mice. Mice were palpated 3 times weekly, and tumors were measured until they reached a target volume of 1,000 mm3, at which point mice were euthanized.
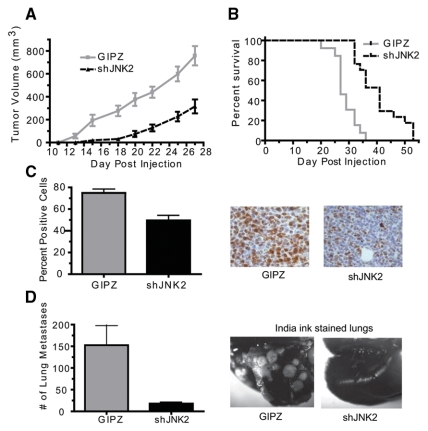
Mice injected with the shJNK2 expressing cells experienced slower tumor growth rates compared to the GIPZ-injected mice (P = 0.0031) (Fig. 2A). The shJNK2 tumor-bearing mice reached the target volume significantly later than those injected with GIPZ cells (41 v. 27 days, respectively; P < 0.0001) (Fig. 2B). Paraffin-embedded tumors were used to measure the proliferation index of cells as marked by Ki-67 expression. A significantly lower fraction of cells stained positive for Ki-67 in shJNK2 tumors compared to GIPZ tumors (52 v. 75%, respectively; P = 0.0028) (Fig. 2C). In vitro proliferation assays showed slightly slower proliferation in shJNK2 (and siJNK, data not shown) expressing cells, indicating that in vivo conditions further augment tumor proliferation differences.
Figure 2.
shJNK2 expression in mammary cancer cells inhibits tumor growth rate and decreases lung metastasis. (A) shJNK2 and GIPZ expressing cells were injected into the mammary fat pad of Balb/c mice. Tumor volume was measured 3 times weekly (Wilcoxon matched pairs test, P = 0.0031). (B) Percentage of survival was determined based on time to reach target tumor volume (Kaplan-Meier survival analysis, P = 0.0001). (C) Immunohistochemical analysis was performed on sections from paraffin-embedded tumors using Ki-67 primary antibody. Positive expressing cells were counted and divided by hematoxylin-stained nuclei (2-tailed Student t test, P = 0.0028). Representative images are provided on the right. (D) Immediately after mice were euthanized, the lungs were perfused with India ink and later destained to visualize unstained metastatic lesions, which were quantified (2-tailed Student t test, P = 0.0011). Examples of India ink–stained lungs are shown.
Metastasis from the orthotopic site encompasses various processes, including cell invasion, extravasation, and intravasation, among others. Using the orthotopic approach, we next evaluated the effect of shJNK2 expression on lung metastasis. When mice were euthanized, intratracheal perfusion was performed with India ink to innumerate lung metastases. Stained lungs from mice bearing shJNK2 tumors showed approximately 85% fewer lung tumor lesions compared to lungs from mice with GIPZ tumors (25 v. 150, respectively; P = 0.0011) (Fig. 2D). These data support an essential role for JNK2 in facilitating both tumor growth and lung metastasis.
ShJNK2 expression inhibits bone metastases and lengthens survival
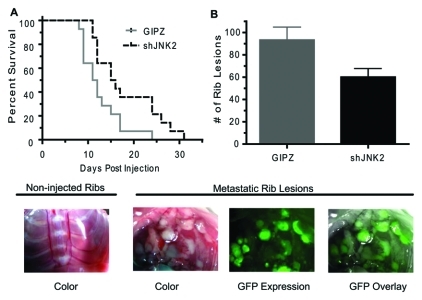
Given the remarkable reduction in lung metastasis observed in the orthotopic tumor model, we then addressed if JNK2 mediates bone metastasis by injecting tumor cells into the left cardiac ventricle of syngeneic mice. Intracardiac cell injection is amenable for studying bone metastasis since tumor cells are directly introduced into the circulation, bypassing intervening steps of metastasis. Mice were monitored daily for target symptoms of morbidity, including but not limited to difficulty breathing, hindered mobility, and ruffled appearance. Symptomatic mice were euthanized. Mice injected with shJNK2 expressing cells experienced significantly longer survival (P = 0.0175) (Fig. 3A) and exhibited 36% fewer metastases to rib bones as evidenced by GFP expression (60 v. 93, respectively; P = 0.0271, unpaired Student t test) (Fig. 3B). These observations strongly support the involvement of JNK2 in breast cancer–related morbidity and bone metastasis.
Figure 3.
shJNK2 expression in tumor cells mediates bone metastasis. (A) shJNK2 or GIPZ 4T.1 cells were intracardiac injected into wild-type mice. Mice were euthanized when they became moribund (Kaplan-Meier, P = 0.0175). (B) After mice were euthanized, rib tumor lesions were counted and confirmed using GFP expression (unpaired Student t test, P = 0.0271). Examples of uninjected ribs versus GFP-positive cancer rib lesions are pictured.
GRB2-associated binding protein 2 (GAB2) expression is reduced in shJNK2 expressing tumors
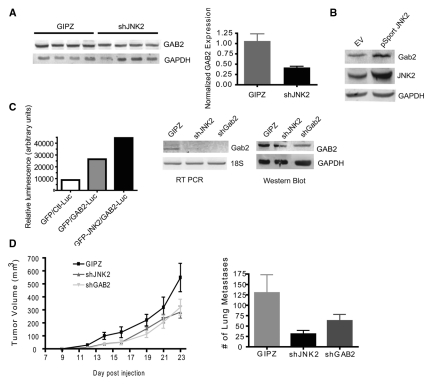
Orthotopic tumors from mice injected with shJNK2 and GIPZ expressing cells were snap-frozen (Fig. 2). RNA was extracted from tumors from each group and reverse transcribed to cDNA for hybridization with NimbleGen mouse microchips (Roche NimbleGen, Madison, WI) to identify potential RTK targets. Microarray analysis revealed a 2-fold reduction in GAB2 mRNA in shJNK2 expressing tumors (data not shown), identifying it as a potential target of JNK2. Subsequently, GAB2 expression differences were verified in tumor lysates at the protein level (P = 0.0269) (Fig. 4A). Figure 4B demonstrates that 2-fold overexpression of human JNK2 in 4T1.2 cells increases GAB2 expression by 2.4-fold, further indicating that GAB2 expression is mediated by JNK2.
Figure 4.
shGAB2 expression inhibits tumor growth and metastasis. (A) Tumor lysates were evaluated for GAB2 expression using Western blot analysis and normalized to the respective GAPDH levels. (B) Parental 4T1.2 cells were transfected with human JNK2-containing plasmid or empty vector. Cell lysates were then evaluated for JNK2 and GAB2 protein expression using GAPDH as a loading control. (C) Jnk2–/– mammary tumor cells expressing GFP or GFP-JNK2 were transfected with a control luciferase (Ctl-Luc) or a Gab2 promoter luciferase (GAB2-Luc) plasmid along with β-galactosidase. Luciferase absorbances were normalized to β-gal absorbances. 4T1.2 cells were transduced with shGAB2 lentivirus and exposed to puromycin. GAB2 expression was compared among GIPZ, shJNK2, and shGAB2 cell populations using Western blot analysis. GAB2 mRNA expression levels were also measured using RT PCR. (D) GIPZ, shJNK2, or shGAB2 expressing cells were injected into the mammary gland of wild-type mice. Tumors were palpated 3 times weekly until reaching target tumor volume, at which time mice were euthanized (post hoc Student t test, shGAB2 v. GIPZ, P = 0.0167). Lungs were then perfused with India ink, and lung tumor lesions were assessed as described above.
To then investigate if GAB2 expression was affected at the transcriptional level, the activity of the GAB2 luciferase promoter was compared to a control promoter containing a minimal sequence.28 Each luciferase plasmid was cotransfected with a β-galactosidase plasmid into a JNK2 knockout mammary tumor cell line stably expressing GFP or GFP-JNK2.29 Figure 4C shows higher activity of the GAB2 promoter when JNK2 is expressed compared to the GFP expressing control cells. To confirm this transcriptional effect on GAB2, RT PCR was performed using GAB2-specific primers. 4T1.2 cells expressing GIPZ and shGAB2 (>70% knockdown achieved) were used as positive and negative controls for GAB2 expression. Figure 4C shows that the shJNK2 expressing cells contain less GAB2 mRNA compared to GIPZ controls, and this change corresponds to a reduction in GAB2 protein.
Given that both JNK1 and JNK2 can share substrates and show redundant or compensatory effects, we next evaluated if JNK1 influences GAB2 expression. To this end, JNK1 expression was inhibited by >70% using the same approach as JNK2 (Suppl. Fig. S1A). Expression of the shJNK1 showed no effect on JNK2 expression. Also, shJNK1 expressing cells had very similar GAB2 expression as the GIPZ control cells. A well-known JNK substrate is cJun. JNK phosphorylates cJun, which in turn induces AP-1 transcription of target genes. To first explore if JNK1 or JNK2 differs in their ability to phosphorylate cJun, we stimulated GIPZ, shJNK1, and shJNK2 cells with serum and evaluated cJun phosphorylation. Serum treatment enhanced cJun phosphorylation in GIPZ cells, whereas both shJNK1 and shJNK2 cells showed partial reduction of phosphorylated cJun (Suppl. Fig. S1B). These data are consistent with the ability of either JNK1 or JNK2 to phosphorylate cJun. We then addressed whether JNKs’ phosphorylation of cJun affects GAB2 expression. To test this question, 4T1.2 cells were transfected with sicJun, which led to a 60% reduction in its expression. However, no significant change in GAB2 expression was observed. Therefore, we conclude that JNK2 regulates GAB2 expression in a cJun-independent fashion.
Knockdown of GAB2 decreases tumor growth rate and lung metastasis
Several studies have recently reported the central role of GAB2 in mammary tumorigenesis including its ability to convey ErbB2- and Src-mediated responses. Furthermore, the gab2 gene is located in close proximity to the cyclin D gene, which is a common amplicon observed in human breast tumors.2 To test if JNK2 conveys its effects on tumor growth and metastasis through GAB2 in our model, GAB2 was silenced by over 70% in the 4T1.2 cell line using shRNA lentiviral vectors (Fig. 4C). ShGAB2, shJNK2, and GIPZ expressing cells were then injected into the mammary fat pad and monitored as described above. Tumors from mice injected with the shGAB2 cells grew more slowly compared to GIPZ tumors (P = 0.0167) (Fig. 4D), similar to the shJNK2 tumors. India ink staining of lungs also revealed fewer lung metastases in both shJNK2 and shGAB2 tumor-bearing mice compared to GIPZ controls (averaging 31, 63, and 130 per mouse, respectively). ShJNK2 groups reached statistical significance (P = 0.025), but shGAB2 did not (P = 0.226) (Fig. 4D). These data suggest that JNK2 affects metastasis, in part, through GAB2.
The roles of Src and PI3K in growth factor–induced JNK
Our overall goal was to evaluate the role of JNK2 in mammary tumor growth and metastasis. GAB2 was of particular interest because of its established importance in breast cancer and because it transduces RTK signaling pathways. Thus, we hypothesized that JNK2 mediates RTK signaling by affecting the expression of GAB2. To better understand how JNK2 influences RTK-mediated tumor growth and metastasis, we first tested the ability of various growth factors to induce phosphorylation of Akt and ERK (pAkt and pERK, respectively). Parental 4T1.2 cells were serum starved and then treated with 10% FBS, HGF, EGF, or insulin (at supraphysiological concentrations to activate both IR and IGF-IR homodimers and heterodimers). Figure 5A shows that FBS induced phosphorylation of both Akt and ERK. HGF treatment also increased pAkt, while insulin induced the most robust and sustained increase in pAkt. Conversely, EGF treatment did not enhance Akt phosphorylation, consistent with our inability to detect EGFR expression in these cells (data not shown). Other than FBS, none of the growth factors tested further increased ERK phosphorylation. These data show that the 4T1.2 cells respond to a variety of ligands important for human breast cancer through activation of the PI3K/Akt pathway, further supporting the relevance of this model to human breast cancer.
Figure 5.
Importance of Src and PI3K in growth factor–induced 4T1.2 cells. (A) Subconfluent cells were serum starved and later treated with either 10% FBS, 40 ng/mL HGF, 100 ng/mL EGF, 5 µg/mL insulin, as indicated. Cells were harvested at the indicated time points, and cell lysates were evaluated for phosphorylated Akt and ERK. (B) Cells were treated as described in A, except they were preincubated with kinase inhibitors for 45 minutes prior to addition of 10% FBS. Cell lysates were used in JNK activity assays, with GST-cJun1-79 as substrate, or in Western blots to evaluate pAkt and pERK. (C) Cells were transfected with siSrc or siJNK. Twenty-four hours later, cells were serum starved overnight and then treated with 10% FBS. Lysates were used in JNK activity assays and Western blot analysis to evaluate Src and JNK2 expression. GAPDH was used to evaluate even loading of samples. (D) The effect of Src and PI3K on 4T1.2 cell viability was evaluated using the MTT assay by inhibiting Src activity with PP2 (10 µM) and LY 294002 (LY, 20 µM).
We then evaluated if FBS stimulation increases JNK activity and, if so, through which upstream kinases. Cells were pretreated with inhibitors of SFKs (Src family kinases, PP2), PI3K (LY 294002), or MEK1 (U0126) for 30 minutes prior to FBS stimulation. Figure 5B shows that FBS induced JNK activity approximately 10-fold, and pretreatment with either PP2 or LY partially inhibited its response. Surprisingly, inhibition of MEK1 led to a robust increase in JNK activity. To confirm that each compound inhibited its expected target, the same lysates were subjected to Western blot analysis of pAkt and pERK. The PP2 inhibited FBS induction of both pAkt and pERK. LY inhibited pAkt and showed a slight increase in pERK. U0126 inhibited pERK, as expected, but induced a strong increase in pAkt. We then used PD980059 (PD) to further evaluate the mechanism by which MEK1 inhibition resulted in increased cJun phosphorylation. To this end, 4T1.2 cells were pretreated with DMSO alone, each inhibitor alone, or PD in combination with either LY or PP2. Figure 5B shows that like U0126, PD also increased serum-induced cJun phosphorylation, but inhibition of either Src or PI3K reverses JNK activation by FBS and PD treatment. Taken together, these data maintain that JNK activity is induced by FBS in a Src- and PI3K-dependent manner in the 4T1.2 mammary tumor cells. Currently, the manner by which MEK1 inhibition increases JNK activity is unknown, but the response is Src and PI3K dependent. Given that both Src and PI3K are upstream of MEK1, this response may be mediated by inhibiting ERKs’ negative feedback of RTKs or adaptor proteins.
PP2 inhibits SFKs but may also affect the activity of other kinases.30 Therefore, we evaluated if reduction of Src expression would mediate a similar response in JNK activity. To this end, 4T1.2 cells were transfected with siRNA directed toward Src (siSrc), which inhibited Src expression by >80% (Fig. 5C). The same cell lysates were used in a JNK activity assay, which showed that siSrc inhibited FBS activation of JNK. Next, the contribution of JNK2 in FBS-induced JNK activity was assessed. Transfection of cells with siJNK inhibited JNK2 p46 and the p54 expression by 80%. SiJNK similarly inhibited JNK1 expression. SiJNK expressing cells experienced a significant reduction in FBS-induced cJun phosphorylation. These data maintain that serum-induced JNK activity is mediated by Src in 4T1.2 cells.
Given the major roles of Src and PI3K in many tumor models, we determined the importance of Src and PI3K in 4T1.2 viability. Subconfluent cells were grown in medium containing 10% FBS and vehicle (DMSO), PP2, or LY for up to 3 days. 4T1.2 cell proliferation was completely blocked by PP2 (Fig. 5D). LY treatment showed a weaker effect on cell proliferation by inhibiting cell viability around 40%, but the cells clearly appeared more flattened, resembling the morphology of the shJNK2 cells (data not shown). These data indicate that Src and PI3K, to a lesser degree, are important for cell proliferation and survival.
Expression of shJNK2 or shGAB2 impairs growth factor–induced pAkt
Data shown thus far suggest that JNK2 mediates many of its responses through regulating the expression of GAB2. If this is the case, then knockdown of JNK2 should result in similar effects as GAB2 knockdown, especially in response to RTK activation. To test this hypothesis, GIPZ, shJNK2, and shGAB2 expressing cells were serum starved, then stimulated with HGF and harvested in a time-dependent fashion. HGF treatment induced pAkt almost 18-fold in the GIPZ cells, but minimal phosphorylation (2- to 2.5-fold) was observed in the shJNK2 and shGAB2 cells (Fig. 6A). This observation is consistent with the ability of JNK2 knockdown to reduce GAB2 expression and subsequent downstream signaling responses. As previously noted in Figure 5A, ERK was constitutively phosphorylated in all samples, and no further increase was seen with HGF treatment. Consistent with this observation, Src was also constitutively phosphorylated on Tyr418 (Fig. 6A), supporting that the Src/Raf/ERK pathway is constitutively active. Reduction of JNK2 or GAB2 expression inhibits HGF-induced phosphorylation of Akt, but not total Akt1 expression (data not shown), suggesting that HGF enhances cell signaling in a GAB2/PI3K/JNK-dependent manner.
Figure 6.
Both shJNK2 and shGAB2 impair growth factor–mediated Akt phosphorylation. GIPZ, shJNK2, and shGAB2 cells were serum starved and then treated with the indicated growth factors. (A) Cells were treated with HGF at indicated time points. Later, lysates were evaluated for pSrc, pERK, and pAkt. GAPDH was used to compare protein loading among samples. (B) Experiments were done as described in A, except cells were treated with Her1. (C) Experiments were done as described in A, except cells were treated with insulin. (D) Effect of Src inhibition on GAB2 tyrosine phosphorylation was tested by immunoprecipitating GAB2 from cell lysates. Western blot analysis was performed using phosphotyrosine and GAB2 primary antibodies. (E) Effects of HGF, Her1, and insulin treatment on GAB2 tyrosine phosphorylation and Src binding to GAB2 were assessed by immunoprecipitation of GAB2 and Western blot analysis using phosphotyrosine, GAB2, and Src primary antibodies.
In addition to HGF/cMet signaling, GAB2 is known to transduce ErbB and Kit responses. Also, a report has described GAB1 phosphorylation in response to insulin,31 but IR and IGF-IR are better known to signal through IRS adaptor proteins. In order to specifically address if knockdown of GAB2 recapitulates JNK2 knockdown in other RTK-induced responses, we exposed GIPZ, shJNK2, and shGAB2 cells to heregulin-1 (Her1, to activate ErbB2/ErbB3 heterodimers) or insulin and then measured pAkt and pERK. Figure 6B and 6C show that like HGF, Her1 and insulin induced pAkt 8- to 48-fold, respectively, but not pERK (data not shown). ShJNK2 expressing cells revealed significant reductions in Her1- and insulin-induced pAkt, whereas pERK was unaltered (Fig. 6B and 6C). Similarly, shGAB2 expressing cells also showed less Akt phosphorylation after Her1 and insulin treatment compared to the GIPZ cells. Together, these data show that reduction of JNK2 or GAB2 inhibits RTK-mediated Akt phosphorylation.
RTK phosphorylation of GAB proteins is critical for recruitment of upstream proteins like PI3K, Grb2, and Src. Previous studies have made observations that GAB2 constitutively binds to Src and that Src tyrosine phosphorylates GAB2.8 To address if Src activity regulates GAB2 tyrosine phosphorylation, 4T1.2 cells were serum starved, and samples were treated with or without PP2 prior to stimulation with FBS. Tyrosine phosphorylation of immunoprecipitated GAB2 was present in the unstimulated (SFM) sample. FBS treatment further increased phosphorylation of GAB2, but this response was not significantly inhibited by PP2 (Fig. 6D). Moreover, we did not observe Src binding to GAB2 in response to FBS.
Others have reported that GAB1, rather than GAB2, mediates HGF/cMet downstream signaling.32 Therefore, we wanted to compare GAB2 tyrosine phosphorylation in response to various growth factors in our model. Serum-starved cells were treated with HGF, Her1, or insulin, and then GAB2 was immunoprecipitated from cell lysates. Surprisingly, HGF and insulin treatments increased GAB2 tyrosine phosphorylation, but Her1 did not (Fig. 6E). Instead, GAB2 showed a mobility shift with Her1 treatment, consistent with another type of posttranslational change, such as serine or threonine phosphorylation. Also of note, Src was only bound to GAB2 in Her1-treated samples. These results differ from a previous publication that reported constitutive binding between Src and GAB2.8 Taken together, we show that GAB2’s ability to transduce RTK signals does not require Src binding or activity. RTK activation by heregulin 1 was the exception in which Src binding to GAB2 was observed but in the absence of GAB2 tyrosine phosphorylation. These studies provide important insights to the regulation of GAB2 function in mammary cancer cells and show that GAB2 function differs in an RTK-dependent fashion.
Systemic jnk2 knockout inhibits metastasis
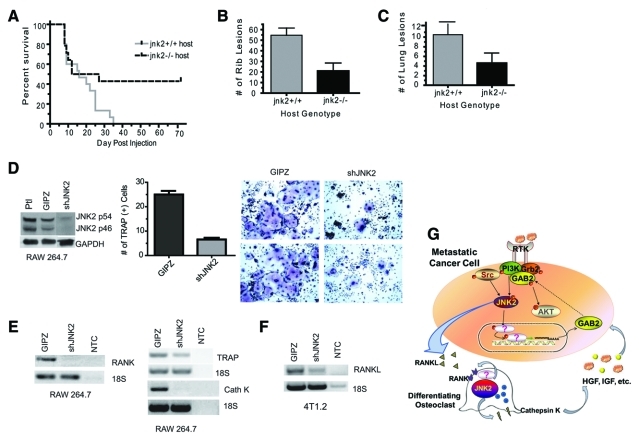
We next hypothesized that JNK2 may also increase bone metastasis at the site of secondary colonization and not only in the early stages of extravasation. The parental 4T1 cell line was originally derived from a spontaneous mouse mammary tumor in a female Balb/c mouse. This allowed us the opportunity to use syngeneic jnk2–/– Balb/c mice to evaluate the contribution of host- or microenvironment-related factors in bone metastasis. For these studies, intracardiac injection of GIPZ expressing 4T1.2 cells was performed in jnk2+/+ and jnk2–/– mice. Jnk2+/+ mice experienced target symptoms significantly earlier than jnk2–/– mice. By day 70 after injection, 43% of jnk2–/– mice remained asymptomatic but were euthanized to evaluate signs of tumor growth (P = 0.0459) (Fig. 7A). When mice were euthanized, lungs were stained with India ink, and ribs were evaluated for GFP expressing bone lesions (due to their ease of visibility and quantification). Metastatic GFP-positive rib lesions were confirmed (and validated with H&E staining) in jnk2–/– mice, showing that cells were successfully injected in both symptomatic and asymptomatic mice. Jnk2+/+ mice experienced 2.6- and 2.2-fold more lung and bone metastases, respectively (P = 0.0043 and P = 0.0224, respectively) (Fig. 7B and 7C). Repeated experiments yielded similar results, showing that loss of jnk2 expression in the host also inhibits metastasis.
Figure 7.
Systemic jnk2 deletion inhibits tumor metastasis, and shJNK2 expression inhibits osteoclast differentiation. (A) 4T1.2 GIPZ expressing cells were injected into either wild-type (jnk2+/+) or jnk2–/– mice in the same fashion as described in Figure 3A (Kaplan-Meier survival analysis, P = 0.0459). (B) Upon harvest, rib tumor lesions were counted and confirmed using GFP expression (Mann-Whitney test, P = 0.0043). (C) Lungs were perfused with India ink and later destained to visualize unstained metastatic lesions (Mann-Whitney test, P = 0.0224). (D) RAW 264.7 cells were infected with viral media collected from packaging cells transduced with shJNK2 or GIPZ lentiviral constructs. Stable cell lines were obtained using puromycin. Knockdown was assessed using Western blot analysis. shJNK2 and GIPZ RAW 264.7 cells were treated with recombinant RANKL (35 ng/mL) for 4 days and then assayed for TRAP staining. TRAP+ cells were quantified and compared (Mann-Whitney test, P = 0.0079). (E) mRNA from GIPZ or shJNK2 expressing RAW 264.7 cells was reverse transcribed to cDNA, and RANK, TRAP, and cathepsin K were amplified using specific primers, as indicated. (F) mRNA from GIPZ and shJNK2 expressing 4T1.2 cells was reverse transcribed, and RANKL was then amplified using specific primers. The negative control included no template (NTC). (G) In mammary tumor cells, JNK2 enhances GAB2 expression. In turn, increased GAB2 enhances RTK induction of Akt and metastasis. Constitutively activate Src sustains ERK activity and transduces growth factor activation of JNK. Metastatic tumor cells metastasize to the bone and enhance osteoclast differentiation by secreting factors such as RANKL. RANKL stimulates RANK in osteoclast progenitor cells to promote differentiation. In osteoclast progenitor cells, JNK2 enhances RANK expression to differentiation and osteolysis through cathepsin K secretion, among other factors. Thus, JNK2 plays dual roles in the cancer cell and the osteoclast to perpetuate tumor progression and bone metastasis.
Bone metastasis occurs commonly in patients with late-stage breast cancer. The bone matrix is a rich source of growth factors, which upon release from the ECM enhance survival and colonization of metastatic tumor cells. However, multiple studies from other investigators indicate that breast cancer cells cannot directly resorb bone.33,34 Metastatic breast cancer cells rely on the resorptive activity of osteoclasts to degrade mineralized bone and release growth factors in their vicinity.35 While exploring the signaling pathways important for invasion and metastasis in our mammary tumor model, we noticed several parallels with the signaling pathways important for differentiation of osteoclasts. These models have shown that RANK/GAB2/JNK pathways are integral for osteoclast differentiation.36 The RANKL/RANK signaling pathway has been characterized in which RANKL is secreted by osteoblasts to induce osteoclast differentiation and activation. RANKL binds and activates RANK (expressed on preosteoclasts), which recruits TRAF6 and GAB2 adaptors to subsequently activate JNK and lead to osteoclast differentiation.36,37
To examine if JNK2 isoforms are essential for osteoclast differentiation, JNK2 was knocked down by 81% in the preosteoclast, monocytic RAW 264.7 cell line (Fig. 7D). As a means to quantify osteoclast formation, multiple assays have been developed to assess TRAP expression as an indicator of osteoclast activity. Osteoclasts secrete TRAP during resorption, thus making TRAP positivity a simple way to verify functionality of an osteoclast. ShJNK2 and GIPZ expressing RAW 264.7 cells were differentiated with RANKL (35 ng/mL) and assayed for TRAP positivity on day 4. Multinucleated cells expressing positive purple stain were counted and compared between groups. JNK2 knockdown in RAW cells led to 72% fewer TRAP-positive differentiated osteoclasts compared to RAW GIPZ cells (n = 5 fields per group, P = 0.0004) (Fig. 7D). Representative images provided in Figure 7D reveal that osteoclasts derived from RAW shJNK2 cells appear morphologically similar to GIPZ expressing control cells. However, RAW shJNK2–derived osteoclasts were smaller in size in comparison to RAW GIPZ–derived osteoclasts.
The current model of the “vicious cycle” of bone metastasis proposes that metastatic tumor cells secrete PTHrP and/or IL-11, which in turn stimulate osteoblasts to synthesize and release RANK ligand to activate RANK on osteoclast precursors. In addition to its effect on differentiation, RANKL enhances osteoclastic bone turnover. As a result, cathepsin K release by osteoclasts further contributes to osteolysis.38,39 While expression of GAB2 did not differ (data not shown), shJNK2 expression in RAW cells was associated with a reduction of RANK expression (Fig. 7E). Reduced activity of RANK likely contributes to the lower TRAP mRNA expression (Fig. 7E) and number of TRAP-positive cells that we observed. Cathepsin K expression was also lower in shJNK2 expressing cells. These data indicate that reduction of JNK2 diminishes osteoclast differentiation along with expression of genes induced during osteoclastic activation.
Lastly, we tested whether secretion of RANKL could be affected in shJNK2 4T1.2 cells. Figure 7F shows that inhibition of JNK2 expression in the 4T1.2 tumor cells decreases RANKL mRNA expression compared to GIPZ cells. Collectively, these data show that shJNK2 expression in tumor cells reduces the synthesis of factors that are attributed to osteoclast differentiation and activation. However, conditioned medium from 4T1.2 shJNK2 or GIPZ cells was insufficient to differentiate RAW cells (data not shown), indicating that RANKL secretion by tumor cells may contribute to osteoclast differentiation, but other sources of RANKL (i.e., from osteoblasts) are necessary for differentiation. In summary, this model supports that JNK2’s primary role for inducing osteoclast differentiation is by regulating RANK expression.
Discussion
Tumor progression is characterized by continued tumor growth and invasiveness. Tumor seeding in distant environments produces macrometastases in which prosurvival factors contribute to metastatic growth. RTK signaling is integral to the metastatic process by enhancing tumor cell invasion along with proliferation and angiogenesis of metastatic lesions. Overt bone and lung metastasis occurs frequently in late-stage breast cancer. With bone metastasis, tumor cells secrete factors that ultimately stimulate osteoclast differentiation and activation. Tumor-associated osteolysis releases growth factors such as IGF-I and HGF from the bone matrix, further stimulating tumor cell proliferation and invasion through activation of tumor-associated RTKs (Fig. 7G). We report herein that impaired RTK signaling (as a result of JNK2 loss) leads to hindered tumor progression and a better outcome in tumor-burdened mice.
JNK2 mediates important functions during tumor development and progression. Ras-induced transformation is severely impaired in jnk2–/– murine embryonic fibroblasts (MEFs), deeming JNK2 essential for oncogenic transformation of normal cells. Subcutaneous injection of the jnk2–/– MEFs resulted in delayed tumor palpation.40 However, the in vivo significance of jnk genes in Ras-mediated transformation was refuted using compound jnk2–/–;jnk1–/– MEFs.41 Using the Polyoma Middle T Antigen tumor model, we observed higher tumor multiplicity but lower tumor proliferation indices in transgenic jnk2–/– mice compared to jnk2+/+ counterparts. JNK2 facilitates cell cycle progression by regulating both p53 phosphorylation and p21Cip1 expression.29,42 Herein, we demonstrate that knockdown of JNK2 expression in highly metastatic mammary tumor cells significantly reduces mammary tumor growth. More remarkable is the powerful reduction in lung and bone metastasis seen with the shJNK2 expressing cells. Microarray analysis of shJNK2 expressing tumors showed a 2-fold reduction in GAB2 expression. In vitro exposure of cells to a variety of growth factors showed similar inhibitory responses between shJNK2 and shGAB2 expression, consistent with the hypothesis that shJNK2, at least in part, affects RTK signaling by inhibiting GAB2 expression. In vivo tumor growth and metastasis data further support this conclusion. JNK2 activation by growth factors occurs in a Src- and PI3K-dependent fashion, both of which bind to GAB2 and convey GAB2-related phenotypes. JNKs’ ability to influence cellular signaling has been reported with other adaptor proteins including IRS1, JIP1 and 3, ShcA, 14-3-3 isoforms, and now GAB2.16 Our findings support that JNK2 also influences RTK signaling by transducing signals, which affect transcription of RTKs and adaptor proteins to facilitate tumor metastasis.
Recent work has supported an important role for GAB2 in breast cancer progression. GAB2 is amplified in a subset of human breast cancers.2 GAB2 overexpression occurs early in breast cancer development43 and is a prognostic indicator for metastatic relapse.5 RTKs such as Kit, cMet, and ErbBs transmit signals through GAB2 to induce PI3K/Akt and Shp/ERK signaling in a variety of different cell types.3 Others have reported that GAB2 cooperates with Src to enhance proliferation and abnormal acinar morphology in human, normal mammary MCF10A cells. In this particular model, Src-dependent invasive responses require PI3K.11 GAB2 expression is also up-regulated by estrogen in breast cancer cells.7 Conversely, the absence of GAB2 strongly inhibits lung metastasis of ErbB2 (Neu)–induced mouse mammary tumors. Our studies show that GAB2 conveys growth factor responses in mammary cancer cells independent of Src binding or phosphorylation. These studies also characterize an important signaling pathway by which tumor cells respond to ligand-induced RTKs through GAB2, Src, PI3K, and JNK2. However, further studies are imperative to better elucidate GAB2 interactions with various RTKs in the presence or absence of activated Src.
Downstream of RTKs, Src functions as an important mediator of growth factor pathways. SFKs are often constitutively active in cancers and can induce tyrosine phosphorylation of RTKs in the absence of ligand. SFKs also mediate cross-talk between RTKs and integrins.44 In this particular model, we noted that Src and ERK phosphorylation were constitutive. Pharmacological inhibition of Src reduced both ERK and Akt phosphorylation and resulted in a potent and rapid reduction on 4T1.2 cell viability. This observation led us to hypothesize that reduction of JNK2 expression either preferentially inhibits Src/PI3K-mediated effects by reducing GAB2 expression and/or that JNK2 functions downstream of Src/PI3K. Activated Src has a well-established role in tumor cell proliferation, migration, and metastasis,44 which may explain the aggressive nature of these tumor cells. However, we did not observe constitutive binding of Src to GAB2. Zhang et al. recently reported the importance of Src in bone metastasis, establishing it as an indicator of poor prognosis and late-onset bone metastasis in breast cancer patients.45 Thus, proteins that mediate Src responses (such as JNK2) may provide new targets for cancer therapy.
While tumor cells frequently overexpress RTKs to enhance survival, proliferation, and metastasis, they also secrete growth factors to influence nontumor cells in their microenvironment to support their continued growth. Using the 4T1.2 model, we were able to evaluate whether systemic knockout of jnk2 in Balb/c mice would influence tumor metastasis. In this model, we observed that systemic jnk2 knockout conveyed a protective effect on tumor progression. Thus, it is feasible that Src may also convey its metastatic responses via GAB2 and JNK2 in the microenvironment. While the specific mechanism for this effect has not been elucidated, it is interesting to note that GAB2 signals through JNK to mediate osteoclast/osteoblast functions in response to RANKL.36 Thus, inhibition or deletion of either of these genes may interfere with tumor cells’ ability to stimulate osteolysis through RANKL/RANK signaling. However, in contrast to our observation in mammary tumor cells, we did not observe a reduction in GAB2 when JNK2 expression was inhibited. Instead, shJNK2 expressing osteoclast precursors showed reduced expression of RANK and markers related to osteoclast differentiation or activation, including TRAP and cathepsin K. In other studies, Gab2–/– mice experienced less mammary tumor metastasis compared to their wild-type controls using the MMTV-Neu transgenic mouse model. GAB2 deficiency also inhibits osteoclastogenesis.36 Thus, the antimetastatic effect of GAB2 deficiency may be mediated either through tumor or host factors.
Mammary epithelial cell and osteoclast differentiation share several common signaling networks that are integral to their differentiation, such as Src, JNK, GAB2, and RANK. Microarray data sets using basal-like B breast cancer cell lines (those expressing several mammary stem cell [MaSC]–related genes) were recently shown to have up-regulated GAB2 signaling, along with EMT, EGF signaling, and down-regulated hTERT.46 Two groups recently reported that progesterone and estrogen induce hormone-sensitive luminal cells to secrete RANKL, which activates RANK, expressed on MaSCs, to increase proliferation of MaSC populations during puberty, diestrus, and pregnancy.47,48 Thus, it is not unexpected that tumor cells may usurp these signaling mechanisms in their microenvironment to sustain their growth. It would be interesting to evaluate if JNK2 regulates RANK expression in mammary stem cells. Lastly, src–/– mice suffer from several associated phenotypes including both mammary and bone abnormalities.49,50 The similarities in cell signaling pathways between tumor cells and osteoclasts suggest that strategically targeting these pathways could afford added benefit at treating or preventing tumor progression.
RTKs have emerged as popular therapeutic targets for cancer treatment in recent years. Drugs targeting ErbB2 activity have provided major improvements in patient outcomes. Unfortunately, resistance to these targeted treatments may occur through up-regulation of Met, ErbB3, or IGF-IR activity, among other mechanisms.51-55 Our studies propose JNK2 as a promising target to reduce breast cancer–mediated lung and bone metastasis. Inhibition of JNK2 expression in the highly metastatic 4T1.2 mammary cancer cells impaired HGF-mediated invasion in vitro and reduced the incidence of metastasis and prolonged survival in tumor-bearing Balb/c mice. In vitro studies further support that reduction in GAB2 or JNK2 impedes downstream signaling of a variety of RTKs important to breast cancer pathogenesis, namely, ErbB2/3, IGF-IR, IR, and cMet. Other researchers have previously reported that the oncogenic and proliferative function of GAB2 relies on GAB2’s binding to and phosphorylation by Src. Herein, we show that the 4T1.2 cells contain constitutively active Src but that GAB2 transduces RTK signals independently of its binding to Src or Src activity. RTK activation by heregulin 1 was an exception in which Src binding to GAB2 was observed in the absence of GAB2 tyrosine phosphorylation. These studies provide important insights to GAB2 function in mammary cancer and show that GAB2 function differs in an RTK-dependent fashion. While the change in GAB2 expression by JNK2 was not potent, it was repeatable using JNK2 overexpression. Further, the reduction of GAB2 expression either through shJNK2 or shGAB2 expression resulted in strong inhibition of Akt phosphorylation in response to several growth factors and reduced lung metastasis, supporting that there was enough change to show a biological effect.
JNK2’s integral role in RTK signaling pathways occurs both in mammary tumor cells as well as in osteoclast differentiation and function, where RANK signaling is enhanced in the latter example. Another aspect supporting JNK2 as an attractive therapeutic target is that jnk2–/– mice are overtly healthy, predicting that toxicities related to its inhibition may be milder than other kinase inhibitors. However, further studies are needed to elucidate the effects of JNK1 on tumor growth and progression to develop the most effective strategy for JNK-targeted cancer treatment.
Materials and Methods
Reagents
The following commercial antibodies were used: JNK2 (Santa Cruz Biotechnology, Santa Cruz, CA), JNK1 (Santa Cruz Biotechnology), phospho-Akt, phospho-cJun, phospho-ERK, cJun (Cell Signaling Technology, Danvers, MA), phospho-Src (BioSource, Carlsbad, CA), GAPDH (Advanced ImmunoChemical Inc., Long Beach, CA), Ki-67 (Neomarkers, Freemont, CA), GAB2 (Millipore, Billerica, MA), Src (Cell Signaling Technology), and phosphotyrosine (Upstate, Billerica, MA). Lentiviral vectors encoding shRNAs (short hairpin RNAs) targeting JNK2 (acc. #NM.016961), GAB2 (acc. #NM.010248), and nonsilencing control (GIPZ) were purchased from Open Biosystems (Huntsville, AL). Human pCMV Sport6 JNK2 was purchased from Open Biosystems. Growth factor–reduced Matrigel was purchased from BD Biosciences. HGF was purchased from PeproTech Inc. (Rocky Hill, NJ). Silencer Select siRNAs targeting JNK (5′-CUAGCAACAUUGUAGUAAATT-3′) and Src (5′-GGCUGAGGAGUGGUACUUUTT-3′) were provided by Life Technologies (Carlsbad, CA), and Signal Silence siRNA targeting cJun was obtained from Cell Signaling Technology (cat. #6204). Humulin R (Lilly, Indianapolis, IN) was used for insulin treatments, and heregulin-1 was purchased from BioVision (Mountain View, CA). RANKL was purchased from Cell Sciences (Canton, MA), and the TRAP kit was purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture, lentiviral infections, and transfections
4T1.2 cells were a generous gift from R. Anderson (University of Melbourne, Australia) and were cultured in α-MEM (GIBCO, Life Technologies, Carlsbad, CA) supplemented with FBS (10% of final volume, BenchMark, Gemini Bio-Products, West Sacramento, CA) and penicillin/streptomycin. RAW 264.7 cells were kindly provided by B.R. Troen (University of Miami, Florida) and cultured with media described for 4T1.2 cells. HEK 293 packaging cells were used to produce viral media upon transfection with shJNK2-, shGAB2-, or GIPZ-containing lentiviral plasmids. 4T1.2 and RAW 264.7 cells were transduced with conditioned media and later treated with 4 µg/mL puromycin to establish stable expressing clones.
Three micrograms each of pCMV Sport6-JNK2 or empty vectors were transfected transiently into parental 4T1.2 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Cells were lysed 48 hours later and assessed for JNK2 and GAB2 protein expression using Western immunoblotting. For JNK and Src siRNA studies, cells were transfected with 30 nM siRNA with Lipofectamine RNAiMax (Invitrogen) following similar instructions. After 24 hours, cells were serum starved overnight and treated with FBS (10% of final volume) before being lysed and subjected to kinase assay. For cJun siRNA studies, cells were transfected with 100 nM siRNA with Lipofectamine 2000 (Invitrogen) as before and lysed after 48 hours. MTT cell viability assay was performed as previously described.42 1400jnk2–/– and 1400jnk2–/– JNK2 re-expressing cells were cultured as previously described29 and cotransfected with a CMVβgal plasmid and either a GAB2-luciferase construct or an empty vector luciferase plasmid generously provided by Dr. Chaussepied.28
In vivo studies
Balb/c mice were maintained according to the University of Texas at Austin Institutional Animal Care and Use Committee guidelines. 1 × 105 4T1.2-derived cells were injected into the #4 mammary fat pad. Mice were palpated 3 times weekly until tumors reached 1,000 mm3. For bone metastasis studies, 1 × 105 cells were injected into the left ventricle of the heart. Mice were then monitored daily for symptoms of distress, including difficulty breathing, ruffled appearance, and limited mobility. Upon appearance of symptoms, mice were euthanized. Mouse lungs were perfused with India ink to quantify metastatic lesions as previously reported.56
Histology and immunohistochemistry
Mammary tumors and rib cages were dissected and fixed with 4% paraformaldehyde overnight at 4°C. Rib cage and long bones were decalcified using decalcification solution (Fisher Scientific, Hampton, NH) for 4 days. Fixed tissues were then processed, paraffin-embedded, and sectioned at 5 µm for H&E staining. Immunohistochemical analysis was performed using Ki-67 antibody at 1:500 dilution. Briefly, sectioned tissues were rehydrated in Citrosolv (Fisher Scientific, Pittsburgh, PA) and serial dilutions of ethanol, incubated with 0.2% Triton-X-100 solution and proteinase K (20 ug/mL) solution for antigen retrieval. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide solution. Slides were blocked with 10% normal goat serum for 2 hours and then incubated with primary antibody overnight at 4°C. Slides were developed using the ABC kit (Vector Laboratories, Burlingame, CA), DAB (Vector Laboratories), and 33% hematoxylin according to the manufacturer’s instructions and subsequently dehydrated in serially increasing ethanol solutions and Citrosolv.
Boyden chamber invasion assay
5 × 104 cells were plated in triplicate in 24-well Millicell hanging cell culture inserts (Millipore) coated with Matrigel (BD Biosciences) in HBSS containing 0.5% BSA. α-MEM containing 10% FBS or HGF (40 ng/mL) was used as the chemoattractant in lower chambers. After 24 hours, media were aspirated, and cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet solution. Inserts were washed with distilled water, at which point all stained cells were counted and averaged between triplicate wells.
Kinase assay
4T1.2 cells were serum starved overnight and pretreated with PP2 (Src inhibitor, 10 µM) or LY-294006 (PI 3-kinase inhibitor, 20 µM) 45 minutes prior to 10% FBS stimulation for 0, 15, 30, and 60 minutes and collected with lysis buffer. Four hundred grams of total protein were incubated with 50 µL c-Jun/Glutathione Sepharose 4B beads (Amersham Biosciences, GE Healthcare, Piscataway, NJ) and 100 µM ATP for 20 minutes at 30°C. The kinase reaction was stopped by adding 4x sample buffer. Kinase reactions were then later probed using phospho-cJun immunoblotting.
Osteoclast differentiation
RAW 264.7–derived cells were plated in 6-well dishes at a density of approximately 1.5 × 105 cells per well in culturing media and 35 ng/mL RANKL (day 0). Media were refreshed with RANKL on day 3. Differences in morphology were visually observed at 10x magnification using a CKX41 Olympus microscope (Tokyo, Japan). Cells were then assessed for positive TRAP staining using the kit following the manufacturer’s instructions.
RT PCR
Total RNA was extracted from 4T1.2- and RAW 264.7–derived cells on day 3 of RANKL treatment (35 ng/mL) using Trizol (Invitrogen) and reverse transcribed to cDNA using Superscript III (Invitrogen) following the manufacturer’s instructions. There was 100 ng cDNA used for each PCR reaction excluding no template control sample, which contained no cDNA. GAB2-specific primers (forward: CTG GAC AAG AAC CAC AAT GC; reverse: AGT CTT TCC TGG AGG TTC AG) were used at 94°C for 1 minute, 48°C for 1 minute, and 72°C for 30 seconds for 30 cycles. RANK-specific primers (forward: CTC TAT GCC CGT GTC CCC TGA AAA; reverse: GGC CGC GAT GTC CCG TCC TT) were used at 94°C for 30 seconds, 59°C for 30 seconds, and 68°C for 1 minute for 35 cycles. RANKL-specific primers (forward: GCC GAG GAA GGG AGA GAA CGA T; reverse: CGC TCG AAA GTA CAG GAA CAG A) were used at 94°C for 30 seconds, 52°C for 30 seconds, and 68°C for 1 minute for 40 cycles. TRAP-specific primers (forward: AGC AGC CAA GGA GGA CTA CGT T; reverse: TCG TTG ATG TCG CAC AGA GG) and cathepsin K–specific primers (forward: TTA ATT TGG GAG AAA AAC CT; reverse: AGC CGC CTC CAC AGC CAT AAT) were used at 94°C for 30 seconds, 56°C for 30 seconds, and 68°C for 1 minute for 30 cycles. 18S-specific primers (forward: GTG ACT CTA GAT AAC CTC GG; reverse: GAC TCA TTC CAA TTA CAG GG) were used as a control for a separate set of samples when assessing each previously named primer set.
Statistical analysis
All statistical analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA). Kaplan-Meier graphs were analyzed using survival analysis. The Student t test was used for comparison of individual groups, and 1-way ANOVA was used for comparison of multiple groups.
Supplementary Material
Acknowledgments
The authors thank Arpi Shresar and Shreya Mitra for technical assistance and dedication to the progression of these studies. They also thank Dr. Robin Anderson for generously providing the 4T1.2 cells, Dr. Bruce Troen for providing RAW 264.7 cells, and Dr. Chaussepied for the control and GAB2 promoter plasmids. Finally, they thank Dr. Alexander Vlassov (Life Technologies) for siRNA reagents, helpful discussions, and scientific expertise.
Footnotes
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by the National Institutes of Health [grant numbers RO1 CA100238 (C.L.V.D.B.), ES T32 ES007247]; and the Department of Defense Breast Cancer Research Program [grant number W81XWH-08-1-0546 (A.N.)]. The content of this information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
References
- 1. Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal. 2009;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bentires-Alj M, Gil SG, Chan R, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nature Med. 2006;12:114-21 [DOI] [PubMed] [Google Scholar]
- 3. Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94:1029-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ke Y, Wu D, Princen F, et al. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene. 2007;26:4951-60 [DOI] [PubMed] [Google Scholar]
- 5. Mira A, Isella C, Renzulli T, et al. The GAB2 signaling scaffold promotes anchorage independence and drives a transcriptional response associated with metastatic progression of breast cancer. Oncogene. 2009;28:4444-55 [DOI] [PubMed] [Google Scholar]
- 6. Brummer T, Schramek D, Hayes VM, et al. Increased proliferation and altered growth factor dependence of human mammary epithelial cells overexpressing the Gab2 docking protein. J Biol Chem. 2006;281:626-37 [DOI] [PubMed] [Google Scholar]
- 7. Daly RJ, Gu H, Parmar J, et al. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene. 2002;21:5175-81 [DOI] [PubMed] [Google Scholar]
- 8. Kong M, Mounier C, Dumas V, Posner BI. Epidermal growth factor-induced DNA synthesis: key role for Src phosphorylation of the docking protein Gab2. J Biol Chem. 2003;278:5837-44 [DOI] [PubMed] [Google Scholar]
- 9. Crouin C, Arnaud M, Gesbert F, Camonis J, Bertoglio J. A yeast two-hybrid study of human p97/Gab2 interactions with its SH2 domain-containing binding partners. FEBS Letters. 2001;495:148-53 [DOI] [PubMed] [Google Scholar]
- 10. Meng S, Chen Z, Munoz-Antonia T, Wu J. Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem J. 2005;391:143-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2007;27:2693-704 [DOI] [PubMed] [Google Scholar]
- 12. Yu M, Luo J, Yang W, et al. The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem. 2006;281:28615-26 [DOI] [PubMed] [Google Scholar]
- 13. Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mamay CL, Mingo-Sion AM, Wolf DM, Molina MD, Berg Van Den. An inhibitory function for JNK in the regulation of IGF-I signaling in breast cancer. Oncogene. 2003;22:602-14 [DOI] [PubMed] [Google Scholar]
- 15. Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896-902 [DOI] [PubMed] [Google Scholar]
- 16. Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbio Mol Bio. 2006;70:1062-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ. Survival signaling mediated by c-Jun NH-terminal kinase in transformed B lymphoblasts. Nature Genetics. 2002;32:201-5 [DOI] [PubMed] [Google Scholar]
- 18. Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature (London). 2002;420:333-6 [DOI] [PubMed] [Google Scholar]
- 19. Lim YP, Wong CY, Ooi LL, Druker BJ, Epstein RJ. Selective tyrosine hyperphosphorylation of cytoskeletal and stress proteins in primary human breast cancers: implications for adjuvant use of kinase-inhibitory drugs. Clin Cancer Res. 2004;10:3980-7 [DOI] [PubMed] [Google Scholar]
- 20. Li JY, Wang H, May S, et al. Constitutive activation of c-Jun N-terminal kinase correlates with histologic grade and EGFR expression in diffuse gliomas. J Neurooncol. 2008;88:11-7 [DOI] [PubMed] [Google Scholar]
- 21. Tsuiki H, Tnani M, Okamoto I, et al. Constitutively active forms of c-Jun NH2-terminal kinase are expressed in primary glial tumors. Cancer Res. 2003;63:250-5 [PubMed] [Google Scholar]
- 22. Cui J, Han SY, Wang C, et al. c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells. Cancer Res. 2006;66:10024-31 [DOI] [PubMed] [Google Scholar]
- 23. Chen N, Nomura M, She QB, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908-12 [PubMed] [Google Scholar]
- 24. Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399-405 [PubMed] [Google Scholar]
- 25. Lelekakis M, Moseley JM, Martin TJ, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163-70 [DOI] [PubMed] [Google Scholar]
- 26. Barr R, Kendrick T, Bogoyevitch M. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002;277:10987-97 [DOI] [PubMed] [Google Scholar]
- 27. Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Transforming growth factor-beta signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol Cancer Res. 2008;6:1521-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaussepied M, Ginsberg D. Transcriptional regulation of AKT activation by E2F. Mol Cell. 2004;16:831-7 [DOI] [PubMed] [Google Scholar]
- 29. Chen P, O’Neal JF, Ebelt ND, et al. Jnk2 effects on tumor development, genetic instability and replicative stress in an oncogene-driven mouse mammary tumor model. PLoS ONE. 2010;5:e10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakshmikuttyamma A, Pastural E, Takahashi N, et al. Bcr-Abl induces autocrine IGF-1 signaling. Oncogene. 2008;27:3831-44 [DOI] [PubMed] [Google Scholar]
- 31. Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560-4 [DOI] [PubMed] [Google Scholar]
- 32. Lock LS, Maroun CR, Naujokas MA, Park M. Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol Biol Cell. 2002;13:2132-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR. Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan Electron Microsc. 1986;(Pt 4):1537-54 [PubMed] [Google Scholar]
- 34. Taube T, Elomaa I, Blomqvist C, Beneton MN, Kanis JA. Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone. 1994;15:161-6 [DOI] [PubMed] [Google Scholar]
- 35. Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609-17 [DOI] [PubMed] [Google Scholar]
- 36. Wada T, Nakashima T, Oliveira-dos-Santos AJ, et al. The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nature Med. 2005;11:394-9 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi N, Kadono Y, Naito A, et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Troen BR. The regulation of cathepsin K gene expression. Ann NY Acad Sci. 2006;1068:165-72 [DOI] [PubMed] [Google Scholar]
- 39. Pang M, Martinez AF, Fernandez I, Balkan W, Troen BR. AP-1 stimulates the cathepson promoter in RAW 264.7 cells. Gene. 2007;403:151-8 [DOI] [PubMed] [Google Scholar]
- 40. Nielsen C, Thastrup J, Bøttzauw T, Jäättelä M, Kallunki T. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res. 2007;67:178-85 [DOI] [PubMed] [Google Scholar]
- 41. Kennedy NJ, Sluss HK, Jones SN, et al. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mingo-Sion A, Marietta P, Koller E, Wolf D, Van Den Berg C. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596-604 [DOI] [PubMed] [Google Scholar]
- 43. Fleuren ED, O’Toole S, Millar EK, et al. Overexpression of the oncogenic signal transducer Gab2 occurs early in breast cancer development. Int J Cancer. 2010;127:1486-92 [DOI] [PubMed] [Google Scholar]
- 44. Finn RS. Targeting Src in breast cancer. Ann Oncol. 2008;19:1379-86 [DOI] [PubMed] [Google Scholar]
- 45. Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kao J, Salari K, Bocanegra M, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PloS One. 2009;4:e6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803-7 [DOI] [PubMed] [Google Scholar]
- 48. Asselin-Labat ML, Vaillant F, Sheridan JM, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798-802 [DOI] [PubMed] [Google Scholar]
- 49. Kim H, Laing M, Muller W. c-Src-null mice exhibit defects in normal mammary gland development and ERalpha signaling. Oncogene. 2005;24:5629-36 [DOI] [PubMed] [Google Scholar]
- 50. Lowe C, Yoneda T, Boyce BF, et al. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci. 1993;90:4485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-43 [DOI] [PubMed] [Google Scholar]
- 52. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977-84 [DOI] [PubMed] [Google Scholar]
- 53. Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001;93:1852-57 [DOI] [PubMed] [Google Scholar]
- 54. Shattuck DL, Miller JK, Carraway KL, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471-7 [DOI] [PubMed] [Google Scholar]
- 55. Wang SE, Xiang B, Guix M, et al. Transforming growth factor {beta} engages TACE and ErbB3 to activate PI3K/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams TM, Medina F, Badano I, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo: role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.