Abstract
Background
Heparin-induced thrombocytopenia (HIT) is an adverse drug reaction caused by antibodies to the heparin/platelet factor 4 (PF4) complex, resulting in thrombocytopenia and prothrombotic state. HIT diagnosis is challenging and depends on clinical presentation and laboratory tests. We investigated the usefulness of clinical scores and heparin/PF4 ELISA optical density (OD) as a diagnostic marker and thrombosis predictor in HIT.
Methods
We analyzed 92 patients with suspected HIT. The heparin/PF4 antibody was measured using a commercial ELISA kit (GTI, USA). For each patient, the 4 T's score and Chong's score were calculated.
Results
Of the 92 patients, 28 were anti-heparin/PF4-seropositive. The 4 T's score and Chong's score showed good correlation (r=0.874). The 4 T's score and OD values showed good performance for diagnosis of the definite and unlikely HIT groups; however, OD levels showed better sensitivity (93.8%) than the 4 T's score used alone (62.5%). Of the 92 patients, 26 developed thrombosis. The OD values were significantly higher in patients with thrombosis than in those without thrombosis (0.52 vs. 0.22, P<0.001). Patients with high OD values (OD>0.4) had an increased risk of thrombosis (adjusted odds ratio 9.44 [3.35-26.6], P<0.001) and a shorter 250-day thrombosis-free survival (32.1% vs. 54.7%, P=0.012).
Conclusions
ELISA OD values in combination with clinical scoring can improve the diagnosis of and thrombosis prediction in HIT. More attention should be paid to the use of clinical scores and OD values as thrombosis predictors in HIT.
Keywords: Heparin-induced thrombocytopenia, Heparin-platelet factor 4 antibody, Clinical scoring system, Optical density value
INTRODUCTION
Heparin-induced thrombocytopenia (HIT) is a distinct clinicopathologic syndrome caused by platelet-activating antibodies that recognize heparin/platelet factor 4 (PF4) complexes. HIT is a potentially serious hypercoagulable state and is associated with a wide spectrum of venous and arterial thrombosis. The early recognition of HIT is critical because thrombotic complication can be life-threatening, and failure to discontinue heparin and initiate an alternative anticoagulant can result in increased morbidity and mortality [1]. The diagnosis of HIT is difficult and primarily relies on clinical criteria and serologic testing for heparin/PF4 antibodies. Various scoring systems have been developed to estimate the probability of HIT based on clinical information. These previously developed clinical scoring systems are useful only for retrospective analysis because these scores use criteria such as response to heparin withdrawal or reexposure to heparin, which limit their applicability for prospective diagnosis [2]. Recently, Warkentin and Heddle [3] developed a new scoring system for the pretest probability of HIT, the 4 T's scoring system (Table 1), which takes advantage of new information regarding the clinical features of HIT and is simple to apply prospectively. The 4 T's scoring system was demonstrated to be accurate when used in conjunction with the serotonin release assay (SRA), and a low score had a high negative predictive value [4]. Laboratory documentation of HIT antibodies has been crucial in determining the clinical scope of HIT syndrome. The SRA is considered the gold standard for HIT diagnosis; however, the SRA cannot be performed in most clinical laboratories. Alternatively, the commercial heparin/PF4 ELISA, which detects heparin/PF4 antibodies, can be easily performed on standard platforms in clinical laboratories and is more widely available. The heparin/PF4 ELISA is known to be very sensitive to the presence of anti-heparin/PF4 antibody [5]; however, it is less specific for the diagnosis of HIT, partly because it can also detect non-pathologic antibodies [6]. Not all patients with anti-heparin/PF4 antibodies develop HIT, and, conversely, the absence of detectable anti-heparin/PF4 does not completely exclude HIT [1]. Although the heparin/PF4 ELISA is designed to report dichotomous results (positive/negative), recent studies have shown that absolute optical density (OD) values have diagnostic and prognostic utility. A previous study reported that increasing OD was associated with an increasing probability of HIT and that higher OD values were associated with an increasing risk of subsequent thrombosis in HIT [7]. We performed a retrospective review of all patients with suspected HIT who underwent heparin/PF4 ELISA at a tertiary care hospital. The aim of this study was to evaluate the clinical utility of clinical scoring systems and the heparin/PF4 ELISA for diagnosing HIT and to investigate whether higher OD values correlate with a higher HIT pretest probability score and higher risk of thrombotic complication and mortality.
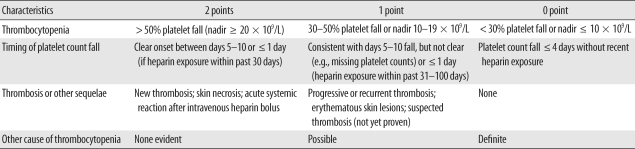
Table 1.
The 4 T's scoring system
Pretest probability score: 6-8 = high; 4-5 = moderate; 0-3 = low. Modified from Greinacher and Warkentin [1].
MATERIALS AND METHODS
1. Patients and methods
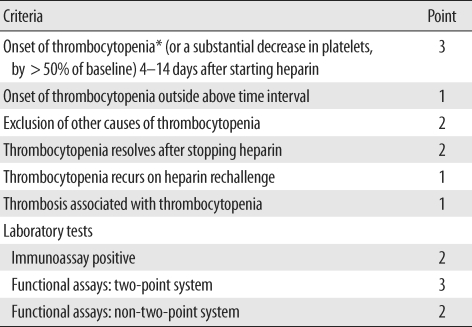
Ninety-two patients who were requested to undergo anti-heparin/PF4 antibody test from October 2007 to July 2009 were the subjects of the present study. The anti-heparin/PF4 antibody level was measured using a commercial ELISA kit (PF4 Enhanced; GTI, Waukesha, WI, USA). Briefly, the solid-phase ELISA microwells provide immobilized PF4: polyvinyl sulfonate (PVS) complexes as a target for the detection of antibodies, because antibodies associated with HIT recognize sites on platelets that are created when PF4 is complexed with heparin or another linear polyanionic compound such as PVS. After ensuring the binding of antibodies recognizing a site on PF4:PVS, the unbound antibodies are washed away. An alkaline phosphatase labeled anti-human globulin reagent (anti-IgG/A/M) is added to the wells and incubated. The unbound anti-IgG/A/M is washed away, and the substrate p-nitrophenyl phosphate is added. After a 30 min incubation period, the reaction is stopped by a sodium hydroxide solution, and the OD at 405 nm is measured in a spectrophotometer. A result was considered positive if the OD value was greater than 0.40, in accordance with both the manufacturer's instructions and the FDA-approved cut-off value. All the procedures were performed according to the manufacturer's guidelines. Then data were reviewed from patients' electronic clinical, laboratory, and radiographic records from initial hospital visits and a follow-up period of up to 10 months. Using the 4 T's score, the pre-test probability of HIT was retrospectively calculated for each patient on the basis of the clinical records at the time the test was ordered by one of the investigators (Table 1) [6]. Retrospective probability scores were calculated using Chong's scoring system (Table 2) [2].
Table 2.
Chong's scoring system
Modified from Chong and Chong [2].
*The presence of thrombocytopenia is mandatory. Thrombocytopenia is defined as a platelet count below 150 × 109/L. If the total point is >7, 5-6, 3-4, and <3, the diagnosis of HIT is considered definite, probable, possible, and unlikely, respectively.
Abbreviation: HIT, heparin-induced thrombocytopenia.
2. Statistical analysis
Data were compared using the Mann-Whitney U test and Kruskal-Wallis ANOVA for continuous variables and the chi-square test for categorical variables. The association of clinical scores and OD values was analyzed using Spearman's correlation coefficient. Sensitivity and specificity were calculated for the heparin/PF4 ELISA and 4 T's scoring system for the definite and unlikely HIT groups by using Chong's scoring system. Thrombosis-free survival and overall survival were estimated using the Kaplan-Meier method, and the difference between curves was determined by using the log-rank test. Heparin/PF4 ELISA OD level and 4 T's score cut-offs for predicting thrombotic complication were determined using ROC curve analysis.
RESULTS
1. Patient characteristics
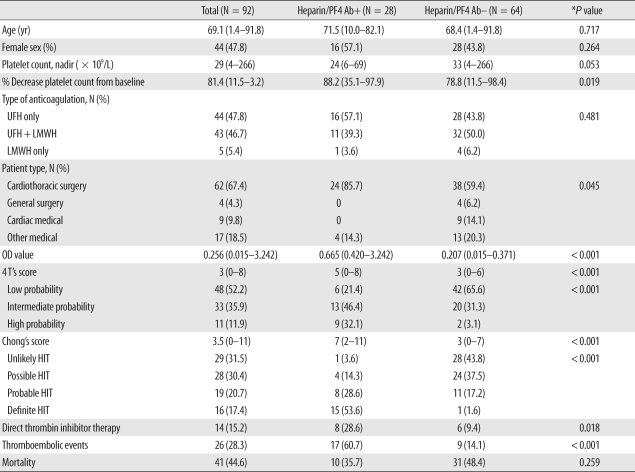
The median age was 69-yr-old (range, 1-92 yr), and 44 patients (47.8%) were female (Table 3). A majority of patients had undergone cardiovascular surgery (62 [67.4%]) or were managed by internal medicine for underlying malignancy (17 [18.5%]). Forty-four patients (47.8%) had received only unfractionated heparin (UFH), 43 (46.7%) both UFH and low molecular weight heparin (LMWH), and 5 (5.4%) LMWH only. Twenty-eight (30.4%) patients were identified as anti-heparin/PF4-seropositive. According to Chong's scoring system, 16 (17.4%) of 92 patients were defined as having definite HIT, 19 (20.7%) as having probable HIT, 28 (30.4%) as having possible HIT, and 29 (31.5%) as unlikely to have HIT. According to the 4 T's scoring system, there were 11 patients (12.0%) with high pretest probability, 33 (35.9%) with intermediate pretest probability, and 48 (52.2%) with low pretest probability. There was good correlation (Spearman correlation, r=0.874, P<0.001) between 4 T's scores and Chong's scores. Heparin was discontinued for all patients after the clinical suspicion of HIT, regardless of the anti-heparin/PF4 test results. Fourteen patients underwent further anticoagulation with direct thrombin inhibitors (11 patients with lepirudin, 2 patients with argatroban, and 1 patient with both lepirudin and argatroban).
Table 3.
Characteristics of 92 patients who underwent the anti-heparin/PF4 antibody ELISA
*P value: chi-square test for categorical variables and Man-Whitney U test for continuous variables.
Data are shown as the median (range) for continuous variables or the number (percentage) for categorical variables unless otherwise indicated. Heparin/PF4 Ab was measured by a commercial ELISA kit, and positive results were defined as optical density (OD) value ≥ 0.4.
Abbreviations: PF4, platelet factor 4; Ab, antibody; UFH, unfractionated heparin; LMWH, low molecular weight heparin; OD, optical density; HIT, heparin-induced thrombocytopenia.
2. Diagnostic performance of HIT scoring systems and heparin/PF4 antibody ELISA OD values
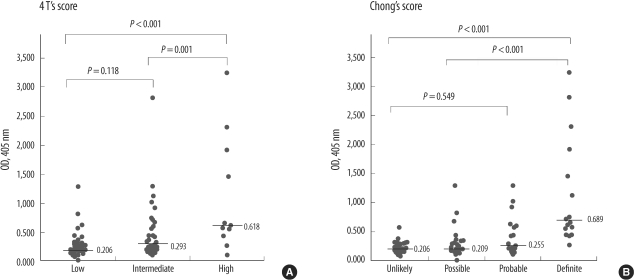
Heparin/PF4 antibody ELISA OD values according to HIT scoring systems are shown in Fig. 1. The OD was significantly different between the 4 T's category of high, intermediate, and low probability (P<0.001). There was a significant difference between the categories of high versus low and intermediate (P<0.001, P=0.001, respectively: Dunn test) but not between the categories of intermediate and low (P=0.118). The OD was significantly different between Chong's scoring category of definite, probable, possible, and unlikely for HIT (P<0.001). There was significant difference between the categories of definite vs. unlikely, definite vs. possible, and definite vs. probable (P<0.001) but not between the categories of probable vs. possible and possible vs. unlikely (P=1.0).
Fig. 1.
Optical density (OD) values according to (A) the 4 T's and (B) Chong's scoring system. Bars represent median OD level. Uppermost P values were calculated using the Kruskal-Wallis test, and pairwise comparisons were performed using the Dunn test.
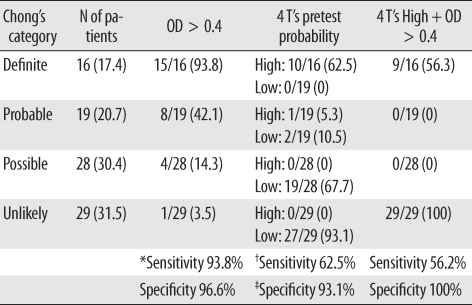
The diagnostic characteristics of the 4 T's score and heparin/PF4 antibody test were compared with Chong's scoring system (Table 4). Using the manufacturer's threshold for a positive test at OD>0.4, only 1 patient was negative for heparin/PF4 antibody in the definite HIT group, and 1 patient was positive for heparin/PF4 antibody in the unlikely HIT group. Therefore, considering the definite and unlikely HIT patients only, the sensitivity and specificity of the heparin/PF4 ELISA with an OD cut-off of 0.4 was 93.8% and 96.6%, respectively. Ten among 11 patients with high pre-test probability according to the 4 T's score were classified as definite HIT by Chong's scoring system. None of the patients with high pretest probability was assessed as unlikely HIT by Chong's scoring system. Therefore, the heparin/PF4 antibody test showed better sensitivity (93.8%) than the 4 T's score used alone (62.5%). When heparin/PF4 antibody was combined with 4 T's high pretest probability, the specificity was 100%, but the sensitivity was only 56.3%.
Table 4.
The 4 T's score and heparin/PF4 ELISA positivity according to Chong's category
*Sensitivity and specificity were defined for the 45 patients in the Definite and Unlikely HIT categories by Chong's scoring system; †Sensitivity of high pretest probability was defined for the patients in the Definite HIT category by Chong's scoring system; ‡Specificity of low pretest probability was defined for the patients in the Unlikely HIT category by Chong's scoring system.
Data are shown as the number (percentage) unless otherwise indicated.
Abbreviations: PF4, platelet factor 4; OD, optical density.
3. Thrombosis and mortality
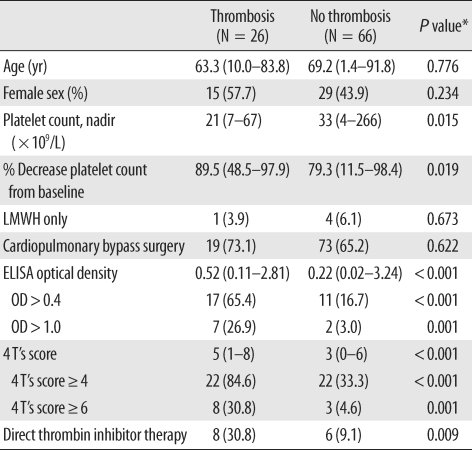
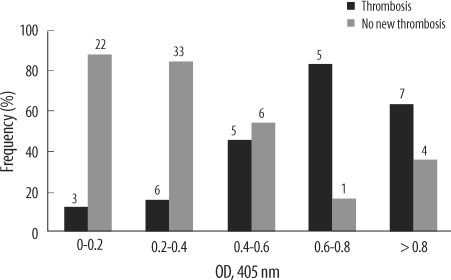
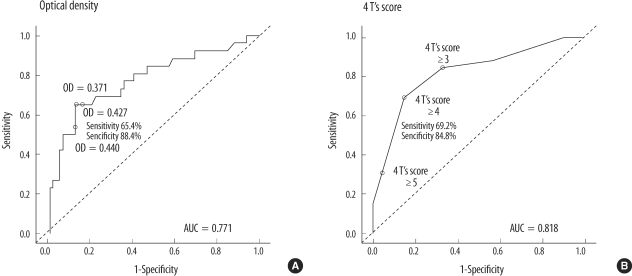
Thromboembolic events were observed in 26 patients (28.3%). In 17 patients (65.4%), arterial thrombosis occurred (6 had thrombosis involving peripheral arteries, 5 intra-atrial or intraventricular thrombi, 4 brain infarction, and 2 splenic infarction), and 9 patients developed venous thrombosis (4 experienced new or progressive pulmonary embolism, 4 peripheral venous stasis or venous gangrene, and 1 recurrent extracorporeal circuit thrombosis). The clinical and laboratory characteristics of all patients who experienced thromboembolic complications were summarized in Table 5. Of the 26 patients, 17 patients (65.4%) tested positive for heparin/PF4 antibody (OD value>0.4), while 9 patients (34.6%) tested negative for heparin/PF4 antibody (OD≤0.4). Thrombocytopenia was more severe in patients who developed thrombosis (platelet nadir 21×109/L, P=0.015). OD values were significantly higher in patients with thrombosis (median, 0.52), compared to those without thrombosis (0.22, P<0.001). More patients in the 4 T's category of intermediate or high pretest probability experienced thromboembolic complication (Table 5). As the OD value increased, the frequency of thromboembolic complication tended to increase (Fig. 2). When the OD cut-offs of 0.4 and 1.0 were considered, 60.7% (17/28) patients with OD>0.4 subsequently developed thromboembolic complications, while 14.1% (9/64) of patients with OD≤0.4 developed thrombosis. Among patients with OD>1.0, 77.8% (7/9) developed thrombosis, and 22.9% (19/83) of patients with OD≤1.0 developed thrombosis. Thus, differences of the thrombosis risk between patients with OD>1.0 and those who with OD≤1.0 were greater, compared with differences between patients with OD>0.4 and those who with OD≤0.4. When the combination of OD values and 4 T's scores were considered, among 22 patients with 4 T's score ≥4 and OD>0.4, 11 patients (68.2%) developed thrombosis, a higher frequency than when 4 T's score ≥4 alone (22/44, 50.0%), or OD>0.4 alone (17/28, 60.7%) were considered. Meanwhile, among 4 patients with 4 T's score ≥6 (high probability) and OD>1.0, 3 patients (75.0%) developed thrombotic complication, the frequency for which was between 72.7% (8/11) when high probability patients were considered alone and 77.8% (7/9) when patients with OD>1.0 considered alone. ROC curves were constructed to determine the best cut-offs for the OD value and 4 T's score for the prediction of thrombosis (Fig. 3). The OD value of 0.427 and 4 T's score of 4 had the best performance as cut-off values. At the OD value of 0.427, the sensitivity to predict thrombosis was 65.4%, and specificity was 88.4%.
Table 5.
Characteristics of patients with and without thromboembolic complication
*P value: chi-square test for categorical variables and Mann-Whitney U test for continuous variables.
Data are shown as the median (range) for continuous variables or the number (percentage) for categorical variables unless otherwise indicated.
Abbreviations: LMWH, low molecular weight heparin; OD, optical density.
Fig. 2.
Frequency of thromboembolic complications according to heparin/PF4 ELISA optical density (OD) level increment. The numbers on each bar indicate the number of patients.
Fig. 3.
ROC curve relating heparin/PF4 ELISA (A) optical density (OD) value and (B) 4 T's scores to occurrence of thromboembolic complication. (A) When the OD cut-off is 0.427, the sensitivity is 65.4%, and the specificity is 88.4%. (B) When the 4 T's score is greater than or equal to 4, the sensitivity is 69.2%, and the specificity is 84.8%.
Abbreviation: AUC, area under curve.
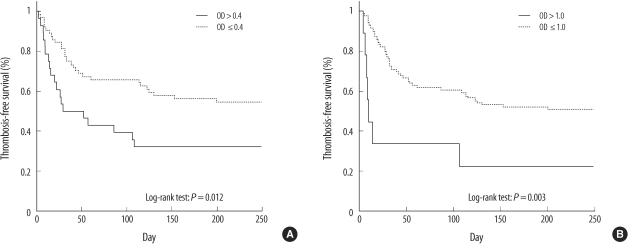
We compared the overall survival and thrombosis-free survival for all the patients according to the OD cut-offs of 0.4 and 1.0 (Fig. 4). Overall mortality of the patients was 44.6%. The overall survivals (OS) were not different between patients with high OD values (OD>0.4 or OD>1.0) and patients with lower OD values (250-day OS rate for patients with OD>0.4 vs. OD≤0.4: 67.9% vs. 56.3%, P=0.246; OD>1.0 vs. OD≤1.0: 55.6% vs. 60.2, P=0.908). The 250-day thrombosis-free survival rate of patients with OD>0.4 was 32.1%, and that of patients with OD≤0.4 was 54.7% (P=0.012) (Fig. 4A). The 250-day thrombosis-free survival rate of patients with OD>1.0 was also significantly lower than that of the patients with lower OD values (22.2% vs. 50.6%, P=0.003) (Fig. 4B).
Fig. 4.
Thrombosis-free survival for patients who underwent heparin/PF4 ELISA for detecting the presence of the heparin/PF4 antibody based on the optical density (OD) cut-off of 0.4 (A) and 1.0 (B).
Patients with OD>0.4 had increased risk of thrombosis, as shown by the odds ratio (OR) for thromboembolic events of 9.44 (95% confidence interval (CI), 3.35-26.6; P<0.001). The OR for patients with OD>1.0 was 11.79 (95% CI, 2.26-61.55; P=0.003). When OD values >1.0 were compared with those with lower positive OD values between 0.4 and 1.0, those with higher OD values tended to have increased risk; however, the increase was not statistically significant (OR, 3.85; 95% CI, 0.6-23.05; P=0.139). With multiple logistic regression analysis, higher OD value was an independent predictor of thromboembolic event. The OR of patients with OD>0.4-after adjusting for age, gender, cardiovascular bypass, and baseline platelet count-was 10.01 (95% CI, 3.26-30.73; P<0.001), and the adjusted OR of patients with OD>1.0 was 12.10 (95% CI, 2.17-67.55; P=0.004). Overall, for every increase of 0.2 OD values in the heparin/PF4 ELISA, the risk of the thromboembolic events increased by OR of 1.39 (95% CI, 1.09-1.78; P=0.007).
DISCUSSION
HIT is a serious complication of heparin treatment, which often leads to life-threatening thrombotic events. Although HIT is a relatively well-known complication, its diagnosis is still challenging because of its variable presentation and the difficulty of proving heparin's effects in patients with many other risk factors [8]. The gold standard for HIT diagnosis is the SRA; however, because this test is technically difficult, time-consuming, and involves the use of radioactive materials, it is usually only performed by a few reference laboratories. Therefore, obtaining SRA results in a timely manner is almost impossible in real practice. Rapid diagnosis of HIT is critical, because timely discontinuation of heparin and use of alternative anticoagulants, such as direct thrombin inhibitors, is known to reduce both morbidity and mortality [8]. Recently, several reports have suggested the usefulness of the clinical pretest probability scoring system, the 4 T's score, in combination with the more rapid and simple enzyme-immunoassay (EIA) for heparin/PF4 antibodies to assess the probability of HIT [9, 10]. Patients with HIT typically have thrombocytopenia 5-10 days after exposure to heparin. HIT-IgG antibodies generally are not detectable before day 5 of heparin treatment, but are readily detectable using sensitive assays when the platelet count first begins to fall because of HIT. EIA for heparin/PF4 antibodies is known to be less specific because it detects all antibodies, including those that do not activate platelets. However, in combination with clinical scoring systems, interpretation of the test results could become more reliable [10, 11]. Although the turnaround time of the heparin/PF4 ELISA may vary depending on the conditions of each laboratory, it can usually be performed far more rapidly and easily than the SRA. Furthermore, another useful aspect of the EIA for heparin/PF4 antibody is that the results can be interpreted quantitatively. Several reports have shown that higher OD values are related to either the higher diagnostic probability of HIT [12-14] or the risk of thrombosis [7, 15]. Other studies have demonstrated that the greater the magnitude of a positive EIA, the greater the likelihood that the patient has heparin-dependent platelet-activating antibodies and, hence, clinical HIT [6, 7, 14, 15]. Zwicker et al. [7] reported that the risk of thrombosis in patients with higher OD values (OD > 1.0) was approximately sixfold higher than that in patients with weak-positive results (0.4-1.0 OD units), and Warkentin et al. [14] found that strong-positive results of heparin/PF4 ELISA were associated with stronger SRA results. The results of our study support the findings of previous studies in that patients with increasing OD values also had a greater risk of thromboembolic complication. Thrombotic events of the arterial system were more frequently observed in this study. Previous prospective studies on HIT showed that HIT patients with cardiovascular disease were more likely to develop arterial thrombosis, whereas venous and arterial thrombotic events occur in approximately equal numbers in medical patients, and venous thrombosis was strongly associated with the postoperative state [16]. The pattern of thrombosis in this study could be thought of as reflecting the characteristics of the study population, with its predominance of cardiovascular surgery patients. In contrast to thrombosis risk, mortality did not differ according to the OD values. One possible explanation for this is that clinicians requested HIT testing for those patients with suspected HIT, with the result that most suspected patients underwent proper HIT management, namely discontinuation of heparin and administration of alternative anticoagulants. With the ROC analysis, the best cut-off for the prediction of thrombosis was an OD value of 0.427 and a 4 T's score of 4. These are close to the currently used cut-offs of OD value 0.4 and 4 T's category of intermediate probability. This finding implies that the current cut-offs can be used as predictors of thrombosis.
Our study had some limitations. First, it was a retrospective study, and hence, an exact assessment of the prevalence and incidence of HIT and its complications was not possible. Second, it was a single-center study of a tertiary university hospital, and cardiovascular surgical patients were predominant; therefore, the study population did not reflect the characteristics of the entire Korean population. Finally, the gold standard laboratory test, the SRA, was not performed; hence, interpretation of the data was limited.
This is the first report of the clinical and laboratory characterization of HIT in the Korean population. Our results were consistent with those of previous studies, and we were able to confirm the usefulness of the heparin/PF4 ELISA and the clinical scoring system for the diagnosis of and thrombosis prediction in HIT in Korean patients. More attention must be paid to the use of clinical scores and OD values as thrombosis predictors in HIT.
Acknowledgements
This work was supported by a grant from the SNUH Research Fund (04-2010-017).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res. 2006;118:165–176. doi: 10.1016/j.thromres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Chong BH, Chong JJ. Heparin-induced thrombocytopenia. Expert Rev Cardiovasc Ther. 2004;2:547–559. doi: 10.1586/14779072.2.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE, Heddle NM. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr Hematol Rep. 2003;2:148–157. [PubMed] [Google Scholar]
- 4.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 5.Greinacher A, Juhl D, Strobel U, Wessel A, Lubenow N, Selleng K, et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost. 2007;5:1666–1673. doi: 10.1111/j.1538-7836.2007.02617.x. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin TE, Sheppard JA, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need. J Lab Clin Med. 2005;146:341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2:2133–2137. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:311S–337S. doi: 10.1378/chest.126.3_suppl.311S. [DOI] [PubMed] [Google Scholar]
- 9.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7:1260–1265. doi: 10.1111/j.1538-7836.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 10.Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T's score and particle gel immunoassay. Br J Haematol. 2008;143:721–726. doi: 10.1111/j.1365-2141.2008.07401.x. [DOI] [PubMed] [Google Scholar]
- 11.Janatpour KA, Gosselin RC, Dager WE, Lee A, Owings JT, Zhou J, et al. Usefulness of optical density values from heparin-platelet factor 4 antibody testing and probability scoring models to diagnose heparin-induced thrombocytopenia. Am J Clin Pathol. 2007;127:429–433. doi: 10.1309/RPE753J4PMG9773Q. [DOI] [PubMed] [Google Scholar]
- 12.Whitlatch NL, Perry SL, Ortel TL. Anti-heparin/platelet factor 4 antibody optical density values and the confirmatory procedure in the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost. 2008;100:678–684. doi: 10.1160/th08-02-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss BM, Shumway NM, Howard RS, Ketchum LK, Reid TJ. Optical density values correlate with the clinical probability of heparin induced thrombocytopenia. J Thromb Thrombolysis. 2008;26:243–247. doi: 10.1007/s11239-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 14.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6:1304–1312. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 15.Mattioli AV, Bonetti L, Carletti U, Ambrosio G, Mattioli G. Thrombotic events in patients with antiplatelet factor 4/heparin antibodies. Heart. 2009;95:1350–1354. doi: 10.1136/hrt.2008.160549. [DOI] [PubMed] [Google Scholar]
- 16.Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–1708. [PubMed] [Google Scholar]