Abstract
Current studies of particulate matter (PM) are confounded by the fact that PM is a complex mixture of primary (crustal material, soot, metals) and secondary (nitrates, sulfates and organics formed in the atmosphere) compounds with considerable variance in composition by sources and location. We have developed a laboratory-based PM that is replicable, does not contain dust or metals and that can be used to study specific health effects of PM composition in animal models. We exposed both neonatal (7 days of age) and adult rats to a single 6-hr exposure of laboratory generated fine diffusion flame soot (DFP; 170 ug/m3), or filtered air. Pulmonary gene and protein expression as well as indicators of cytotoxicity were evaluated 24 hours after exposure. Although DFP exposure did not alter airway epithelial cell composition in either neonates or adults, increased LDH activity was found in the bronchoalveolar lavage fluid of neonates indicating an age-specific increase in susceptibility. In adults, 16 genes were differentially expressed as a result of DFP exposure while only 6 genes were altered in the airways of neonates. Glutamate cytsteine ligase protein was increased in abundance in both DFP exposed neonates and adults indicating an initiation of antioxidant responses involving the synthesis of glutathione. DFP significantly decreased catalase gene expression in adult airways, although catalase protein expression was increased by DFP in both neonates and adults. We conclude that key airway antioxidant enzymes undergo changes in expression in response to a moderate PM exposure that does not cause frank epithelial injury and that neonates have a different response pattern than adults.
Keywords: Bronchiolar, lung development, particulate matter and antioxidant
INTRODUCTION
Over a quarter of the U.S. population (28%) lives in areas with unhealthful short-term levels of PM (ALA, 2009). Fine particles, less than 2.5 μm (PM2.5), have been linked to the development of respiratory infections, exacerbation of asthma and increased risk of hospitalization and premature death (ALA, 2009). Although air quality standards have been in place for over a decade, many states and counties are at non-attainment levels for either coarse PM10 (USEPA, 2008) and/or fine PM2.5 (USEPA, 2009).
Compared to adults, young physically active children are a high risk group for adverse effects from PM (Oosterlee et al., 1996). The developing lung may be susceptible due to the continual and extensive differentiation and maturation of over 40 cell types during perinatal development. Children have higher minute ventilation and are aerobically active outdoors. Their small body size and smaller mean airway diameter with increased air exchange can result in increased particle deposition and total dose (Branis et al., 2009). Even in healthy individuals, a single short term exposure to PM induces lung inflammation (Ghio et al., 2000; Samet et al., 2009). Children who live in areas with high levels of short term fine particulate pollution, primarily from vehicular traffic (i.e. near roadways), have increased morbidity and mortality from respiratory illnesses such as bronchitis and pneumonia in a dose-dependent manner (Ciccone et al., 1998). Additionally, doctor diagnosed constrictive airway disease (asthma) is significantly greater in children exposed to traffic generated air pollution (van Vliet et al., 1997). The focus of the current study was the conducting airways of the lung, a region that is the target of deposited particulate matter and that is the primary site for constrictive airway diseases such as asthma. The conducting airways undergo substantial shifts in antioxidant protein expression in the postnatal period (Fanucchi et al., 2000). The biologic mechanisms by which fine PM causes conducting airway health effects are still largely unknown.
PM is a complex mixture that varies greatly by location, source, time of day, season and climate. This complicates the systematic study of health effects. Outdoor PM contains allergens, metals, soil and carbonaceous particles (soot) generated from combustion. Hydrocarbons and polycyclic aromatic hydrocarbons (PAH) may be bound to the surfaces of soot particles as a result of incomplete combustion. Hydrocarbons associated with soot undergo chemical changes upon mixing and aging in the outdoor environment. In addition to binding to the surfaces of particles, PAHs are also incorporated within PM (Billet et al., 2007) and some are also free in the vapor phase of the atmosphere. The dominant contributor to the overall concentration of particles in urban and metropolitan areas is vehicle combustion exhaust (Pey et al., 2009). However, the nature of these particles changes with driving conditions, fuel type, vehicle size and engine technology. Madden points out that evolving regulations – and the concomitant response in technology – have caused considerable changes in the nature of diesel emissions (Madden, 2008). Due to the variability in engine emissions, it is desirable to include a standardized surrogate for toxicity testing of combustion-generated aerosols and associated PAH. In response to this need, we have used a diffusion flame system (Pinkerton et al., 2008) to generate diffusion flame particulate matter (DFP). DFP are combustion-derived fine soot particles composed primarily of elemental carbon with a low polycyclic aromatic hydrocarbon (PAH) content that are generated in a laboratory. These allow modification of PM composition (i.e. PAH content, metals, additional pollutant gases) as well as repeated experiments to define mechanisms of effect. The ability to reproducibly compare biologic responses solely from a particulate source without potential confounders like weather, variation in ambient air quality, allergens, or metals present in field samples is key to conducting reproducible mechanistic studies, especially in animals at early stages of postnatal development.
A common response to atmospheres containing combustion products including PM and PAHs is lung oxidative stress (Billet et al., 2007; Na and Cocker, 2005). This may be due to the generation of reactive oxygen species (ROS), oxygen-containing compounds including superoxides, peroxides and hydroxyl radicals within the PM (Becker et al., 2005). In the adult lung, the presence of oxidative stressors upregulates key antioxidant enzyme systems (Harding R, 2004). Several enzymes including the peroxidase, peroxiredoxin, and the superoxide dismustase families, as well as catalase and glutamate-cysteine ligase (the rate limiting step in glutathione synthesis) can be induced by oxidative stress (Claiborne et al., 1999; Weisiger and Fridovich, 1973). Toxicologic studies have shown that these systems work in concert to reduce oxidants as well as maintain cellular homeostasis.
We hypothesized that neonatal (7 day postnatal age) and adult rat airways will respond differentially to DFP exposure. We further hypothesized that an inability to alter the normal enzyme expression pattern to maintain homeostasis would be responsible for increased neonatal airway epithelial susceptibility to fine PM. In the current study, we compared cytotoxicity, oxidative stress and dextoxification responses in the airways of neonatal and adult Sprague Dawley rats exposed to DFP or filtered air (FA). The goals of this study are threefold: 1) to generate an inhalation exposure atmosphere containing PM that is low in PAH and high in elemental carbon, 2) to define the effect of inhalation of a low level of fine PM on cytotoxicity and antioxidant systems of the airways and 3) to determine whether the nature of the cytotoxic and antioxidant response varies by age.
METHODS
Flame and particle characterization
Diffusion flame particles (DFP) were generated using a co-annular diffusion flame burner. The burner consists of a 7.1mm tube (inner diameter) surrounded by an 88.9mm concentric outer tube (inner diameter). The burner is enclosed in Pyrex tubing to isolate the burner from ambient air. Ethylene was metered through the inner tube at 220–235cm3/min using mass flow controller (model 647C flow control unit and model 1179A flow control valve, MKS Instruments, Andover, MA). Filtered and dried air was metered at 30L/min using a Fisher and Porter variable area flowmeter (Andrews Glass, Vineland, NJ) and delivered around the circumference of the burner chamber.
DFP are then channeled to a mixing chamber via stainless steel flex tubing, where they are combined with clean air and introduced to the inhalation exposure chamber. DFP combined with CBR (chemical/biological/radiological treatment) filtered room air entered the exposure chamber were the subject animals were exposed whole body. Chamber CO levels were monitored using a Teledyne-API Model 300E CO analyzer (San Diego, CA) and was calibrated with an NIST traceable span gas of 202.4 ppm CO diluted in ultra-pure air to 10 ppm CO for calibration (Scott-Marrin Inc., Riverside, CA). Chamber NOx levels were monitored (Dasibi 2108 Chemiluminescence NOx Analyzer, Glendale, CA). DFP was collected directly from the exposure chamber for analysis though ports in the chamber wall. Particle number concentration was determined using a condensation particle counter (CPC, TSI model 3775, Shoreview, MN). Particle size distribution was determined using a scanning mobility particle sizer (SMPS) (model 3080 electrostatic classifier with model 3081 differential mobility analyzer, and a model 3020 CPC, TSI, Shoreview, MN).
DFP mass concentration was determined by collecting particles from the chamber on glass fiber filters (Pallflex Emfab™ 47mm filters, Ann Arbor, MI) placed in a filter housing (BGI, Waltham, MA). The sampling flow rate is set at 20L/min air flow rate driven by a vacuum source downstream of the flow. Collection was performed for the duration of the exposure. Total particulate mass was determined gravimetrically (Sartorius AG MC5 microbalance, Goettingen, Germany). Mass concentrations were also determined for the filtered air control chamber. DFP for elemental carbon to organic carbon ratio (EC/OC) analysis was collected on 47mm glass fiber filters (Pallflex Tissuequartz™, Ann Arbor, MI) as described above. The EC/OC ratio was determined using a method previously described (Herner et al., 2005; Robert et al., 2007). Particle and vapor phase PAH speciation was performed by the Desert Research Institute (DRI, Reno, NV). DFP were collected on Pallflex Tissuequartz™ 47mm filters (Ann Arbor, MI) and vapor phase organic compounds were collected on XAD resin supplied by DRI (Reno, NV).
Transmission Electron Microscopy (TEM)
DFP were sampled via an electrostatic precipitator (ESP) similar in design to that described previously by (Morrow and Mercer, 1964). Particles were sampled from the exposure chamber using ¼″ inch conductive tubing and drawn through the ESP using a vacuum pump. The sampling flow rate was 100cm3/min and the ion current was set to 3.5 μA. The morphology of soot particles were analyzed by transmission electron microscopy (TEM, Phillips CM-12, LaB6 cathode, operated at 120 kV) and carbon coated copper grids were used for particle sampling (300 mesh, lacey carbon type-A substrate, Ted Pella Inc. Redding, CA).
Animals and exposure protocol
Adult (2.5 months) and newborn postnatal male Sprague Dawley rats with accompanying dams were obtained from Harlan Laboratories and allowed to acclimate in CBR (chemical/biological/radiological) filtered air until newborn pups reached 7 days of age. Animals were acutely exposed in a whole-body chamber to either 6 hours of filtered air (FA) or an atmosphere of 170μg/m3 DFP. Two identical custom built exposure chambers were used for the exposure experiments, one chamber housed DFP exposed animals and the other housed age matched filtered air control animals (Hinners et al., 1968). The stainless steel chambers have a volume of 3.8m3. A mixing chamber located at the top of the animal chamber where CBR filtered room air was mixed with DFP. Chambers were maintained at −0.3 inches of H2O gauge pressure and temperature were maintained between 22.2°C and 24.4°C. The filtered air flow rate through the chamber was set at 30 air exchanges per hour. During exposure, adult rats were housed in stainless steel wire cages. Due to size differences, 7 day old postnatal rats were housed with lactating females in polycarbonate cages with wire lids; shredded Kimwipes® (Kimberley-Clark, Neenah, WI) were used for bedding during the exposure period. Cages were arranged in the chamber in a single level. Adult rats were provided with Laboratory Rodent Diet (Purina Mills, St. Louis, MO) and water ad libitum. All animal experiments were performed under protocols approved by the University of California Davis IACUC in accordance with NIH guidelines. Animals were necropsied 24 hours following cessation of the 6 hour exposure. All animals were euthanized through intraperitoneal injection of an overdose of pentobarbital (150 mg/kg). At necropsy, tracheas were cannulated, thorax opened and lung removed en bloc for processing.
Lactate Dehydrogenase cytotoxicity assay
Bronchoalveolar lavage fluid (BALF) was collected through recovery of intratracheal instillations of Hank’s Buffered Salt Solution (Gibco, Carlsbad, CA) at 35μl/g body weight concentration. Lactate dehydrogenase (LDH) activity in the BALF was detected using a LDH Cytotoxicity Assay Kit (Cayman Chemical Company, Ann Harbor, MI) following manufacturer instructions. LDH activity (mU) was normalized to protein concentration using a Bradford Protein Assay (Bio-Rad, Hercules, CA).
Histology
Lungs were inflation-fixed with 37% formaldehyde vapor bubbled under 30 cm hydrostatic pressure for 1 hour as described previously (Hammond and Mobbs, 1984; Wilson et al., 2001). The tracheas were tied off and the lungs immersed in 1% paraformaldehyde and stored at 4°C until embedment into Araldite 502 (Electron Microscopy Sciences, Hatfield, PA). Sections (2.0 μm) were stained with a solution containing 1% methylene blue, 1% azure II and 0.5% sodium borate for high resolution histological and morphometric analysis.
Morphometry
The abundance of normal and cytotoxic (vacuolated) airway epithelial cells was analyzed using high resolution images and morphometric procedures previously used and discussed in detail by (Hyde et al., 1990; Plopper et al., 1992). All the measurements were made using 2.0μm resin sections. Midlevel (intrapulmonary generation 3–4) main axial pathway airway epithelial cells were counted. The entire circumference of each defined airway generation was imaged at 60× magnification and a minimum of 10 fields were sampled for counting using a random number table and a uniform random sampling scheme (Howard and Reed, 1998). The volume densities (Vv) of five categories of cells (basal, mucous, nonciliated, ciliated, and vacuolated) were defined by point (P) and intercept (I) counting of bronchial epithelial vertical profiles using a cycloid grid and Stereology Toolbox (Morphometrix, Davis, CA) on collected images. Vv was calculated using the formula:
where Pp is the point fraction of Pn, the number of test points hitting the structure of interest, divided by Pt, the total points hitting the reference space (epithelium). The surface area of epithelial basement membrane per reference volume (Sv) is determined by point and intercept counting and calculated using the formula:
where Io is the number of intersections with the object (epithelial basal lamina) and Lr is the length of the test line in the reference volume (epithelium). The thickness of the epithelium, or volume per unit area (Vs) of basal lamina (μm3/μm2), was calculated using the formula for arithmetic mean thickness (τ):
RT-profiler arrays on microdissected airways
For RNA isolation, lungs were filled to capacity with and stored in RNAlater (Ambion, Austin, TX) at −20°C until microdissection. RNAlater stabilized intrapulmonary airways from the lobar bronchus to the terminal bronchioles were dissected free from the surround parenchyma as described in (Baker et al., 2004). Airway enriched RNA was isolated using Tri Reagent® (Molecular Research Center, Inc., Cincinnati, OH) following the manufacturer’s protocol based on the method detailed in (Chomczynski and Sacchi, 1987). Quantification of gene expression was performed using RT2 SYBR® green qPCR master mix (SABiosciences, Frederick, MD) on RT2 qPCR Arrays (cat# PARN-003, PARN-065) strictly following manufacturer instructions (SABiosciences, Frederick, MD). A complete list of genes assayed is available (Supplemental Tables 3S, 4S and 5S). Results were calculated using the comparative Ct method (Livak and Schmittgen, 2001) and RT2 Profiler PCR Array Data Analysis (SABiosciences, Ferderick, MD) using Hypoxanthine-guanine phosphoribosyltransferase (HPRT) as the reference gene. HPRT was chosen as the reference gene due to its consistency and the low variance between groups assayed an independent experiment (Supplemental Table 6S). Results are expressed as a fold change in gene expression relative to filtered animals of the same age, unless otherwise stated.
Immunohistochemistry
Lung tissue prepared for immunohistochemical analysis were inflated with 37% formaldehyde vapor bubbled under 30cm hydrostatic pressure for 1 hour and stored in 1% paraformaldehyde for less than 24 hours prior to processing and paraffin embedment. Paraffin sections were immunostained using the following antibodies: rabbit anti-glutamate cysteine ligase (Neomarkers, Fremont, CA) at 1:600, rabbit anti-glutathione S-transferase mu isoform (Novocastra, Bannockburn, IL) at 1:1200, sheep anti-catalase (The Binding Site, San Diego, CA) at 1:3000, rabbit anti-glutathione peroxidase-1 (Abcam, Cambridge, MA) at 1:1000 and mouse anti-PCNA (Dako, Carpinteria CA) at 1:600 using methods as previously described (Van Winkle et al., 1996). Sections from all groups were stained simultaneously to minimize variability in between runs. The concentration of primary antibody was determined through a series of dilutions to optimize staining density and minimal background and to allow for detection of either increases or decreases in protein expression with DFP exposure. The procedure was performed according to manufacturer instructions (Vector Labs, Burlingame, CA) with several alterations as outlined. Following tissue hydration, endogenous peroxidase activity was quenched with a 10% solution of hydrogen peroxide. To eliminate nonspecific primary antibody binding, tissue sections were blocked with bovine serum albumin. Primary antibodies were allowed to incubate at 4°C overnight. Signal was visualized using the Vectastain ABC kit and nickel chloride enhanced 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical, St. Louis, MO) as the chromagen. Controls included substitution of primary antibody with phosphate buffered saline to ensure specific positive staining.
Statistics
All data are reported as the mean ± standard error of the mean (S.E.M.) unless otherwise stated. Comparison between groups was done using two-way factorial analysis of variance (ANOVA) with Fisher’s Protected Least Significant Difference post hoc method to determine significance. In cases where a significant age by exposure interaction were observed, each factor was analyzed separately using a one-way ANOVA, followed by post-hoc analysis using PLSD. All statistical functions were performed using Statview (SAS, Cary, NC). A value of P < 0.05 was considered statistically significant.
RESULTS
Characterization of Diffusion Flame generated atmosphere
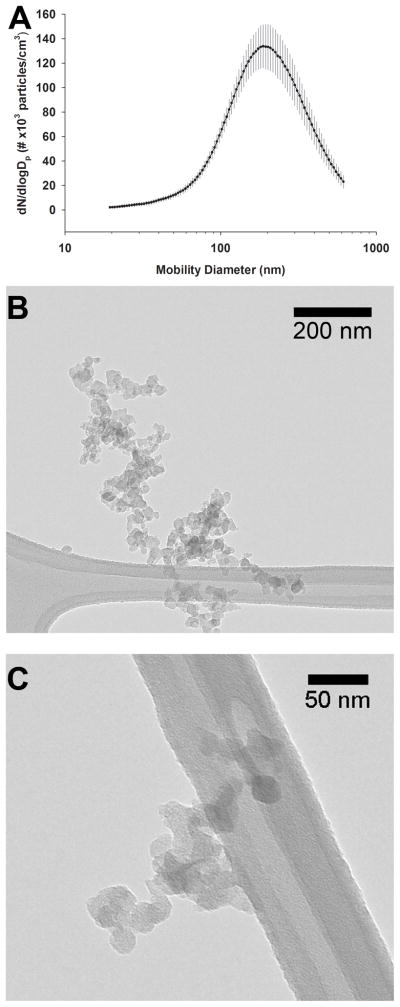
Rats were whole body exposed for 6 hours to a diffusion flame generated atmosphere; dams were exposed with their pups. The atmosphere contained both particles and gases. Both were characterized (Figure 1, Supplemental Table 1S and 2S) and the abundance reported is the average of two collections. There was a mean concentration of 4.4 × 104 ± 1.0 × 104 particles/cm3 (mean ± SD) based on CPC measurements over duration of exposure and a mass concentration for the of 170 ± 7 μg/m3 (mean ± SD) based on filter measurement. These particles were high in elemental carbon and had an EC/OC ratio of 8.6. The geometric mean mobility diameter was 192.8 ± 1.9 nm (geometic mean ± geometric SD) (Figure 1A). DFP exposure chamber CO levels were within 0.2 ppm of the FA chamber levels, with quantification below 0.2 ppm limited by instrument accuracy. Chamber NOx levels had an exposure concentration of 0.15 ppm NO and 0.07 ppm NO2. The amount of total PAH attached to PM was 19 ng/m3 and the amount of gas phase PAH was 225 ng/m3. The 20 most abundant vapor phase and particulate phase PAHs are listed (Supplemental Table 1S and 2S). In general, biphenyls and naphthalene-like compounds dominated the vapor phase and pyrenes dominated the particulate phase. Typical morphologies of DFP are shown (Figure 1B, C) where the particles are composed of 20–40 nm round primary particles forming larger fractal aggregates. The particle morphology resembled diesel exhaust (Chen et al., 2005).
Figure 1.
Particle characterization. Mobility size distribution of the particles in the exposure chamber indicates a geometric mean particle size of 192 nm (A) Values are expressed as mean ± SD. Electron micrographs (B, C) of the particle morphology indicate that the particles varied in shape and that they consisted of primary particles 20–40 nm in diameter and formed larger fractal aggregates.
Cytotoxicity of Diffusion Flame Particles
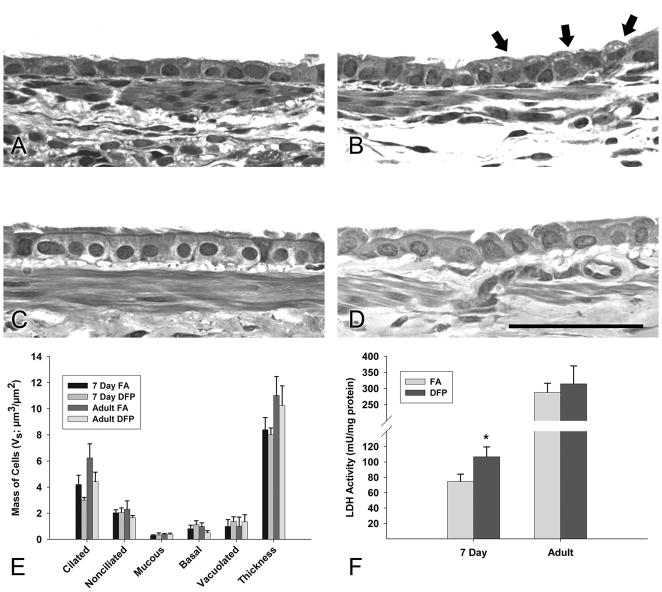
To determine whether exposure to diffusion flame particles (DFP) causes overt cytotoxicity in the airway epithelium, we compared morphologic changes, including the mass of cytotoxic cells containing vacuoles, in neonatal and adult rats 24 hours after an acute 6 hour DFP exposure (Figure 2). The epithelium in adult rats was morphologically similar to neonatal rats (compare Figure 2A with Figure 2C). Very few vacuolated cells were observed in adult DFP exposed rats (Figure 2D) but focal areas of vacuolated cells was found in the large airways of the neonatal rats (Figure 2B). Using high resolution microscopy on 2.0 μm resin sections and morphometric approaches, we measured the airway epithelium for morphometric changes in cell mass (Vs). Basal, mucous, nonciliated, ciliated and vacuolated cells were counted (Howard and Reed, 1998). In neonatal rats reared in filtered air (FA), ciliated and nonciliated cells populated the majority of the simple cuboidal epithelium in the large airways (Figure 2E). After DFP exposure, there was a trend towards a decrease in ciliated cells in neonatal rats, abundance of nonciliated cells was not affected by exposure. Adult rats were morphologically similar to postnatal rats in their abundance of ciliated and nonciliated cells (Figure 2C). DFP exposure also insignificantly decreased ciliated cells in adults while nonciliated cell mass remained identical. Basal, mucous, and total epithelial thickness were unaffected by DFP exposure in large airways (Figure 2E). When we measured a more global marker of lung cell injury, lactate dehydrogenase (LDH) activity, in bronchoalveolar lavage fluid (BALF), we found significantly increased LDH activity in DFP exposed neonatal rats (Figure 2F) but DFP exposed adult rats had no change in BALF LDH activity. Basal levels of LDH release were higher in FA adult rats than in neonatal rats.
Figure 2.
Morphologic changes in 7 day postnatal and adult rats 24 hours following DFP exposure. Resin sections were analyzed at 60× magnification for histologic changes. (A) In 7 day old postnatal rats reared in filtered air, a simple cuboidal epithelium of mostly ciliated and nonciliated cells was observed in the proximal airways. (B) After DFP exposure, more vacoulated cells were present (indicated by arrows). The epithelium of the large conducting airways was morphologically similar for filtered air (C) and DFP exposed (D) adult rats. Scale bar is 50μm. (E) Cell morphologies of five types: basal, mucous, nonciliated, ciliated, and vacuolated were quantified using point and intercept counts in large intrapulmonray airways. Calculated cell masses are shown (Vs). Although vacoulated cell mass increased and ciliated cell mass decreased following DFP exposure compared to filtered air controls, interactions between exposures were insignificant in both ages. Basal, mucous, and nonciliated cell mass in addition to epithelial thickness remained relatively consistent between exposure groups. Data are mean ± SEM (n=6 rats/group). (F) Lactate dehydrogenase (LDH), a marker for cytotoxicity, was quantified in bronchioalveolar lavage fluid. Significantly more LDH was detected in DFP exposed 7 day old rats, but was not changed in adult animals. Data are plotted as means ± SEM (n=6 rats/group). * = P <0.05, as compared to filtered air controls of the same age.
Oxidative Stress and Antioxidant Response
To determine oxidative stress and antioxidant responses in the conducting airways of animals exposed to DFP, we measured gene transcription and defined protein location in neonatal and adult rats. Using ‘Oxidative Stress and Antioxidant Defense’ and ‘Stress and Toxicity PathwayFinder’ RT2 RT-profiler qPCR arrays (SABiosciences, Frederick, MD), we quantified a total of 162 genes in RNA from microdissected airway trees. Of these genes, the mean expression levels of 65 genes were determined to be statistically significantly different either between ages or with exposure (Figure 3, Tables 3S–5S in the online supplement). However the pattern of changes was dissimilar between neonatal and adult rats exposed to PM. Six genes were significantly increased by DFP exposure in neonatal rats (Table 1). Most of these genes were classified as antioxidant and oxidative stress response genes in the peroxiredoxin and superoxide dismutase families. A complete list of fold changes for DFP exposed compared to FA for neonatal rats is available in the online supplement (Table 3S). Compared to neonatal rats, adults had a more robust response to DFP exposure; 17 genes were significantly changed as a result of exposure (Table 2). Similar to neonatal rats, most changed genes were antioxidant and oxidative stress response genes in the peroxiredoxin and peroxidase families. Fold changes with DFP exposure for the adult rats is available in the online supplement (Table 4S). 33 genes were significantly different within the airways by age and these are listed in the online supplement (Table 5S) and included genes involved in cell proliferation (PCNA) and metabolism (P450s).
Figure 3.
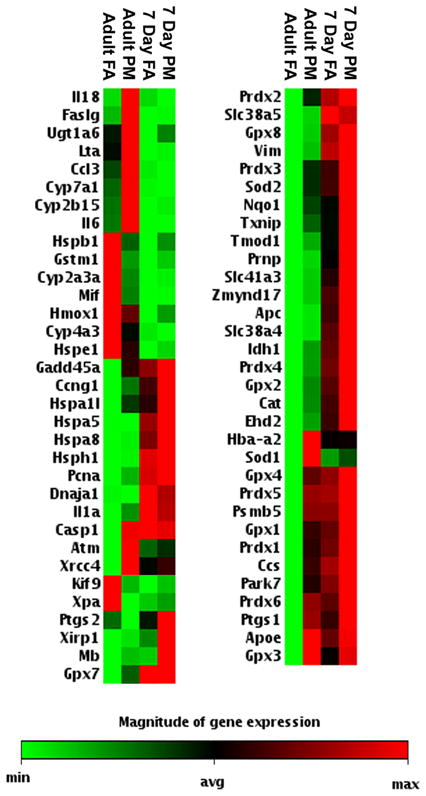
Heatmaps of all genes that were differentially expressed in the airways of rats as a combination of age or exposure. RNA was isolated from microdissected intrapulmonary airways obtained from RNAlater stabilized lung tissue and quantified using the RT2 qPCR Array platform (SABiosciences, Frederick, MD). All filtered air adult genes were compared against 7 day old filtered air controls using HPRT as the reference gene. Each age exposed to DFP was also compared to its respective age matched FA control tissue. A heat map of all genes that were differentially expressed at a P value less than 0.05 is shown. The relative magnitude of expression is indicated on a spectrum ranging from minimum (green) to the maximum detected (red). Expression patterns in 65 genes differed significantly as a combination of age and/or exposure effects (n=3–5 rats/group).
Table 1.
DFP-induced alterations in airway gene expression in 7-day old rats.
| Symbol | Refseq | Gene Name | Fold Regulation | P-value | Classification |
|---|---|---|---|---|---|
| Tmod1 | NM_013044 | Tropomodulin 1 | 1.96 | 0.014 | Antioxidant / Oxidative Stress Response |
| Prdx3 | NM_022540 | Peroxiredoxin 3 | 1.57 | 0.036 | Antioxidant / Oxidative Stress Response |
| Sod2 | NM_017051 | Superoxide dismutase 2, mitochondrial | 1.52 | 0.049 | Antioxidant / Oxidative Stress Response |
| Sod1 | NM_017050 | Superoxide dismutase 1, soluble | 1.18 | 0.032 | Antioxidant / Oxidative Stress Response |
| Xirp1 | XM_236702 | Xin actin-binding repeat containing 1 | 3.33 | 0.012 | Cell Structure |
| Mb | NM_021588 | Myoglobin | 9.98 | 0.022 | Oxygen Transporter |
Table 2.
DFP-induced alterations in airway gene expression in adult rats.
| Symbol | Refseq | Gene Name | Fold Regulation | P-value | Classification |
|---|---|---|---|---|---|
| Kif9 | XM_001077207 | Kinesin family member 9 | 2.18 | 0.044 | Antioxidant / Oxidative Stress Response |
| Prdx1 | NM_057114 | Peroxiredoxin 1 | −3.87 | 0.039 | Antioxidant / Oxidative Stress Response |
| Ptgs1 | NM_017043 | Prostaglandin-endoperoxide synthase 1 | −4.46 | 0.029 | Antioxidant / Oxidative Stress Response |
| Psmb5 | XM_341314 | Proteasome (prosome, macropain) subunit, beta type 5 | −4.67 | 0.047 | Antioxidant / Oxidative Stress Response |
| Prdx2 | NM_017169 | Peroxiredoxin 2 | −5.18 | 0.018 | Antioxidant / Oxidative Stress Response |
| Prdx5 | NM_053610 | Peroxiredoxin 5 | −7.45 | 0.047 | Antioxidant / Oxidative Stress Response |
| Cat | NM_012520 | Catalase | −10.87 | 0.039 | Antioxidant / Oxidative Stress Response |
| Gpx7 | XM_216473 | Glutathione peroxidase 7 | −14.39 | 0.044 | Antioxidant / Oxidative Stress Response |
| Gpx1 | NM_030826 | Glutathione peroxidase 1 | −28.79 | 0.042 | Antioxidant / Oxidative Stress Response |
| Gpx6 | NM_147165 | Glutathione peroxidase 6 | −52.42 | 0.031 | Antioxidant / Oxidative Stress Response |
| Gpx3 | NM_022525 | Glutathione peroxidase 3 | −214.71 | 0.046 | Antioxidant / Oxidative Stress Response |
| Vim | NM_031140 | Vimentin | −13.37 | 0.049 | Cell Structure |
| Atm | XM_236275 | Ataxia telangiectasia mutated homolog (human) | 4.6 | 0.013 | DNA Damage and Repair |
| Xpa | XM_216403 | Xeroderma pigmentosum, complementatio n group A | 1.69 | 0.000 | DNA Damage and Repair |
| Il18 | NM_019165 | Interleukin 18 | 3.76 | 0.045 | Inflammation |
| Ccnc | XM_342812 | Cyclin C | 1.67 | 0.025 | Proliferation and Carcinogenesis |
| Fmo2 | NM_144737 | Flavin containing monooxygenase 2 | −15.15 | 0.013 | Xenobiotic metabolism |
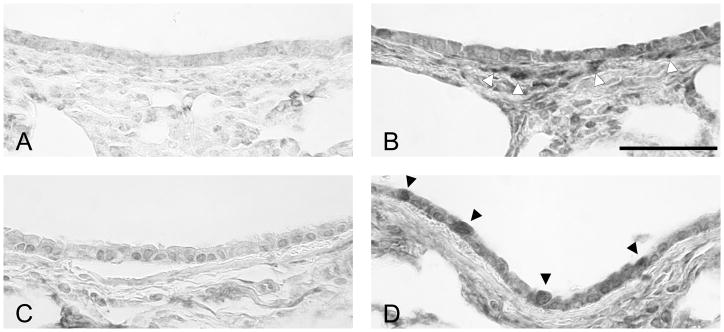
Since there were genes that were significantly changed in response to DFP in the antioxidant and oxidative stress response categories, we examined the abundance and spatial distribution of several key proteins and enzymes that are involved in oxidative stress responses. Glutathione (GSH) is well known to be key for both xenobiotic conjugation and plays an active role as an electrophilic scavenger. We examined the abundance and spatial distribution of glutatmate-cysteine ligase (GCL) protein, an oxidative stress-inducible rate-limiting enzyme in GSH synthesis (Figure 4). Immunohistochemical staining in large bronchioles and bronchi was diffuse and was found in both the airway epithelium and associated interstitium in neonatal (Figure 4A) and adult (Figure 4C) FA exposed control animals. In general, GCL protein expression was distributed uniformly by airway level in the neonatal FA animals but tended to be more abundant in large airways in the adult FA animals and less abundant in terminal bronchioles (data not shown). After DFP exposure, GCL protein was more abundantly expressed in both the airway epithelium in both Clara and ciliated cells and in adjacent interstitial cells compared to age matched FA exposed (compare 4B with 4A and 4D with 4C). Intensely immunopositive cells in the airway wall differed in its spatial localization between the two ages. In neonatal rats, GCL positive cells were in the peribronchiolar interstitium, while few cells in the epithelium were positive (Figure 4B). Conversely, in DFP exposed adults rats, GCL positive cells were abundant in the airway epithelium, and few were seen in the subepithelial region (Figure 4D). In animals exposed to DFP, GCL was less abundant in the terminal bronchioles of neonates while the adults had heavy apical staining in the TB cells (data not shown).
Figure 4.
Histochemical staining of glutamate-cysteine ligase (GCL). Paraffin sections from filtered air (A and C) or DFP exposed (B and D) neonatal (A and B) or adult rats (C and D) were immunostained for GCL. GCL protein abundance was low in filtered air animals regardless of age (compare 7 day postnatal FA in A with adult FA in C). Twenty-four hours after the cessation of DFP exposure, GCL protein expression was markedly increased in both ages (B and D), but the staining patterns differed between the two ages. In DFP exposed 7 day old neonates (B), GCL protein was abundant in subepithelial cells (white arrowheads). In contrast, GCL protein was immunolocalized primarily within the airway epithelium in exposed adults (black arrowheads, D). Scale bar for A, B, C, and D (shown in B) is 50μm.
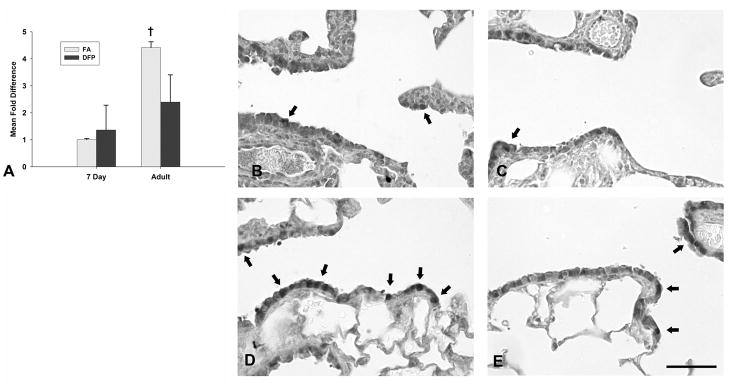
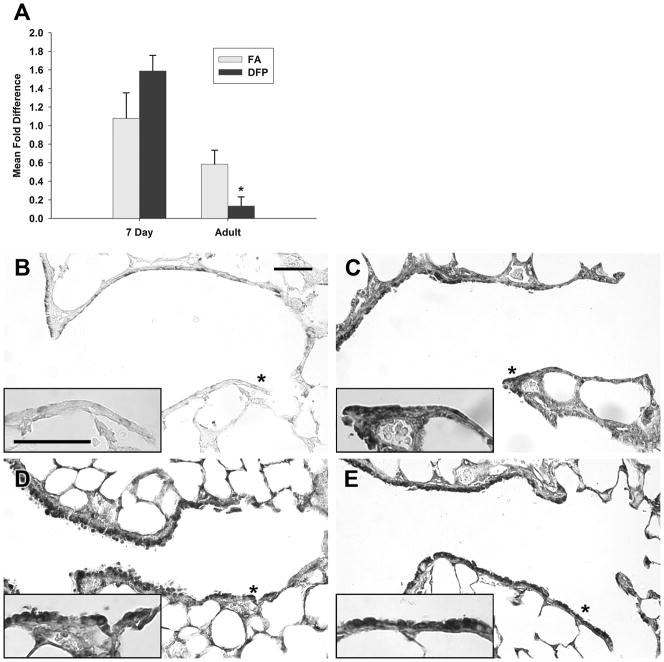
Glutathione S-transferases (GST) catalyze the conjugation of GSH into electrophilic compounds for detoxification. We examined the gene expression and immunohistochemical localization of glutathione S-transferase mu isoform (GSTμ) protein (Figure 5), an enzyme that has been shown to be capable of detoxifying carcinogens, environmental toxins and products of oxidative stress (Hayes and Pulford, 1995), and that was differentially expressed in the qPCR array. Gene expression was calculated using the comparative Ct method from raw data obtained from RT2 qPCR arrays. Fold differences from all groups are compared against FA neonatal rats set to 1 (Figure 5A). Basally, FA adult rats had over a 4 fold increase of GSTμ transcripts compared to neonatal rats (†, P<0.05). This was confirmed by immunohistochemical staining that showed substantially more GSTμ protein containing positive cells in the terminal bronchiolar epithelium of FA adult (Figure 5D) compared to FA neonatal rats (Figure 5B). In general, GSTμ protein was more abundant in the airway epithelium than the parenchyma and was localized to both Clara and ciliated cells. Larger airways had more abundant protein expression than more distal airways in both neonates and adults (data not shown). Subsequent to DFP exposure, both GSTμ gene and protein expression remained unchanged in neonatal rats (Figure 5C), but gene expression trended downwards (P=0.07) and this correlated with fewer GSTμ positive cells in the adults (Figure 5E).
Figure 5.
Gene expression (A) and immunohistochemical staining (B–E) of glutathione S-transferase mu isoform (GSTμ) in the airways of filtered air (B and D) or DFP exposed (C and E) neonatal (B and C) or adult rats (D and E). Basally, FA adults had significantly greater GSTμ gene expression in microdissected airways than 7 day old FA neonates (A). Following DFP exposure, GSTμ gene expression remained unchanged in neonates and trended downwards in DFP exposed adults, but was not statistically significant (P=0.07). Gene expression of GSTμ was calculated using the comparative Ct method and displayed as a mean fold difference ± SEM (n=3–5 rats/group) compared against FA 7 day postnatal animals using HPRT as the reference gene. † = P <0.05, as compared to FA 7 day postnatal controls. Immunohistochemical staining for GSTμ protein indicates a similar pattern compared with gene expression data. GSTμ protein was diffusely localized to the terminal bronchiolar airway epithelium in both FA (B) and DFP exposed (C) 7 day old neonates with a few cells containing more abundant staining (arrows). In contrast, cells with increased GSTμ protein immunolocalization were more abundant in FA adult rats (D) and the number of cells with abundant GST μ protein was decreased in adult animals after DFP exposure (E). Scale bar in E for B, C, D and E is 50μm.
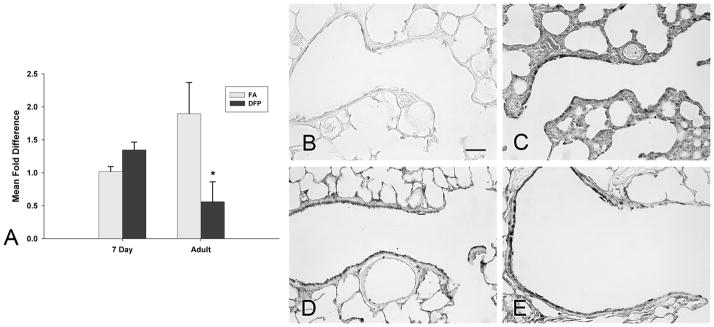
The distribution and abundance of catalase protein and gene expression was determined in the airway epithelium of neonate and adult rats (Figure 6). Catalase gene expression was less in FA adult rats than in FA neonatal rats (Figure 6A). DFP exposure significantly decreased catalase gene expression in the adult rats compared to adult filtered air control animals (*, P< 0.05). Catalase protein expression was robust in the airway epithelium of FA neonatal rats but was less abundant in the distal portion of the terminal bronchiole (Figure 6B). After DFP exposure, neonatal catalase protein staining remained similar to FA controls in the proximal airway epithelium but was increased in the peribronchiolar interstitium and in the epithelium at the terminal bronchiole alveolar duct junction (Figure 6C). Larger airways had more abundant protein expression than more distal airways in both neonates and adult FA exposed animals (data not shown). Catalase protein was more abundant throughout the lung in the adult animals (Figure 6D). Adult rats had significantly decreased levels of catalase gene expression subsequent to DFP exposure, but this was not in agreement with the protein immunolocalization which was increased in abundance in the apex of Clara cells in the most distal airway epithelium (Figure 6E).
Figure 6.
Gene expression (A) and immunohistochemical staining (B–E) of catalase in the airways of filtered air (B and D) or DFP exposed (C and E) neonatal (B and C) or adult rats (D and E). Gene expression of catalase (A) in microdissected airways was calculated using the comparative Ct method and displayed as a mean fold difference ± SEM (n=3–5 rats/group) compared against FA 7 day postnatal animals using HPRT as the reference gene. After DFP exposure, catalase gene expression remained unchanged in neonates, while a significant decrease in gene expression was found in adults. * = P <0.05, as compared to filtered air controls of the same age. Immunohistochemical staining for catalase protein in FA 7 day neonates was localized to the airway epithelium (B). After DFP exposure (C), catalase staining remained intense within the airway epithelium but increased in other lung compartments. Compared to FA neonates, catalase protein in adult FA exposed rats (D) was increased in abundance. After DFP exposure (E), intense catalase positive staining could be observed within the terminal bronchiole, as denoted by asterisks, in the airway epithelium. Areas with asterisks are pictured in the high magnification insets in D and E respectively. Scale bars are 50μm.
The distribution and abundance of glutathione peroxidase 1 (gpx1) gene and protein expression was defined in the airways of neonatal and adult rats (Figure 7). Glutathione peroxidase gene expression was significantly decreased in adult rat airways by exposure to DFP (Figure 7A). Levels of glutathione peroxidase gene expression were similar between neonatal and adult rats. However, the localization of protein in the airways of neonatal and adult rats was quite different. Glutathione peroxidase protein was more abundant in adult airway epithelium (staining was darker and more cells had dark staining indicating protein presence) and other regions of the lung including the interstium, alveoli and vasculature (Figure 7B and D). Glutathione peroxidase protein was found in the neonatal airway epithelium at all airway levels following DFP exposure (Figure 7C). In adults exposed to DFP, glutathione peroxidase was also localized in the epithelium of larger bronchi and bronchioles but there was less expression of the protein in the most distal portions of the terminal bronchioles when compared to the adult FA group (Figure 7E).
Figure 7.
Gene expression (A) and immunohistochemical staining (B–E) of glutathione peroxidase 1 in the airways of filtered air (B and D) or DFP exposed (C and E) neonatal (B and C) or adult rats (D and E). Gene expression of glutathione peroxidase 1 (A) in microdissected airways was calculated using the comparative Ct method and displayed as a mean fold difference ± SEM (n=3–5 rats/group) compared against FA 7 day postnatal animals using HPRT as the reference gene. After DFP exposure, glutathione peroxidase 1 gene expression remained unchanged in neonates, while a significant decrease in gene expression was found in adults. * = P <0.05, as compared to filtered air controls of the same age. Protein expression in FA 7 day neonates was faint (B) but increased markedly with DFP exposure (C). Compared to FA neonates, protein expression in adult FA exposed rats (D) was higher. After DFP exposure (E), intense glutathione peroxidase 1 positive staining was observed in the airway epithelium but was diminished in the most distal portion of the terminal bronchiole. Scale bars are 50μm.
DISCUSSION
The current study found that even a low dose of fine soot particles can cause a biological response in neonatal and adult rats when specific lung regions are considered in the approach. This study differs from other studies of lung gene expression following particulate matter exposure (Heidenfelder et al., 2009) due to the specific focus on conducting airways of postnatal and adult rats, instead of whole lung. We defined significant gene expression changes in the conducting airways, the lung region that is involved in airway diseases such as chronic bronchitis, asthma and cancer. Air pollution, specifically particulate matter exposures, has been shown to influence the development of these diseases, especially then the exposures occur during early childhood.
We used a diffusion flame system, such has been described in detail previously (Pinkerton et al., 2004; Yang et al., 2001; Zhong et al.; Zhou et al., 2003), to expose both postnatal and adult rats to a single acute (6hr) DFP soot exposure. In the current study, we used a particle atmosphere with a concentration of 170 μg/m3 for 6 hrs and a particle geometric mean mobility diameter of 192 nm with an EC/OC ratio of 8.6. Our single 6 hour exposure is an achievable “real world” exposure level that corresponds to an acute exposure that, when averaged over 24 hours, would result in an average level of 42.5 μg/m3. This is similar to a level within the San Joaquin Valley Air Basin, which in had greater than 42 days above the NAAQS PM2.5 standard of 35 μg/m3 for a 24 hr average (CARB, 2009). The EC/OC ratio of 8.6 indicated that the particles were composed primarily of elemental carbon soot. Sprague Dawley rats were chosen as the animal model due to their previous use in many pulmonary toxicologic studies involving particulate matter (Dye et al., 2001; Sherratt et al., 1988; Theophilus et al., 2007) and because their large size (compared to mice) facilitates assessment of both airway growth (Lee et al. submitted) and airway microdissection.
The DFP atmosphere was generated in a similar manner to previous studies of iron containing diffusion flame soot that used longer exposure times (3 days) to particles of a similar size range but a higher concentration (250 μg/m3) in older animals (10d of age and young adult rats) (Zhou et al., 2003). Our studies differ from these previous studies principally in the exposure duration and the lack of metals. Metals can accentuate toxicity by contributing to oxidant stress. A robust oxidant stress and cytotoxicity response was noted in the previous studies of diffusion flame soot that contained iron. However, the previous studies of diffusion flame soot without iron found no adverse respiratory health effects in adult rats exposed to 250 μg/m3 (Zhong et al., 2010). While the 10 day old rats in the previous study were exposed to soot containing iron, they were not exposed to diffusion flame soot lacking iron (Pinkerton et al., 2008). We found that, even in the absence of frank morphologic changes indicative of cytotoxicity, oxidant stress was present in the adult. The airways of adult rats had significant changes in airway gene expression indicating a response to DFP soot exposure (17 genes) at a lower dose than what was used in the previous study. In neonates, there was an increase in LDH activity indicative of cytotoxicity and also some gene expression changes in airways. The study by Heidenfelder et al studied the effect of concentrated ambient particulates on gene expression changes in whole rat lung and found no changes due to 493 μg/m3 CAPS exposure alone (Heidenfelder et al., 2009). However, when groups exposed to CAPS and ovalbumin were assessed the gene expression results were more sensitive than the BALF cell type and number. Our results agree with these findings, that gene expression changes are a sensitive marker of pulmonary effects, but also indicate that improvement in detection can likely be gained for studying biologic responses in the lung tissue by comparing specific lung subcompartments.
In contrast to adult rats, neonatal rats exhibited a cytotoxic response to DFP soot that included significant increases in LDH activity in BALF and focal regions of epithelial cells with small vacuoles in the large airways (not significantly different from controls by morphometry). The reason the morphometric measure of vacuolated cells was not significant may have been partly due to the very small number of regions where the cells contained vacuolization, indicating a mild response that when averaged over the entire airway segment was not significant. Previous studies did not examine the effect of soot lacking metals in the early postnatal rat (Pinkerton et al., 2008). The reason neonatal rats are more susceptible to cytotoxicity from DFP soot remains a mystery but two points should be made: 1) that the cytotoxicity was not widespread and was mild and 2) that the composition of the DFP atmosphere or physicochemical properties of the particles themselves could be contributing to this differential susceptibility by age. Previous studies of bioactivated PAH mediated cytotoxicity in young mice, rats and rabbits have indicated that neonatal animals are much more susceptible to cytotoxicity by compounds that require metabolic activation to form a toxic compound, such as naphthalene, 1-nitronaphthalene and 4-ipomeanol (Fanucchi et al., 1997; Fanucchi et al., 2004; Plopper et al., 1994; Smiley-Jewell et al., 2000). The reason for this is still undefined but our current study would suggest that the same paradigm applies to neonatal rats exposed to DFP containing large amounts of elemental carbon but with only small amounts of PAH. This raises the possibility that the airways of young animals are susceptible to perturbation through a unique mechanism or a novel target. Future studies that compare PM with different sizes and PAH compositions would help determine the contributing factors to neonatal susceptibility to PM.
Interestingly the gene expression data shows that airways of adult rats had a greater number of significant gene changes (17) than neonates (6) out of 162 total assessed in response to DFP exposure. It may be that animals in the process of postnatal lung development and growth have a limited ability to respond to environmental perturbation by deviating from their normal developmental pattern. This inability to respond may be related to enhanced cytotoxicity and perturbation of growth. Further studies are needed to determine whether this cytotoxic effect, as well as lack of an antioxidant response, holds true for particles with different sizes and different amounts of associated PAHs, in animals where cytochrome P450 monooxygenase activity has been chemically blocked or in animals of different ages or strains. A limitation of our study is that it examines a single timepoint following exposure, 24 hrs. This timepoint was selected based on the likelihood of cytotoxicity in high resolution sections being visible at this time. However, it is possible, even likely, that gene expression changes following an acute exposure follow different temporal patterns depending on the gene, its regulation, mRNA stability etc. This has been shown for whole lung gene expression in spontaneously hypertensive rats following exposure to 10mg/kg intratracheally instilled urban PM extract as assessed by oligonucleotide array analysis (Kooter et al., 2005). The maximum number of gene expression changes were found at 2–6 hours following instillation. It is quite possible that our gene expression changes noted at 24 hours could have missed significant decreases or increases that occurred after the 6 hr inhalation interval. Future studies are needed to determine the temporal pattern of gene expression changes in the airways specifically following inhalation exposure to PM.
Not surprisingly a number of genes were differentially expressed between 7 day postnatal and adult FA rats (33). This is to be expected when comparing airways of actively growing and differentiating neonates with adults. Most previous studies of developmental differences in gene expression have not examined airways specifically, but instead measured gene expression changes in whole lung or studied only protein expression. In agreement with many previous studies, we found that adult rats compared to neonates had decreased expression of genes involved in growth and proliferation and apoptosis (cyclin G1, PCNA, caspase 1), an increase in cytochrome P450s (cyp2a3a, cyp2b15, cyp4a3 and cyp7a1) as well as GSTμ and a decrease in 3 members of a family of genes for the solute carrier family (see supplemental data Table 5S). The solute carrier family of genes are known to transport a number of substances (Sundberg et al., 2008). Solute carrier family members, slc38a4, slc38a5 and slc41a3, were all significantly decreased in adult rat airways compared to neonatal rat airways. The SLC38 genes are part of a family of Na+ dependent neutral amino acid transporters, a process that would be especially important in a developing system with increased needs for protein synthesis and the need to regulate cell volume.
Previous studies of iron containing soot in neonatal rats found significant reduction in cell proliferation in the centriacinar region of the lung (Pinkerton et al., 2008), and this was thought to be related to subsequent lung remodeling. Lung remodeling and impaired epithelial repair has also been noted in the conducting airways of postnatal animals exposed to bioactivated cytotoxicants (Smiley-Jewell et al., 2000; Smiley-Jewell et al., 1998) but was found not to be related to reduced cell proliferation (Smiley-Jewell and Plopper, 2003). In the current study, PCNA gene expression, a marker of cell proliferation in the airways, was not significantly changed by DFP exposure in either postnatal or adult rats compared to age matched controls, and there were no marked differences in PCNA protein expression as well (Supplemental data, Figure S). The only gene in the cell proliferation category that was significantly changed was found in adult rats, cyclin C (cnnc). However, it is important to point out that the centriacinar region was not examined for PCNA gene expression in this study and so the immediate and long term impact of fine particle exposure on the centriacinar lung region remains a fertile area of research for future studies.
We investigated the pattern of protein expression of the antioxidant enzymes GCL, GSTμ, catalase, and glutathione peroxidase; as examples of genes that had differing expression patterns in our array data and GCL as an indicator of GSH status. Protein expression of GCL, the rate limiting enzyme in GSH synthesis, was increased in the airways and the associated interstitium of both DFP exposed neonatal and adult rats indicating an antioxidant response to DFP exposure. This is in agreement with previous studies of carbon black effects on whole lung gene expression where a 50 mg/m3 exposure increased mRNA expression of GCL in rats (Carter et al., 2006). Previous studies of iron containing soot in neonatal rats found significant oxidative stress (Pinkerton et al., 2008). The current study confirms that DFP also caused oxidative stress in neonatal rats. We found that GSTμ gene expression was significantly increased with age but that protein expression did not have as stark an age related change. This is in agreement with previous studies of GST activity and protein expression with postnatal age in mice, where activity increased with age and protein expression was a bit less linear (Fanucchi et al., 2000).
We also examined the gene and protein expression changes for catalase, an oxidative stress responsive enzyme responsible for catalyzing the decomposition of hydrogen peroxide (H2O2) into molecular oxygen and water. H2O2 can be formed directly through reactive oxygen species (ROS) reactions found in particulate matter (Chung et al., 2006), and could also be generated enzymatically through superoxide dismutase mediated reactions intracellularly. Catalase protein expression within the airway epithelium and the associated interstitium was markedly increased in neonatal rats exposed to DFP and was more abundant in adult rats compared to neonates. Further there was a slight increase in the abundance of cells with intense catalase protein immunolocalization in the DFP exposed adult rats. Protein expression data for catalase did not match the gene expression data which indicated a significant decrease in adult rats exposed to DFP and in FA adults compared to FA neonates. We do not know the mechanism for this mismatch but disagreement between gene and protein data is not uncommon (Pascal et al., 2008) and could reflect changes in protein or mRNA stability or protein translational regulation. In agreement with our protein expression findings, catalase has been shown to increase in activity with postnatal maturation in the rat lung (Chen and Frank, 1993). Interestingly, exposure of normal human bronchial epithelial cells to butadiene soot, as well as exposure of adult rats to diesel exhaust particles, has been shown to significantly decrease catalase enzyme activity (Kennedy et al., 2009; Rengasamy et al., 2003). Reduction of catalase activity in the rats was thought to be related to the carbonaceous core of the diesel exhaust, a feature that is well preserved in our DFP exposure particles (Rengasamy et al., 2003). We did not measure catalase activity but our gene expression data indicates that DFP exposure in adults significantly downregulates catalase gene expression. It would be interesting to determine in future studies if catalase protein expression is decreased at later timepoints (> 24 hrs post exposure) and what the effect of DFP exposure is on catalase activity in both neonates and adults.
Four members of the glutathione peroxidase family demonstrated significantly reduced gene expression in response to DFP exposure in adult rats. The key role of glutathione peroxidases is protection from oxidant damage as they reduce lipid peroxides and detoxify hydrogen peroxide. We assessed the protein distribution of glutathione peroxidase 1 (Gpx1) as an example of this group. Expression of the gpx1 gene was significantly decreased > 25 fold in DFP compared to FA exposed adult rats (Table 2). Allelic variants of gpx1 have been found to protective from lung cancer (Raaschou-Nielsen et al., 2007; Rosenberger et al., 2008), allelic loss of this gene is common in colon cancer (Hu et al., 2005) and the gene product is posttranslationally regulated (Rhee et al., 2005). The effect of decreases in the gpx1 gene in a more chronic study and its relation to tumor formation could be a fruitful area for future research. Additional timepoints and studies of protein stability are needed to put the decreased gene expression seen in the adults exposed to DFP into context as there is moderate disagreement with the level of protein expression detected in the tissue. The gene expression data was a sensitive indicator of exposure effect in the adult animals with DFP exposure. This underscores that neither protein expression nor gene expression can be used solely but that both can provide indications of potential areas for additional study.
CONCLUSIONS
We exposed both neonatal and adult rats to DFP soot particles and found that even a modest, acute exposure to particulate matter induced significant changes in gene expression in both neonatal and adult rat airways. These changes were found at exposure levels below those used in previous studies that included primarily whole lung expression data as endpoints (Heidenfelder et al., 2009; Pinkerton et al., 2008) and underscore the value of site-specific evaluation of responses when evaluating inhaled toxicant effects. The current study also indicates that gene expression responses are sensitive indicators of effect of PM exposure. Further we found that the basal levels and the airway specific response to DFP exposure of both the neonatal and adult rat airways were significantly different for certain antioxidant genes and proteins. Of the genes that were significantly changed by DFP exposure in the airways of neonates and adults there are no genes (0/162) in common by age with exposure. This reiterates the common assertion that young animals are not “little adults” and that they have a unique capability to respond, or not respond, to toxic challenges and that this is related to their stage of development and can be quite a bit different than the response in adult animals. We conclude that 1) neonates are more susceptible to cytotoxicity from particulate matter and are less capable of altering gene expression in response to a toxic challenge than adults and 2) that airway gene expression changes are sensitive markers of biologic effect of PM in both neonates and adults, even in the absence of frank cytotoxicity.
Supplementary Material
Figure S: Gene expression (A) and immunohistochemical staining (B–E) of proliferating cell nuclear antigen (PCNA) in the airways of filtered air (B and D) or DFP exposed (C and E) neonatal (B and C) or adult rats (D and E). Gene expression of PCNA was calculated using the comparative Ct method and data is shown as mean fold difference ± SEM (n=3–5 rats/group) compared against FA 7 day postnatal animals using HPRT as the reference gene. (A) Basally, gene expression of PCNA was significantly less in FA adults as compared with 7 day postnatal animals. PCNA expression remained unchanged following DFP exposure in either age group. † = P <0.05, as compared to FA 7 day postnatal controls. Substantial epithelial (arrowheads) and interstitial (white asterisks) immunohistochemical staining were observed in 7 day FA neonates in along all airway levels (B). After DFP exposure (C), PCNA positive cells were found to be reduced relative to FA neonates. However, staining intensity remained the same in positive cells. Compared to FA neonates, PCNA staining in adult FA animals were significantly reduced both in quantity and intensity (D). After DFP exposure, clusters of positive cells were observed in midlevel airways (E). Scale bar in E for B, C, D and E is 50μm.
Acknowledgments
We are grateful to the following people for their skilled technical assistance during exposures, sample collection and processing: Brian Tarkington, Louise Olson, Patricia Edwards, Judy Shimizu, and Trenton Combs. We thank Michael Kleeman’s laboratory at UC Davis for EC/OC sample analysis and Barbara Zielinska at DRI for filter PAH speciation. Finally, we would like to acknowledge Kent Pinkerton, Michael Kleeman and Jesse Charrier for reading and editing the manuscript.
Footnotes
DECLARATION OF INTEREST
Support for the University of California at Davis core facilities used in this work: the Cellular and Molecular Imaging Core Facility (ES005707) and the inhalation exposure facility at the California National Primate Research Center (RR00169) is acknowledged. Although the research described in the article has been funded primarily by the United States Environmental Protection Agency through grant RD-83241401-0 to the University of California, Davis, it has not been subject to the Agency’s required peer and policy review and, therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred. The project described was also supported in part by Award Number P42ES004699 from the National Institute of Environmental Health Sciences. Ms. Sutherland’s effort was supported by a National Institute of Environmental Health Sciences training grant T32 ES007059. Mr. Chan’s effort was partially supported by a traineeship with the Superfund Basic Sciences Research Program at UC Davis (P42 ES04699). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- ALA. American Lung Association State of the Air: 2009. American Lung Association; New York, NY: 2009. [Google Scholar]
- Baker GL, Shultz MA, Fanucchi MV, Morin DM, Buckpitt AR, Plopper CG. Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol Sci. 2004;77:135–141. doi: 10.1093/toxsci/kfh002. [DOI] [PubMed] [Google Scholar]
- Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YCT. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environmental Health Perspectives. 2005;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet S, Garcon G, Dagher Z, Verdin A, Ledoux F, Cazier F, Courcot D, Aboukais A, Shirali P. Ambient particulate matter (PM2.5): physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549) Environ Res. 2007;105:212–223. doi: 10.1016/j.envres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Branis M, Safranek J, Hytychova A. Exposure of children to airborne particulate matter of different size fractions during indoor physical education at school. Building and Environment. 2009;44:1246–1252. [Google Scholar]
- CARB. Air Quality Data and Statistics for PM2.5 in San Jaoquin Valley air basin for 2009: Days above standard California Air Resources Board (CARB) 2009. [Google Scholar]
- Carter JM, Corson N, Driscoll KE, Elder A, Finkelstein JN, Harkema JN, Gelein R, Wade-Mercer P, Nguyen K, Oberdorster G. A comparative dose-related response of several key pro- and antiinflammatory mediators in the lungs of rats, mice, and hamsters after subchronic inhalation of carbon black. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2006;48:1265–1278. doi: 10.1097/01.jom.0000230489.06025.14. [DOI] [PubMed] [Google Scholar]
- Chen Y, Frank L. Differential gene expression of antioxidant enzymes in the perinatal rat lung. Pediatric research. 1993;34:27–31. doi: 10.1203/00006450-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shah N, Braun A, Huggins F, Huffman G. Electron microscopy investigation of carbonaceous particulate matter generated by combusion of fossil fuels. Energy and Fuels. 2005;19:1644–1651. [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung MY, Lazaro RA, Lim D, Jackson J, Lyon J, Rendulic D, Hasson AS. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environmental Science & Technology. 2006;40:4880–4886. doi: 10.1021/es0515957. [DOI] [PubMed] [Google Scholar]
- Ciccone G, Forastiere F, Agabiti N, Biggeri A, Bisanti L, Chellini E, Corbo G, Dell’Orco V, Dalmasso P, Volante TF, Galassi C, Piffer S, Renzoni E, Rusconi F, Sestini P, Viegi G. Road traffic and adverse respiratory effects in children. SIDRIA Collaborative Group. Occup Environ Med. 1998;55:771–778. doi: 10.1136/oem.55.11.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, 3rd, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- Dye JA, Lehmann JR, McGee JK, Winsett DW, Ledbetter AD, Everitt JI, Ghio AJ, Costa DL. Acute pulmonary toxicity of particulate matter filter extracts in rats: coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect. 2001;109(Suppl 3):395–403. doi: 10.1289/ehp.01109s3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi MV, Buckpitt AR, Murphy ME, Plopper CG. Naphthalene cytotoxicity of differentiating Clara cells in neonatal mice. Toxicology and applied pharmacology. 1997:96–104. doi: 10.1006/taap.1997.8119. [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Buckpitt AR, Murphy ME, Storms DH, Hammock BD, Plopper CG. Development of phase II xenobiotic metabolizing enzymes in differentiating murine clara cells. Toxicology and applied pharmacology. 2000;168:253–267. doi: 10.1006/taap.2000.9020. [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Day KC, Clay CC, Plopper CG. Increased vulnerability of neonatal rats and mice to 1-nitronaphthalene-induced pulmonary injury. Toxicology and applied pharmacology. 2004;201:53–65. doi: 10.1016/j.taap.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Hammond TG, Mobbs M. Lung oedema--microscopic detection. J Appl Toxicol. 1984;4:219–221. doi: 10.1002/jat.2550040411. [DOI] [PubMed] [Google Scholar]
- Harding R, PKE, Plopper CG. The Lung - Development, Aging and the Environment. Elsevier Academic Press; San Diego, CA: 2004. p. 403. [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-Transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Heidenfelder BL, Reif DM, Harkema JR, Cohen Hubal EA, Hudgens EE, Bramble LA, Wagner JG, Morishita M, Keeler GJ, Edwards SW, Gallagher JE. Comparative microarray analysis and pulmonary changes in Brown Norway rats exposed to ovalbumin and concentrated air particulates. Toxicol Sci. 2009;108:207–221. doi: 10.1093/toxsci/kfp005. [DOI] [PubMed] [Google Scholar]
- Herner JD, Aw J, Gao O, Chang DP, Kleeman MJ. Size and Composition Distribution of Airborne Particulate Matter in Northern California: I--Particulate Mass, Carbon, and Water-Soluble Ions. Journal of the Air & Waste Management Association (1995) 2005;55:30–51. doi: 10.1080/10473289.2005.10464600. [DOI] [PubMed] [Google Scholar]
- Hinners R, Burkart J, Punte C. Animal inhalation exposure chambers. Arch Environ Health. 1968:194–206. doi: 10.1080/00039896.1968.10665043. [DOI] [PubMed] [Google Scholar]
- Howard C, Reed M. Unbiased Stereology. Springer-Verlag; New York: 1998. p. 246. [Google Scholar]
- Hu Y, Benya RV, Carroll RE, Diamond AM. Allelic loss of the gene for the GPX1 selenium-containing protein is a common event in cancer. The Journal of nutrition. 2005;135:3021S–3024S. doi: 10.1093/jn/135.12.3021S. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Plopper CG, St George JA, Harkema JR. Morphometric Cell Biology of Air Space Epithelium. In: Schraufnagel DE, editor. Electron Microscopy of the Lung. Vol. 48. Marcel Dekker; New York: 1990. pp. 71–120. [Google Scholar]
- Kennedy CH, Catallo WJ, Wilson VL, Mitchell JB. Combustion products of 1,3-butadiene inhibit catalase activity and induce expression of oxidative DNA damage repair enzymes in human bronchial epithelial cells. Cell biology and toxicology. 2009;25:457–470. doi: 10.1007/s10565-008-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter I, Pennings J, Opperhuizen A, Cassee F. Gene expression pattern in spontaneously hypertensive rats exposed to urban particulate matter (EHC-93) Inhalation toxicology. 2005;17:53–65. doi: 10.1080/08958370590885717. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madden MC. Complex issues with examining diesel exhaust toxicity: is the task getting easier or harder? Exp Toxicol Pathol. 2008;60:135–140. doi: 10.1016/j.etp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Morrow PE, Mercer TT. A Point-to-Plane Electrostatic Precipitator for Particle Size Sampling. American Industrial Hygiene Association Journal. 1964;25:8–14. doi: 10.1080/00028896409342547. [DOI] [PubMed] [Google Scholar]
- Na K, Cocker DR. Organic and elemental carbon concentrations in fine particulate matter in residences, schoolrooms, and outdoor air in Mira Loma, California. Atmospheric Environment. 2005;39:3325–3333. [Google Scholar]
- Oosterlee A, Drijver M, Lebret E, Brunekreef B. Chronic respiratory symptoms in children and adults living along streets with high traffic density. Occup Environ Med. 1996;53:241–247. doi: 10.1136/oem.53.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal LE, True LD, Campbell DS, Deutsch EW, Risk M, Coleman IM, Eichner LJ, Nelson PS, Liu AY. Correlation of mRNA and protein levels: cell type-specific gene expression of cluster designation antigens in the prostate. BMC genomics. 2008;9:246. doi: 10.1186/1471-2164-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pey J, Querol X, Alastuey A, Rodriguez S, Putaud JP, Van Dingenen R. Source apportionment of urban fine and ultra-fine particle number concentration in a Western Mediterranean city. Atmospheric Environment. 2009;43:4407–4415. [Google Scholar]
- Pinkerton KE, Zhou Y, Zhong C, Smith KR, Teague SV, Kennedy IM, Menache MG. Mechanisms of particulate matter toxicity in neonatal and young adult rat lungs. Research report (Health Effects Institute) 2008:3–41. discussion 43–52. [PubMed] [Google Scholar]
- Pinkerton KE, Zhou YM, Teague SV, Peake JL, Walther RC, Kennedy IM, Leppert VJ, Aust AE. Reduced lung cell proliferation following short-term exposure to ultrafine soot and iron particles in neonatal rats: key to impaired lung growth? Inhalation toxicology. 2004;16(Suppl 1):73–81. doi: 10.1080/08958370490443123. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Laboratory investigation; a journal of technical methods and pathology. 1992;67:553–565. [PubMed] [Google Scholar]
- Plopper CG, Weir AJ, Nishio SJ, Chang A, Voit M, Philpot RM, Buckpitt AR. Elevated susceptibility to 4-ipomeanol cytotoxicity in immature Clara cells of neonatal rabbits. J Pharmacol Exp Ther. 1994;269:867–880. [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Sorensen M, Hansen RD, Frederiksen K, Tjonneland A, Overvad K, Vogel U. GPX1 Pro198Leu polymorphism, interactions with smoking and alcohol consumption, and risk for lung cancer. Cancer letters. 2007;247:293–300. doi: 10.1016/j.canlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Rengasamy A, Barger MW, Kane E, Ma JK, Castranova V, Ma JY. Diesel exhaust particle-induced alterations of pulmonary phase I and phase II enzymes of rats. Journal of toxicology and environmental health. 2003;66:153–167. doi: 10.1080/15287390306403. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxidants & redox signaling. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Robert MA, VanBergen S, Kleeman MJ, Jakober CA. Size and Composition Distributions of Particulate Matter Emissions: Part 1--Light-Duty Gasoline Vehicles. Journal of the Air & Waste Management Association (1995) 2007;57:1414–1428. doi: 10.3155/1047-3289.57.12.1414. [DOI] [PubMed] [Google Scholar]
- Rosenberger A, Illig T, Korb K, Klopp N, Zietemann V, Wolke G, Meese E, Sybrecht G, Kronenberg F, Cebulla M, Degen M, Drings P, Groschel A, Konietzko N, Kreymborg KG, Haussinger K, Hoffken G, Jilge B, Ko YD, Morr H, Schmidt C, Schmidt EW, Tauscher D, Bickeboller H, Wichmann HE. Do genetic factors protect for early onset lung cancer? A case control study before the age of 50 years. BMC cancer. 2008;8:60. doi: 10.1186/1471-2407-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Rappold A, Graff D, Cascio WE, Berntsen JH, Huang YC, Herbst M, Bassett M, Montilla T, Hazucha MJ, Bromberg PA, Devlin RB. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med. 2009;179:1034–1042. doi: 10.1164/rccm.200807-1043OC. [DOI] [PubMed] [Google Scholar]
- Sherratt AJ, Culpepper BT, Lubawy WC. Relative Participation of the Gas-Phase and Total Particulate Matter in the Imbalance in Prostacyclin and Thromboxane Formation Seen Following Chronic Cigarette-Smoke Exposure. Prostaglandins Leukotrienes and Essential Fatty Acids. 1988;34:15–18. doi: 10.1016/0952-3278(88)90019-1. [DOI] [PubMed] [Google Scholar]
- Smiley-Jewell SM, Liu FJ, Weir AJ, Plopper CG. Acute injury to differentiating Clara cells in neonatal rabbits results in age-related failure of bronchiolar epithelial repair. Toxicologic pathology. 2000;28:267–276. doi: 10.1177/019262330002800206. [DOI] [PubMed] [Google Scholar]
- Smiley-Jewell SM, Nishio SJ, Weir AJ, Plopper CG. Neonatal Clara cell toxicity by 4-ipomeanol alters bronchiolar organization in adult rabbits. The American journal of physiology. 1998;274:L485–498. doi: 10.1152/ajplung.1998.274.4.L485. [DOI] [PubMed] [Google Scholar]
- Smiley-Jewell SM, Plopper CG. Proliferation during early phases of bronchiolar repair in neonatal rabbits following lung injury by 4-ipomeanol. Toxicology and applied pharmacology. 2003;192:69–77. doi: 10.1016/s0041-008x(03)00258-8. [DOI] [PubMed] [Google Scholar]
- Sundberg BE, Waag E, Jacobsson JA, Stephansson O, Rumaks J, Svirskis S, Alsio J, Roman E, Ebendal T, Klusa V, Fredriksson R. The evolutionary history and tissue mapping of amino acid transporters belonging to solute carrier families SLC32, SLC36, and SLC38. J Mol Neurosci. 2008;35:179–193. doi: 10.1007/s12031-008-9046-x. [DOI] [PubMed] [Google Scholar]
- Theophilus EH, Shreve WK, Ayres PH, Garner CD, Pence DH, Swauger JE. Comparative 13-week cigarette smoke inhalation study in Sprague-Dawley rats: Evaluation of cigarettes with two banded cigarette paper technologies. Food and Chemical Toxicology. 2007;45:1076–1090. doi: 10.1016/j.fct.2006.12.031. [DOI] [PubMed] [Google Scholar]
- USEPA. US EPA Green Book - Counties Designated Nonattainment. Vol. 2008 2008. [Google Scholar]
- USEPA. US EPA Green Book - Particulate Matter (PM-2.5) Nonattainment Areas (1997 Standard) Vol. 2009 2009. [Google Scholar]
- van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res. 1997;74:122–132. doi: 10.1006/enrs.1997.3757. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Isaac JM, Plopper CG. Repair of naphthalene-injured microdissected airways in vitro. Am J Respir Cell Mol Biol. 1996;15:1–8. doi: 10.1165/ajrcmb.15.1.8679213. [DOI] [PubMed] [Google Scholar]
- Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- Wilson HH, Chauhan J, Kerry PJ, Evans JG. Ethanol vapour-fixation of rat lung for immunocytochemistry investigations. J Immunol Methods. 2001;247:187–190. doi: 10.1016/s0022-1759(00)00314-8. [DOI] [PubMed] [Google Scholar]
- Yang G, Teague SV, Pinkerton K, Kennedy I. Synthesis of an ultrafine iron and soot aerosol for the evaluation of particle toxicity. Aerosol Sci Tehnol. 2001;35:759–766. [Google Scholar]
- Zhong CY, Zhou YM, Smith KR, Kennedy IM, Chen CY, Aust AE, Pinkerton KE. Oxidative injury in the lungs of neonatal rats following short-term exposure to ultrafine iron and soot particles. Journal of toxicology and environmental health. 2010;73:837–847. doi: 10.1080/15287391003689366. [DOI] [PubMed] [Google Scholar]
- Zhou YM, Zhong CY, Kennedy IM, Leppert VJ, Pinkerton KE. Oxidative stress and NFkappaB activation in the lungs of rats: a synergistic interaction between soot and iron particles. Toxicology and applied pharmacology. 2003;190:157–169. doi: 10.1016/s0041-008x(03)00157-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S: Gene expression (A) and immunohistochemical staining (B–E) of proliferating cell nuclear antigen (PCNA) in the airways of filtered air (B and D) or DFP exposed (C and E) neonatal (B and C) or adult rats (D and E). Gene expression of PCNA was calculated using the comparative Ct method and data is shown as mean fold difference ± SEM (n=3–5 rats/group) compared against FA 7 day postnatal animals using HPRT as the reference gene. (A) Basally, gene expression of PCNA was significantly less in FA adults as compared with 7 day postnatal animals. PCNA expression remained unchanged following DFP exposure in either age group. † = P <0.05, as compared to FA 7 day postnatal controls. Substantial epithelial (arrowheads) and interstitial (white asterisks) immunohistochemical staining were observed in 7 day FA neonates in along all airway levels (B). After DFP exposure (C), PCNA positive cells were found to be reduced relative to FA neonates. However, staining intensity remained the same in positive cells. Compared to FA neonates, PCNA staining in adult FA animals were significantly reduced both in quantity and intensity (D). After DFP exposure, clusters of positive cells were observed in midlevel airways (E). Scale bar in E for B, C, D and E is 50μm.