Abstract
Objective
To examine the course of mood symptoms following induction onto naltrexone, we examined change in total and symptom clusters of depression in newly abstinent opioid-dependent individuals being treated with depot naltrexone (Depotrex; BIOTEK).
Method
In a series of opioid-dependent patients (N=34) treated with naltrexone maintenance and behavioral therapy, mood was assessed with the 17-item Hamilton Depression Scale and subscale scores at baseline, and after naltrexone induction at 2- and 4-weeks post-baseline, using GEE models.
Results
Patients demonstrated high baseline affective burden and significant improvement of depression scores over a 4-week period post baseline (F2,66=8.88, p=0.0004). Somatic and cognitive-affective subscale scores significantly declined, as well as 7 individual item scores. By contrast, “late insomnia” score significantly increased by 2 weeks post-baseline, but did not remain significantly increased at 4 weeks post-baseline.
Conclusion
Naltrexone induction and maintenance in newly abstinent opioid-dependent individuals does not appear to be associated with the onset or worsening of depression; however, it may be associated with sleep impairment early in treatment.
Keywords: naltrexone, depression, opioid dependence, Hamilton Depression Rating Scale
Introduction
Naltrexone, a mu-, kappa-, and delta-opiate receptor antagonist with greatest affinity for the mu receptor (1), can be prescribed for treating opiate dependence (2). However, to date, naltrexone has been under-utilized as a maintenance treatment for opioid dependence, in part because of poor retention and compliance with the oral formulation (3–5). One study of opiate-dependent patients treated with naltrexone found baseline depression a weak predictor of dropout (6), however, another did not replicate this finding (7). By contrast, for smoking cessation, depression at baseline was found to predict improved treatment outcomes (8).
If baseline depression may predict poor treatment retention in naltrexone maintenance, then depression arising during the course of treatment may also be associated with drop out. Studies looking at the association between naltrexone treatment and emergence of depression have been mixed. A review of the association between naltrexone and dysphoria also found the link to be inconclusive (9). On the one hand, studies have demonstrated that oral naltrexone is capable of inducing fatigue, nausea, sleepiness, restlessness, and dysphoria in non-opiate users for a few hours acutely post ingestion (10–12). Upon cessation of the drug (11), some subjects have reported feeling “much better” On the other hand, a double-blind randomized controlled trial comparing naltrexone to disulfiram and placebo for the treatment of alcohol dependence found that naltrexone was associated with improved depressive symptoms over 12 weeks in patients with baseline current depression (13).
While it is known that poor retention is associated with naltrexone treatment, and that baseline depression is possibly associated with treatment drop out, it remains unclear whether naltrexone treatment for opiate dependence is associated with emergence of new depressive symptoms. The aim of this study is to examine the course of depressive symptoms in opiate-dependent individuals who have been inducted onto naltrexone maintenance following detoxification. There is evidence that in the context of naltrexone treatment, baseline depressive symptoms may predict better treatment outcomes, but baseline history of depression may be associated with poor retention. Therefore, we will seek to determine whether an association exists between either baseline history of treated depression, or baseline symptoms of depression, and worsening depressive symptoms, using the 17-item Hamilton Depression Scale (HAM-D) to examine the change in the total score (severity) of depression at 2 and 4-weeks from baseline. We will also explore the distinction between opiate-induced depressive symptoms and possible naltrexone-induced symptoms by assessing changes in individual items in the Ham-D. The results of this study may inform the clinical decision of whether to initiate naltrexone treatment in opiate-dependent subjects at risk for depression.
Materials and Methods
Behavioral Naltrexone Therapy (BNT) is a manual-based therapy designed to foster compliance with naltrexone following inpatient naltrexone induction and opiate detoxification. BNT outpatient treatment includes elements of Motivational Interviewing, Cognitive Behavioral counseling methods, voucher incentives, and involvement of a significant other to support the treatment and help monitor adherence to naltrexone (14). Treatment-seeking opiate-dependent patients (N=125) were screened and received baseline assessments at the Columbia University Substance Treatment and Research Services (STARS). Patients accepted into the trial were admitted for inpatient detoxification and induction onto oral naltrexone on an inpatient research unit at New York State Psychiatric Institute (NYSPI). They were randomized to receive a single injection of active versus placebo depot naltrexone prior to discharge following detoxification. In addition, all patients continued oral naltrexone maintenance for the duration of their participation in this 6-month trial. All patients were offered twice weekly outpatient therapy sessions at which Behavioral Naltrexone Therapy was delivered. Individuals were excluded from participation in the study if they were diagnosed with an impairing DSM-IV Axis I disorder such as current psychosis, suicidal ideation, or drug dependence other than prescription opioids, nicotine or caffeine. Individuals with diagnosed mood disorders were not excluded so long as their maintenance medications, or mood symptoms, would not interfere with the study treatments and procedures, as determined by clinical psychiatric interview. The Hamilton Depression Rating Scale (Ham-D) was administered at baseline and bi-weekly throughout the 24 week study. This study was approved by the IRB of NYSPI.
Study Sample
For the purposes of this secondary analyses, n=34 BNT patients included in this sample who: 1) completed at least 4 weeks of outpatient maintenance; 2) completed a Ham-D at 2- and 4-weeks post-baseline 3) were randomized to active depot naltrexone prior to discharge from the inpatient unit. The last criterion was chosen to ensure compliance on the naltrexone regimen. Results from a recent randomized, open-label study comparing naltrexone treatment with methadone maintenance for opiate dependence demonstrated that patients who were adherent to naltrexone treatment exhibited fewer symptoms of depression than those who were non-adherent (15).
A review of the patients’ psychiatric history was conducted to assess the baseline prevalence of mood disorder (primary or substance-induced). Prior to study entry, all patients were assessed by a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (SCID)(16–17), conducted by a Masters-level therapist, and a clinical interview by a study psychiatrist. Patients were considered having a baseline depressive disorder if they 1) carried a formal diagnosis of major depressive disorder or dysthymia, 2) were diagnosed with substance-induced depression that required formal inpatient or outpatient treatment, or 3) met criteria for either major depression or dysthymia upon clinical interview and/or SCID at study entry.
Measures and Statistical Analyses
The primary outcome was the Hamilton Depression Rating Scale (Ham-D) total score (18–19). The Ham-D is a widely used, validated standardized assessment of various domains of depression, including mood, cognition, vegetative symptoms, and insomnia. While the total score of the scale provides insight regarding patients’ overall depressive burden, it does not distinguish between somatic items, which may be increased as a result of opioid withdrawal, and cognitive items, which may represent signs of primary depression. Therefore, secondary outcomes include somatic and cognitive-affective symptoms. Specifically, the items from the 17-item Ham-D were assigned to either a “somatic” or a “cognitive-affective” symptom category. These assignments were based on a prior meta-analysis of factor analyses of these items that resulted in loading into four categories: anxiety, depression, insomnia, and somatic (20). For the purpose of our study, insomnia and somatic items were collectively referred to as “somatic” while anxiety and depression items were collectively “cognitive-affective.” “Somatic” items included: loss of libido; somatic/gastrointestinal; weight loss; insomnia early; insomnia middle; insomnia late; somatic symptoms general. “Cognitive-affective” items included: depressed mood; work & activities; feelings of guilt; suicide; anxiety psychic; anxiety somatic; hypochondriasis; insight; psychomotor retardation; psychomotor agitation.
Data Analyses
All analyses were two-sided with a significance level of 0.05. The primary outcome, the total HAM-D score, was assessed using a generalized estimated equation and an identity link function (GEE). GEEs allow for an uneven number of observations over time and provide efficient and robust estimates of the parameters of interest. The total Ham-D score was modeled as a function of history of mood disorder, and time, with the baseline HAM-D score used as reference. In the case of a significant finding in the change in the total HAM-D score, we assessed whether the change was driven by somatic or cognitive-affective symptoms in two secondary sub-group analyses. Specifically, we conducted two GEE models and analyze the effect of time, using somatic HAM-D scores and cognitive-affective HAM-D scores as the outcomes. Furthermore, to identify Ham-D items that substantially changed over time, we analyzed all 17 items individually by conducting multiple GEE models with a log-link function, due to their count nature, to compare their scores 2- and 4-weeks post-baseline relative to baseline.
Results
To date, 34 subjects maintained on depot naltrexone have completed Ham-D assessments at baseline, and 2 and 4 weeks post-baseline. This cohort is described in Table 1. The cohort is predominantly male (91%), mostly identifying as either Hispanic (53%) or non-Hispanic White (38%), mean age 37.2 (range=21–51 years old, SD=8.7 years), with mean baseline use of 5.9 bags of heroin daily (range=1–20 bags daily, SD=3.3 bags). Forty-four percent (n=15) of the cohort met criteria for either a formal history of diagnosed and treated depressive disorder, or diagnosed on clinical interview and/or SCID for probable depressive disorder. Of 15 these patients, 3 entered the study on stable antidepressant regimens that were continued. Within the first 4 weeks of the study, 2 other patients from this group, with prior histories of treatment, began antidepressant treatment under the supervision of the study psychiatrists.
Table 1.
Baseline demographic and clinical characteristics of randomized patients (N=34)
| Mean (SD) | |
|---|---|
| Age | 37.2 (8.7) |
| Heroin use (bags/day) | 5.9 (3.3) |
| n (%) | |
| Gender | |
| Male | 31 (91.2%) |
| Female | 3 (8.8%) |
| Race | |
| White | 13 (38.2%) |
| Black | 3 (8.8%) |
| Hispanic* | 18 (52.9%) |
| History of depression** | 15(44.1%) |
may identify as White or Black Hispanic
Current/prior formal treatment for primary depressive disorder or Substance-Induced Mood Disorder, OR diagnosed on clinical interview and/or SCID with likely history of primary mood disorder with no prior diagnosis or treatment
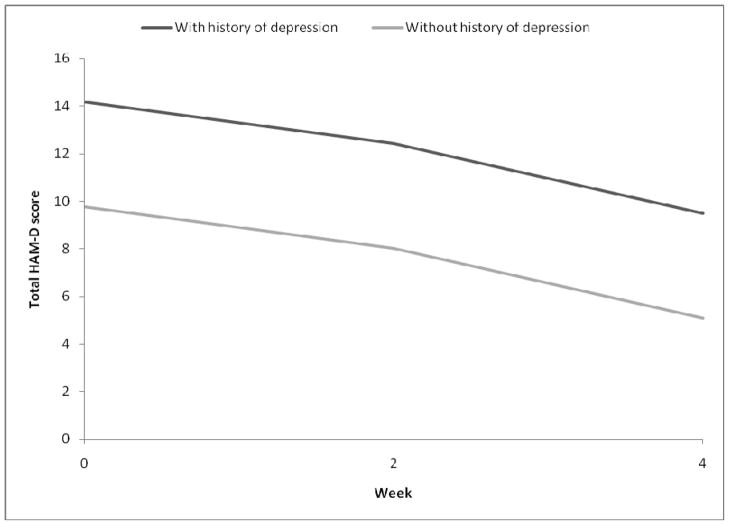
Results from the GEE model (Table 2) for total HAM-D score indicated that there was a significant time effect (F2,66=9.45, p=0.0002) and a significant effect of history of depression (F1,32=12.88, p=0.001), suggesting that history of depression was predictive of the overall total HAM-D score throughout the 4-week period studied. The beta estimates from the model suggested that participants who had a history of depression had an overall higher total HAM-D score (mean difference of 4.4) throughout the study period than participants who did not. Nonetheless, as illustrated in Figure 1, the significant time effect suggested that on average, all participants experienced a decrease of total HAM-D score by Week 2 (−1.74, SE 0.93), and by Week 4 (−4.68, SE=1.09). An interaction between time and history of depression was tested and found to be insignificant, and was therefore not included in the final model. When similar GEE models were run on somatic HAM-D scores (F2,66=5.53, p=0.006) and cognitive-affective HAM-D scores (F2,66=8.15, p=0.0007), a similar time effect was found, suggesting that component HAM-D scores indeed decreased over time (see Table 3).
Table 2.
Generalized linear regression (GEE) estimates for total HAM-D scores during the study period
| Total | |||
|---|---|---|---|
| Effect | _ (SE) | Statistics | p-value |
| Intercept# | 9.76 (1.00) | <0.0001 | |
| Time* | F2,66 = 9.45 | 0.0002 | |
| Week 2 | −1.74 (0.93) | ||
| Week 4 | −4.68 (1.09) | ||
| History of mood disorder## | 4.40 (1.23) | F1,32= 12.88 | 0.001 |
Baseline was used as reference.
Represents baseline Ham-D score of patients without diagnosed Depression
Represents mean additional score at baseline patients with Depression have above those without
Figure 1.
Total HAM-D scores over time, by history of depression
Table 3.
Generalized linear regression (GEE) estimates for HAM-D scores during the study period, by symptom category
| Somatic | Cognitive-Affective | |||||
|---|---|---|---|---|---|---|
| Effect | _ (SE) | Statistics | p-value | _ (SE) | Statistics | p-value |
| Intercept# | 4.68 (0.48) | 5.08 (0.66) | ||||
| Time* | F2,66= 5.53 | 0.006 | F2,33= 8.15 | 0.0007 | ||
| Week 2 | −0.92 (0.52) | −0.82 (0.59) | 0.17 | |||
| Week 4 | −1.92 (0.58) | −2.74 (0.71) | ||||
| History of mood disorder## | 1.93 (0.56) | F1,32= 11.98 | 0.002 | 2.49 (0.82) | F1,32= 9.29 | 0.005 |
Baseline was used as reference.
Represents baseline Ham-D score of patients without diagnosed Depression
Represents mean additional score at baseline patients with Depression have above those without
To explore the underlying individual items that contributed to the significant decrease in total and categorized HAM-D scores, we conducted additional GEE models on all 17 individual items comprising the HAM-D scale. Eight items (5 somatic and 3 cognitive-affective) were found to change significantly over time. The five somatic items that showed significant time effect were “Libido,” “Decreased appetite,” “Weight loss, “Late insomnia,” and “General somatic.” The three cognitive-affect items that displayed a significant time trend were “Work & Activities,” “Guilt,” and “Anxiety-psychic.” “Late insomnia” was the only item to worsen significantly at 2 weeks post-baseline. Four items (libido, decreased appetite, weight loss, and guilt) improved by 2 weeks post-baseline with sustained significant improvement by 4 weeks post-baseline. Three items (work and activities, general somatic and anxiety-psychic) significantly improved by 4 weeks post-baseline, but not at 2 weeks post-baseline.
Discussion
This cohort of 34 mostly Hispanic and Caucasian male heroin users presented a high baseline affective burden. Forty-four percent (n=15) presented with formal past diagnoses or treatment for a primary depressive disorder, a substance-induced depression severe enough to warrant independent treatment, or a likely primary depressive disorder, albeit previously undiagnosed. This finding is consistent with studies documenting that 26%–54% of treatment-seeking opiate-dependent patients have comorbid depression (11). The cohort’s baseline mean Ham-D score of 11.7 (range=0–25, SD= 5.4) suggests that more than half the cohort met criteria for at least mild depression (score>8) upon entry to the study. By 4 weeks post-baseline, this cohort treated with depot naltrexone had significant declines in mean total, somatic, and cognitive-affective Ham-D scores. Among Ham-D items with significant change, only “late insomnia” worsened, while the other 7 items with significant change all improved by 4 weeks post-baseline, while many improved by 2 weeks post-baseline with sustained improvement at 4 weeks. These data are consistent with literature suggesting “sleeplessness” as a component of naltrexone treatment.
Interestingly, when the total cohort was subdivided into two groups based upon prior or current treatment of depressive symptoms and those with likely diagnosable primary depressive disorders based on interview, and those without histories of treated depressive symptoms and without primary depressive disorders based on interview, we found that the former group had significantly higher mean Ham-D scores at baseline, 2-weeks and 4-weeks into the study compared to the total group and those patients without likely primary depressive disorders. Furthermore, even though the mean Ham-D scores of the patients with histories of depression were significantly higher at the three study points, their scores declined significantly as a function of time between the study points. This improvement was seen in their total Ham-D scores, as well as their Somatic and Cognitive-affective subscale scores.
There are several limitations in this study. It is an exploratory study, with a relatively small (N=34) mostly male (91%) cohort, and may not be generalizable to other populations. Furthermore, it utilizes multiple analyses, possibly contributing to Type I error. These findings would need to be replicated in independent samples. Additionally, while the BNT manualized therapy does not focus on the identification and treatment of depressive symptoms, it includes ancillary work on managing depressive symptoms once identified. There is also a probability that the supportive, structured milieu of manualized therapy in a clinical setting may have contributed to improvement in mood. The initiation of antidepressant treatment in 2 patients of the 15 patients with prior histories of antidepressant treatment may have skewed the results in favor of improved depressive symptoms. All patients were permitted oral trazodone 50–100mg as needed for insomnia for the first 2 weeks of the study. However these doses are generally considered to lack therapeutic efficacy as an antidepressant. Lastly, the subjects selected for these analyses were those who remained in treatment for the first 4 weeks of the study, and were compliant with the baseline, Week 2 and Week 4 Ham-D assessments. It was determined that Ham-D scores improve with time in patients compliant with baseline, week 2 and week 4 visits, maintained on an injection of depot naltrexone, regardless of their histories of primary depressive disorder; However, it was not determined what happens to Ham-D scores of those patients maintained on an injection of depot naltrexone, who were not compliant with these followup assessments. As stated in the introduction to this paper, there is some evidence suggesting that baseline depression may reduce treatment retention. An association between noncompliance with the study’s assessment protocols and worsening depression could not be ruled out.
Conclusion
Major depressive disorder is a common comorbidity among individuals with opiate dependence. By one estimate, approximately 54% of patients in treatment for opiate dependence also had a lifetime diagnosis of major depressive disorder (21). A study of opiate dependence treatment in Australia demonstrated that 25.8% of patients met criteria for major depressive disorder, compared to 16.3% of opiate-dependent individuals not in treatment (22). The same study demonstrated that among opiate-dependent patients enrolled in treatment, those with comorbid major depression were five times more likely to have attempted suicide in the past 12 months than addicts without depression (22). Using the 5-item Beck Depression Inventory, it was found that addicts entering opiate treatment at a methadone clinic had statistically similar scores to individuals being admitted to psychiatric inpatient units after surviving suicide attempts, and 25% of the patients in methadone maintenance treatment continued to meet criteria for current major depressive disorder (23). The same study demonstrated that while those individuals applying for treatment had higher depression scores than those in current treatment or post-treatment and abstinent, all three cohorts had higher scores than did non-opiate users (23). In another study, 152 patients screening for entry into a methadone maintenance treatment program were found to be at least “mildly depressed” using four separate depression rating scales (24).
Depression in opiate-dependent patients has been shown to improve with initiation of methadone treatment (25). This improvement of depression may be associated with the methadone itself, or the elimination of the daily stresses of obtaining, using, and withdrawing from opiates as the patients’ lives stabilize in methadone treatment (26). Furthermore, depression unresponsive to methadone treatment has improved with antidepressant treatment in opiate-dependent patients (26–28)
Several studies have attempted to clarify the particular qualities of opiate-induced depression (29–33). The symptoms of opiate-induced depression are both somatic and cognitive/affective. Somatically, patients manifest fatigue (32) and sleep impairment (33). In terms of cognitive-affective symptoms, individuals experience decreased motivation, anhedonia, decreased libido, indecisiveness, social withdrawal, hypochondriasis, guilt and a sense of failure (29–21, 33). They feel irritable, annoyed at others, lonely and sad (32). These symptoms overlap significantly with those of a major depressive episode. In opiate-dependent patients, depressive symptoms may be manifestations of opiate-induced depression, an underlying major depressive disorder, opiate intoxication or withdrawal. Often it is difficult to determine the cause of the depression (28).
In summary, there are several similarities and differences between the symptoms of opiate-induced depression and those reportedly induced by naltrexone. Cognitively and affectively, both manifest dysphoria, irritability, and confusion/decreased alertness. With respect to somatic symptoms, both demonstrate fatigue and impaired sleep. However, naltrexone-induced depression is also associated with nausea and decreased appetite, symptoms not seen in opiate-induced depression. Furthermore opiate-induced depression is characterized by anhedonia, guilt, social isolation, decreased libido and hypochondriacal preoccupation, not associated with reported naltrexone-induced depression (10–12, 29–33)
These data suggest that naltrexone treatment is not associated with worsening depressive symptoms, especially in the context of compliant treatment via depot formulation. This finding holds true even among those patients with known histories of treated depression or those diagnosed with a primary depressive disorder upon interview. Furthermore, while Hamilton Depression Scale symptoms associated with opiate withdrawal (decreased appetite, decreased weight) improved, other items associated more specifically with opiate-induced depression also improved (anhedonia, guilt, libido). Since naltrexone treatment was not associated with worsening of depression scores, even among those patients with histories of diagnosed and/or treated depression, naltrexone should still be considered a viable pharmacological option when treating opioid-dependent patients currently diagnosed with, or at risk for, depression. Nevertheless, insomnia frequently complicates the course of antagonist maintenance in early abstinence, and this persistent symptom deserves therapeutic intervention to promote treatment retention and reduce the risk of relapse.
These results highlight the association of naltrexone treatment with reduced depressive symptoms. This finding is not a causal relationship, and therefore antidepressant effects should not be attributed to naltrexone. While mean Ham-D scores did decrease in this naltrexone-treated sample, individual patients may experience worsening depression while on naltrexone treatment. Therefore, opiate-dependent patients maintained on naltrexone should be monitored regularly for mood throughout the course of treatment.
Acknowledgments
The authors acknowledge the support of NIDA R01 010746 and K24 022412.
References
- 1.Wang D, Sun X, Sadee W. Different effects of opiate antagonists on mu-, delta-, and kappa-opiate receptors with and without agonist pretreatment. The Journal of Pharmacology and Experimental Therapeutics. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor PG, Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Annals of Internal Medicine. 2000;133:40–54. doi: 10.7326/0003-4819-133-1-200007040-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri M, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction. 2002;97:1241–1249. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 5.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews. 2006;(1):Article number: CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MA, Rothenberg JL, Vosburg SK, Church SH, Feldman SJ, Epstein EM, Kleber HD, Nunes EV. Predictors of retention in naltrexone maintenance for opioid dependence: analysis of a stage I trial. American Journal on Addictions. 2006;15:150–159. doi: 10.1080/10550490500528464. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter KM, Jiang H, Sullivan MA, Bisaga A, Comer SD, Raby WN, Brooks AC, Nunes EV. Betting on change: modeling transitional probabilities to guide therapy development for opioid dependence. Psychology of Addictive Behaviors. 2009;23:47–55. doi: 10.1037/a0013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh Z, Epstein A, Munisamy G, King A. The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. Journal of Addictive Diseases. 2008;27(1):65–72. doi: 10.1300/J069v27n01_07. [DOI] [PubMed] [Google Scholar]
- 9.Miotto K, McCann M, Basch J, Rawson R, Ling W. Naltrexone and dysphoria: fact or myth? The American Journal on Addictions. 2002;11:151–160. doi: 10.1080/10550490290087929. [DOI] [PubMed] [Google Scholar]
- 10.Mendelson JH, Ellingboe J, Keuhnle JC, Mello NK. Effects of naltrexone on mood and neuroendocrine function in normal adult males. Psychoneuroendocrinilogy. 1979;3:231–236. doi: 10.1016/0306-4530(78)90013-6. [DOI] [PubMed] [Google Scholar]
- 11.Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug and Alcohol Dependence. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- 12.Crowley TJ, Wagner JE, Zerbe G, Macdonald M. Naltrexone-induced dysphoria in former opioid addicts. American Journal of Psychiatry. 1985;142(9):1081–1084. doi: 10.1176/ajp.142.9.1081. [DOI] [PubMed] [Google Scholar]
- 13.Petrakis I, Ralevski E, Nich C, Levinson C, Carroll K, Poling J, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and current depression. Journal of Clinical Psychopharmacology. 2007;27(2):160–165. doi: 10.1097/jcp.0b13e3180337fcb. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. Journal of Substance Abuse Treatment. 2002;23(4):351–60. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 15.Dean AJ, Saunders JB, Jones RT, Young RM, Connor JP, Lawford BR. Does naltrexone treatment lead to depression? Journal of Psychiatry and Neuroscience. 2006;31(11):38–45. [PMC free article] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 17.First Michael B, Spitzer Robert L, Miriam Gibbon, Williams Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; Nov, 2002. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 20.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 21.Brady KT, Myrick H, Sonne S. Co-occurring addictive and affective disorders. In: Graham AW, Schultz TK, Mayo-Smith MF, et al., editors. Principles of Addiction Medicine. 3. Chevy Chase, MD: American Society of Addiction Medicine; 2003. pp. 1277–1286. [Google Scholar]
- 22.Teesson M, Havard A, Fairbairn S, Ross J, Lynskey M, Darke S. Depression among entrants to treatment for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence, correlates and treatment seeking. Drug and Alcohol Dependence. 2005;78:309–315. doi: 10.1016/j.drugalcdep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Mintz J, O’brien CP, Woody G, Beck AT. Depression in treated narcotic addicts, ex-addicts, nonaddicts, and suicide attempters. American Journal of Drug and Alcohol Abuse. 1979;6(4):385–396. doi: 10.3109/00952997909007051. [DOI] [PubMed] [Google Scholar]
- 24.Steer RA, Emery GD, Beck AT. Correlates of self-reported and clinically assessed depression in male heroin addicts. Journal of Clinical Psychology. 1980;56(3):798–800. doi: 10.1002/1097-4679(198007)36:3<798::aid-jclp2270360337>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Strain EC, Stitzer ML, Bigelow GE. Early treatment time course of depressive symptoms in opiate addicts. Journal of Nervous and Mental Disease. 1991;179:215–221. doi: 10.1097/00005053-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, Ocepek-Welikson K, Koenig T, Brady R, McGrath PJ, Woody G. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Archives of General Psychiatry. 1998;55:153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- 27.Woody GE, O’Brien CP, Rickels K. Depression and anxiety in heroin addicts: a placebo-controlled study of doxepin in combination with methadone. American Journal of Psychiatry. 1975;132:447–450. doi: 10.1176/ajp.132.4.447. [DOI] [PubMed] [Google Scholar]
- 28.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biological Psychiatry. 2004;56:793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Haertzen CA, Hooks NT. Changes in personality and subjective experience associated with the chronic administration and withdrawal of opiates. The Journal of Nervous and Mental Disease. 1969;148(6):606–614. doi: 10.1097/00005053-196906000-00004. [DOI] [PubMed] [Google Scholar]
- 30.McNamee HB, Mirin SM, Kuehnle JC, Meyer RE. Affective changes in chronic opiate use. British Journal of Addiction to Alcohol and Other Drugs. 1976;71:275–280. doi: 10.1111/j.1360-0443.1976.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 31.Shaw BF, Steer RA, Beck AT, Schut J. The structure of depression in heroin addicts. British Journal of Addiction to Alcohol and Other Drugs. 1979;74(3):295–303. doi: 10.1111/j.1360-0443.1979.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 32.Steer RA, Schut J. Affect dimensions of male heroin addicts admitted for inpatient detoxification. The International Journal of the Addictions. 1981;16(2):341–348. doi: 10.3109/10826088109038833. [DOI] [PubMed] [Google Scholar]
- 33.Blatt SJ, Rounsaville B, Eyre SL, Wilber C. The psychodynamics of opiate addiction. The Journal of Nervous and Mental Disease. 1984;172(6):342–352. doi: 10.1097/00005053-198406000-00005. [DOI] [PubMed] [Google Scholar]