Abstract
The description of itch (formally known as pruritus) as an “unpleasant sensation that elicits the desire or reflex to scratch” (Ikoma et al., 2006) is immediately familiar. Research in the field of pruritoception has added to our understanding of this area of sensory neurobiology as it pertains to both normal and pathological conditions. In particular, much progress has been made on the mechanisms and circuits of itch, which we review here.
Introduction
Itch can be acutely generated, but it is also produced clinically by any number of causes. Pruritoceptive itch occurs through activation of peripheral nerve fibers (e.g., from insect bites or poison ivy). Neuropathic itch results from neuronal damage (e.g., shingles), whereas psychogenic itch is of psychiatric origin (e.g., obsessive-compulsive disorder) (Yosipovitch and Samuel, 2008).
Attempts to characterize pruritoception must incorporate nociception as pain is necessary to describe itch comprehensively. Itch is operationally defined by its requirement for scratching, a mildly noxious stimulus that inhibits itch sensation. It appears that itch can be induced by inhibition of pain circuitry as seems to occur during opioid administration (Szarvas et al., 2003). Itch or pain can result from different types of stimuli, such as chemical or mechanical, and the two modalities share certain cellular and molecular mechanisms. They may also serve similar protective functions. Nociception often produces an avoidance response to minimize damage, whereas pruritoception leads to scratching that can remove irritants like insects from the skin. However, itch sensation normally occurs well after insect bites or contact with poison ivy, and thus the unpleasantness of itch may act as a delayed warning system to avoid future insults.
Several theories have been put forth to explain how itch is detected and the manner in which pain circuitry inhibits and otherwise overlaps with the itch pathway. Certain components of this pathway, down to the level of molecules in some cases, have been identified. We will evaluate the theories and assess their validity in the context of these results.
At the periphery, a number of recent studies have helped uncover the molecules and mechanisms involved in itch transduction. This work began with histamine, the most well-characterized pruritogen, and has since been extended to include a variety of other compounds. Nonhistaminergic forms of itch have been discovered, leading to a broader understanding of how itch is detected peripherally. This has also provided additional targets for therapeutics as numerous medical conditions involving pruritus are not amenable to antihistamine treatment (Twycross et al., 2003).
How Is Itch Encoded?
Given that scratching an itch is the most fundamental aspect of pruritoception, the theories proposed to model itch are defined by how pain relates to itch. Beginning with intensity theory, put forth based on experiments from nearly a century ago (von Frey 1922; Lewis et al., 1927), several theories have been offered.
Intensity theory requires neurons that are activated by both painful and itchy stimuli (McMahon and Koltzenburg 1992). As the name suggests, these cells would discriminate between the two depending upon the intensity of stimulation produced. The same neuron could be weakly or strongly activated, producing the sensation of itch or pain, respectively. However, later work in humans has made this theory untenable. If intensity of stimulation is increased, then an itchy sensation should transition to a painful one, but this was not observed (Tuckett 1982). Similarly, a lower intensity of a painful stimulus does not turn into itch (Ochoa and Torebjörk 1989; Handwerker et al., 1991).
Another theory termed labeled line requires separate populations of neurons for whom detection of itch or pain is mutually exclusive. Work by Schmelz and colleagues (1997) in humans identified a promising set of histamine-sensitive C fibers in humans. This population, consisting of fibers with low conduction velocities, high activation thresholds, and no sensitivity to mechanical stimuli, exhibited properties that were noticeably different from other known nociceptors.
However, the same group (Schmelz et al., 2003) went on to show that these supposed itch-only fibers were activated by nociceptive stimuli, notably capsaicin. Johanek and colleagues (2007) later identified fibers responding to itch induced by cowhage, but not histamine, and these were also activated by capsaicin. A recent study from Imamachi et al. (2009) confirms that seemingly nociceptive neurons are vital for itch detection. Intrathecal injection of capsaicin to selectively ablate TRPV1+ fibers from the dorsal root ganglia (DRG) led to substantial deficits in the behavioral scratch response to pruritogens. Collectively, these data argue against the labeled line model by demonstrating that purported pruritoceptors also respond to algogens.
The correct model should incorporate neurons that sense both nociceptive and pruritogenic stimuli but can nonetheless differentiate the two. Peripheral neurons that are candidate pruritoceptors often detect painful stimuli as well (Schmelz et al., 2003; Johanek et al., 2007; Shim et al., 2007; Namer et al., 2008; Liu et al., 2009). On the other hand, nociceptors that are not activated by pruritogens (histaminergic or otherwise) are seen, suggesting that pruritoceptors may constitute a subset of the nociceptor population. A form of population-based coding with sensation produced by activation of a specific array of neurons (McMahon and Koltzenburg 1992) would fit this observation.
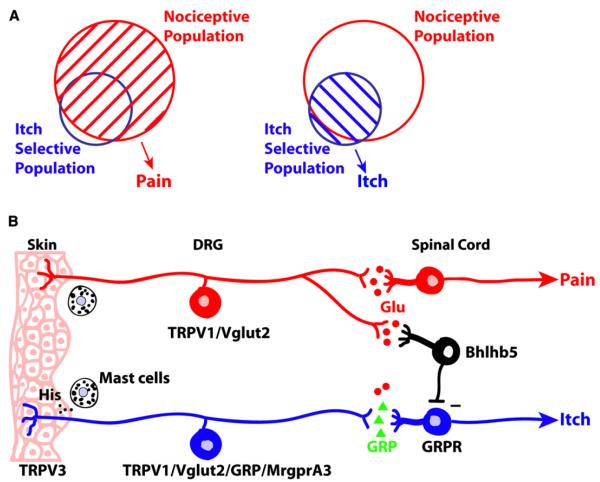
Itch-responsive neurons within the larger nociceptor population may then be considered selective for itch and would produce this sensation upon activation. However, if a painful stimulus also activates the larger nociceptive population, this greater response would mask the itch-responsive subset and produce the sensation of pain (Figure 1A). In this manner, inhibition of the itch pathway occurs via the nociceptive-only neurons. This hypothesis, termed the selectivity model (Handwerker 1992; McMahon and Koltzenburg 1992; Simone et al., 2004), explains the commonalities of itch and pain while accounting for their opposing actions.
Figure 1. The Selectivity Theory of Itch.
(A) Diagram of pain versus itch perception according to the selectivity model. Pain sensation is produced upon activation (denoted by stripes) of the larger nociceptive population, which includes many itch-selective neurons. If the smaller itch-selective subset is activated on its own, the lack of inhibition by nociceptors produces the perception of itch. The nonoverlapping itch neurons could include the unconfirmed itch-specific cells of the labeled line model. (B) First- and second-order connections in the pain and itch pathways. The illustration shows one potential combination of interactions among primary sensory neurons, interneurons, and projection neurons that is consistent with the selectivity model but others are possible as well. For example, instead of or in addition to those from peripheral nociceptors, synaptic contacts may also exist from nociceptive spinal neurons to interneurons. Mast cell release of histamine alone is depicted for clarity, although other pruritogens, e.g., 5HT, tryptases, and interleukins, are likely to play a role as well. A minus sign indicates the inhibitory synapse between the interneuron and itch-selective spinal cord neuron.
The Selectivity Model at the Periphery
Two studies in this issue of Neuron provide supporting evidence for the selectivity model by identifying a population of peripheral neurons that appears to include the itch-selective subset (Lagerström et al., 2010; Liu et al., 2010). Liu et al. endorse a theory they term population coding, which is equivalent to the selectivity theory nomenclature used by Lagerström et al. as well as this minireview. The gene for the vesicular glutamate transporter VGLUT2 was deleted from DRG neurons with a variety of DRG-specific Cre mouse lines. Variation in Cre expression can account for some differences in behavioral phenotypes between the two groups’ mice, and the results are otherwise similar. As expected, removal of VGLUT2 in conditional knockout mice impaired glutamatergic signaling in the targeted nociceptive neurons to produce thermal pain deficits. Strikingly, upon reaching ~2 months of age, most mice had developed skin lesions resulting from spontaneous scratching. Evoked itch initiated by injection of compound 48/80 or other pruritogens is also elevated when VGLUT2 expression is absent. In addition, it would be worthwhile to test whether the mice are affected in models of chronic itch, which could reveal the role of VGLUT2 in clinical itch states. Compared to the itch phenotype, the pain deficits observed in these mice are mild. Compensation for the loss of synaptic input early in development could contribute to these lesser pain deficits. The partial reduction in the pain response may sufficiently reduce nociceptive inhibition of itch to generate a strong itch phenotype.
The studies report a high degree of overlap between the VGLUT2+ population and gastrin-releasing peptide+ (GRP) and MrgprA3+ cells, which have previously been characterized as itch-sensitive neurons (Sun and Chen, 2007; Sun et al., 2009; Liu et al., 2009). They show that VGLUT2 expression also coincides with a subset of TRPV1+ cells consistent with the TRPV1+ neuron ablation study described earlier (Imamachi et al., 2009). Notably, each report demonstrates that both histaminergic and nonhistaminergic itch are affected. Further experiments, including ablation of putative itch-selective subpopulations, e.g., MrgprA3+ or GRP+ cells, are needed to firmly establish a particular requirement for these neurons in itch.
One prediction of the selectivity model (Figure 1) is that activating the itch-selective subset alone without concomitant activation of the remaining, nociceptive TRPV1+ cells should generate itch, even if this is done with an algogen. Liu et al. (2010) provide indirect evidence for this in an experiment in which capsaicin is injected into the cheek before the mice have developed skin lesions. In the VGLUT2-Nav1.8-Cre knockout mice, i.e., with nociceptors silenced by disrupting glutamatergic transmission, capsaicin induces itch rather than pain sensation. Eliminating the “nociceptor-only” population, i.e., those nociceptors that do not also respond to pruritogens, could extend these results if capsaicin can induce itch in the absence of inhibition from the deleted nociceptor population. Lagerström et al. (2010) observe a reduction in the itch response to topical capsaicin application, but this could be due to desensitization of pain and itch fibers 2 hr after capsaicin treatment. Although most evidence supports the selectivity model at the DRG level, it is possible that an unidentified subset of DRG neurons exists that is dedicated to a specific type of itch but not pain pathway.
Considering the Selectivity Theory of Itch in the Spinal Cord
The expected central targets for pruritoceptors, a subset of C fibers, are dorsal horn neurons located in the upper laminae of the spinal cord. Studies have investigated their potential role as second-order neurons in the itch circuit.
Simone et al. (2004) saw that most monkey spinothalamic tract (STT) neurons were capsaicin sensitive and a subset of these also responded to histamine. Work in rat (Jinks and Carstens 2002) found capsaicin also activated dorsal horn neurons responding to the itch mediator serotonin 5HT. Experiments in mouse showed that itch-selective spinal neurons, activated by histamine and 5HT as well as SLIGRL-NH2, an agonist for the PAR2 receptor, responded to nociceptive stimuli including heat and mustard oil (Akiyama et al., 2009a, 2009b). The inhibition of itch by pain was shown in monkey by Davidson et al. (2007a), who observed that peripheral scratching inhibited histamine- or cowhage-sensitive STT neurons. They later found a group of histamine- and capsaicin-responsive cells that were inhibited by scratching upon application of the former but not latter compound (Davidson et al., 2009). These data from different animal models suggest the dual activation of neurons by either itchy or painful stimuli occurs centrally as predicted by selectivity theory. However, there is still debate about the applicability of selectivity versus labeled line theory to itch circuitry in the spinal cord.
In 2001, an STT population responsive to histamine was seen in cat spinal cord, providing early evidence for a potential itch-specific population (Andrew and Craig, 2001). Certain STT neurons responded only to histamine and not mustard oil, a painful stimulus. The later discovery of a role for the gastrin-releasing peptide receptor (GRPR) in itch (Sun and Chen, 2007; Sun et al., 2009) may also mark an itch-specific subset. This receptor is found in the spinal cord dorsal horn, and GRP, the putative ligand, is expressed in some small-diameter DRG neurons. Evoked nonhistaminergic itch, but not pain, is significantly decreased in GRPR mutant mice. When GRPR+ cells are ablated, both histaminergic and nonhistaminergic itch behavior are lost, whereas pain sensitivity is intact. Importantly, in GRPR-ablated lamina I dorsal horn, NK-1+ neurons, most of which are STT neurons required for both pain and itch behavior (see below), are still present, suggesting that GRPR+ and STT neurons comprise two separate populations. The GRPR+ neurons are candidates for the itch-specific spinal cord neurons postulated by labeled line theory. Electrophysiological recordings of these neurons demonstrating that they respond to itchy but not painful stimuli are needed to rule out the possibility that GRPR+ cells play a dispensable role in the pain pathway. It is also important to determine whether GRPR+ cells are projection neurons or interneurons.
Another dorsal horn population expresses NK-1, a G protein-coupled receptor (GPCR) demonstrated to play a critical role in itch produced by 5HT in rat (Carstens et al., 2010). Removal of NK-1+ neurons also leads to pain deficits (Mantyh et al., 1997; Nichols et al., 1999), suggesting this cell population contains the putative pruritoceptors of the selectivity model while also contributing to nociception. It will be interesting to see whether NK-1 overlaps with GRPR in the rat spinal cord and, conversely, whether NK-1 ablation in mouse matches the rat phenotype.
This also raises the important issue of species differences in pruritoception. Even rats, the closest model system to mice, are unique in that histamine does not induce a behavioral scratch response (Jinks and Carstens 2002). At the level of both DRG (Johanek et al., 2008; Namer et al., 2008) and spinal cord (Davidson et al., 2007b) in humans and nonhuman primates, there seem to be separate C fibers for histaminergic and nonhistaminergic (namely cowhage-induced) itch. Mice, however, demonstrate some overlap with respect to activation by these compounds (Akiyama et al., 2009a, 2009b), highlighting another important species difference that must be acknowledged.
The role of spinal interneurons has been the subject of limited study, but a paper from Ross et al. (2010) identifies a subset of these cells involved in itch. The transcription factor Bhlhb5 is required for development of some dorsal horn neurons. Ablation of this gene from an inhibitory interneuron population marked by Pax2 leads to the development of skin lesions at ~2 months of age in these mice. These results, which parallel those of the VGLUT2 studies, offer a place for spinal interneurons in the itch circuit and call for their further investigation.
A preliminary picture of the pain and itch circuitry emerges from the collective DRG and spinal cord data (Figure 1B). This shows how the selectivity model can explain itch and specifies the molecular identity of certain critical neurons in the pathway.
Molecular Mechanisms of Itch Signaling
Historically, humans were the animal model of choice for the itch field, but this was a limitation with respect to molecular studies. Rodent models, especially the mouse (Kuraishi et al., 1995), allowed for targeted manipulation of itch-selective neurons and insight into itch signaling for both histaminergic and nonhistaminergic forms of itch.
Primary sensory neurons are responsible for the transduction of itch induced by pruritogens, which often act directly upon receptors within these fibers. Another step may be involved if the pruritogen first activates a nonneuronal cell type, which then releases pruritogen(s) that will consequently signal through primary fibers. Both are common means of generating itch, but the majority of studies have investigated the former to look for the receptors targeted by particular pruritogens and their concomitant signaling mechanisms.
The histamine H1 receptor has been considered the main mediator for itch generated by histamine, although the H4 receptor may also be important (Bell et al., 2004). The H1 receptor couples with phospholipase C β3 (PLCβ3) (Han et al., 2006) and the erstwhile purely nociceptive (Caterina et al., 2000) ion channel TRPV1 (Shim et al., 2007; Imamachi et al., 2009). Cowhage is thought to produce a nonhistaminergic form of itch through PAR2 and/or PAR4 (Reddy et al., 2008), and both are known to play a role in pain sensation (Ossovskaya and Bunnett 2004). Other PAR2 agonists including the aforementioned SLIGRL-NH2 (Shimada et al., 2006) and the protease tryptase (Ui et al., 2006) are demonstrably pruritogenic. Intriguingly, TRPV1 may also play a role here as PAR2 activation is known to sensitize TRPV1 (Amadesi et al., 2004; Dai et al., 2004).
5HT acts upon GPCRs of the 5HTR family in the context of itch, and although this also requires PLCβ3, TRPV1 is not involved (Imamachi et al., 2009). The GPCR MrgprA3 is the target for another notable pruritogen, chloroquine, a well-known antimalarial drug that often produces noncompliance from the side effect of severe pruritus (Liu et al., 2009). Thus, the participation of GPCRs and TRP channels, which come up repeatedly, may help guide future studies to elucidate itch transduction pathways.
Indirect activation of secondary cell types by pruritogens is not as well characterized. Mast cells are associated with itch (Sugimoto et al., 1998) given that they release several pruritogens (Harvima et al., 2008) upon degranulation, and other cells of the immune system may also play a role (Ikoma et al., 2006). Another source is the skin, on which keratinocytes, to take one example, contain potential itch-related molecules like TRPV3 and TRPV4 (Chung et al., 2004). A TRPV3 mutation has been linked to allergic and pruritic dermatitis in mice that is thought to originate from expression in keratinocytes (Yoshioka et al., 2009).
Although much has been discovered about itch signaling peripherally, fewer studies have focused on the molecules involved at the spinal level. GRPR and NK-1 provide starting points for investigation while other markers and mediators will uncover molecular pathways for itch in the spinal cord.
Neurotransmission in the Itch Circuit
Another critical finding of the two papers from Lagerström et al. (2010) and Liu et al. (2010) is that glutamatergic transmission is not required for signaling in itch-selective neurons. Removal of the VGLUT2 transporter impairs spontaneous postsynaptic currents (Liu et al., 2010), although a more thorough characterization of the electrophysiological deficits in knockout mice from both studies is crucial to confirm the loss of glutamatergic signaling. Although it may still play a modulatory role, direct transmission with glutamate appears to be dispensable for itch given that the knockouts show increased itch responses. The data thus imply another neurotransmitter is required for transmitting the itch signal from the periphery. GRP is an appealing candidate, although its role must be tested directly.
Spontaneous itch emerges in these studies without pruritic stimuli present and in which the only functional deficit is impaired glutamatergic output from sensory afferents. Lagerström et al. (2010) found that antihistamines blocked spontaneous itch in their VGLUT2 mutant mice. Given that exogenous histamine was not used to induce scratching, it is likely that internal sources activated the peripheral fibers. This suggests some kind of input is needed to activate itch-selective neurons in order to release neurotransmitter and eventually produce an itch percept. Activation of these fibers by other nonpruritic stimuli could also generate the sensation of itch in the absence of VGLUT2.
Conclusions
Itch is a recognizable phenomenon in both clinical and nonpathological conditions, and recent work has helped shed light on the molecular players and circuitry involved in signaling this important sensory modality. The discovery of afferent fiber populations responsive to histamine, the canonical pruritogen, was later extended to encompass other compounds in the catalog of pruritogens, which are also capable of generating nonhistaminergic forms of itch. After the intensity and labeled line models initially put forth to explain how itch is encoded, selectivity theory gained favor after early studies in humans were expanded to include other primate and mammalian species. The selectivity model hypothesizes the existence of pruritoceptors that are part of a larger set of nociceptors in which activation of the whole group versus that of the itch-selective subset elicits the perception of pain or itch, respectively.
Deletion of VGLUT2 from certain mouse DRG neuron populations produces spontaneous itch and enhances both histaminergic and nonhistaminergic varieties of evoked itch. VGLUT2+ cells express molecular markers of previously identified itch-selective neurons but appear to be part of the larger nociceptor population (Imamachi et al., 2009; Liu et al., 2009; Shim et al., 2007; Sun and Chen 2007). Liu et al. (2010) demonstrate that glutamatergic inactivation of nociceptors via removal of VGLUT2 leads to capsaicin-evoked itch instead of pain, providing evidence for the selectivity theory peripherally. Centrally, the dorsal horn neurons that follow DRG fibers in the itch pathway have been identified with molecular and electrophysiological studies (Jinks and Carstens 2002; Simone et al., 2004; Akiyama et al., 2009a, 2009b; Davidson et al., 2009; Sun et al., 2009). These exhibit properties similar to those of the peripheral pruritoceptors as predicted by the selectivity model. However, the labeled line theory of encoding itch in both DRG and dorsal horn neurons remains viable. The remarkable molecular diversity of DRG neurons (Basbaum et al., 2009) means an itch-specific subpopulation may still be revealed. Labeled line at the spinal cord level is also possible especially with the striking behavioral phenotype found in the GRPR+ neuron ablation mice (Sun et al., 2009).
Experiments to investigate the role of spinal interneurons are underway and will require further study to characterize what is likely a crucial part of the itch circuit. The remainder of the circuit, including central elements such as those in the thalamus and cortex, has been examined (Drzezga et al., 2001) but how these regions connect to other parts of the itch pathway has thus far been the subject of limited study (Craig and Andrew 2002).
An explosion of recent work has shed light on the molecules and cells involved in the transduction of itchy stimuli. These studies have identified receptor targets and signaling components utilized by a number of pruritogens. Detailed accounts of these pathways remain to be uncovered along with features such as how the molecular narratives fit with our understanding of itch circuitry. This includes specifying how neurotransmission occurs to signal itch, which has been partially elucidated by recent work (Lagerström et al., 2010; Liu et al., 2010) demonstrating that standard glutamatergic transmission is not essential.
REFERENCES
- Akiyama T, Carstens MI, Carstens E. J. Neurophysiol. 2009a;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. J. Neurosci. 2009b;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, et al. J. Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Nat. Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JK, McQueen DS, Rees JL. Br. J. Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. J. Biol. Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Craig AD, Andrew D. J. Neurophysiol. 2002;87:1902–1914. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. J. Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Acta Derm. Venereol. 2007a;87:475. [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr J. Neurosci. 2007b;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Nat. Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Pain. 2001;92:295–305. doi: 10.1016/s0304-3959(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Handwerker HO. APSJ. 1992;1:135–138. [Google Scholar]
- Handwerker HO, Forster C, Kirchhoff C. J. Neurophysiol. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Arch. Dermatol. Res. 2008;300:461–478. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. Nat. Rev. Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. Proc. Natl. Acad. Sci. USA. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. J. Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. J. Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. J. Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Eur. J. Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Ölund C, Smith C, Mendez JA, Chen Z-F, et al. Neuron. 2010;68(this issue):529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Grant RT, Marvin HM. Heart. 1927;14:139–160. [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Samad O. Abdel, Zhang L, Duan B, Tong Q, Lopes C, Ji R-R, Lowell BB, Ma Q. Neuron. 2010;68(this issue):543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. J. Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. J. Physiol. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. J. Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. J. Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. J. Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. J. Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Eur. J. Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr J. Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Eur. J. Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarvas S, Harmon D, Murphy D. J. Clin. Anesth. 2003;15:234–239. doi: 10.1016/s0952-8180(02)00501-9. [DOI] [PubMed] [Google Scholar]
- Tuckett RP. J. Invest. Dermatol. 1982;79:368–373. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Eur. J. Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- von Frey M. Arch. Neerl. Physiol. Homme Anim. 1922;7:142–145. [Google Scholar]
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, Sakata T, Horikawa T, Arimura A. J. Invest. Dermatol. 2009;129:714–722. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Samuel LS. Dermatol. Ther. 2008;21:32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]