Abstract
Growth hormone (GH) controls hepatic physiology to a large extent through the transcription factor signal transducers and activators of transcription (STAT) 5. Here, we focus on lessons learned from the physiology and pathophysiology of mice with disrupted Ghr and Stat5 loci. We discuss that hepatosteatosis and hepatocellular carcinoma observed in the absence of STAT5 can be explained in part through an aberrant activation of STAT1 and STAT3, which in themselves promote cell proliferation and survival. We also argue that STAT5 can be a context-specific tumor suppressor as it negatively regulates cell cycle progression. Lastly, we discuss promiscuity between STAT members that permits a given cytokine receptor to activate different STATs and thereby elicit context-dependent biological responses.
Keywords: hepatosteatosis, hepatocellular carcinoma, growth hormone, STAT5, gene knockout
Introduction
Although cytokines can employ a diverse set of signaling components to convey their messages, members of the signal transducers and activators of transcription (STAT) appear to be their preferred venue of communication. These latent transcription factors are recruited to membrane-bound cytokine receptors and phosphorylated by tyrosine kinases from the JAK family at a single tyrosine residue. Phosphorylation of specific tyrosine residues facilitates the dimerization of STATs and subsequent nuclear translocation, a prerequisite to function as transcription factors. The peloton of STATs consists of 6 bona fide members, STATs 1 to 6, and the 2 isoforms of STAT5, STAT5A and STAT5B (referred to as STAT5 throughout this review). The 2 highly conserved STAT5 proteins, which serve to a large extent identical or overlapping functions, are encoded by 2 juxtaposed genes. In hepatocytes, growth hormone (GH) is the most prominent activator of STAT5, and their respective functions have been studied extensively in humans and mice. In humans, a large number of mutations in the GH-STAT5 machinery have been scouted, and in mice, mutations in the Ghr and Stat5 loci have been introduced into the germline and specifically into the liver genome. These loss-of-function mutations have been explored on a histological, functional, and genomic level. Here, we focus on how genetically modified mice shaped our understanding of cytokine signaling in hepatocytes, normal physiology, and disease. In particular, we discuss the molecular and functional consequences obtained upon loss of GH-STAT5 signaling.
Genetics of GHR-STAT5 Signaling
Dissecting molecular pathways emanating from the GHR
Germline manipulations in mice generating loss of the growth hormone receptor (GHR) as well as truncations and amino acid substitutions provided pivotal insight into GH biology,1-5 and liver-specific deletion of the Ghr 6 clarified to what extent the defects were cell autonomous, of systemic or of local nature. The GHR harbors 10 intracellular tyrosine residues that upon JAK2-mediated phosphorylation serve as docking sites for signaling components, including SOCS 1 and 3, SHC, SHP2, CIS, and STAT54 (Fig. 1A). In a set of elegant genetic experiments, Mike Waters and colleagues3 have introduced several mutations into the endogenous mouse Ghr gene and thereby assigned specific molecular components to defined physiological functions. These experiments have delineated unique and redundant functions of modules in the JAK2-STAT5 pathway. In mutant 569 (Ghr-569), the GHR was truncated at amino acid 569, and tyrosine residues 539 and 545 had been converted to phenylalanine (Fig. 1B). This mutant had retained 5 tyrosine residues, including docking sites for SOCS1 and SOCS3 and for SHC and SHP2, and residual STAT5 activation (~30%) was observed in liver tissue.4 In contrast, mutant 391 (Ghr-391) only retained 2 tyrosine residues (Fig. 1B), docking sites for SOCS1 and SOCS3, and it was unable to convey any detectable STAT5 activation.4 STAT3 activity observed with this mutant might be directly controlled by JAK2 and not require a tyrosine docking site. In the third mouse model, Box1 of the GHR, which is essential for JAK2 interaction, was mutated and inactivated (Fig. 1B).1 Homodimeric class 1 cytokine receptors can employ both JAK and Src family kinases (SFK), and a targeted mutation inactivating JAK2 binding to the GHR specifically shed light onto the relative importance of JAK2 versus SFKs.1 GH-induced activation of JAK2, STAT3, STAT5, and Akt was completely abrogated in GHR Box1 mutant mice, while the Src and ERK1/2 pathways appeared to be intact.1 The severity of postnatal growth retardation and adult-onset obesity in the absence of GH-mediated JAK2 signaling was comparable to that observed in germline Ghr-null mice. Circulating IGF-1 levels were greatly reduced in these mice, emphasizing the importance of the GHR-JAK2-STAT5 pathway in Igf-1 transcription. As expected from the absence of the negative IGF-1 loop, circulating GH levels were increased, which in itself could further activate Src and ERK1/2 signaling. The main difference between the GHR Box1 mutation and the 2 truncations described above is the absolute loss of STAT5 signaling in the former one.
Figure 1.
Structure of the growth hormone receptor (GHR) and emanating signaling pathways. (A) Intracellular domain structure of the GHR. Ten intracellular tyrosine residues are phosphorylated (p-Y) by JAK2 upon GH binding to its receptor. STAT5 docks to 4 p-Y residues (*; high-affinity p-Y sites interacting with STAT5).4 (B) Dissecting molecular signaling pathways emanating from the GHR. Mutant Ghr-569 is truncated at amino acid 569, and tyrosine residues 539 and 545 had been converted to phenylalanine. Thirty percent of STAT5 activation was retained by this mutant. Mutant Ghr-391 only retained the tyrosine residues binding SOCS1 and 3. Increased STAT3 phosphorylation observed with this mutation is possibly the result of direct JAK2 activation. JAK2 binding was impaired in the Box1 mutation. GH-induced activation of JAK2, STAT3, and STAT5 was completely abolished in this mutant.1 Dark circle = p-Y residues; light circle in mutant 569 = Y-F substitutions; light tetragon in mutant Box1 = mutation of JAK2 binding site in Box1; pale circle in mutant Box1 = nonphosphorylated tyrosine residues. While STAT5 activation and increased expression of STAT5 downstream targets Igf1 and Socs2 were obtained with the Ghr-569 mutation, STAT1 activation and elevated expression of respective target genes (e.g., Pparγ) were observed upon GH activation of Ghr-369. Similarly, GH activated preferentially STAT1 and STAT3 in hepatocytes lacking STAT5.7
Gene expression profiling of liver tissue from the different mutant mice permitted the assignment of gene classes to distinct signaling modules. Of the 90 GH-regulated hepatic genes, 52% were subject to STAT5 control, 27% were regulated by Src/ERK, and 14% were under the direct control of JAK2.1 However, it is necessary to keep in mind that some of the gene expression changes observed in the absence of STAT5, for example, PPARγ, are likely the result of increased STAT1 and STAT3 activity7 and will be discussed later.
Liver-specific deletion of the Stat5a/b locus
From the 2 STAT5 members, STAT5B is more abundant in the liver than STAT5A, and in mice, loss of the Stat5b gene resulted in severely reduced body growth8 and impaired liver metabolism.9-11 In contrast, loss of STAT5A had little impact on liver metabolism but severely disrupted mammary alveolar differentiation, which impacted lactation performance.12 Deletion of the entire Stat5a/b locus from the germline of mice resulted in perinatal lethality,13 which excluded the implementation of metabolic studies. However, studies on mice with a hepatocyte-specific deletion of the Stat5a/b locus7,14 provided in depth insight into the hepatoprotective role of these transcription factors and their responsibility in controlling liver metabolism.
Hepatosteatosis
Nonalcoholic fatty liver disease (NAFLD) has been clinically associated with GH deficiency and was reversed by GH administration in at least one patient.15 Moreover, NAFLD in patients carrying disabling GHR mutations (Laron syndrome) is not linked to the degree of obesity and cannot be reversed by chronic IGF-1 treatment.16 Mouse genetics has shed light onto mechanisms that link loss of GH and STAT5 signaling to NAFLD. Liver-specific loss of GHR6 or STAT57,17 signaling resulted in hepatosteatosis, which in some cases was exacerbated by the introduction of additional genetic18 or pharmacological17 lesions. Research on the different Ghr and Stat5 mutant mice helped to define the signaling modules key to GH’s contribution in preventing hepatosteatosis.1,2,6,7,17 Genetic loss of specific signaling modules was linked to changing gene expression patterns that helped to propose a plausible mechanism. As a note of caution, mice carrying the partially disabled Ghr genes were generated through a knock-in approach,1,2 and these mutations were passed on through the germline, thus affecting every cell. In contrast, in other studies, Alb-Cre transgenic mice were used to delete the Stat5 and Ghr loci specifically in liver tissue.6,7,17
The 3 GHR mutants displayed specific signaling deficiencies (Fig. 1B). While mutant Ghr-569 exhibited a partial loss of STAT5, mutants Ghr-391 and Box1 were unable to activate STAT5 using standard detection systems.2 Box1 mutant was unique in that it is unable to bind JAK2 and thus not competent in activating any JAK2-dependent pathway including those mediated by STATs 1, 3, and 5. Paucity of STAT5 signaling in Ghr-391 mice resulted in hepatosteatosis starting at 4 months of age and increased levels of alanin transaminase (ALT) and aspartate transaminase (AST) by 1 year of age. Hepatosteatosis in mice lacking STAT5 specifically in the liver was more severe than in Ghr-null and Ghr-391 mutant mice,2,7 which correlated with increased triglyceride levels in the former model. Gene expression profiling pointed to a molecular mechanism explaining the hepatosteatosis. Notably, increased fatty acid (FA) uptake by livers can be explained by the greatly elevated levels of CD36 in the absence of STAT5,2,7 and ChIP assays established that STAT5 binds to the Cd36 gene promoter and probably suppresses transcription.2 It is likely that elevated hepatic PPARγ levels7 also contribute to hepatosteatosis. While adenovirus-mediated overexpression of PPARγ in mice supports steatosis,19 loss of PPARγ reduced steatosis in AZIP mice.20,21
Although evidence supports the notion that many of the genes identified to be deregulated in hepatocytes from mutant mice with lower or no STAT5 activity, such as SOCS2, are bona fide STAT5 targets, the altered expression of others might be due to secondary events. Levels and activity of STATs 1 and 3 were increased in the absence of STAT5 (Fig. 2), which correlated with increased expression of respective targets, including the STAT1 target Pparγ and the STAT3 target cyclin D1.2,7 Concomitant loss of STAT5 and STAT1 restored cell proliferation but not the emergence of hepatosteatosis.7 In addition, lower expression of the STAT5 target gene Socs3, which resulted in excessive STAT3 activation, could contribute to the steatosis phenotype as liver-specific SOCS3-null mice develop steatosis.22 In summary, the hepatoprotective contribution of GH can be explained by STAT5’s contribution to ensure low levels of CD36 and thereby restricting lipid uptake as well as restraining PPARγ levels.
Figure 2.
Balancing the cell cycle through STAT3 and STAT5. GH-induced STAT5 activation triggers elevated transcription of Cdk2nb and Cdkn1a, encoding the cell cycle inhibitors p15 and p21, respectively. STAT5 activates these genes through binding to promoter-bound GAS motifs. STAT5 suppresses STAT3 signaling through the induction of SOCS3 levels. In the absence of STAT5, the expression of cell cycle inhibitors is decreased. Reduced SOCS3 levels result in increased STAT3 activation and the concomitant induction of STAT3 target gene CCND1 (cyclin D1), which contributes to increased cell cycle progression. Increased STAT3 activation also results in increased Bcl-x expression, providing a survival advantage. Loss of STAT5 also enhances expression and activation of STAT1 and corresponding downstream targets. Solid lines = signaling venues in control cells in the presence of STAT5; dotted lines = signaling venues adopted in the absence of STAT5.
Hepatocellular Carcinoma: Balancing the Cell Cycle through STAT3 and STAT5
In hematopoietic stem cells (HSCs), and their emerging lineages, STAT5 promotes cytokine-induced cell proliferation and survival.23 Notably, excessive and aberrant STAT5 activity has been linked to the development of leukemia. Ablation of STAT5 in established BCR-ABL–induced leukemia in mice results in complete remission,24 demonstrating a critical role for this transcription factor in the maintenance of hematopoietic disorders. Similarly, expression of a constitutively active form of STAT5 in mouse mammary tissue leads to pregnancy-independent functional alveolar growth and development of breast cancer.25 Loss of STAT5A from various mouse models of breast cancer modulates cancer progression,26-28 further supporting the notion that STAT5 positively controls cell proliferation and transformation. Likewise, constitutive STAT5 activity has been observed in a variety of solid tumors, including prostate.29
The development of fibrosis and acceleration of hepatocellular carcinoma (HCC) in mice with a liver-specific deletion of STAT5 upon CCl4 treatment were therefore an unexpected observation.7,17,18,30 This observation placed STAT5 for the first time into the category of tumor suppressors. In addition to liver, loss of STAT5 in mouse embryonic fibroblasts (MEFs) also led to enhanced cell proliferation.30 Context-specific functions of STAT5 can partly be explained through differential target gene expression, namely cyclin D1 and Bcl-x in promoting cell proliferation and survival, respectively. However, establishment of fibrosis and HCC in the absence of STAT5 is likely the result of several deregulated entry points (Fig. 2). In the liver and MEFs, STAT5 directly activates the Cdkn1a and Cdkn2b genes,30 and reduced levels of the encoded cell cycle inhibitors p15 and p21 in the absence of STAT5 correlate with enhanced cell proliferation. In addition, excessive activation of STAT3 and STAT1 in the absence of STAT5 yields additional cell proliferation and survival cues through the activation of successive genetic cascades.7,17 Lastly, reduced expression of SOCS3 in the absence of STAT5 results in enhanced STAT3 signaling with concomitant increase of cyclin D1.7
Reduced levels of SOCS3 in human HCC are the result of impaired expression of aberrantly methylated SOCS3 genes, and molecular clinicians have recognized the inverse relationship between SOCS3 and STAT3 in the progression of HCC.35-37 This inverse relationship and the resulting consequences on liver metabolism and pathology have been studied in mice lacking the Socs3 gene specifically in the liver.31,32 Loss of SOCS3 yields increased levels of STAT3 signaling, with a secondary consequence of elevated TGF-β levels and enhanced chemical-induced liver fibrosis.32 Intriguingly, enhanced liver fibrosis and CCl4-induced HCC development in liver-specific STAT5-null mice were also linked to increased TGF-β levels.17 In this context, increased TGF-β levels are the outcome of one of STAT5’s noncanonical functions. STAT5, through its N-terminus, binds TGF-β, which reduces its stability.17 In the SOCS3-null mouse model, it was suggested that STAT3 acted as a transcriptional activator of the Tgfβ1 gene.32 The development of fibrosis and HCC upon the deletion of STAT5 from the liver can be attributed to the derailment of several molecular pathways. First and foremost, increased STAT3 activation and TGF-β levels observed in the absence of STAT5 are likely drivers in the development of liver fibrosis and HCC.17 STAT5 likely executes its liver-protective task in synergy with other components, as supported by the combined absence of STAT5 and the multidrug resistance gene 2 (Mdr2), which results in early and severe liver fibrosis and the concomitant blunting of pertinent hepatoprotective genes, including Egfr, hepatocyte nuclear factor 6 (Hnf6), Lifr, and Prlr.18
Excessive STAT3 activation appears to be the common denominator in liver-specific STAT5- and SCOS3-null mice, suggesting that STAT3 is a driver in chemical-induced liver disease observed in these models. This proposal is supported through the use of an independent mouse model based on the hepatoprotective role of the NF-κB venue.33 Liver-specific loss of the NF-κB activating kinase IKKB results in accelerated HCC development, which coincides with STAT3 activation.33 Ablation of STAT3 prevented HCC formation, thus conceptually supporting the negative crosstalk between NF-κB and STAT3.
Plasticity of GH-STAT Signaling
In general, cytokine receptors display promiscuity as they can differentially activate more than one member of the STAT family. It can be predicted that molecular consequences observed in cells exposed to cytokines will reflect the integration of individual signals imposed by each activated STAT member. For example, if most of the STAT activity upon cytokine stimulation is composed of STAT5, cells will experience a STAT5-driven biology. If the activation profile shifts to STAT1 or STAT3, biological consequences will shift accordingly (Figs. 2 and 3). The observation that cytokine receptors display an inherent promiscuity and activate context-dependent redundant and unique STAT signals has implications for the quality of cytokine signaling in any given cell type as the biological output likely depends on the relative abundance of the different STAT members. Liver-specific loss of the Stat5 locus exemplified the consequences of a shifting STAT-signaling web.2,7,17,30 In control liver (and in MEFs), STAT5 activates the Cdkn2b and Cdkn1a genes, which negatively impacts cell cycle progression and curtails cell proliferation. In addition, GH-STAT5–controlled expression of Socs3 suppresses STAT3 signaling, another proponent of cell proliferation through its downstream target cyclin D1 (Fig. 2).
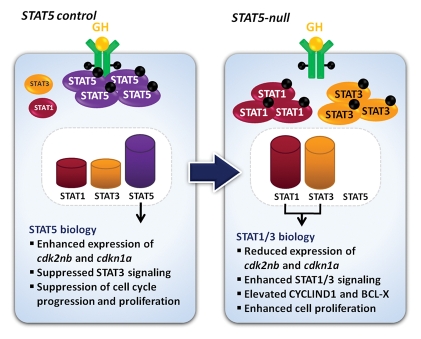
Figure 3.
Plasticity of GH-STAT signaling. In control cells, GH preferentially activates STAT5 and thereby promotes a “STAT5 biology” (left panel). However, in the absence of STAT5, STAT1 and STAT3 can be recruited and activated by the GHR, and GH can elicit a STAT1/3 biology as characterized by the activation of respective target genes. Thus, a concentration shift of different STAT members can result in a changing biology induced by a given cytokine.
In summary, STAT5 controls the cell cycle in a context-specific fashion through primary (transcriptional) and secondary (restricting other STATs) events. Notably, loss of STAT5 creates a void on phosphotyrosine residues of the GHR, which is filled by STAT1 and STAT3, and their aberrant activation induces hepatosteatotic and proliferative programs. Thus, reduced or loss of expression of a single member of the STAT family has far-reaching consequences that go beyond the loss of regulation of direct target genes. In the absence of a specific STAT, the corresponding receptor can now recruit and activate other members of this family. This concept has been extended to STAT6, which is activated by IL-7 only in the absence of STAT5, and its function is at least partially redundant with STAT5.34
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by the NIH (Intramural Program) and the WCU Project [grant number R33-10059] through the NRF, South Korea.
References
- 1. Barclay JL, Kerr LM, Arthur L, et al. In vivo targeting of the growth hormone receptor (GHR) Box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol Endocrinol. 2010;24:204-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barclay JL, Nelson CN, Ishikawa M, et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181-92 [DOI] [PubMed] [Google Scholar]
- 3. Rowland JE, Kerr LM, White M, Noakes PG, Waters MJ. Heterozygote effects in mice with partial truncations in the growth hormone receptor cytoplasmic domain: assessment of growth parameters and phenotype. Endocrinology. 2005;146:5278-86 [DOI] [PubMed] [Google Scholar]
- 4. Rowland JE, Lichanska AM, Kerr LM, et al. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol. 2005;25:66-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94:13215-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui Y, Hosui A, Sun R, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504-13 [DOI] [PubMed] [Google Scholar]
- 8. Udy GB, Towers RP, Snell RG, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333-51 [DOI] [PubMed] [Google Scholar]
- 10. Clodfelter KH, Miles GD, Wauthier V, et al. Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics. 2007;31:63-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179-86 [DOI] [PubMed] [Google Scholar]
- 13. Cui Y, Riedlinger G, Miyoshi K, et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engblom D, Kornfeld JW, Schwake L, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21:1157-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takahashi Y, Iida K, Takahashi K, et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132:938-43 [DOI] [PubMed] [Google Scholar]
- 16. Laron Z, Ginsberg S, Webb M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion: preliminary report. Growth Horm IGF Res. 2008;18:434-8 [DOI] [PubMed] [Google Scholar]
- 17. Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J Exp Med. 2009;206:819-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blaas L, Kornfeld JW, Schramek D, et al. Disruption of the growth hormone–signal transducer and activator of transcription 5–insulin like growth factor 1 axis severely aggravates liver fibrosis in a mouse model of cholestasis. Hepatology. 2010;51:1319-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) over expression. J Biol Chem. 2003;278:498-505 [DOI] [PubMed] [Google Scholar]
- 20. Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268-76 [DOI] [PubMed] [Google Scholar]
- 22. Sachithanandan N, Fam BC, Fynch S, et al. Liver-specific suppressor of cytokine signaling-3 deletion in mice enhances hepatic insulin sensitivity and lipogenesis resulting in fatty liver and obesity. Hepatology. 2010;52:1632-42 [DOI] [PubMed] [Google Scholar]
- 23. Wierenga AT, Vellenga E, Schuringa JJ. Maximal STAT5-induced proliferation and self-renewal at intermediate STAT5 activity levels. Mol Cell Biol. 2008;28:6668-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoelbl A, Schuster C, Kovacic B, et al. Stat5 is indispensable for the maintenance of bcr/abl-positive leukaemia. EMBO Mol Med. 2010;2:98-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vafaizadeh V, Klemmt P, Brendel C, et al. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010;28:928-38 [DOI] [PubMed] [Google Scholar]
- 26. Miermont AM, Parrish AR, Furth PA. Role of ERalpha in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis. Carcinogenesis. 2010;31:1124-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Humphreys RC, Hennighausen L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999;10:685-94 [PubMed] [Google Scholar]
- 28. Humphreys RC, Hennighausen L. Transforming growth factor alpha and mouse models of human breast cancer. Oncogene. 2000;19:1085-91 [DOI] [PubMed] [Google Scholar]
- 29. Tan SH, Dagvadorj A, Shen F, et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68:236-48 [DOI] [PubMed] [Google Scholar]
- 30. Yu JH, Zhu BM, Wickre M, et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology. 2010;52:1808-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogata H, Kobayashi T, Chinen T, et al. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179-93 [DOI] [PubMed] [Google Scholar]
- 32. Ogata H, Chinen T, Yoshida T, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520-30 [DOI] [PubMed] [Google Scholar]
- 33. He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park JH, Adoro S, Guinter T, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niwa Y, Kanda H, Shikauchi Y, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406-17 [DOI] [PubMed] [Google Scholar]
- 36. Elliott J. SOCS3 in liver regeneration and hepatocarcinoma. Mol Interv. 2008;1:19-21 [DOI] [PubMed] [Google Scholar]
- 37. Riehle KJ, Campbell JS, McMahan RS, et al. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91-103 [DOI] [PMC free article] [PubMed] [Google Scholar]