Abstract
While it has been established that Treg cells can down-modulate an immune response, no study has addressed if the observed increase in Treg cells in aged mice is related to the decreased and delayed specific CD8 T cell responses seen following primary influenza infection. In this study, phenotypic characteristics and function of Treg cells were analyzed in young (4–6 months) and aged (18–22 months) mice prior to and during the course of primary influenza infection. Upon infection, aged, but not young, mice have a significant expansion of Treg cells. In addition, Treg cells of aged mice demonstrate both a higher percentage and higher expression per cell of CD69 both at baseline and during infection compared to young mice. However, Treg cells isolated from young and aged mice comparably suppress CD8 T cells and suppression is dose dependent. These results suggest that the increase in the percentage of Treg cells in aged mice may contribute to the diminished CD8 T cell response to primary influenza infection.
Keywords: Regulatory T cells, Aging and Influenza
1. Introduction
Immunosenescence defines the decline in the immune response that occurs with aging. These changes have been associated with increased incidences of infection, malignancy and disease (Derhovanessian, Solana et al. 2008).While age-altered changes have been noted in almost every component of the immune response: antigen presenting cells (APCs), B cells, T cells, as well as antibody and cytokine production (Miller 1996), the most notable changes have been demonstrated in T cell responses (Negoro, Hara et al. 1986; Deng, Jing et al. 2004; Jiang, Gross et al. 2007). These deceases in T cell responses are observed both in vitro and in vivo. For example, our laboratory has demonstrated that following influenza infection aged mice have both a reduced and delayed response, measured by specific CD8 T cell expansion and IFN-γ production (Po, Gardner et al. 2002). While it is clear that aging negatively affects the immune response, the exact mechanisms of these changes are not well understood.
Treg cells are a subpopulation of T cells which are responsible for maintaining immunological self-tolerance and reducing tissue damage following an immune response (Shevach 2002). Sakaguchi et al. demonstrated that a subpopulation of CD4 T cells constitutively expresses the IL-2Rα chain (CD25) and that these cells demonstrate suppressive capabilities (Sakaguchi, Sakaguchi et al. 1995). However, since all lymphocytes have the ability to up-regulate CD25 following activation, in order to bind growth cytokine IL-2 with high affinity, this marker alone cannot be used to identify Treg cells. Foxp3 (Forkhead winged helix protein 3) is an intracellular molecule expressed solely by Treg cells. Foxp3 is required for the development and function of Treg cells and absence of this molecule either by genetic defect, molecular manipulation or selective depletion results in the absence of Treg cells (Fontenot, Gavin et al. 2003; Jiang, Lau et al. 2003; Lahl, Loddenkemper et al. 2007). The importance of foxp3 in Treg cell function is demonstrated in scurfy mice and its human related IPEX (immune dysregulation, polyendocrinopathy, entropathy, X-linked) syndrome. These diseases have both been linked to genetic mutation in the protein coding for foxp3. Scurfy mice and IPEX patients suffer from multi-organ lymphoproliferative disorder and usually die early in life. While the importance of Treg cells in maintaining immunological tolerance to self cannot be minimized, the mechanisms used to exert tolerance may interfere with a response to foreign antigen (Suvas, Kumaraguru et al. 2003; Dittmer, He et al. 2004).
Treg cell percentage and number have been shown to increase with aging (Nishioka, Shimizu et al. 2006; Zhao, Sun et al. 2007). It has been postulated that the accumulation of fully functional Tregs in aged mice diminishes the ability of aged mice to mount a robust immune response. Lages et al. demonstrated that Treg cells from aged mice suppress the production of IFN-γ by effector T cells during Leishmania major infection and that depletion of Tregs prevents reactivation of the infection (Lages, Suffia et al. 2008). Similar studies have demonstrated that Treg cells can reduce tumor immunity in aged mice (Sharma, Dominguez et al. 2006). These studies collectively demonstrate that Treg cells may contribute to immunosenescence and discerning their function may lead to a better understanding of the decline in T cell responses seen in aging.
While the phenotype of Treg cells in aged mice has been analyzed in several studies using both in vitro and in vivo systems, no study has addressed whether or not Treg cells play a role in the reduced response to primary virus infection that is seen in aging. In the current study, we investigated possible phenotypic differences in Treg cells of aged mice before and after influenza infection to determine if such changes influence the CD8 effector T cell response to primary virus infection. Since the assessment of functional capacity of Treg cells of aged mice has produced conflicting results (Sakaguchi, Sakaguchi et al. 1995; Sharma, Dominguez et al. 2006; Lages, Suffia et al. 2008), we wanted to examine further the functional capacity of Treg cells isolated from young and aged mice. The results of these studies not only confirmed that there is a significant increase in Treg cells in aged mice, but also that the percentage of Treg cells increases further after infection in aged but not young mice. In contrast, there is no difference in the suppressive abilities of Treg cells isolated from young and aged mice. Our findings suggest that this increase in Treg cells in aged mice may interfere with the primary immune response to influenza.
2. Methods and Materials
2.1. Mice
Four-month and 18-month old wild type Balb/c and C57Bl/6 mice were purchased from the NIA at Harlan Sprague Dawley (Indianapolis, IN). Six to 8 week old P14 (GP33–41 TCR-Tg) mice (Ashton-Rickardt et al, 1994) were purchased from Taconic Farms (Hudson, NY), and 6 to 8 week old Balb/c ByJ Cl.1 Thy1.1, Clone-4 (HA518–526 TCR-Tg) mice (Cl-4) (Kreuwel et al., 2001) were purchased from Jackson laboratory (Bar Harbor, Maine). All mice were maintained in AAALAC-approved barrier facilities at Drexel University (Philadelphia, PA). Mice were allowed to acclimate for at least one week in our animal facilities prior to use. Mice exhibiting enlarged spleens or tumors were eliminated from this study. All experiments involving mice were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of Drexel University.
2.2. Virus Infection
Influenza A-Puerto Rico/8/34 (PR8; H1N1) viruse was propagated in specific-pathogen-free eggs and stored at −80 °C for subsequent use. Mice were infected intravenously (i.v.) with 300 ul of sterile saline containing 300 Hemagglutination units (HAU) of PR8.
2.3. Ex-vivo stimulation
Splenocytes were isolated from young and aged mice using 0.83% ammonium chloride and resuspended in RPMI-1640 containing 10% FB, L-glutamine, 2-b-mercaptoethanol and gentamycin. Splenocytes were stimulated with 10−6 M NP366–374 peptide for influenza infected C57Bl/6 mice or 10−6 M HA518–522 peptide for influenza infected Balb/c mice and stimulated for 5 hours at 37 °C with 5% CO2 . Following surface and intracellular staining, cells were analyzed using flow cytometry.
2.4. Treg Isolation
Isolated splenocytes were resuspended in MACS buffer (PBS, 0.5%BSA) and stained with an antibody cocktail, which recognizes CD8, CDc11 and NK1.1 and micro-beads, to negatively select for CD4+T cells. Following CD4 selection, remaining splenocytes are stained with an antibody which recognize CD25 and micro-beads. The double selection method allows for the selection of cells, which are postive for both CD4 and CD25. CD4+CD25+ enrichment was performed using MACS isolation, enrichment was better than 70% in all experiments, and some experiments yielded enrichment percentages greater than 90%.
2.5. Suppression Assay
Responder cells were isolated from TCR Tg mice and labeled with the cytoplasmic dye CFSE. 1.5×105 total TCR Tg splenocytes per well were co-cultured with Tregs at a Treg cell: Tg splenocyte ratio of either 1:1 or 1:10 in a 96-well plate containing 10−6 M of GP33–41 peptide for responder cells from P14 mice or HA518–526 for responder cells from Cl-4 mice. Plates were incubated for 72 hours at 37°C with 5% CO2. Percentage of CFSElo CD8 T cells were obtained by gating on CD8 T cells as the parent population then gating on all CD8 T cells with an CFSE MFI <103.
2.6. Flow Cytometry
2×106 cells were stained for surface markers using mAb (anti-CD4, CD8, CD44, CD62L, CD69, CD25 and CTLA-4) purchased from BD Pharmigen. Intracellular staining was performed using anti-foxp3 mAb purchased from Ebioscience following fixation wash buffers (cytofix and cytoperm kit purchased from Ebioscience). Intracellular IFN-γ antibody was purchased from BD Pharmingen; cells were fixed with fixation buffer purchased from BD. Flow cytometry was performed on FACS Canto and data was analyzed using FlowJo Software.
2.7. Statistical Analysis
All statistical analyses were performed using Student’s t test. Significant differences were determined at the level of p < 0.05. Results are expressed as mean ± SD. Correlation was calculated using pearson’s correlation coefficient.
3. Results
3.1. Expansion of CD4+foxp3+ and CD4+CD25+foxp3+ T cells in response to PR8 virus infection in vivo
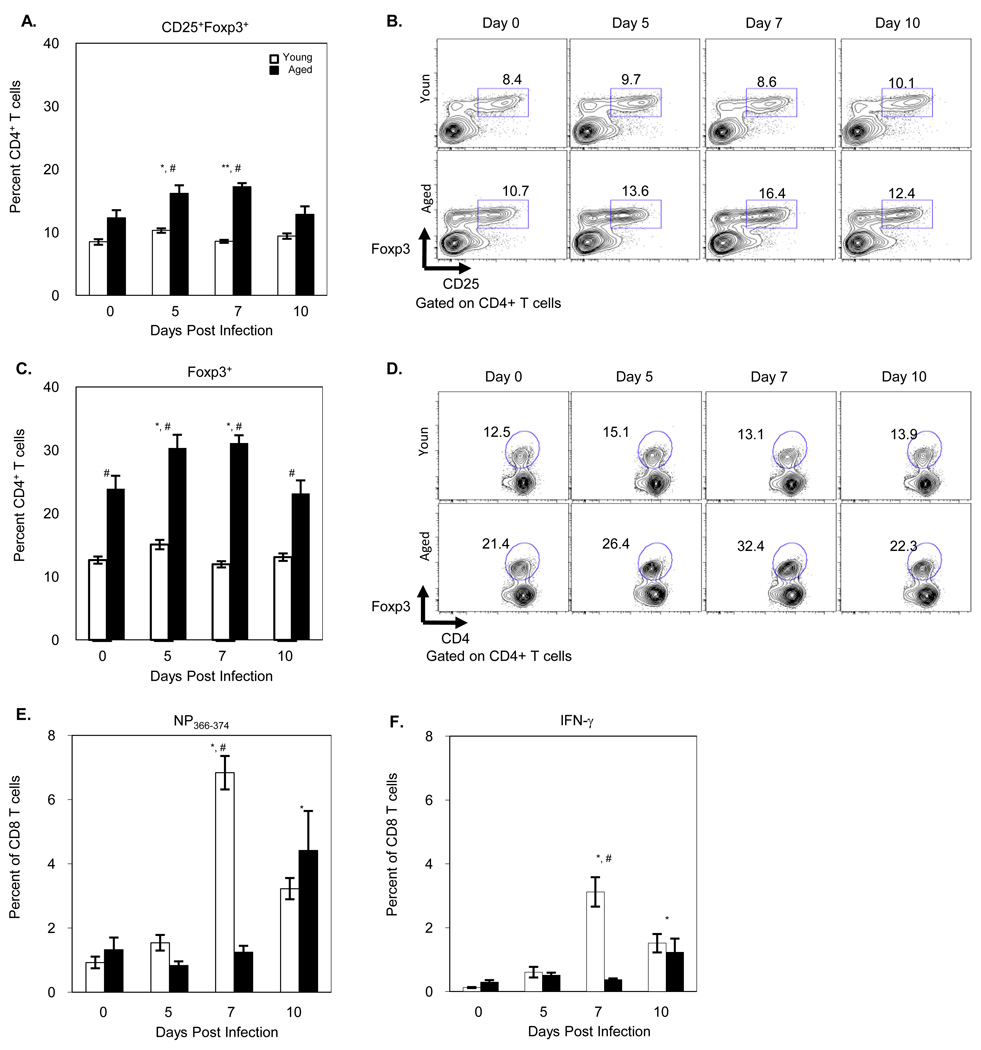
We have previously demonstrated that aged mice have a delayed and reduced CD8 T cell response to influenza infection (Po, Gardner et al. 2002). To examine the possible contribution of Treg cells to this change, young and aged B6 mice were infected with 300 H.A.U. of PR8 influenza virus. Splenocytes were obtained at varying times after infection and stained with antibodies that recognize Treg cells as well as specific CD8 T cells. While there was no significant change in the percentage of CD4+CD25+foxp3+ or CD4+foxp3+ T cells in young mice following infection, significant expansion of Treg cells was observed in aged mice (Figure 1A–D). Notably at the peak of both specific CD8 T cells (NP366–374) expansion (Figure 1E) and function (IFN-γ) (Figure 1F) in young mice (Day 7), aged mice demonstrated the peak expansion of Treg cells and limited specific CD8 T cell response. Interestingly the percentage of Treg cells returned to pre-infection levels in aged mice on day 10, the time at which significant expansion of specific CD8 T cells and IFN-γ production is observed in aged mice. These results suggest that the decrease and the delay in specific CD8 T cell expansion and function in aged mice may be related to the increased number and percentage of Treg cells not only at baseline but also the expanded percentage after viral infection.
Figure 1. Expansion of Treg cells in response to influenza infection.
Young and aged B6 mice were i.v. infected with 300 H.A.U PR8. On Days 5, 7 and 10 post-infection, splenocytes were isolated, stained with CD4, CD25 and foxp3 antibodies and for virus specific CD8 cells with NP366–374 and IFN-γ and assessed by flow cytometry A) Percentage of CD4 T cells that are CD25 and foxp3 positive. B) Representative figure of CD25+foxp3+ CD4 T cells following influenza infection in young and aged mice. C) Percentage of CD4 T cells positive for foxp3. D) Representative figure of foxp3+ CD4 T cells following influenza infection in young and aged mice. E) Percentage of CD8 T cells positive for NP366. F) Percentage of CD8 T cells positive for IFN-γ. Values represent the mean ± S.E., n=6 per day per age group. *p<0.05, **p<0.01, ***p<0.001 compared to Day 0. # p<0.05 comparing young and aged in same day. Two independent experiments were combined.
Several reports describe two subsets of Treg cells 1) naturally occurring Treg cells (nTregs) and 2) induced Treg cells (iTregs) (Shevach and Stephens 2006). NTreg cells are naturally occurring Treg cells produced in the thymus of both mice and humans. These cells begin to express both CD25 and foxp3 intrathymically and account for 5–10% of the total CD4 T cell population (Workman, Szymczak-Workman et al. 2009). In addition to naturally arising Treg cells it has been demonstrated that in various conditions naïve CD4 T cells can convert to foxp3 expressing CD4 T cells that can suppress effector cell function. For example, Chen et al demonstrated that in the presence of anti-CD3, anti-CD28 and TGF-β naïve CD4 T cells can be induced to express CD25 and foxp3 and have suppressive capabilities (Chen, Jin et al. 2003). Further, Wahl and colleagues were also able to demonstrate that these in vitro induced Treg cells were able to suppress immune responses (Chen, Jin et al. 2003).
In order to determine whether the expanded Treg cells seen after primary infection in aged mice were due to increased conversion of naïve CD4 T cells to Treg cells, we compared the ability of naïve CD4 T cells isolated from young and aged mice to convert to iTreg cells upon in vitro culture with anti-CD3, anti-CD28 and TGF-β. After depletion of CD4+CD25+ cells by MACS, CD4+CD25+foxp3+ represented about 3% of the CD4 population of both young and aged mice at initiation of culture. While neither young nor aged CD4 T cells demonstrated a significant increase in CD4+CD25+foxp3+ cells at 24 hr, by 48 hr 15% of CD4 T cells isolated from young mice had converted to Treg cells, while only 5% of naïve CD4 T cells isolated from aged mice had converted (p < 0.05). No additional conversion was seen in either culture at 72 hr. Due to low conversion of naïve CD4 T cells from aged mice into Treg cells, we concluded that the expanded Treg cells seen in aged mice after infection was a result of expansion of nTreg cells, rather than conversion to iTreg cells.
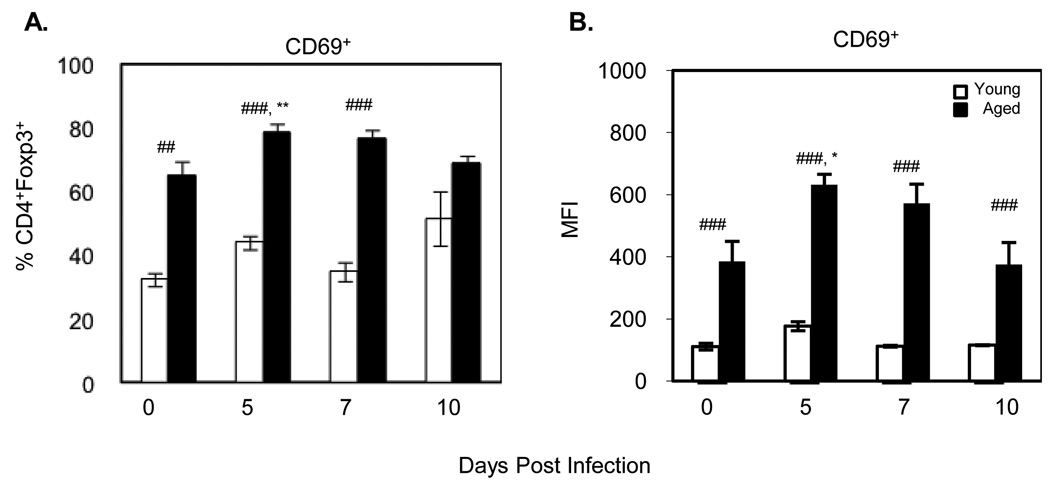
We also investigated whether or not the Treg cells were more activated by assessing both the percentage and level of expression of CD69 following influenza infection. Although CD69 has been recently described as a possible regulatory marker (Han, Guo et al. 2009), CD69 is also a marker of activation in response to antigen challenge (Cosulich, Rubartelli et al. 1987). Our previous data demonstrated that CD69 activation occurs early usually peaking during the first 24 hours after infection on CD8 T cells (data not shown). Analysis of Treg activation revealed that CD4+foxp3+ Treg cells in aged mice demonstrate a significantly increased percentage of CD69 expression at baseline compared to Treg cells of young mice and infection further significantly increases the percentage of CD4+foxp3+ Treg cells of aged, but not young mice, that express CD69 (Figure 2A). This increase is apparent not only in the percent positive but also in the level of expression of CD69 as determined by MFI which is also significantly higher at baseline in aged mice and increases following influenza infection (Figure 2B).
Figure 2. Activation of Treg cells in response to influenza infection.
On Days 5, 7 and 10 post-infection, CD4+foxp3+ Treg cells were analyzed by flow cytometry for expression of the activation marker CD69. Data represents the mean ± S.E., n=3 mice per day per group. *p<0.05, **p<0.01, ***p<0.001 comparing responses to uninfected of same age. #p<0.05, ##p<0.01, ###p<0.001 comparing the responses of young vs aged on same day. Two experiments were combined.
3.2. Phenotypic characteristics of Treg cells in aged compared to young mice
To further explore whether or not Treg cells play a role in the reduced immune response to viral infection of aged mice, we wanted to characterize the Treg cells seen both at baseline and after infection. While a number of studies on Treg cells and aging report increased percentages, we also wanted to study other markers implicated in the function of Treg cells, which have not been well characterized in aged mice.
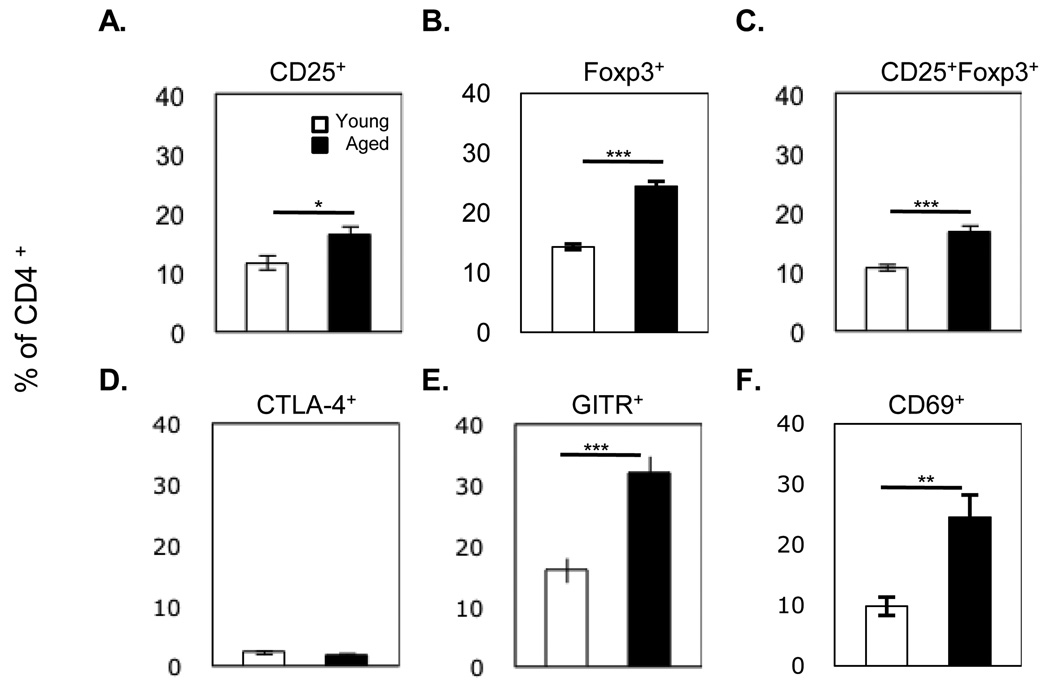
Many studies examining Treg cells identify Treg cells as CD4+CD25+ T cells (Sakaguchi, Sakaguchi et al. 1995). Our data confirm previous studies demonstrating that aged mice have a significant increase in this population (Figure 3A: young vs. aged 11.6 ± 1.2% vs. 16.2 ± 1.5% p<0.05). Co-expression of both CD25 and foxp3 on CD4 T cells has also been used to define Treg cells (Nishioka, Shimizu et al. 2006). Analysis using these two markers demonstrated a similar increase of Treg cells in aged compared to young mice (Figure 3C: young vs. aged 10.6 ± 0.5% vs. 16.5 ± 1.1% p<0.001). However, we observed the largest difference between young and aged mice in the percentage of CD4+foxp3+ T cells (Figure 3B: young vs. aged 14.3 ± 0.5% vs. 24.4 ± 0.8% p<0.001).
Figure 3. Characterization of CD4 T cells in the spleens of young and aged mice.
Splenocytes were isolated from young and aged C57BL/6 mice then stained with antibodies associated with Treg cells (CD4, CD25 and foxp3) and assessed by flow cytometry. Values represent the mean ± S.E., n=7–30 mice per group. *p<0.05, **p<0.01, ***p<0.001.
Several surface receptors of Treg cells have been implicated in the down-regulation of activated T cells. Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is expressed by a subpopulation of T cells and has been shown to negatively affect the immune response. When CTLA-4 is deleted or blocked by antibody, mice have been reported to develop autoimmunity (Friedline, Brown et al. 2009) or to exhibit enhanced tumor immunity (Leach, Krummel et al. 1996). We found no statistical difference between young and aged mice in the percentage of CD4 T cells that expressed CTLA-4 (Figure 2D).
It has been reported that Treg cells constitutively express Glucocorticoid-induced TNF-related receptor (GITR). However the importance of this receptor in Treg cells function is questionable. While addition of anti-GITR completely abrogates suppression, the mechanism of inhibition by this molecule remains controversial (Shevach and Stephens 2006). Further, CD4+CD25+ Treg cells from GITR−/− mice are able to suppress CD4 effector T cells at levels similar to Treg cells isolated from GITR+/+ (Stephens, McHugh et al. 2004). The proportion of CD4 T cells expressing GITR is significantly increased in aged mice (Figure 3E: young vs aged 15.8 ± 2.0% vs 32.0 ± 2.7% p<0.001) and most closely reflects the percentage of CD4+foxp3+.
CD69 is recognized as an early activation marker. Recently Han et al. have demonstrated that a sub-population of CD4 T cells which constitutively express CD69 have regulatory capabilities (Han, Guo et al. 2009). These cells do not express CD25 or foxp3 and therefore were proposed as a new subset of Treg cells. We found that aged mice have an increased proportion of CD4+CD69+ T cells compared to young mice, (Figure 3F: 22.5 ± 3.7% vs. 8.6 ± 1.5%, respectively, p<0.01).
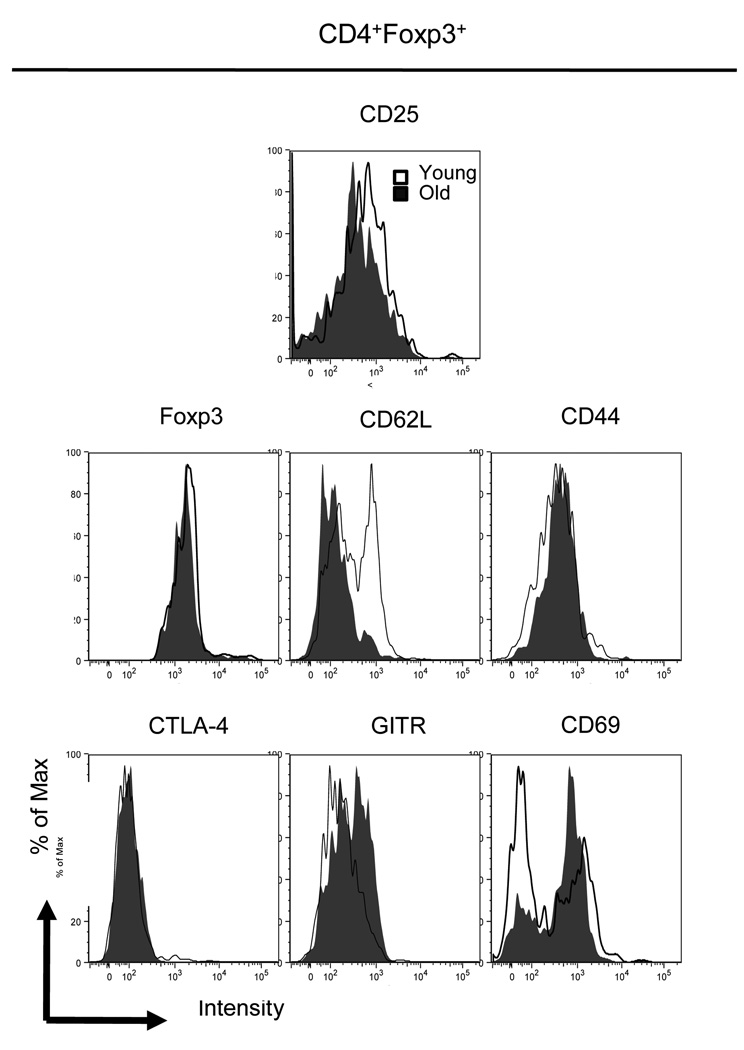
Since many of the markers used to identify Treg cells are molecules that may be involved in their function, we also assessed age-associated differences in the levels of expression of these molecules using mean fluorescence intensity (MFI) (Figure 4 and Table 1). Traditionally it has been acknowledged that CD25 is a marker of Treg cells. However, CD25 is also up-regulated on activated T cells. Similarly CD69, CTLA-4 and GITR are all increased on T cells upon activation. In contrast foxp3 protein is found only in Treg cells. Therefore we defined Treg cells as CD4+foxp3+ T cells and examined the expression of activation markers on these cells.
Figure 4. Expression of Treg cell markers of young vs aged mice.
CD4+foxp3+ Treg cells were analyzed for their expression of surface receptors related to their function. All CD4 cells expressing foxp3 were analyzed for their expression of foxp3, CD25, CTLA-4, GITR, CD69, CD44 and CD62L. Representative experiment repeated twice.
Table 4. 1. Mean Fluroscent intensity of functional markers on CD4+foxp3+ Treg cells.
CD4+foxp3+ were analyzed for their expression of CD25, foxp3, CTLA-4, GITR, CD44 and CD62L. Experiment repeated twice with n=3 young and aged mice in each experiment.
| B6 | ||
|---|---|---|
| Young | Aged | |
| CD25 | 450 ± 78.2 | 206 ± 32 a |
| Foxp3 | 579 ± 5.4 | 537 ± 1.2 |
| CTLA-4 | 49.8 ± 9.6 | 51.7 ± 11.6 |
| GITR | 206 ± 11.3 | 302 ± 10.7 b |
| CD69 | 201 ± 21.3 | 405 ± 21.5 a |
| CD62L | 307 ± 6.2 | 147 ± 13.6 b |
| CD44 | 366 ± 12.7 | 444 ± 58 |
p<0.05,
p<0.001
Treg cells of young and aged mice express foxp3 and CTLA-4 at similar levels. CD25 (IL-2Rα), believed to interfere with effector T cells binding to the proliferative cytokine IL-2, demonstrate an increased expression on Treg cells of young mice compared to their older counterparts (450 ± 78.2 vs 206 ± 32 p<0.05). In contrast, the expression of CD69 is significantly decreased in young compared to aged mice.
The migration of Treg cells can have a significant impact on function in vivo. Similar to effector T cells, Treg cells express CD62L which could play an important role in Treg trafficking. Treg cells of aged mice demonstrated a significant reduction in CD62L expression compared to young mice, 147 ± 13.6 vs. 307 ± 6.2 (p<0.01). CD44 marker has also been implicated as a marker on Treg cells (Liu, Soong et al. 2009); no differences were noticed between Treg cells of young vs aged mice. While the percentage of Treg cells (4+25+) were significantly increased in aged compared to young mice; aged mice had significantly decreased expression of CD25 compared to young mice. In contrast the aged mice had significantly increased expression of CD69, GITR and CD62L. Other important Treg cell functional markers, CD44, foxp3 and CTLA-4, showed no age-altered difference in their expression patterns.
3.3. CD4+CD25+ Treg cells from young and aged mice suppress specific Tg splenocytes at similar levels
Our results demonstrate that influenza infection of aged mice induces an increase in the percentages of Tregs. However, since there are conflicting studies regarding whether or not Treg cells isolated from young and aged mice suppress T cells at a similar level (Sharma, Dominguez et al. 2006; Lages, Suffia et al. 2008), we wanted to examine the ability of Treg cells from aged mice were able to suppress specific CD8 T cell responses. We addressed this question using an in vitro suppression assay.
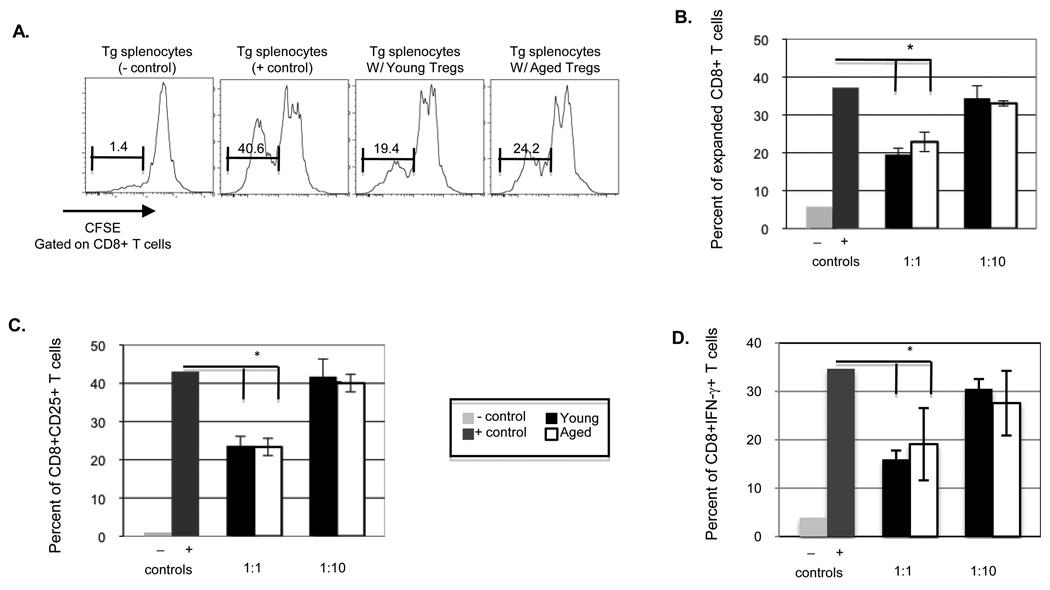
While foxp3 is the only molecule uniquely expressed on Treg cells, its intracellular location makes it impossible to utilize to sort cells. Thus the CD25 surface receptor is commonly used to isolate Treg cells. CD4+CD25+ were isolated from young and old B6 mice. Splenocytes from TCR Tg mice were used as responder cells. Ideally we would have used a Tg mouse model whose TCR recognize influenza antigen on a B6 background. However since such mice are not commercially available, we utilized B6 mice recognizing LCMV (P14). CD4+CD25+ Treg cells were co-cultured with isolated Tg splenocytes labeled with CFSE at Treg cell:Tg splenocytes ratios of 1:1 and 1:10 and then stimulated with GP33–41 peptide. 72 hours post stimulation co-cultures were stained with antibodies that recognize CD8 T cell activation (CD25) and assessed for the expansion and activation of CD8 T cells. As shown in Figure 5, addition of Treg cells from young and aged mice significantly suppress specific CD8 T cell responses at similar levels. Specifically the addition of Treg cells significantly reduces the expansion of specific CD8 T cells, demonstrated by increased CFSE expression in co-cultures containing Treg cells. Addition of Treg cells to these cultures also reduced CD8 T cell IFN-γ production, demonstrating a loss of function, as well as loss of the ability to expand (Figure. 5D). Further the addition of Treg cells significantly reduced the percentage of CD8 T cells which express activation marker CD25 (Figure. 5C). This suppression was dose dependent since addition of Treg cells at a ratio of 1:10 did not have a significant impact of CD8 T cell activation, expansion or function.
Figure 5. Young and aged Treg cells of mice suppress Tg splenocytes at similar levels.
CD4+CD25+ Treg cells were isolated from young and aged B6 mice and co-cultured with P14 Tg splenocytes at a ratio of 1:1 or 1:10 (Treg cell;Tg splenocyte). A) Representation of Tg splenocytes stimulated in the absence and presence of young or aged enriched CD4+CD25+ cells. B) CD8 T cell expansion assessed by CFSE; C) Activation of CD8 T cells assessed by percent CD25 expression. D) Functional activity of CD8 T cells assessed by percent IFN-γ expression. Data represents the mean ± S.E., n=3. Similar results were obtained in two experiments.
While influenza specific Tg mice are not available on a B6 background they are available on a Balb/c background (Clone-4 (CL-4)). Since several studies have reported differences in the function of Treg cells isolated from different mice strains (Chen, Oppenheim et al. 2005), it was important to determine whether are not the similarities seen in Treg cell function in young and aged mice is a strain dependent phenomena. In addition we wanted to ensure that these results were not unique for P14 Tg mice or LCMV suppression. Therefore suppression of influenza specific Tg splenocytes was also evaluated using Treg cells from young and aged Balb/c mice. CL-4 influenza specific Tg splenocytes respond to influenza peptide HA518–526. Experiments using CL-4 Tg mice had similar results confirming that Treg cells from young and aged mice suppress specific CD8 T cells at similar levels (data not shown).
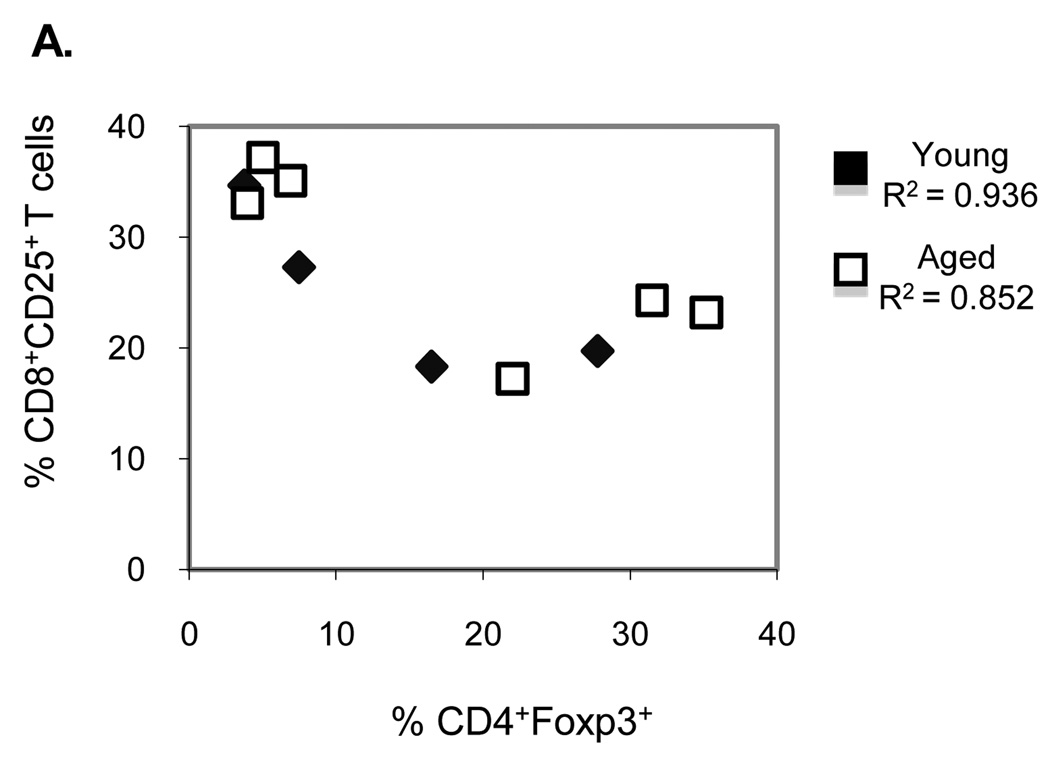
We further analyzed the dose dependency of Treg cells by comparing the activation of P14 CD8 T cells in the presence of CD4+foxp3+ Treg cells. Correlation between the percentages of CD4+foxp3+ T cells with activated CD8+CD25+ T cells in the culture suggests that activation of CD8 T cells is inversely related to the percentage of Treg cells (correlation co-efficient of 0.936 vs. 0.852, young vs. aged respectively, p<0.01; Figure 6A). This dose dependance was also apparent when comparing IFN-γ production by CD8 T cells in the presence of Treg cells: in the presence of an increased percentage of Treg cells there was reduced percentage of IFN-γ producing CD8 T cells.
Figure 6.
Correlation between CD4+foxp3+ Treg cells and activated CD8+CD25+ T cells. Following 72 hours of stimulation co-cultures were stained with antibodies that recognize Treg cells and CD25+ CD8 T cells. Results from B6 and P14 co-cultures. Data represents n=6 per age group. Results were obtained in two similar experiments.
The results from these experiments further support the hypothesis that Treg cells isolated from young and aged mice suppress at similar levels (Sharma, Dominguez et al. 2006). While the individual function of individual Treg cells of aged mice may not be a major factor in the reduced immune response seen with increased age, the increased percentages of Treg cells found in aged mice could be a potential barrier for the specific CD8 T cell response.
4. Discussion
Po et al. demonstrate that the specific CD8 T cell response to primary influenza infection is altered in aged mice, as shown by decreased and delayed CD8 T cell expansion as well as specific CD8 T cell function as assessed by IFN-γ production (Po, Gardner et al. 2002). While many studies link the age-altered changes in T cells to intrinsic defects, we hypothesized that the extrinsic environment of aged mice may also contribute to the decrease. Specifically we hypothesized that this decreased CD8 T cell expansion and function may be linked to the increase in Treg cells seen in aged mice.
Since Treg cell percentage is increased in aged mice (Nishioka, Nishida et al. 2008), we wanted to study the effects of influenza infection on this population of CD4 T cells. As early as 5 days after infection we see a significant increase in the percentage of Treg cells in aged mice while young mice maintain a similar percentage of Treg cells throughout the course of infection (Figure 1). The expansion of Treg cells in aged mice could provide a potential hurdle for the immune response to overcome, as other studies have demonstrated Treg cells can interfere with the in vivo immune response (Zelinskyy, Dietze et al. 2009). It is not until day 10 when the percentage of Treg cells in aged mice returns to basal level that the CD8 T cells begin to expand and respond to influenza infection by producing IFN-γ. Interestingly after influenza infection, Treg cells have an increase state of activation as demonstrated by increasing percentages and increased expression of CD69 both before and during infection. We hypothesize that this early and continued expansion of Treg cells in aged mice may interfere with expansion and function of specific CD8 T cell.
Several studies have compared the percentages of Treg cells. As previously discussed Treg cells have been defined differently based on their surface phenotype as being: CD4+CD25+, CD4+foxp3+ or CD4+CD25+foxp3+. In this report we are able to conclude that Treg cells are significantly increased in aged (>18 months) compared to young (4–6 months) mice in all three of the previously defined populations (Figure 1). Han et al. proposed that a new subset of Treg cells, CD4+CD69+, were also able to suppress effector T cell function (Han, Guo et al. 2009). We, therefore, examined this subpopulation of CD4 T cells. We found that total CD4+CD69+ T cells as well as CD4+CD69+foxp3− Treg cells are also increased in aged mice compared to young (young vs aged, 8.9 ± 1.6% vs 19.4 ± 2.3%). These results provide an interesting set of data which shows that the regulatory component of the immune response is significantly changed in aging.
While there is clearly an increase in Treg cells with age, the question is whether their increased percentage alone or in conjunction with enhanced suppressive function contribute to the age-associated decline in response. Our results from in vitro suppression assays agree with those of Sharma et al. and Nishioka et al. which suggest that there is no difference in per cell function of Treg cells of aged compared to young mice (Nishioka, Shimizu et al. 2006; Sharma, Dominguez et al. 2006). In addition, we show that Treg cells suppress CD8 T cell activation in a dose dependent manner (Figure 8). The dose dependent suppression of effector function, demonstrated by the significant R2 values seen in correlation studies, is critical when there is increasing percentages found in aged mice both prior to and during and immune response to influenza infection. This increased percentage of Treg cells may raise the threshold of activation required to produce a strong immune response to foreign antigen.
Although we report no significant difference in the suppressive function of Treg cells in a suppression assay, our results from MFI shows that aged mice have an increased expression of GITR, whose ligand is found on APCs. The GITR ligand usually binds to T cells during an immune response and this binding enhances antigen presentation as well as activation of APCs and T cells. The constitutive expression of GITR on Treg cells allows them to bind to APCs as well and to interfere with antigen presentation and T cell activation. It has been reported that G3c, a mAb which recognizes GITR, breaks the hypoproliferative state of Treg cells and cause them to expand both in vivo and in vitro (Nishioka, Nishida et al. 2008). Therefore we postulate that binding of Treg cells to APCs via GITR can both down-regulate an immune response by interfering with antigen presentation and can also lead to the expansion of Treg cells. GITR should be further analyzed as a mechanism, which leads to the expansion of Treg cells following influenza infection in aged mice.
The results of this study strongly suggest that the presence of increasing percentages of activated Treg cells in aged mice is a contributing factor in the decreased and delayed CD8 T cell during primary influenza infection.
Research Highlights.
Upon influenza infection, aged, but not young, mice have a significant expansion of Treg cells.
Treg cells of aged mice demonstrate both a higher percentage and higher expression per cell of CD69 both at baseline and during infection compared to young mice.
Treg cells from young and aged mice comparably suppress CD8 T cells and suppression is dose dependent.
These results suggest that the increase in the percentage of Treg cells in aged mice may contribute to the diminished CD8 T cell response to primary influenza infection.
Abbrevations used
- HAU
hemagglutination unit
- LCMV
lymphocytic choriomeningitis virus
- Tg
transgenic
- NP
NP366–374/Db
- HA
HA518–526/Kd
- GP
GP33–41/Db
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Chen W, Jin W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Oppenheim JJ, et al. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25− responder T cells than C57BL/6 mice. J Leukoc Biol. 2005;78(1):114–121. doi: 10.1189/jlb.0604341. [DOI] [PubMed] [Google Scholar]
- Cosulich ME, Rubartelli A, et al. Functional characterization of an antigen involved in an early step of T-cell activation. Proc Natl Acad Sci U S A. 1987;84(12):4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Jing Y, et al. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172(6):3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Solana R, et al. Immunity, ageing and cancer. Immun Ageing. 2008;5:11. doi: 10.1186/1742-4933-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer U, He H, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20(3):293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, et al. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Friedline RH, Brown DS, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206(2):421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Guo Q, et al. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gross D, et al. Aging affects initiation and continuation of T cell proliferation. Mech Ageing Dev. 2007;128(4):332–339. doi: 10.1016/j.mad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lau LL, et al. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171(8):4352–4358. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- Lages CS, Suffia I, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, et al. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Liu T, Soong L, et al. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol Direct. 2009;4:40. doi: 10.1186/1745-6150-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Negoro S, Hara H, et al. Mechanisms of age-related decline in antigen-specific T cell proliferative response: IL-2 receptor expression and recombinant IL-2 induced proliferative response of purified Tac-positive T cells. Mech Ageing Dev. 1986;36(3):223–241. doi: 10.1016/0047-6374(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Nishida E, et al. In vivo expansion of CD4+Foxp3+ regulatory T cells mediated by GITR molecules. Immunol Lett. 2008;121(2):97–104. doi: 10.1016/j.imlet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, et al. CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3+ T cells in aged mice. J Immunol. 2006;176(11):6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Po JL, Gardner EM, et al. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123(8):1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- Sharma S, Dominguez AL, et al. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177(12):8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6(8):613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- Stephens GL, McHugh RS, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173(8):5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- Suvas S, Kumaraguru U, et al. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Szymczak-Workman AL, et al. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66(16):2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Dietze KK, et al. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114(15):3199–3207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- Zhao L, Sun L, et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81(6):1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]