Abstract
Background
Mu opiate receptor agonism has been associated with weight gain, while mu antagonists have been associated with weight neutrality, or even weight loss.
Aim
This study examined the course of weight changes in opiate-dependent patients over the first six months of treatment in methadone (agonist) versus naltrexone (antagonist) maintenance.
Design
A retrospective chart review was conducted on 36 opiate-dependent patients maintained on methadone (n=16) or naltrexone (n=20).
Outcome measures and analyses
The primary outcome measure was change in body weight from baseline to 3 months and 6 months into treatment. ANOVA was used to compare mean weights between the methadone- and naltrexone-maintained patients. Secondarily, mean percent weight changes from baseline to 3 months, and baseline to 6 months into treatment were compared using Student’s T-test.
Results
Weight at baseline, 3 months and 6 months into treatment did not differ significantly between the two groups, and neither did percent weight change from baseline to 3 months, and baseline to 6 months. At 3 months, n=16 methadone patients had a mean weight increase of 1.86% (SD 7.22%) compared to n=20 BNT patients with an increase of 4.63% (SD 6.49%),. At 6 months, n=16 methadone patients had a mean weight increase compared to baseline of 3.67% (SD 9.52%) compared to n=20 BNT patients, who demonstrated a mean increase of 6.69% (SD 7.56%). No association was found between baseline weight, defined as “low” or “high” relative to group medians, and percent gain within and between treatment groups.
Conclusion
This study did not detect a statistically different course of weight gain between methadone and naltrexone maintenance treatment for opiate-dependent patients.
Keywords: methadone, naltrexone, weight, opiate dependence
INTRODUCTION
Opiate addiction is a significant current public health problem in the U. S. that negatively affects patients’ lives in medical, social, occupational and legal domains. It is estimated that 1 million Americans meet criteria for opiate addiction (1). Medically supervised treatment of these individuals with the opiate agonist methadone results in considerable reductions in morbidity and mortality (2). However, methadone-maintained patients have demonstrated significant weight gain while in treatment (3, 4). Preclinical trials have also found that chronic exposure to mu opiate agonism has been associated with weight gain (5–7). Naltrexone, a mu opiate antagonist, is an alternative treatment for less severely dependent opioid addicts, and has also demonstrated effectiveness in this population (8). Furthermore, mu antagonists have been associated with weight loss in preclinical (9) and clinical trials (5). Yet several clinical trials of naltrexone have failed to demonstrate either significant weight loss or significant weight gain (10–13). While the aforementioned studies were conducted in either normal or obese subjects, to date there are no published clinical data on the effects of naltrexone maintenance treatment on the weight of opioid-dependent subjects.
Weight gain is a significant health problem in the United States. According to the National Health and Nutrition Examination Survey (NAHANES) 2005–2006, 34.3% of US citizens 20 years old or older are considered obese, with body mass index (BMI) of 30 or more (14). Weight gain is a significant problem for opiate-dependent patients. An autopsy study of Swedish intravenous drug users found that 36% of heroin users were overweight (BMI>25), 43.1% of methadone treated patients were overweight, and that most of the obese (BMI 30.0 to 39.9) IV drugs users (27.5%) were methadone maintained patients (15). Being overweight or obese is associated with increased risk for developing coronary artery disease, insulin-resistant diabetes mellitus, osteoarthritis, various cancers (16). Given that drug abusers already face many health risks, these study findings highlight the importance of addressing weight management during the course of both agonist and antagonist maintenance for opioid dependence.
The aim of this study was to examine the possible association between naltrexone treatment and weight changes in patients being treated for opioid dependence. The hypothesis was that naltrexone maintenance treatment would lead to significantly less weight gain than methadone maintenance in opioid-dependent patients. A chart review was conducted comparing total weight during the first 6 months of treatment in methadone-maintained opioid-dependent patients (n=16) and naltrexone-maintained opioid-dependent patients (n=20). Baseline, 3-month, and 6-month data on weight status were obtained for comparison.
METHODS
Design and Sample
A chart review was conducted on a total of 36 opiate-dependent former patients: 16 methadone-treated patients from the Addiction Institute of New York’s Opioid Treatment Program, and 20 naltrexone-treated patients from the Behavioral Naltrexone Therapy (BNT) trial conducted at Columbia University’s Substance Abuse and Treatment Service (STARS) outpatient research clinic. Patients treated at the Addiction Institute’s Opioid Treatment Program received individual and/or group supportive counseling focused on relapse prevention and treatment compliance. Opioid-dependent participants enrolled in the BNT trial were inducted onto oral naltrexone for a 6-month maintenance course. Patients received twice-weekly BNT, a manual-based therapy incorporating elements of Motivational Interviewing, cognitive behavioral counseling, voucher incentives, and Network Therapy (17).
Charts were chosen for review among those which met the following criteria for patients: 1) were no longer attending and had been formally discharged from their treatment site, 2) had been in treatment for at least 6 months, 3) provided baseline, 3 month- and 6-month weights. While both facilities afford their patients regular clinical follow-up care, neither facility offers a treatment program or therapy specifically aimed at weight loss, diet or nutrition.
The research design and data collection plan were approved by the Institutional Review Boards of both New York State Psychiatric Institute and St. Luke’s-Roosevelt Hospital. Waiver of consent for study participation was obtained from both IRBs, as the patients had been formally discharged, and were sufficiently de-identified. Charts reviewed from the Addiction Institute Opiate Clinic included patients treated between the years 1998 and 2009. Charts reviewed from STARS BNT clinical research program included patients treated in 2002 to 2007.
Facilities and Procedures
Addiction Institute
The Addiction Institute of New York’s Opioid Treatment Program provides outpatient methadone treatment for up to 300 patients. The Opioid Treatment Program, located at Roosevelt Hospital in New York City, is a comprehensive treatment program for individuals with an opioid addiction. In addition to the administration of methadone or buprenorphine, a full range of clinical services are offered, including group and individual counseling, psychiatric services, medical and medication management, and vocational counseling for individuals with an opioid addiction.
STARS
The Substance Treatment and Research Service of Columbia University (STARS) is an outpatient research site. STARS consists of two outpatient locations where treatment is provided to participants enrolled in NIDA-funded trials for substance abuse or dependence. At any given time, approximately 4–7 trials are recruiting participants. Trials focus on state-of-the-art pharmacological and manual-based psychotherapeutic treatments of alcohol and other substances of abuse. Data for this study were collected from treatment-seeking opiate-dependent patients who were accepted into the BNT trial, then detoxified and inducted onto oral naltrexone and possibly intramusclular depot naltrexone as well.
Statistical Analyses
Chi-square analyses were used to compare proportional variables pertaining to the baseline demographics of the methadone-treated and naltrexone-treated samples. The Student’s T-test was used to compare parametric baseline means between the two samples. ANOVA was used to detect significant differences at baseline and follow-up regarding weight between the methadone and naltrexone groups. Significance level was considered at α=0.05, and all tests were 2-sided.
RESULTS
Baseline demographic data pertaining to the methadone-treated and naltrexone-treated cohorts are summarized in Table 1. Both groups were comprised predominantly of white or Hispanic men, who were domiciled, and had a high school education. When using Chi-square analyses to detect differences in proportions, only employment status (p=0.05) reached significance, revealing that the methadone-treated patients were less likely to be employed than the naltrexone-treated patients. There were no significant group differences regarding baseline amount of heroin or percentage using intravenous heroin, as well as baseline alcohol, cigarette, cocaine, or marijuana use.
Table 1.
Baseline demographics and drug use
| Naltrexone n=20 | Methadone n=16 | |
|---|---|---|
| Age in years (SD) | 37.45 (9.25) | 41.18 (9.49) |
| Male n(%) | 16(80.0) | 11(68.7) |
| Race n(%) | ||
| White | 10 (50.0) | 9 (56.2) |
| Hispanic | 5 (25.0) | 5 (31.3) |
| African-American | 4 (20.0) | 2 (12.5) |
| Other | 1 (5.0) | 0 (0.0) |
| Employed* n (%) | 10 (50.0) | 3 (18.8) |
| Domiciled n (%) | 20(100) | 16(100) |
| Highest level of education n (%) | ||
| grade school | 6 (30.0) | 3 (18.8) |
| high school | 5 (25.0) | 7 (43.7) |
| at least some college | 9 (45.0) | 6 (37.5) |
| Diabetes n (%) | ||
| no diagnosis | 19 (95.0) | 16 (100.0) |
| Diagnosed, untreated | 0 (0.0) | 0 (0.0) |
| Diagnosed, treated | 1 (5.0) | 0 (0.0) |
| Hypertension n (%) | ||
| no diagnosis | 18 (90.0) | 13 (81.2) |
| diagnosed, untreated | 0 (0.0) | 2 (12.5) |
| diagnosed, treated | 2 (10.0) | 1 (6.3) |
| Mood disorder n (%) | ||
| no diagnosis | 11 (55.0) | 11 (68.8) |
| diagnosed, untreated | 8 (40.0) | 2 (12.5) |
| diagnosed, treated | 1 (5.0) | 3 (18.7) |
| Psychotic disorder n(%) | ||
| no diagnosis | 19 (95.0) | 15 (93.7) |
| diagnosed, untreated | 1 (5.0) | 0 (0.0) |
| diagnosed, treated | 0 (0.0) | 1 (6.3) |
| Daily heroin bags^ (SD) | 6.03 (3.59) | 8.18 (5.76) |
| IV heroin ^ n(%) | 9(45.0) | 11(68.8) |
| Daily drinks of alcohol (SD) | 0.50 (1.00) | 0.56 (1.63) |
| Daily packs cigarettes(SD) | 0.68 (0.44) | 0.57 (0.51) |
| Daily $’s on cocaine (SD) | 3.80 (10.82) | 2.06 (7.48) |
| Daily marijuana joints (SD) | 0.42 (0.99) | .13 (0.34) |
significance of difference between groups p<=0.5
n=11 methadone and n=18 naltrexone patients used heroin at baseline
Baseline diagnoses of diabetes, hypertension, mood disorder and psychotic disorder were explored, as all can be associated with weight gain. The two groups did not differ significantly with respect to these diagnoses. While diabetes, hypertension, and psychotic disorders are present in a small minority of the patients, mood disorders (major depressive disorder, dysthymia, and bipolar disorder) were more frequently diagnosed in the naltrexone-treated patients, but this difference did not reach statistical significance (p=0.12).
While the majority of patients in both groups were seeking treatment for heroin dependence, it should be noted that 4 of 16 methadone patients (25%) were using oral or IV opioids other than heroin at baseline (one was using an oxycodone product, two were using methadone purchased from the street, and two had transferred from other methadone programs). Of the naltrexone-treated patients, 2 of 20 (10%) were using hydrocodone products rather than heroin. However, these differences were not significant (χ2 = 1.44, p>.05).
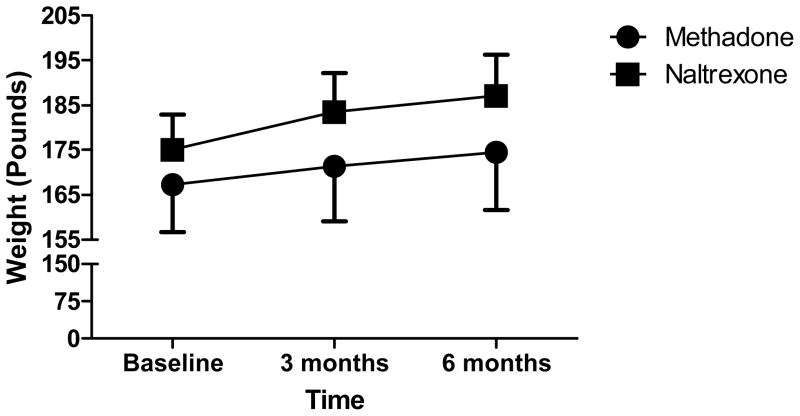
Figure 1 demonstrates that weight at baseline, 3 months and 6 months into treatment did not differ significantly between the two groups, F(1, 34)=0.59, p=0.45. A weight-by-treatment interaction was calculated using Huynh-Feldt correction due to lack of sphericity within the data. There was no significant interaction between treatment and weight, F(1.37, 46.50) = 0.79, p=41. Percent weight change from baseline to 3 months, and baseline to 6 months, did not differ between the two groups. At 3 months, n=16 methadone patients had a mean weight increase of 1.86% (SD 7.22%) compared to n=20 BNT patients with an increase of 4.63% (SD 6.49%); t(30.59)=−1.19, p=0.24. At 6 months, n=16 methadone patients had a mean weight increase compared to baseline of 3.67% (SD 9.52%) compared to n=20 BNT patients, who demonstrated a mean increase of 6.69% (SD 7.56%); t(28.27)=−1.03, p=0.31.
Figure 1.
Weight Across 6 Months of Opioid Maintenance
Note: Error bars depict the standard error of the mean.
To assess whether baseline weight would affect weight gain outcomes within and between treatment groups, patients were subdivided into “low weight” and “high weight” categories depending on whether their baseline weight measured below or above their treatment group’s median weight respectively. The median weight for the methadone-treated group was 164 lbs (74.4kg), and the naltrexone-treated group’s median weight was 167 lbs (75.7kg). The percentage of weight gained from baseline to 3 months, and from baseline to 6 months, did not differ between “low weight” and “high weight” patients within treatment groups. Furthermore, within weight categories, the percentage of weight gained from baseline to 3 months, and baseline to 6 months, did not differ between treatments.
Given the small differences in weight change over 6 months that was found between these treatment groups, a power analysis was undertaken to determine the number of patients that would be needed per group to observe a valid significant difference. Using the means and standard deviations between both groups at 6 months, such a study would have to include 232 patients per group to achieve adequate power (0.80) to detect these differences.
DISCUSSION
Both the naltrexone-maintained patients and the methadone-maintained patients were statistically similar across most baseline demographic, medical, psychiatric, and substance use items. At baseline, methadone patients were significantly less likely to be employed than the naltrexone patients. Furthermore, our hypothesis that there would be a significant difference in weight gain, with the methadone-maintained group demonstrating greater weight gain, was not supported. Notably, both groups gained approximately 10 pounds (add kg) of mean weight over 6 months of treatment.
This study has several limitations. The methadone maintenance treatment cohort used as a comparison, while statistically similar to the naltrexone-treated group regarding many variables, was comprised of an independent group of patients not originally recruited for research. Furthermore, one cannot account for differences in the facilities’ staff and procedures which may affect outcomes. Future studies might recruit patients clinically eligible for either agonist or antagonist maintenance and randomly assign individuals from the same recruitment cohort to either naltrexone or methadone maintenance. Body mass index (BMI) could not be measured in many cases due to absence of recorded height in the chart. In order to maximize data for analyses, weight in pounds and percent weight change from baseline were used. The present analyses may have benefited from larger sample sizes with fewer missing data points.
There are several additional comparisons that can be made between methadone- and naltrexone-treated opioid dependent cohorts in future studies. The current study could have benefited by comparing other secondary outcomes regarding weight gain in addition such as changes in blood pressure, hemoglobin A1c, cholesterol and triglycerides, as well as abdominal girth. We considered examining data on blood pressure as a secondary outcome to weight gain; however, too many data points were missing from the methadone-maintenance group to allow for adequate statistical comparison. Literature suggests that opioid dependence is associated with derangements in glycemic control similar to that of non-insulin dependent diabetes mellitus (18–20). Furthermore, naltrexone administration has been associated with decreased hemoglobin A1c in obese human subjects (21). The clinical literature associating opiate antagonists with weight loss is somewhat tenuous, with several clinical trials failing to observe significant weight loss (22–25). However, there is stronger evidence in the literature pertaining to changes in dietary preference. Preclinical (26–34) and clinical (35–38) data suggest that exposure to mu-opiate agonists is associated with a predilection for fatty and sweet (“palatable”) foods. Additionally, exposure to mu-opiate antagonists such as naltrexone is associated with a reduction in intake of fatty and sweet foods without necessarily leading to weight loss in both preclinical (27–30, 39–40) and clinical (41–43) trials. Further studies in this area may benefit from examining changes in dietary habits among opioid-dependent patients initiating methadone maintenance treatment versus naltrexone maintenance treatment. There is already a literature describing methadone-maintained patients’ shift in dietary preference toward sweet and fatty foods and away from more nutritious options (35, 44). In one study, gender influenced weight loss in obese subjects randomized to daily placebo, 50mg or 100mg of naltrexone. Female subjects lost a mean of 1.7 kg (3.7 lb) by the end of the study, while no effect was found in male subjects (45). The present study included too few female subjects to allow for adequate statistical comparison to control for gender.
Naltrexone maintenance therapy represents a viable alternative treatment to methadone maintenance for opiate dependence, especially for opioid addicts presenting for treatment early in the course of addiction and for those addicted to prescription opioids. Preclinical and clinical data suggest that maintenance on mu opiate agonists such as methadone is associated with weight gain, possibly due to changes in diet with increased preference for sweet and fatty foods. Preclinical and clinical data (in non-opiate-dependent human subjects) suggest that antagonist maintenance is typically a weight-neutral, or even weight-diminishing, treatment condition. The present study could find no significant difference in mean weight change among opiate dependent patients treated with either methadone or naltrexone maintenance therapy without any specific dietary counseling.
The negative results of this study may be explained by neurobiological and psychosocial factors. In addition to mu opiate receptor activity, agonism of kappa and delta opiate receptors have also been associated with hyperphagia (46–47). Naltrexone is an antagonist at the mu receptor (Ki 0.5nM), the kappa receptor (Ki 0.9nM) and at the delta opiate receptor (Ki 10nM) (48). Perhaps patients undergoing naltrexone maintenance treatment experience comparable weight gain to their methadone maintained counterparts because they receive hedonic effects from palatable foods through the kappa and delta opiate receptor pathways due to differential receptor blockade. Studies exploring the effects of naltrexone on weight change have used non-opiate-dependent lean or obese subjects (5, 10–13). It has been demonstrated that opiate-dependent individuals generally maintain poor diets, and often lead peripatetic lifestyles, constantly on the move from “fix” to “fix” (49). Perhaps stabilization in a structured clinical setting leads to a diversion of resources to food rather than drugs, and a diminution of “exercise” spent wandering for drugs and an increase in sedentary activities. These psychosocial changes may have a greater effect on weight changes in opiate dependent patients than the differential effect of mu agonists or antagonists on the central nervous system.
Opiate dependent patients in treatment may have few supportive resources and coping skills. “Fast food” and sweets may be used for comfort during abstinence, and may also be used for primary subsistence. High-calorie foods tend to be less expensive to healthier food options. Treatment providers focus on opiate addicts’ path toward, and maintenance of, abstinence. Emphasis should also be placed on this vulnerable population’s primary medical care, including maintenance of proper weight.
Lastly, potential weight gain associated with chronic use of mu-opiates has special implications for pain management providers, since chronic opioid therapy is an acceptable strategy for management of noncancer pain (50). Providers should be wary when treating pain secondary to both osteoarthritis often associated with obesity (51) and peripheral neuropathy associated with Type-2 diabetes (52). Potential weight gain from chronic mu-opiate administration to treat these pain conditions may eventually compound the severity of the pain, necessitating a cycle of escalating doses of opiate medication. This is particularly dangerous for obese patients, who may be more susceptible to respiratory suppression from mu-agonists as they are already prone to developing sleep apnea (53).
CITATIONS
- 1.Borg L, Kravets I, Kreek MJ. The pharmacology of long-acting as contrasted with short-acting opioids. In: Ries Richard K, Fiellin David A, Miller Shannon C, Saitz Richard., editors. Principles of Addiction Medicine. 4. Philadelphia: Lipincott Williams & Wilkins; 2009. pp. 117–131. [Google Scholar]
- 2.Institute of Medicine Committee. Prevention of HIV Infection among Injecting Drug Users in High Risk Countries: Preventing HIV Infection among Injecting Drug Users in High Risk Countries. Washington, DC: The National Press; 2006. [Google Scholar]
- 3.Rajs J, Petersson A, Thiblin I, Olsson-Mortlock C, Fredriksson A, Eksborg S. Nutritional status of deceased illicit drug addicts in Stockholn, Sweden—a longitudinal medicolegal study. The Journal of Forensic Science. 2004;49(2):1–10. [PubMed] [Google Scholar]
- 4.Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Substance Use and Misuse. 2007;42:1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson RL. Opioid regulation of food intake and body weight in humans. Federation Proceedings. 1987;46(1):178–182. [PubMed] [Google Scholar]
- 6.Levine AS, Atkinson RL. Opioids in the regulation of food intake and energy expenditure. Federation Proceedings. 1987;46(1):159–161. [PubMed] [Google Scholar]
- 7.Mohs ME, Watson RR, Leonard-Green T. Nutritional effects of marijuana, heroin, cocaine, and nicotine. Journal of the American Dietetic Association. 1990;90:1261–1267. [PubMed] [Google Scholar]
- 8.Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 9.Yuan CS, Wang CZ, Attele A, Zhang L. Methylnaltrexone reduced body weight gain in ob/ob mice. Journal of Opioid Management. 2009;5(4):213–218. doi: 10.5055/jom.2009.0023. [DOI] [PubMed] [Google Scholar]
- 10.Maggio CA, Presta E, Bracco EF, Vasselli JR, Kissileff HR, Pfohl DN, Hashim SA. Naltrexone and human eating behavior: a dose-ranging inpatient trial in moderately obese men. Brain Research Bulletin. 1985;14(6):657–661. doi: 10.1016/0361-9230(85)90115-7. [DOI] [PubMed] [Google Scholar]
- 11.Malcolm R, O’Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C. A controlled trial of naltrexone in obese humans. International Journal of Obesity. 1985;9(5):347–353. [PubMed] [Google Scholar]
- 12.Pfohl DN, Allen JI, Atkinson RL, Knopman DS, Malcolm RJ, Mitchell JE, Morley JE. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Research Monograph. 1986;67:66–72. [PubMed] [Google Scholar]
- 13.Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D. High-dose naltrexone therapy and dietary counseling for obesity. Biological Psychiatry. 1987;221(1):35–42. doi: 10.1016/0006-3223(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 14.Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1976–80 through 2005–2006. National Health and Nutrition Examination Survey (NHANES) (rest of reference?)
- 15.Rajs J, Petersson A, Thiblin I, Olsson-Mortlock C, Fredriksson A, Eksborg S. Nutritional status of deceased illicit drug addicts in Stockholn, Sweden—a longitudinal medicolegal study. The Journal of Forensic Science. 2004;49(2):1–10. [PubMed] [Google Scholar]
- 16.NIH, NHLBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. HHS, PHS; 1998. pp. 12–19. [PubMed] [Google Scholar]
- 17.Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. Journal of Substance Abuse Treatment. 2002;23(4):351–60. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 18.Reed JL, Ghodse AH. Oral glucose tolerance and hormonal response in heroin-dependent males. British Medical Journal. 1973;2:582–585. doi: 10.1136/bmj.2.5866.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willenbring ML, Morely JE, Krahn DD, Carlson GA, Levine AS, Shafer RB. Psychoneuroendocrine effects of methadone maintenance. Psychoneuroendocrinology. 1989;14(5):371–379. doi: 10.1016/0306-4530(89)90007-3. [DOI] [PubMed] [Google Scholar]
- 20.Ceriello A, Giugliano D, Passariello N, Quatraro A, Dello Russo P, Torella R, D’Onofrio F. Impaired glucose metabolism in heroin and methadone users. Hormone & Metabolic Research. 1987;19(9):430–433. doi: 10.1055/s-2007-1011844. [DOI] [PubMed] [Google Scholar]
- 21.De Marinis L, Mancini A, Valle D, Bianchi A, De Luca AM, Fulghesu AM, Villa P, Mancuso S, Lanzone A. Influence of chronic naltrexone treatment on growth hormone and insulin secretion in obese subjects. International Journal of Obesity & Realted Metabolic Disorders. 1997;21(11):1076–1081. doi: 10.1038/sj.ijo.0800519. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson RL, Berle LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL. Effects of long-term therapy with naltrexone on body weight in obesity. Clinical Pharmacology & Therapeutics. 1985;38(4):419–422. doi: 10.1038/clpt.1985.197. [DOI] [PubMed] [Google Scholar]
- 23.Maggio CA, Presta E, Bracco EF, Vasselli JR, Kissileff HR, Pfohl DN, Hashim SA. Naltrexone and human eating behavior: a dose-ranging inpatient trial in moderately obese men. Brain Research Bulletin. 1985;14(6):657–661. doi: 10.1016/0361-9230(85)90115-7. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D. High-dose naltrexone therapy and dietary counseling for obesity. Biological Psychiatry. 1987;221(1):35–42. doi: 10.1016/0006-3223(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 25.Malcolm R, O’Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C. A controlled trial of naltrexone in obese humans. International Journal of Obesity. 1985;9(5):347–353. [PubMed] [Google Scholar]
- 26.Dum J, Gramsch CH, Herz A. Activation of hypothalamic B-endorphin pools by reward induced by highly palatable food. Pharmacology, Biochemistry, and Behavior. 1983;18:443–447. doi: 10.1016/0091-3057(83)90467-7. [DOI] [PubMed] [Google Scholar]
- 27.Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Research. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- 28.Koch JE, Glass MJ, Cooper ML, Bondar RJ. Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neuroscience. 1995;66(4):951–957. doi: 10.1016/0306-4522(95)00001-y. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE, Bless EP, Swanson CJ. Investigation into the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. The Journal of Pharmacology and Experiemntal Therapeutics. 1996;278(3):1499–1507. [PubMed] [Google Scholar]
- 30.Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the entral tegmental area region of rats. Physiology and Behavior. 2002;76:107–116. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 32.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet LJ, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 33.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Molecular Brain Research. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Behavioral Reviews. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zador D, Lyons Wall PM, Webster I. High sugar intake in a group of women on methadone maintenance in South Western Sydney, Australia. Addiction. 1996;91(7):1053–1061. [PubMed] [Google Scholar]
- 36.Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Substance Use and Misuse. 2007;42:1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- 37.Morabia A, Fabre J, Chee E, Zeger S, Orsta E, Robert A. Diet and opiate addiction: a quantitative assessment of the diet of non-institutionalized opiate addicts. British Journal of Addiction. 1989;84:173–180. doi: 10.1111/j.1360-0443.1989.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 38.Bogucka-Bonikowska A, Baran-Furga H, Chmielewska K, Habrat B, Scinska A, Kukwa A, Koros E, Kostowski W, Polanowska E, Bienkowski P. Taste function in methadone-maintained opioid-dependent men. Drug and Alcohol Dependence. 2002;68:113–117. doi: 10.1016/s0376-8716(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 39.Gosnell BA, Krahn DD. The effects of continuous naltrexone infusions on diet preferences are modulated by adaptation to the diets. Physiology and Behavior. 1992;51(2):239–244. doi: 10.1016/0031-9384(92)90136-p. [DOI] [PubMed] [Google Scholar]
- 40.Sahr AE, Sindelar DK, Alexander-Chacko JT, Eastwood BJ, Mitch CH, Statnick MA. Activation of mesolimbic dopamine neurons during novel and daily limited access to palatable food is blocked by the opioid antagonist LY255582. American Journal of Physiology -Regulatory, Integrative and Comparative Physiology. 2008;295:R463–R471. doi: 10.1152/ajpregu.00390.2007. [DOI] [PubMed] [Google Scholar]
- 41.Yeomans MR, Wright P, Macleod HA, Critchley JAJH. Effects of nalmefene on feeding in humans. Psychopharmacology. 1990;100:426–432. doi: 10.1007/BF02244618. [DOI] [PubMed] [Google Scholar]
- 42.Drewnowski A, Krahn D, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces consumption of sweet high-fat foods in obese and lean female binge eaters. American Journal of Clinical Nutrition. 1995;61:1206–1212. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- 43.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behavior. Neuroscience and Behavioral Reviews. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 44.Kolarzyk E, Chrostek MJ, Pach D, Janik D, Kwiatkowski J, Szurkowska M. Assessment of daily nutrition ratios of opiate-dependent persons before and after 4 years of methadone maintenance treatment. Przeglad Lekarski. 2005;62(6):368–372. [PubMed] [Google Scholar]
- 45.Atkinson RL, Berle LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL. Effects of long-term therapy with naltrexone on body weight in obesity. Clinical Pharmacology & Therapeutics. 1985;38(4):419–422. doi: 10.1038/clpt.1985.197. [DOI] [PubMed] [Google Scholar]
- 46.Czyzyk Traci A, Nogueiras Ruben, Lockwood John F, McKinzie Jamie H, Coskun Tamer, Pintar John E, Hammond Craig, Tschöp Matthias H, Statnick Michael A. Opioid receptors control the metabolic response to a high-energy diet in mice. The FASEB Journal. 2010;24:1151–1159. doi: 10.1096/fj.09-143610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruegg H, Yu W, Bodnar RJ. Opiate receptor subtype agonist-induced enhancements of sucrose intake are dependent upon sucrose concentration. Physiology & Behavior. 1997;62(1):121–128. doi: 10.1016/s0031-9384(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Sun X, Sadee W. Different effects of opiate antagonists on mu-, delta-, and kappa-opiate receptors with and without agonist pretreatment. The Journal of Pharmacology and Experimental Therapeutics. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- 49.Sapira JD. The narcotic addict as a medical patient. American Journal of Medicine. 1968;45:555–588. doi: 10.1016/0002-9343(68)90172-1. [DOI] [PubMed] [Google Scholar]
- 50.Chou R, Fanciullo G, Fine P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal Of Pain: Official Journal Of The American Pain Society. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guh D, Zhang W, Bansback N, Amarsi Z, Birmingham C, Anis A. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(88):1–20. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsay T, Rodgers B, Savath V, Hettinger K. Treating diabetic peripheral neuropathic pain. American Family Physician. 2010;82(2):151–158. [PubMed] [Google Scholar]
- 53.Peppard P, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA: The Journal Of The American Medical Association. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]