Abstract
The glycoprotein hormone erythropoietin (EPO) is a key regulator in the production of red blood cells. EPO is produced mainly in the embryonic liver and kidney of adults. Other organs are also known to express varying amounts of EPO. In our study, we have analyzed the epigenetic regulation of EPO in human cancer cell lines by DNA methylation assays, chromatin immunoprecipitation, RT-PCR, and promoter analysis under different growth conditions. Moreover, the growth-related effects of ectopic EPO expression were analyzed in a head and neck cancer cell line. We found frequent DNA hypermethylation of the CpG island promoter and enhancer of EPO in different cancer cell lines. Aberrant methylation of EPO promoter was observed in primary lung, head and neck, breast, and liver cancers. Hypermethylation of EPO was associated with a decreased expression of EPO in cancer cells. Treatment of cancer cell lines with 5-aza-2′-deoxycytidine (Aza), an inhibitor of DNA methylation, reactivated EPO expression under hypoxia. In contrast, in the liver cancer cell line HepB3, the EPO promoter was unmethylated, and a high EPO expression was observed independently of Aza treatment. Moreover, in vitro hypermethylation of the EPO promoter and enhancer reduced expression of a reporter gene under normoxia and hypoxia. Induction of EPO under hypoxia was accompanied by increased histone H3 acetylation and reduced histone H3 lysine 9 trimethylation. In a head and neck cancer cell line, which exhibited low EPO levels, ectopic expression of EPO significantly enhanced proliferation under normoxia and hypoxia. In summary, we show that hypermethylation of regulatory sequences of EPO is frequently observed in tumors and that this aberrant methylation induces epigenetic silencing of EPO in cancer cells.
Keywords: erythropoietin, hypoxia, cancer, epigenetics, DNA methylation, chromatin
Introduction
Erythropoietin (EPO) is a 34-kDa glycoprotein that is mainly produced in the fetal liver and adult kidney.1,2 Additionally, differential expression of EPO has been shown in other nonhematopoietic tissues such as the brain, placenta, testis, and female genital section, but also in tumors.3-8 EPO is induced by a low oxygen level and is responsible for the regulation of the daily production of 2 × 1011 red blood cells.9 Proliferation and differentiation of erythrocytes are controlled through EPO in adult bone marrow. Because of its potential to correct anemia, EPO has been prescribed to cancer patients.10,11 Although recombinant human EPO significantly reduces the risk of blood transfusions in cancer patients, clinical studies have reported a decreased survival following recombinant EPO treatment in patients with different cancer types including breast cancer and head and neck squamous cell carcinoma (HNSCC).12-14
Expressions of EPO and its receptor (EPOR) have been described in various cancers.7,15 However, in some tumors, EPO expression is reduced.16-18 Recent data suggest that EPO and EPOR are involved in several growth-stimulating pathways including proliferation, angiogenesis, invasion, and protectiveness against injury of the heart and brain.19-23 An EPO-mediated invasion through the Janus kinase (JAK) signal transducer and activator of the transcription signaling pathway has been demonstrated using HNSCC cells.24 It has been reported the EPO stimulates proliferation of renal cancer cells.25 It has been proposed that EPO acts in a paracrine fashion in the central nervous system and might function as a protective factor against hypoxia-induced damage of neurons.26 EPO is also responsible for an angiogenic response in several cancers.27,28 In prostate cancer, EPO regulates an autocrine/paracrine signaling pathway that regulates growth and survival of cancer cells.29
EPO gene expression is under the control of inhibitory (GATA-2, NF-κB) and stimulatory (hypoxia-inducible factors [HIFs], hepatocyte nuclear factor) transcription factors.30 Induction of EPO occurs under hypoxic conditions, and the EPO gene is activated through binding of the hypoxia-induced factor 1 α (HIF1A) and aryl hydrocarbon receptor nuclear translocator (ARNT; also termed HIF1B) to the enhancer and promoter, respectively.31,32 The binding site of HIF1A has been reported to be in the enhancer region located at the 3′ region of the EPO gene.32 It has been reported that DNA methylation of human erythropoietin occurs at the promoter and enhancer region of HeLa cells.17 The methylation of these regions inhibits transcription of EPO.16-18 However, the methylation of EPO was not analyzed in cancer cells in detail.
Epigenetic alterations of tumor suppressor genes contribute essentially to tumor development and tumor progression in cancers. Epigenetic gene silencing is mediated by aberrant methylation of CpG island promoters.33 One of the most frequent hypermethylated genes is the RASSF1A tumor suppressor.34-36 Promoter hypermethylation has been studied as a biomarker system for the diagnosis and detection of early cancers and can likely be used as a prognostic factor in malignant diseases.37 Demethylation agents, such as 5-azacytidine and 5-aza-2′-desoxycytidine (Aza), that inhibit DNA methyltransferases and revert the methylation status of silenced genes, are currently being tested in the treatment of cancers.38,39
The aim of our study was to investigate the epigenetic status of the EPO gene in different tumor cell lines, primary tumor samples, and normal tissues. Therefore, the promoter and enhancer methylation was analyzed. Additionally, DNA methylation–dependent expression of EPO under Aza treatment and hypoxia using reporter assays was investigated. Here, we report that EPO is frequently hypermethylated in human cancers and that this reduces its expression significantly.
Results
Reduced EPO expression in human cancers
In our study, we investigated the epigenetic regulation of EPO expression in human tumors. It has been reported that EPO is expressed mainly in the kidney but is also found in other tissues. We first analyzed the mRNA levels of EPO in normal human tissues using real-time PCR (Fig. 1A). High levels of EPO were found in the kidney and liver; however, EPO mRNA was also present in placenta and to a lesser amount in the heart, brain, lung, and skeletal muscle (Fig. 1A). Subsequently, EPO expression was analyzed in different tumor cancer cell lines including HepB3 (liver cancer), Hep2 (larynx carcinoma), RPMI-2650 (nasal septum carcinoma), HeLaS3 (cervix carcinoma), A549 (lung adenocarcinoma), FTC236 (follicular thyroid cancer), PATU-S and HUP-T3 (pancreatic carcinomas), and MZ1851 and MZ2865 (renal cancers) by real-time PCR (Fig. 1B). Compared to HepB3, EPO expression was reduced in all of the cancer cell lines analyzed.
Figure 1.
EPO expression in normal human tissues (A) and cancer cell lines (B). RNA was isolated from different human tissues and cancer cell lines and reverse transcribed. EPO expression was determined by real-time PCR and normalized to GAPDH. (A) The expression of EPO in normal tissues was plotted relative to the expression in placenta (= 1). (B) EPO expression was analyzed in HepB3 (liver cancer), Hep2 (larynx carcinoma), RPMI2650 (nasal septum carcinoma), HeLaS3 (cervix carcinoma), A549 (lung adenocarcinoma), FTC236 (follicular thyroid cancer), PATU-S and HUP-T3 (pancreatic carcinomas), and MZ1851 and MZ2865 (renal cancers) and plotted relative to the expression in HepB3 (= 1).
Methylation of the EPO promoter occurs frequently in human cancers
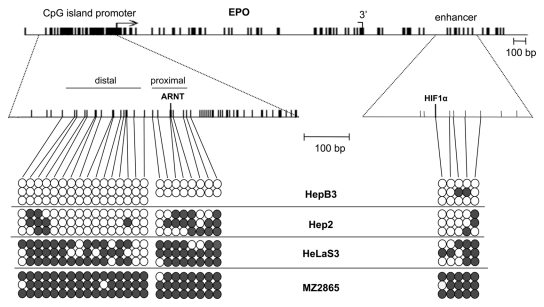
DNA hypermethylation of CpG island promoters is associated with gene inactivation in carcinogenesis. We therefore analyzed the CpG frequency of the EPO gene and observed a CpG island in its promoter region (Fig. 2). The gene body and enhancer were not located in CpG-rich regions. To correlate the aberrant expression of EPO in cancer cell lines with epigenetic deregulation, we investigated the aberrant methylation of the EPO gene by bisulfite sequencing in HepB3, Hep2, HeLaS3, and MZ2865 (Fig. 2). In HepB3, the promoter was unmethylated, and methylation of the enhancer was infrequent (Fig. 2). In contrast, the CpG island promoter was hypermethylated in Hep2 cells that exhibited low EPO expression. Similarly, HeLaS3 and MZ2865 showed severe promoter and enhancer hypermethylation. These data were also confirmed by combined bisulfite restriction analysis (COBRA) for several cancer cell lines (Suppl. Fig. S1). We revealed hypermethylation of the EPO promoter in 6 lung cancer cell lines (A549, HCC15, SK-MES1, DV-90, CRL5886, and H358), 3 breast cancer cell lines (ZR751, T47D, and MDAMB231), several renal cancer cell lines (MZ1851 and MZ2865), thyroid cancer cell line FTC238, and 3 head and neck cancer cell lines (Hep2, HNSCC-14C, and RPMI2650) (data not shown) (Fig. 2 and Suppl. Fig. S1).
Figure 2.
Structure and methylation of the EPO gene. The transcription initiation (arrow) and termination sites (3′) of EPO are depicted. Vertical lines indicate CpGs, and the ARNT and HIF1α binding sites are shown. Methylation of CpGs distal and proximal to the ARNT site and enhancer were analyzed in HepB3, Hep2, HeLaS3, and MZ2865 cells by bisulfite analysis. PCR products were cloned and sequenced. White and gray dots indicate unmethylated and methylated Cs, respectively.
We then analyzed the methylation of EPO in primary liver samples, head and neck squamous cell cancer (HNSCC), lung and breast tumors, and matching normal samples by COBRA (Fig. 3 and Table 1). DNA hypermethylation of the enhancer was observed in HNSCC and matching normal tissue with the same frequency (100%) (Fig. 3 and Table 1). However, the distal promoter region was more frequently methylated in tumor tissues compared to normal samples (44/75 = 57% v. 15/42 = 36%; P < 0.05, Fisher exact test). For example, methylation of the promoter was observed in HNSCC T1, T2, and T3; however, only the normal tissue N2 showed significant methylation of the distal promoter region (Fig. 3). In contrast, all tumors (T1, T2, and T3) and normal (N1, N2, and N3) tissues exhibited enhancer hypermethylation (Fig. 3). Tumor-specific hypermethylation of the EPO promoter was also observed for liver, breast, and lung samples (Table 1 and Suppl. Fig. S1). These results indicated that a CpG island promoter methylation of EPO occurs preferentially in tumor cells.
Figure 3.
Methylation of EPO in human primary tissues. The methylation status of 3 head and neck tumors (T1, T2, and T3) and matching normal tissue (N1, N2, and N3) was analyzed by combined bisulfite restriction analysis (COBRA). Three different regions (enhancer, distal, and proximal promoter) were amplified and digested with BstUI (B), HypCH4IV (H), and TaqI (T) or mock digested (-). Products were resolved on 2% gels together with a 100-bp ladder (M).
Table 1.
Methylation of EPO in Primary Human Tissues
| Sample (region) | Tumor | Normal tissue | Tumor v. normala |
|---|---|---|---|
| Head and neck (enhancer) | 100% (17/17) | 100% (18/18) | P = 1 |
| Head and neck (proximal) | 50% (7/14) | 11% (2/18) | P < 0.05 |
| Head and neck (distal) | 76% (13/17) | 58% (11/19) | P = 0.3 |
| Lung (distal) | 41% (14/34) | 0% (0/6) | P = 0.07 |
| Liver (distal) | 100% (11/11) | 75% (3/4) | P = 0.26 |
| Breast (distal) | 46% (6/13) | 8% (1/13) | P = 0.07 |
Two-tailed Fisher exact test.
EPO induction is suppressed by its hypermethylation
Subsequently, we tested if the observed hypermethylation of the EPO promoter affects its regulation under hypoxia. Therefore, we analyzed the expression of EPO and a control (carbonic anhydrase IX [CAIX]) under normoxia and hypoxia in HepB3, Hep2, and HeLaS3 cells after treatment for several days with an inhibitor of DNA methyltransferase (10 µM Aza; 5-aza-2′-desoxycytidine). In HepB3 cells, which harbor an unmethylated EPO promoter, EPO expression was strongly induced (25x) under hypoxia independently of Aza (Fig. 4). However, in Hep2 cells that exhibited promoter methylation, EPO induction under hypoxia was very weak (2x) and increased dramatically after Aza treatment (Fig. 4). In HeLaS3, which also harbor methylated promoter sequences, no hypoxia-induced EPO expression was found. Once again, the EPO expression dramatically increased under hypoxia when the cells were treated with 10 µM Aza. Since expression of EPO is regulated by HIF1A, we confirmed HIF1A expression for all 3 cancer cell lines (Suppl. Fig. S2). EPOR was also detected in these cell lines (Suppl. Fig. S2). The expression of CAIX was induced by hypoxia in HepB3, Hep2, and HeLaS3. Taken together, this indicates that promoter hypermethylation of EPO strongly affects hypoxia-induced expression of EPO.
Figure 4.
Expression of EPO and CAIX in cancer cell lines under different growth conditions. Cancer cells were treated for 4 days with 10 µM of 5-aza-2′-desoxycytidine (Aza) and hypoxia (1% O2). RNA was isolated and reverse transcribed. Expression of EPO and CAIX was determined from 3 independent experiments by real-time PCR, normalized to GAPDH, and plotted relative to the values obtained in 0 µM Aza and normoxia (= 1).
We also analyzed the chromatin modifications at the promoter and enhancer under normoxia and hypoxia. For Hep2 cells, which exhibit a partially methylated promoter, histone H3 acetylation and histone H3 lysine 9 trimethylation were analyzed by ChIP (Fig. 5). For both the promoter and enhancer, an increased acetylation of histone H3 and decreased histone H3 lysine 9 trimethylation were found in hypoxia compared to normoxia. This indicates that activation of EPO under hypoxia is accompanied by chromatin changes.
Figure 5.
Changes of chromatin modification at the EPO promoter and enhancer under hypoxia. Chromatin was crosslinked by treating Hep2 cells with 1% formaldehyde. For the chromatin immunoprecipitation, anti-histone H3, anti-histone H3K9me3, and anti-histone H3ac were utilized. Precipitated DNA for the EPO promoter and enhancer was analyzed by real-time PCR. The values obtained for the precipitated anti-histone H3K9me3 and anti-histone H3ac were normalized against histone H3 precipitation and plotted relative to the values obtained in normoxia (= 1).
Next, we analyzed the expression of a luciferase reporter gene under the control of the EPO promoter or enhancer (Fig. 6). To do this, a 426-bp fragment of the EPO promoter and/or 185-bp fragment of the EPO enhancer was cloned into pRL null and was in vitro methylated with a CpG methyltransferase. Unmethylated and methylated constructs were transfected into HepB3, Hep2, and HeLaS3 cells and were analyzed under normoxia and hypoxia for luciferase expression (Fig. 6). The promoter activated transcription only in Hep2 cells. Interestingly, the EPO enhancer upregulated luciferase expression dramatically under hypoxia by activating the EPO promoter or a cryptic promoter in all 3 cell lines (Fig. 6). However, such a hypoxia-induced activation was not observed for all in vitro methylated constructs. These data suggest that aberrant methylation of regulatory sequences of EPO greatly reduces gene expression.
Figure 6.
Epigenetic regulation of a reporter gene under the control of the EPO promoter and enhancer. A 426-bp fragment of the EPO promoter and the 185-bp fragment of the EPO enhancer were cloned into pRL null. Constructs were in vitro methylated with SssI methyltransferase. The cells were cotransfected with the indicated constructs and the pGL3 promoter vector in HepB3, Hep2, and HeLaS3 cells. After a 24-hour incubation at hypoxia or normoxia, the Renilla luciferase reporter expression (R) was measured with the dual-luciferase reporter assay system and normalized for firefly luciferase expression (F). Expression was plotted relative to the values obtained using the pRL-null control under normoxia (R/F = 1).
Ectopic expression of EPO enhances growth
In order to analyze the functional consequence of epigenetic silencing of EPO in cancer cells, EPO was ectopically expressed in Hep2 cells, which exhibit low endogenous EPO expression. The full-length cDNA was cloned into a GFP- and a FLAG-tagged vector and transfected in Hep2 cells (Suppl. Fig. S3). Expression of green fluorescent EPO was predominantly found in the cytoplasm (Suppl. Fig. S3). Additionally, colony formation was analyzed under G418 selection in Hep2 cells (Fig. 7A). After 3 weeks of G418 selection, significantly more colonies were detected in the EPO-transfected Hep2 cells compared to vector-transfected ones (P < 0.04, t test) (Fig. 7A). We also analyzed the cell growth of 3 clones overexpressing EPO and controls (Fig. 7B and 7C). Under normoxia, the clones that ectopically express EPO exhibited a 40% higher proliferation rate than controls (P < 0.02, t test) (Fig. 7B). A similar trend (35%) was also observed under hypoxia (Fig. 7B). Additionally, the anchorage-independent growth of these 3 EPO overexpression clones and controls was investigated (Fig. 7C). Under normoxia, the ectopically EPO-expressing clones formed 60% larger colonies compared to controls (P < 0.01, t test). Larger colonies were also detected under hypoxia (Fig. 7C). In summary, these data suggest that the proliferation rate is significantly enhanced in cells that exhibited high EPO expression.
Figure 7.
Ectopic expression of EPO enhances growth in Hep2 cells. (A) The 614-bp full-length ORF of EPO was cloned in pCMV-Tag1, and 1 µg of plasmid DNA and vector control were transfected in the HNSCC cell line Hep2. Cells were grown under normoxia or hypoxia and selected with 1.25 mg/mL G418 for 3 weeks. Colonies were stained with Giemsa, and colony numbers were determined in triplicates. (B) Three clones re-expressing EPO and 3 control clones were picked, and the growth rate was analyzed. Therefore, 50,000 cells/mL (= 1) each were plated in 6-well plates with 1 mg/mL G418, and cell numbers were counted after 48 hours of normoxia or 72 hours of hypoxia. (C) Anchorage-independent growth was analyzed in 0.3% soft agar. Three stable transfected clones were each seeded in 0.3% agarose. Experiments were performed in triplicates with 5,000 cells per plate under selection with 1.25 mg/mL G418. The colony sizes of 50 clones were measured using a microscope after 4 weeks.
Discussion
In our study, we have analyzed the epigenetic regulation of EPO in different cancer cell lines and primary tissues. Here, we report that EPO is frequently epigenetically downregulated by the hypermethylation of its regulatory sequences. We observed that the EPO CpG island promoter is aberrantly methylated in cancer cell lines and primary tumors. To the best of our knowledge, this is the first comprehensive study that has analyzed the hypermethylation of EPO in primary tumors as well as in normal tissues from different entities. It has already been previously reported that both the EPO promoter and enhancer are hypermethylated in different cancer cell lines.16,17 However, these studies were limited to 4 neuroblastoma cell lines, HeLa, and 2 hepatocellular cancer cell lines.16-18 In our study, we report that EPO hypermethylation is also frequently found in renal cell cancer, head and neck cancer, lung cancer, pancreatic cancer, and other cancer cell lines. Moreover, we show that the promoter of EPO is more frequently methylated in tumor tissues compared to normal tissue originating from cancer patients. This indicates a tumor-specific hypermethylation of EPO.
It has previously been reported that several tumor suppressor genes (e.g., RASSF1A) are frequently hypermethylated in different cancer entities.33-36 However, EPO does not represent a tumor suppressor gene, and therefore, its tumor-specific aberrant methylation shows that genes with growth-promoting functions are hypermethylated in cancer as well. In several normal epithelial cells, EPO is not constitutively expressed like a housekeeping gene or a tumor suppressor gene but is only activated under hypoxic conditions. EPO is transcribed at a very low basal rate in several normal cells.40 Due to epigenetic alterations that occur during tumorigenesis, silencing of the poorly expressed EPO gene is established and then permanently maintained by its hypermethylation. Reactivation of EPO is abolished due to these epigenetic modifications when hypoxic conditions occur. We have reported that epigenetic inactivation of a tumor suppressor gene occurs during senescence in normal epithelial cells.41 We show that the basal levels and hypoxia-induced expression of EPO are both reduced in cancer cells (Hep2 and HeLaS3) that exhibit aberrant methylation. When these cells are treated with the DNMT inhibitor Aza, both the basal and hypoxia-regulated expressions of EPO are restored (Fig. 4). In contrast, in HepB3 cells that harbor an unmethylated promoter and enhancer, this Aza-dependent effect was not observed (Fig. 4). This indicates that aberrant methylation is causal for the silencing of EPO. It has been previously reported that HeLa cells exhibit promoter and enhancer hypermethylation.17,18 However, the influence of Aza treatment with respect to EPO expression has not been tested before. It has been shown that the methylation of regulatory sequences directly blocks the binding of transcription factors (e.g., HIF1A).17,18
The only cell line that exhibits high levels of basal EPO expression is the hepatocellular cancer HepB3. HepB3 cells harbor an unmethylated promoter and enhancer (Fig. 2), which has previously been reported.17,18,40 It is interesting to note that the luciferase reporter gene was regulated independently of the cell lines utilized and that HIF1A mRNA was present in all cells (Fig. 6 and Suppl. Fig. S2). This indicates that in cells that harbor a methylated EPO gene, the hypoxia-dependent signaling pathways are intact and independent of the Aza treatment as well. Promoter-activated transcription was seen only in Hep2 cells and not in HeLa and HepB3. In Hep2 cells, the composition of basal transcription factors might be different compared to the other 2 cell lines.
In Hep2 cells, which exhibit a partially methylated promoter and enhancer sequence, a weak induction of EPO under hypoxia was observed (Fig. 4). Hep2 cells show lower expression of EPO compared to HeLa and MZ2865 cells (Fig. 1). However, DNA methylation was less frequent in the promoter and enhancer regions in Hep2 (Fig. 2). Additionally, we observed repressive chromatin modifications at both regions (Fig. 5). Histone deacetylation and methylation are also involved in the silencing of EPO expression and may be responsible for the low EPO expression observed in Hep2 cells. Since repressive chromatin modifications are reversible (Fig. 5), it is interesting to note that Hep2 cells exhibited a higher hypoxia-induced EPO expression compared to HeLa cells (Fig. 4). This induction was accompanied by altered chromatin modifications and increased histone H3 acetylation (Fig. 5). It has been reported that HIF1A interacts with histone acetyltransferases such as CBP/p300.42,43 It is conceivable that the basal expression is controlled by the promoter alone while the hypoxia-induced expression is a result of the interplay between promoter and enhancer. In cancer cells, both the promoter and enhancer are methylated, and the hypoxia-induced expression of EPO is repressed. DNA methyltransferases are often overexpressed in tumor cells. For example, DNMT1 and DNMT3b, which are responsible for the maintenance of methylation and de novo methylation, respectively, are upregulated in tumors.44-47
We showed that the ectopic expression of EPO promotes clonogenicity, proliferation, and anchorage-independent growth under normoxia and hypoxia (Fig. 7) in the larynx carcinoma cell line Hep2. It has already been reported that EPO is involved in several growth-promoting pathways of cells including angiogenesis, proliferation, and invasion. An EPO-mediated invasion has been demonstrated in HNSCC cells.19 It has been reported that EPO stimulates the growth of renal cancer and prostate cancer cells.25,29 These growth-stimulating functions of EPO are consistent with our data obtained for the larynx cancer cell line Hep2 under hypoxia and normoxia.
In summary, we report that EPO is frequently hypermethylated in human cancers, including primary tumors and cancer cell lines. Promoter and enhancer hypermethylation of EPO repressed its hypoxia-induced expression and basal expression. Epigenetic silencing of EPO was restored through the DNA methlytransferase inhibitor Aza. EPO exhibits growth-promoting features including proliferation, clonogenicity, and anchorage-independent growth. These data suggest that an aberrant cancer-specific methylation could also affect the CpG island promoter of genes with potential growth-promoting function and that this in turn could cause an upregulation of such genes in cancer therapies that result in the demethylation of genomic DNA.
Materials and Methods
Cell lines and primary tissues
Cancer cell lines were cultured under recommended conditions.36,48-50 Kidney cancer cell lines (MZ1851 and MZ2865) were obtained from Professor B. Seliger (University of Halle-Wittenberg).51 Hep2, HeLaS3, MZ1851, and HepB3 were cultured in DMEM containing 10% FCS and 1% PS in 5% CO2 and 16% O2 (normoxia) or 1% O2 (hypoxia). There was 10 µM of 5-aza-2′-deoxycytidine (Aza) added daily to cancer cells for 4 days without replacing media.
Primary human cancers and matching normal tissue samples were obtained from patients of the City of Hope Medical Center or the University of Halle-Wittenberg and were previously described.34,36,52 The local committee of medical ethics approved the use, and all patients gave their consent.
Combined bisulfite restriction analysis and bisulfite sequencing
Methylation of the promoter and enhancer of EPO was analyzed using combined bisulfite restriction analysis and bisulfite sequencing. The DNA of patient samples and cell lines were isolated by a standard phenol/chloroform extraction and NaAc/EtOH precipitation. The bisulfite-treated DNA was amplified with primers specific for the distal and proximal promoter region and the enhancer of EPO (Suppl. Table S1). For the distal promoter region and enhancer, a seminested PCR using an internal primer was performed (Suppl. Table S1). There were 20 to 50 ng of PCR products digested with 10 U of BstUI, HpyCH4IV, or TaqI (New England Biolabs, Beverly, MA) according to the manufacturer’s protocol and analyzed on 2% TBE agarose gels. For bisulfite sequencing, PCR products were cloned in pGEM-T-Easy (Promega, Heidelberg, Germany) and sequenced (SeqLab, Göttingen, Germany).
RNA expression analysis
Total RNA from cancer cell lines (HepB3, Hep2, RPMI2650, HeLaS3, A549, FTC236, PATU-S, HUP-T3, MZ1851, and MZ2865) was isolated using TRIzol-Reagent (Invitrogen, Karlsruhe, Germany) followed by a chloroform extraction and an isopropanol precipitation. The quality of the RNA was controlled on 1% agarose gels. For reverse transcription, 1 µg RNA was incubated with oligo-dT and hexameric primers with MMLV reverse transcriptase (Promega) for 1 hour at 42°C in 20 µL. Subsequently, 2 µL of cDNA was analyzed by real-time PCR (Rotor-Gene 2000, Corbett Life Science, Sydney, Australia) with SYBR green. The primer pairs for EPO, EPOR, HIF1a, GAPDH, and CAIX are listed in Supplementary Table S1. Results were normalized to GAPDH. Furthermore, we analyzed the mRNA expression of EPO in several normal tissues including the heart, brain, lung, skeletal muscle, and kidney (cDNA panel, Clontech, Palo Alto, CA) and liver and placenta (pooled RNAs from 3-5 normal tissue probes).
Reporter assay
For the reporter assay, the EPO promoter and/or the EPO enhancer was cloned into the pRL-null vector (Promega). A 426-bp fragment of the EPO promoter was amplified with primers 5′-GAAGATCTGCGGAACTCAGCAACCCAGGCATCT and 5′-CCCAAGCTTCGGGAGGACAGCGCGGTGCGGC from genomic DNA of HepB3 and cloned into the BglII and HindIII site of pRL null. A 185-bp fragment of the EPO enhancer was amplified with primers 5′-GGATCCTGTCCCACTCCTGGCAGCAGTG and 5′-GGCTCCATTCAAGGCCTCACCGGATCC and cloned in the BamHI site downstream of the polyA sequence of pRL null in SCS110 strains, which lack both dam and dcm activity. All constructs were verified by sequencing (SeqLab). Constructs were in vitro methylated with SssI methyltransferase (NEB, Frankfurt, Germany). The cells were transfected with 250 ng and 100 ng of the construct using Nanofectin (PAA, Pasching, Germany) for Hep2 and HEKfectin (Bio-Rad, München, Germany) for HeLaS3 and HepB3, respectively. For normalization, 100 ng and 250 ng of pGL3 promoter vector (Promega) was cotransfected, respectively. After a 24-hour incubation at hypoxia or normoxia, the reporter expression was measured with the dual-luciferase reporter assay system (Promega).41
Chromatin immunoprecipitation
Chromatin was crosslinked by incubation of 106 Hep2 cells for 10 minutes at 37°C with 1% formaldehyde. Cells were lysed in SDS for 10 minutes on ice, and the DNA was fragmented into products of 200 to 450 bp by sonication for 5 to 10 seconds 2 to 3 times on ice. For the precipitation, the following antibodies were used: anti-histone H3 (ab1791, Abcam, Cambridge, UK), anti-histone H3K9me3 (ab8898, Abcam), and anti-histone H3ac (06-599, Upstate, Billerica, MA). The DNA-protein-antibody complexes were precipitated with salmon sperm DNA/Protein A Agarose (Upstate). The amount of precipitated DNA was analyzed by real-time PCR. Results of anti-histone H3K9me3 and anti-histone H3ac were normalized against anti-histone H3 precipitation.
EPO transfection, proliferation, and colony formation assay
To generate the full-length cDNA of EPO, cDNA from human kidney was amplified with primers 5′-AGATCTTGATGGGGGTGCACGAATGTCCTG and 5′-TGGTGGATATGCCCAGGTCGACACAC flanking the ORF of EPO. The 614-bp full-length ORF of EPO was ligated into pT-Adv (Clontech) and subsequently cloned in pCMV-Tag1 (Stratagene, La Jolla, CA) and pEYFP-C2 (Clontech). All constructs were verified by sequencing (SeqLab). Subsequently, 1 µg of plasmid DNA and vector control were transfected in the HNSCC cell line Hep2 using Nanofectin (PAA). Cells were selected with 1.25 mg/mL G418 (PAA) for 3 weeks. Colonies were stained with Giemsa, and colony numbers were determined. Clones re-expressing EPO and vector controls were picked, and the growth rate was analyzed. In order to do so, 50,000 cells/mL each were plated in 6-well plates with 1 mg/mL G418, and cell numbers were counted. In order to investigate the proliferation in soft agar, stably transfected cells were seeded in 0.3% agarose. Experiments were performed in triplicates with 5,000 cells per plate under selection with 1.25 mg/mL G418. Colony size was measured using a microscope after 4 weeks, and the size of 50 colonies was determined with Motic Images Plus 2.0 (Motic, Wetzlar, Germany).
Statistics
All statistical correlations were performed using SPSS 15.0 (Chicago, IL).
Supplementary Material
Footnotes
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by the Deutsche Krebshilfe [grant number 109179]; UGMLC (LOEWE); and DFG [grant number DA552] to Reinhard Dammann.
References
- 1. Dame C, Fahnenstich H, Freitag P, et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood. 1998;92:3218-25 [PubMed] [Google Scholar]
- 2. Eckardt KU, Ratcliffe PJ, Tan CC, Bauer C, Kurtz A. Age-dependent expression of the erythropoietin gene in rat liver and kidneys. J Clin Invest. 1992;89:753-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conrad KP, Benyo DF, Westerhausen-Larsen A, Miles TM. Expression of erythropoietin by the human placenta. FASEB J. 1996;10:760-8 [DOI] [PubMed] [Google Scholar]
- 4. Magnanti M, Gandini O, Giuliani L, et al. Erythropoietin expression in primary rat Sertoli and peritubular myoid cells. Blood. 2001;98:2872-4 [DOI] [PubMed] [Google Scholar]
- 5. Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. A novel site of erythropoietin production: oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269:19488-93 [PubMed] [Google Scholar]
- 6. Yasuda Y, Okano M, Nagao M, et al. Erythropoietin in mouse avascular yolk sacs is increased by retinoic acid. Dev Dyn. 1996;207:184-94 [DOI] [PubMed] [Google Scholar]
- 7. Acs G, Acs P, Beckwith SM, et al. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561-5 [PubMed] [Google Scholar]
- 8. Dagnon K, Pacary E, Commo F, et al. Expression of erythropoietin and erythropoietin receptor in non-small cell lung carcinomas. Clin Cancer Res. 2005;11:993-9 [PubMed] [Google Scholar]
- 9. Fried W. Erythropoietin and erythropoiesis. Exp Hematol. 2009;37:1007-15 [DOI] [PubMed] [Google Scholar]
- 10. Steensma DP, Loprinzi CL. Erythropoietin use in cancer patients: a matter of life and death? J Clin Oncol. 2005;23:5865-8 [DOI] [PubMed] [Google Scholar]
- 11. Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26:3607-13 [DOI] [PubMed] [Google Scholar]
- 12. Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255-60 [DOI] [PubMed] [Google Scholar]
- 13. Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459-60 [DOI] [PubMed] [Google Scholar]
- 14. Tovari J, Pirker R, Timar J, Ostoros G, Kovacs G, Dome B. Erythropoietin in cancer: an update. Curr Mol Med. 2008;8:481-91 [DOI] [PubMed] [Google Scholar]
- 15. Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332-9 [DOI] [PubMed] [Google Scholar]
- 16. Rossler J, Stolze I, Frede S, et al. Hypoxia-induced erythropoietin expression in human neuroblastoma requires a methylation free HIF-1 binding site. J Cell Biochem. 2004;93:153-61 [DOI] [PubMed] [Google Scholar]
- 17. Wenger RH, Kvietikova I, Rolfs A, Camenisch G, Gassmann M. Oxygen-regulated erythropoietin gene expression is dependent on a CpG methylation-free hypoxia-inducible factor-1 DNA-binding site. Eur J Biochem. 1998;253:771-7 [DOI] [PubMed] [Google Scholar]
- 18. Yin H, Blanchard KL. DNA methylation represses the expression of the human erythropoietin gene by two different mechanisms. Blood. 2000;95:111-9 [PubMed] [Google Scholar]
- 19. Lai SY, Childs EE, Xi S, et al. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442-9 [DOI] [PubMed] [Google Scholar]
- 20. Ribatti D. Erythropoietin and tumor angiogenesis. Stem Cells Dev. 2010;19:1-4 [DOI] [PubMed] [Google Scholar]
- 21. Yasuda Y, Fujita Y, Matsuo T, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021-9 [DOI] [PubMed] [Google Scholar]
- 22. Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14-31 [DOI] [PubMed] [Google Scholar]
- 23. Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells from clinic to bench: a critical review. Biochim Biophys Acta. 2010;1806:82-95 [DOI] [PubMed] [Google Scholar]
- 24. Mohyeldin A, Lu H, Dalgard C, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7:537-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647-57 [DOI] [PubMed] [Google Scholar]
- 26. Marti HH, Gassmann M, Wenger RH, et al. Detection of erythropoietin in human liquor: intrinsic erythropoietin production in the brain. Kidney Int. 1997;51:416-8 [DOI] [PubMed] [Google Scholar]
- 27. Nico B, Annese T, Guidolin D, Finato N, Crivellato E, Ribatti D. Epo is involved in angiogenesis in human glioma. J Neurooncol. 2011;102:51-8 [DOI] [PubMed] [Google Scholar]
- 28. Ribatti D, Nico B, Perra MT, et al. Erythropoietin is involved in angiogenesis in human primary melanoma. Int J Exp Pathol. 2010;91:495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ, Feldman L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol Cancer Res. 2009;7:1150-7 [DOI] [PubMed] [Google Scholar]
- 30. Jelkmann W. Control of erythropoietin gene expression and its use in medicine. Methods Enzymol. 2007;435:179-97 [DOI] [PubMed] [Google Scholar]
- 31. Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways: demonstration of competition and compensation. J Biol Chem. 1999;274:12115-23 [DOI] [PubMed] [Google Scholar]
- 32. Wang GL, Semenza GL. Molecular basis of hypoxia-induced erythropoietin expression. Curr Opin Hematol. 1996;3:156-62 [DOI] [PubMed] [Google Scholar]
- 33. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415-28 [DOI] [PubMed] [Google Scholar]
- 34. Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet. 2000;25:315-9 [DOI] [PubMed] [Google Scholar]
- 35. Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer: from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796:114-28 [DOI] [PubMed] [Google Scholar]
- 36. Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep. 2009;22:1519-26 [DOI] [PubMed] [Google Scholar]
- 37. Brena RM, Huang TH, Plass C. Quantitative assessment of DNA methylation: potential applications for disease diagnosis, classification, and prognosis in clinical settings. J Mol Med. 2006;84:365-77 [DOI] [PubMed] [Google Scholar]
- 38. Jain N, Rossi A, Garcia-Manero G. Epigenetic therapy of leukemia: an update. Int J Biochem Cell Biol. 2009;41:72-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oki Y, Issa JP. Review: recent clinical trials in epigenetic therapy. Rev Recent Clin Trials. 2006;1:169-82 [DOI] [PubMed] [Google Scholar]
- 40. Fandrey J, Bunn HF. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993;81:617-23 [PubMed] [Google Scholar]
- 41. Strunnikova M, Schagdarsurengin U, Kehlen A, Garbe JC, Stampfer MR, Dammann R. Chromatin inactivation precedes de novo DNA methylation during the progressive epigenetic silencing of the RASSF1A promoter. Mol Cell Biol. 2005;25:3923-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kallio PJ, Okamoto K, O’Brien S, et al. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17:6573-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Semenza GL. Physiology meets biophysics: visualizing the interaction of hypoxia-inducible factor 1 alpha with p300 and CBP. Proc Natl Acad Sci U S A. 2002;99:11570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan H, Zhao ZJ, Cheng J, Su XW, Wu QX, Shan YF. Overexpression of DNA methyltransferase 1 and its biological significance in primary hepatocellular carcinoma. World J Gastroenterol. 2009;15:2020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hino R, Uozaki H, Murakami N, et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766-74 [DOI] [PubMed] [Google Scholar]
- 46. Roll JD, Rivenbark AG, Jones WD, Coleman WB. DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol Cancer. 2008;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J, Bhutani M, Pathak AK, et al. Delta DNMT3B variants regulate DNA methylation in a promoter-specific manner. Cancer Res. 2007;67:10647-52 [DOI] [PubMed] [Google Scholar]
- 48. Dammann R, Schagdarsurengin U, Liu L, et al. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22:3806-12 [DOI] [PubMed] [Google Scholar]
- 49. Schagdarsurengin U, Gimm O, Dralle H, Hoang-Vu C, Dammann R. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid. 2006;16:633-42 [DOI] [PubMed] [Google Scholar]
- 50. Schagdarsurengin U, Wilkens L, Steinemann D, et al. Frequent epigenetic inactivation of the RASSF1A gene in hepatocellular carcinoma. Oncogene. 2003;22:1866-71 [DOI] [PubMed] [Google Scholar]
- 51. Seliger B, Handke D, Schabel E, Bukur J, Lichtenfels R, Dammann R. Epigenetic control of the ubiquitin carboxyl terminal hydrolase 1 in renal cell carcinoma. J Transl Med. 2009;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dammann R, Yang G, Pfeifer GP. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001;61:3105-9 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.