Summary
Regulating transcription under different conditions is vital to all organisms. As Escherichia coli shifts from exponential to stationary growth, regulation of transcription is achieved in large part by the tight binding of 6S RNA to Eσ70, RNA polymerase with the σ70 specificity subunit. Ribo-sequestration of Eσ70 by 6S RNA serves to down-regulate σ70-dependent transcription, which is needed for exponential growth. This facilitates transcription from promoters dependent on stationary phase sigma, σs. Previous work has suggested that the 6S RNA binding to Eσ70 simply mimics the Eσ70/promoter interaction. In this issue of Molecular Microbiology, Klocko and Wassarman demonstrate that many of the contacts between residues within σ70 region 4 and 6S RNA are distinct from those between region 4 and promoter DNA. Several residues that interact with 6S RNA are ones previously known to interact with protein activators of Eσ70. Their work adds 6S RNA to the growing list of factors that can regulate Eσ70 by interacting with region 4.
Keywords: 6S RNA, sigma, transcription, polymerase, promoter
The regulation of transcription is essential for the correct development of any organism. Features of the transcription machinery and the processes that control transcription are conserved throughout biology, bolstering Jacques Monod’s contention regarding Escherichia coli and elephants (Monod & Jacob, 1961). All multi-subunit RNA polymerases have a core of enzymatic activity plus a specificity factor that recognizes and binds promoter DNA. In bacteria, a core RNA polymerase consisting of the subunits ββ’α2ω associates with a σ specificity factor to generate holoenzyme (Eσ). Protein activators and repressors and small RNAs can modulate Eσ activity under various conditions depending on cell requirements for survival and growth.
Primary σ factors, such as E. coli σ70, predominate during exponential growth while alternate σ factors are used during different growth conditions or in times of stress (reviewed in (Gruber & Gross, 2003, Paget & Helmann, 2003)). The degree of sequence conservation amongst the hundreds of σ factors that have been identified is remarkable. Most contain conserved Regions 2, 3, and 4; primary σ factors contain the N-terminal Region 1 as well. Specific interactions between promoter DNA elements and residues in σ70 Regions 1.2, 2, 3, and 4 facilitate promoter recognition and/or melting of double-stranded DNA (reviewed in (Hook-Barnard & Hinton, 2007)). For example, residues within σ70 Region 4 (Figure 1A) interact with the -35 promoter recognition element (consensus TTGACA) ((Campbell et al., 2002) and references therein). Region 4.2 residues that make direct contact with base determinants within the -35 element lie in the second helix of a classic, DNA-binding helix-turn-helix (H-T-H) motif (Figure 1A and B).
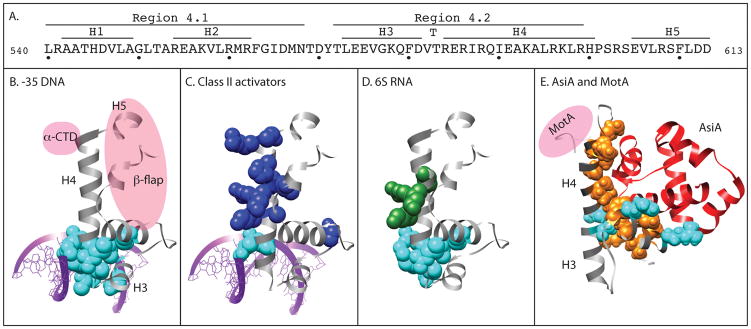
Figure 1.
Panel A: amino acid sequence and secondary structure of E. coliσ70 region 4 (residues 540 to 613). (Gruber and Gross, 2003; Lambert et al. 2004).
Panels B-D: Structure of region 4 of T. aquaticusσA (gray) bound to -35 element DNA as observed by x-ray crystallography (Campbell et al., 2002).
B. purple, -35 element DNA; spacefill residues, σ residues that contact -35 element DNA; pink ovals, β-flap portion of the β subunit and the α subunit-CTD. σ/DNA contacts are from Campbell et al., 2002 except that the residue corresponding to E. coli K593 is not denoted as a contact because it did not affect σ/DNA binding in Klocko and Wasserman (this issue).
C. spacefill residues, σ residues that have been found to contact specific Class II activators: cyan, coincident with DNA-binding residues; blue, distinct from DNA-binding residues. λcI: R588, K593, K596; FNR: K593, R596, K597, H600, R603; Ada: K593, K597, R603; RhaS: K593, R599; RhaR: R599.
D. spacefill residues, σ residues that contact 6S RNA: cyan, coincident with DNA-binding residues; green, distinct from DNA binding residues. (See Klocko and Wassarman, this issue).
Panel E: Structure of region 4 of E. coli σ70 (gray) bound to the bacteriophage T4 co-activator AsiA (red) as observed by NMR (Lambert et al., 2004). Pink oval, the position of the activator MotA. Spacefill residues, σ residues that contact AsiA: cyan, coincident with DNA-binding residues; orange, distinct from DNA-binding residues.
When a bacterial cell population shifts from exponential growth into stationary phase, there is a general promoter usage switch from σ70-dependent promoters to σs-dependent promoters. The amount of σ70-transcription significantly decreases though the level of σ70 in the cell stays relatively constant. This observation was explained in part by the finding that the anti-sigma factor Rsd can sequester σ70, preventing its association with core (Jishage & Ishihama, 1998, Mitchell et al., 2007). In the last decade, Wassarman and colleagues have described the efficient ribo-sequestration of Eσ70 by 6S RNA, contributing significantly to our understanding of gene regulation in stationary phase growth (reviewed in (Wassarman, 2007)). In this issue of Molecular Microbiology, Klocko and Wassarman elucidate the interaction of 6S RNA with σ70 Region 4, revealing how the interaction with 6S RNA differs in molecular detail from the interaction with the -35 DNA.
6S RNA (reviewed in (Wassarman, 2007)) is a highly abundant RNA species that accumulates as cells enter stationary phase. Most 6S RNA is found associated with Eσ70 in a specific, stable complex. The interaction with 6S RNA further stabilizes the core/σ70 interaction. The presence of 6S RNA specifically inhibits Eσ70 transcription in vivo and in vitro, thus defining 6S RNA as an inhibitor of σ70-dependent transcription. Remarkably, the bound 6S RNA becomes a template for transcription when NTP concentrations are relatively high. As 6S RNA is transcribed, σ70 is released from the transcription complex. This is similar to the release of σ70 as polymerase transitions from the initiating complex to a typical elongation complex. Thus, it has been proposed that the Eσ70/6S RNA complex serves to sequester σ70 during stationary phase, down-regulating σ70-transcription at the time when σs transcription is required. The increase in intracellular NTP pools as cells encounter good growth conditions would then facilitate transcription from the bound 6S RNA template, leading to the release of σ70.
It has been proposed that 6S RNA functions as a molecular mimic of σ70 promoter DNA. The predicted structure of 6S RNA shows a mostly ds RNA with a central bulge, an unpaired region of 17 nucleotides on the template strand and 13 nucleotides on the nontemplate strand (see Fig. 1, Klocko and Wassarman, this issue). The bulge resembles the “open” promoter DNA in a stable complex with Eσ70, in which unpaired DNA from -11 to around +3 reveals the start site of transcription (reviewed in (Hook-Barnard & Hinton, 2007)). Analyses using crosslinking and Fe-generated OH radical cleavage have established that the 6S RNA bulge lies at the active site in contact with σ70, β, and β’, as would be expected for an Eσ70 template. Thus, the bulge would represent the unwound DNA from the -10 element through the start of transcription, and a portion of the upstream sequence would contribute the “-35 element”. Indeed, deletion of σ70 Region 4.2, which interacts directly with -35 element DNA, significantly decreases Eσ70 binding to 6S RNA (Cavanagh et al., 2008). However, the proposed structure of the 6S RNA upstream of the template bulge neither resembles the sequence of a -35 element nor is it fully double-stranded. The differences between the conformations of ds RNA versus ds DNA (A-form versus B-form), though, might accommodate the sequence variation. The finding that sensitivity to inhibition by 6S RNA is promoter specific provides a more compelling argument against 6S RNA operating as a molecular mimic of DNA. Promoters with a poor match to the canonical -35 element or promoters containing an extended -10 sequence irrespective of their -35 sequence are selectively inhibited by 6S RNA (Cavanagh et al., 2008, Trotochaud & Wassarman, 2004). In contrast, neither the overall promoter binding strength nor the strength of the -10 element are indicative of 6S RNA sensitivity. How this promoter selectivity is achieved is not understood.
In this issue of Molecular Microbiology, Klocko and Wassarman have tested the theory that 6S RNA is a promoter mimic. They reasoned that a comparison of the molecular interactions used by σ70 Region 4.2 with promoter DNA versus 6S RNA would determine whether 6S RNA simply replaces the promoter DNA. To do this, the authors employed EMSA to investigate the effect of single amino acid substitutions within Region 4.2 on the binding of Eσ70 to promoter DNA or 6S RNA. Their work shows that while some of the residues that interact with each nucleic acid are coincident, several are not, and the consequence of an amino acid mutation can vary dramatically. For example, alanine substitutions at two Region 4.2 residues that are thought to interact directly with the -35 DNA, R584 and E585, have different effects on binding to promoter DNA or 6S RNA. R584A is more deleterious for 6S RNA binding, while E585A is more deleterious for binding to promoter DNA. Furthermore, while alanine substitutions at more C-terminal residues (K593, R596, K597, R599) do not significantly affect DNA binding, they do decrease binding to 6S RNA.
Several positively charged residues within Region 4.2 define a patch needed for 6S RNA interaction. Introduction of more overall positive charge (A592K or E585A) improves 6S RNA binding. In addition, various substitutions at K593 and K597 highlight the importance of these positive charges. K → R substitutions at either of these positions do not have any effect, and a K → Q or H change decreases binding to 6S RNA like that seen with the alanine substitution. Furthermore, a K593 → E change eliminates binding. None of these substitutions affect binding to the promoter DNA. In a clever experiment, Klocko and Wassarman show that the binding of the Eσ70 K597H mutant to 6S RNA can be restored to wild type levels by lowering the pH, which converts histidine from an uncharged to a positively charged residue.
The work presented by Klocko and Wassarman represents the first molecular characterization of a riboregulator in a complex with a region of σ70. Several other proteins are known to bind to both RNA and DNA, such as TFIIIA, a transcription factor of eukaryotic pol III; the bacteriophage T4 single-stranded DNA 32 protein; and the p53 and Drosophila Bicoid proteins, transcription activators of eukaryotic pol II. Although DNA and RNA share many chemical features, most nucleic acid binding proteins recognize either DNA or RNA with high specificity. Understanding how σ70 Region 4 can make specific contacts with the DNA -35 element and with 6S RNA will offer insights into other proteins with the unique capacity to interact with both DNA and RNA.
Klocko and Wassarman’s work also provides another example of the inherent plasticity and multifunctional nature of σ70 Region 4. Early work suggested that the H-T-H within Region 4.2 simply provided a classic motif for interacting with the major groove of the -35 element DNA. However, it is now apparent that σ70 Region 4 is a malleable domain that can interact with multiple partners. When σ70 is bound to core, residues within Region 4.1 and 4.2 contact the -35 element DNA, while other portions of Region 4 and the far C-terminus of σ70 (Helix 5) interact with the β-flap, a structural component of the β subunit (Figure 1B) (Vassylyev et al., 2002, Kuznedelov et al., 2002). This interaction is needed to position Region 4 correctly within holoenzyme, allowing simultaneous contact between Region 4 and the -35 element and between σ70 Region 2 and the -10 element. In addition, R603 within Region 4 can also interact with the polymerase α-CTD at promoters in which the α-CTD binds immediately upstream of the -35 element (Figure 1B) (Ross et al., 2003). Thus, within the holoenzyme, flexibility of Region 4 accommodates various interactions.
The plasticity of Region 4 is further evident through its interactions with a variety of regulatory factors. Class II activators of E. coli polymerase, such as Ada, FNR, RhaR, RhaS, and lambda cI, target overlapping subsets of residues within the DNA binding helix (helix 4) to make direct contact with polymerase (Figure 1C) (discussed in (Bonocora et al., 2008). These activators bind immediately upstream of the -35 element, so it is thought that the additional contact between the activator and Region 4 helps to reinforce the interaction of Region 4 with the DNA. The wide variety of interacting partners suggests that Region 4 is a flexible domain that can be adapted for many purposes. However, the most dramatic example of Region 4 flexibility is the total remodelling of this domain by the bacteriophage T4 protein AsiA (Figure 1E) (Lambert et al., 2004). The resulting conformational change allows the T4 activator, MotA, to interact with σ70 residues that normally interact with the β-flap (Bonocora et al., 2008). In their paper, Klocko and Wassarman characterize yet another example of the functional flexibility ofσ70 Region 4 – in this case, by using residues that under other circumstances would interact with DNA or transcription activators but now interact with 6S RNA (Figure 1D). Their findings argue that the Region 4/6S RNA interaction is designed to inhibit transcription from specific promoters: those with a weak -35 element or an extended -10 sequence. Although it is not yet clear how this is accomplished, the ability of Region 4 to assume various conformations suggests that 6S RNA might be able to hold Region 4 in a conformation that is more or less inhibitory depending on the promoter type. With this new work, we gain an even greater appreciation for the ability of cells to use a small, malleable element to control a wide spectrum of regulatory outcomes.
References
- Bonocora RP, Caignan G, Woodrell C, Werner MH, Hinton DM. A basic/hydrophobic cleft of the T4 activator MotA interacts with the C-terminus of E.coli sigma70 to activate middle gene transcription. Mol Microbiol. 2008;69:331–343. doi: 10.1111/j.1365-2958.2008.06276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, Trester-Zedlitz ML, Darst SA. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9:527–539. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- Cavanagh AT, Klocko AD, Liu X, Wassarman KM. Promoter specificity for 6S RNA regulation of transcription is determined by core promoter sequences and competition for region 4.2 of sigma70. Mol Microbiol. 2008;67:1242–1256. doi: 10.1111/j.1365-2958.2008.06117.x. [DOI] [PubMed] [Google Scholar]
- Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- Hook-Barnard IG, Hinton DM. Transcription Initiation by Mix and Match Elements: Flexibility for Polymerase Binding to Bacterial Promoters. Gene Regulation and Systems Biology. 2007;481:275–293. http://la-press.com/article.php?article_id= [PMC free article] [PubMed]
- Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc Natl Acad Sci U S A. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–857. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- Lambert LJ, Wei Y, Schirf V, Demeler B, Werner MH. T4 AsiA blocks DNA recognition by remodeling sigma(70) region 4. Embo J. 2004;23:2952–2962. doi: 10.1038/sj.emboj.7600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Oshima T, Piper SE, Webster CL, Westblade LF, Karimova G, Ladant D, Kolb A, Hobman JL, Busby SJ, Lee DJ. The Escherichia coli regulator of sigma 70 protein, Rsd, can up-regulate some stress-dependent promoters by sequestering sigma 70. J Bacteriol. 2007;189:3489–3495. doi: 10.1128/JB.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Jacob F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Paget MS, Helmann JD. The sigma70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W, Schneider DA, Paul BJ, Mertens A, Gourse RL. An intersubunit contact stimulating transcription initiation by E coli RNA polymerase: interaction of the alpha C-terminal domain and sigma region 4. Genes Dev. 2003;17:1293–1307. doi: 10.1101/gad.1079403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotochaud AE, Wassarman KM. 6S RNA function enhances long-term cell survival. J Bacteriol. 2004;186:4978–4985. doi: 10.1128/JB.186.15.4978-4985.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- Wassarman KM. 6S RNA: a regulator of transcription. Mol Microbiol. 2007;65:1425–1431. doi: 10.1111/j.1365-2958.2007.05894.x. [DOI] [PubMed] [Google Scholar]