Abstract
Introduction and Objectives
The clinical significance of ductal prostatic carcinoma has not been well defined. We utilized a population-based cancer registry to identify a large group of ductal carcinoma cases to characterize the impact of the ductal subtype on the presentation and survival of men with prostate cancer.
Methods
A national cancer registry was used to identify incident cases of ductal and acinar adenocarcinomas from 1996–2006. Clinicopathologic variables were analyzed and Cox multivariate survival analysis performed. PSA values were available for the years 2004–2006, and these were used to assess for differences in Gleason grade and serum PSA levels between ductal and acinar cancers at the time of diagnosis.
Results
A total of 442,881 acinar cases and 371 ductal cases were identified. Ductal cases were more likely to present with distant disease (12% vs. 4%, p<0.001) and to be poorly differentiated (50% vs. 32%; p<0.001). Ductal histology was associated with a 30% lowering of the geometric mean PSA (adjusted coeff.=0.7, 95% CI 0.6–0.8) and a more than two-fold increased odds of having a PSA<4.0 ng/ml (OR 2.4, 95% CI 1.4–4.0) independent of other clinicopathologic variables. For those with non-distant disease at diagnosis, ductal histology was associated with a 2.4-fold (CI 1.5–3.8) increased disease-specific mortality.
Conclusions
In the largest series of this histologic subtype, ductal cancers were more likely to present with advanced stage cancer and a lower PSA, suggesting that timely detection of the disease is a significant challenge. In addition, those with loco regional disease were more likely to die of their disease.
Keywords: Prostate, ductal, SEER, PSA
INTRODUCTION
Prostate cancer (PCa) is a disease with a variable clinical course. The histologic Gleason score is an important factor in risk stratification. The role of variant (non-acinar) PCa histologies on outcomes is established for some (e.g., small cell carcinoma) but not all subtypes.1 Ductal adenocarcinoma of the prostate is a histologic subtype initially described in 1967 with clinical implications that are still not well understood.2 Its prevalence in prostatectomy and biopsy specimens has ranged from 0.4–0.8% for pure ductal and up to 5% for mixed ductal adenocarcinoma.3–6 Histologically, it is characterized by the presence of tall, pseudostratified columnar epithelium with abundant cytoplasm in a papillary or cribriform-papillary pattern. It can occur as a pure form or, more often, admixed with the typical acinar pattern carcinoma. Due to its appearance, ductal adenocarcinoma was previously thought to arise from Mullerian remnants and possess unique clinical features related to its origin.7 While further analyses have shown that these tumors do arise from prostatic tissue,8, 9 speculation regarding the unique clinical features of this tumor remains.
There has been significant debate regarding whether or not the presence of ductal prostate cancer carries distinct prognostic implications, but recent evidence has suggested that it may be more aggressive than acinar adenocarcinoma.10–13 However, due to the rarity of the subtype, this observation has been difficult to confirm, as most studies have been limited to small, single-institution series. In this study, we utilized a population-based cancer registry to identify a large group of patients with ductal carcinoma and better characterize the impact of the ductal subtype on the presentation and survival of men with PCa.
METHODS
Data source and study population
The Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify the study cohort. SEER collects incident cancer and survival data from seventeen population-based cancer registries covering approximately 26% of the United States population. Data from 1996–2006 from all 17 SEER registries were included (San Francisco-Oakland SMSA, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), rural Georgia, Utah, Metropolitan Atlanta, Alaska, San Jose-Monterey, Los-Angeles, Kentucky, Louisiana, New Jersey, and Greater California).
Cases were identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes for the prostate. Ductal (8500–8503) and acinar (8550, 8140) cases were identified by ICD-O-3 histology code.
Data collection and coding
Demographic data collected included age, race, tumor registry, and year of diagnosis. Age was categorized into 5-year age groups, and race was categorized as white, black or other. Pathologic data included primary T-stage (clinical stage was used if pathologic stage was not available), SEER historic stage (localized/regional, distant), nodal status (negative, positive, not-performed), metastatic status (present/absent), and tumor grade (well, moderately, poorly/undifferentiated, unknown). Primary treatment was recorded as radical prostatectomy (RP), radiation therapy (XRT), or other/missing.
For tumor grade, the SEER grading system was used as specific Gleason grades were not recorded prior to 2004. The SEER grading system uses “well differentiated”, “moderately differentiated”, and “poorly differentiated”, corresponding to Gleason scores “2–4”, “5–7”, and “8–10”, respectively. Gleason score 7 was moved from “moderately differentiated” to “poorly differentiated” with cases diagnosed after January 1, 2003. For the years 2004–2006, PSA and Gleason data were also available. Serum PSA was categorized as <4.0, 4.0–9.9, and ≥10 ng/ml. Gleason score was categorized as ≤6, 7, and 8–10. In patients who underwent RP, the final Gleason grade was utilized. Surgical margin status was not available, precluding assessment of this parameter. Disease-specific survival (DSS) was calculated starting at the date of diagnosis to the date of death due to PCa. If death was not observed, patients were censored at the date of last follow-up or at the time of death due to non-PCa causes.
Statistical Analysis
Demographic and pathologic data were compared between ductal and acinar cases with chi-squared tests. The annual incidence of ductal carcinoma was determined using SEER*Stat 6.6.2 sofware. Annual incidence rates were age-adjusted to the 2000 United States standard population. The annual percentage change was calculated using weighted least squares methods. Kaplan-Meier survival curves were generated to compare the unadjusted DSS experience between ductal and acinar cases. Unadjusted and multivariable Cox regression was performed to evaluate the disease-specific mortality risk. Covariates included in the survival analysis were age, grade, clinical stage, nodal status, treatment modality, and race, and robust standard errors were used. There was evidence for effect modification between histology and stage (interaction p-value = 0.01); therefore, mortality risk estimates were reported separately for localized/regional and distant stage disease. We found no evidence for effect modification by treatment (RP vs. XRT for localized disease, interaction p-value = 0.60). Hazard ratios (HR) are presented along with their 95% confidence intervals (95% CI).
A separate analysis was limited to those diagnosed from 2004–2006 as PSA, Gleason score, and clinical stage were available for these years. To explore whether those with ductal tumors were more likely to have lower PSA levels, two regression models were created. First, a multivariate linear regression analysis was performed predicting serum PSA. PSA was log-transformed given its non-normal distribution. The predictor of interest was histology (ductal vs. acinar) adjusting for race, age, Gleason grade, and clinical stage (T1c, cT2, cT3/4). A second multivariate logistic model was created where the outcome was a PSA < 4.0 ng/ml adjusting for the same covariates as in the linear model. Statistical analyses were conducted using Stata software, Version 11 (Stata, Inc., College Station, TX).
RESULTS
A total of 371 patients with ductal adenocarcinoma were identified along with 442,881 patients with acinar adenocarcinoma. Table 1 shows the differences in demographic and pathologic data. Ductal cases were more commonly seen in men over 70-years (54% vs. 44%, p<0.001), and there were no differences in the distribution by race. Ductal cases received either RP or XRT less commonly than acinar cases (55% vs. 66%, p < 0.001). Ductal cases more commonly presented with poorly differentiated (50% vs. 32%, p <0.001) and distant disease (12% vs. 4%, p <0.001) compared to acinar cases. Those with ductal tumors more commonly had extracapsular disease (12% vs. 3%, p < 0.001) by clinical staging. Gleason grade and clinical T stage, available from 2004–2006, were higher in ductal compared to acinar cases (Table 2). Additionally, among the 137,865 acinar cases and 130 ductal cases who underwent RP and therefore had complete pathologic information available, ductal cases were more likely to be poorly differentiated (52% vs. 32%, p<0.001). In RP patients, ductal cases were also more likely to extracapsular disease at the time of RP (p<0.001). Ductal and acinar cases did not differ significantly in the rates of lymph node metastases (2.3% vs. 1.9%, p=0.74). The annual incidence of ductal tumors increased from 0.21 per 1,000,000 persons in 1995, to 0.55/1,000,000 persons in 2000, to 0.96 per 1,000,000 persons in 2007. The annual percentage change was 6.78 (95% CI 3.53 – 10.13, p < 0.05).
Table 1.
Demographic and pathologic characteristics and treatment by tumor type.
| Patient characteristics | Acinar carcinoma | Ductal carcinoma | p-value* | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total patients | 442881 | 99.9% | 371 | 0.1% | |
| Race | |||||
| White | 354,417 | 80% | 296 | 80% | |
| Black | 53,974 | 12% | 38 | 10% | |
| Other | 34,490 | 8% | 37 | 10% | 0.18 |
| Age (yrs) | |||||
| <55 | 39,304 | 9% | 27 | 7% | |
| 55–60 | 53,233 | 12% | 25 | 7% | |
| 60–65 | 70,045 | 16% | 47 | 13% | |
| 65–70 | 85,105 | 19% | 69 | 19% | |
| >70 | 194,757 | 44% | 201 | 54% | <0.001^ |
| Stage | |||||
| SEER historic stage | |||||
| Localized/Regional | 410,838 | 93% | 306 | 82% | |
| Distant | 16,764 | 4% | 46 | 12% | <0.001 |
| Clinical T-stage | |||||
| cT1 | 187,893 | 45 | 130 | 40 | <0.001 |
| cT2 | 214,110 | 52 | 155 | 48 | |
| cT3+ | 12,584 | 3 | 39 | 12 | |
| Pathologic T-stage‡ | |||||
| pT2 | 106,433 | 80 | 81 | 62 | <0.001 |
| pT3a | 17,457 | 13 | 27 | 21 | |
| pT3b/pT4 | 8,575 | 6 | 23 | 18 | |
| Grade** | |||||
| Well to moderately diff. | 290,958 | 66% | 119 | 32% | |
| Poorly differentiated | 136,528 | 31% | 185 | 50% | <0.001 |
| Primary treatment | |||||
| RP | 137,589 | 31% | 130 | 35% | |
| XRT | 155,568 | 35% | 73 | 20% | |
| Other | 149,724 | 34% | 168 | 45% | <0.001 |
p values are univariate from Chi-squared tests.
p-value from trend test
Limited to those undergoing radical prstatectomy
Until January 1, 2003, “well to moderately differentiated” and “poorly differentiated” correspond to Gleason scores “2–7” and “8–10”. Following this, Gleason score 7 was classified as “poorly differentiated.” Note: percentages do not add up to 100% due to missing data.
Table 2.
Gleason grade and clinical stage by tumor type for patients diagnosed from 2004–2006.
| Patient characteristics | Acinar carcinoma | Ductal carcinoma | p-value* | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total patients | 146,366 | 99.9% | 146 | 0.1% | |
| Gleason grade | |||||
| ≤6 | 69,445 | 47% | 21 | 14% | |
| 7 | 50,647 | 35% | 21 | 14% | |
| 8–10 | 21,606 | 15% | 68 | 47% | <0.001 |
| Clinical stage | |||||
| T1 | 77,767 | 53% | 51 | 35% | |
| T2 | 59,207 | 40% | 57 | 39% | |
| T3–4 | 4,279 | 3% | 23 | 16% | <0.001 |
p values are univariate from Chi-squared test.
Note: percentages do not add up to 100% due to missing data.
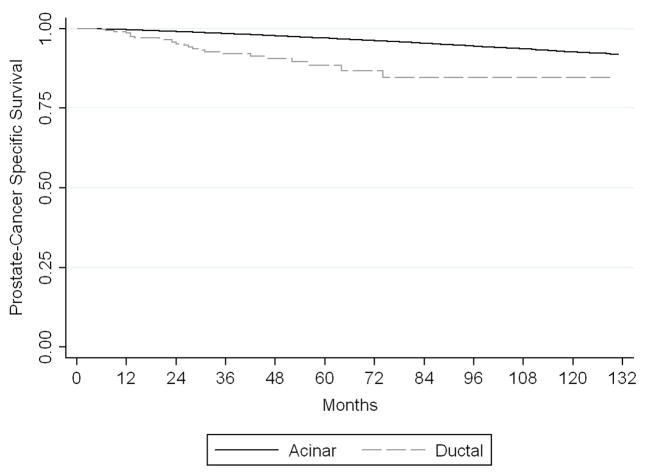
There was a significant difference in disease-specific survival between ductal and acinar patients with localized or regional disease (Figure 1). In the univariate analysis (Table 3), those with non-distant disease with ductal histology had a risk of PCa-specific mortality (PCSM) almost 4-times greater than those with acinar tumors (HR 3.9, 95% CI 2.6–5.8). After adjusting for potential confounders (age, race, grade, clinical T-stage, nodal status, and treatment modality), the PCSM risk was attenuated but still 2.2-fold higher for those with ductal histology (95% CI 1.4–3.5). No differences in survival were seen between ductal and acinar patients with distant disease in either the univariate or multivariate models. PCSM was too infrequent in the subgroup of patients diagnosed from 2004–2006 for a survival analysis to be performed.
Figure 1.
Kaplan-Meier analysis for disease-specific survival by tumor histology in patients with localized or regional cancer. Patients with ductal adenocarcinoma had a significantly lower prostate cancer-specific survival than patients with acinar adenocarcinoma (p<0.001).
Table 3.
Risk of disease-specific mortality for ductal versus acinar adenocarcinoma.
| Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | |
| Localized/Regional | ||||||
| Acinar | 1 | referent | ||||
| Ductal | 3.9 | 2.6 – 5.8 | <0.001 | 2.2 | 1.4–3.5 | 0.001 |
| Distant | ||||||
| Acinar | 1 | referent | ||||
| Ductal | 1.0 | 0.6 – 1.45 | 0.8 | 0.8 | 0.2–1.4 | 0.6 |
Multivariate controls for age, race, grade, clinical T stage, nodal status, treatment modality
The multivariate models for PSA controlled for age, gleason sum, clinical stage and race, and these showed significant differences in serum PSA levels at the time of diagnosis in ductal vs. acinar cases. In the logistic model for the odds of having a PSA <4.0 ng/ml, those with ductal histology were 2.4-times more likely to have a PSA <4.0 ng/ml compared to those with acinar tumors (OR 2.4, 95% CI 1.4–4.0, p = 0.001). In the linear model, (Table 5), those with ductal cancer had a 30% lower geometric mean PSA compared to those with acinar adenocarcinoma (ratio of geometric means (RGM) = 0.7, 95% CI 0.6 – 0.8, p <0.001).
DISCUSSION
Historically, ductal PCa was thought to be less aggressive than adenocarcinoma;2, 7, 14 however, current evidence suggests that these patients are at an increased risk of disease progression.10–13 A number of findings in this study support the hypothesis that ductal carcinoma is an aggressive PCa subtype. First, ductal adenocarcinomas were significantly more likely to be poorly differentiated and have metastatic diseases than acinar adenocarcinomas. Second, in the subset of patients undergoing RP — and therefore with complete pathologic information — both grade and stage were significantly higher in those with ductal cancer. Third, patients with localized or regional ductal cancer had a greater than two-fold increased risk of PCSM compared to patients with acinar carcinoma after adjusting for relevant clinical and pathologic variables. These findings suggest that the detection of ductal carcinoma on pathology independently predicts a worse overall prognosis.
A potentially important finding is the apparent decreased PSA secretion of ductal carcinomas. Evidence for decreased secretion of PSA by ductal prostate cancers has been reported in smaller series. In a study of 46 patients with metastatic prostate cancer and a PSA ≤ 2 ng/ml, 9 (20%) were found to have ductal histology — a much higher percentage than would be expected based on the incidence of ductal cancer.15 Additionally, progression of disease in a patient with ductal cancer with no evidence of biochemical recurrence was also observed in another small institutional cohort.16 Although our study could not assess PSA recurrences after primary therapy, we were able to explore PSA levels at presentation. After adjusting for relevant clinical and pathologic factors including stage and grade, the mean PSA levels were 30% lower in patients with ductal cancer. While others have found similar PSA levels between ductal and acinar cases,17 no prior study has assessed PSA expression within a multivariate analysis. We also assessed the likelihood of patients with ductal cancers having a PSA <4.0 ng/ml, a commonly used cut-point in determining whether or not a prostate needle biopsy should be performed. In the multivariable analysis, patients with ductal carcinoma were 2.4 times more likely to have a PSA below 4.0 ng/ml, suggesting these cancers are less likely to be detected by PSA screening than acinar adenocarcinomas. This difference in serum PSA could be related to some ductal cancers being identified during endoscopic resection of a urethral polyp, but it may also be due to the pattern of tumor growth within the prostatic ducts leading to a relative increase in luminal PSA secretion and decrease in serum PSA secretion. Additionally, while PSA expression itself may be reduced in ductal tumors, further studies are necessary to assess this hypothesis. The decreased likelihood of identifying these tumors by PSA screening may play a role in the increased aggressiveness of ductal cancers due to a delay in diagnosis.
The unique attributes of ductal prostate cancer have been demonstrated in multiple studies. Unlike acinar adenocarcinoma, ductal cancers may present as a urethral polyp and are sometimes diagnosed on transurethral biopsy.4, 18, 19 However, ductal cancers diagnosed on urethral biopsy are only rarely confined to the urethra.18 Additionally, cancers with ductal histology are generally found to have an acinar component as well.3, 5 In a recently published study, Tu etal.20 showed that, in their cohort of patients, there was a correlation between the composition of the ductal carcinoma (pure versus mixed) and the overall mortality and risk of metastasis. The mixed ductal prostate cancers appeared to be associated with an increased risk of metastasis and increased overall mortality compared with pure ductal carcinomas. They reported a median 8.9 year overall survival in their cohort of 50 patients who underwent surgery for mixed ductal carcinoma versus 13.8 years in 25 patients with pure ductal histology. The presence of any amount of ductal histology has also been found to be a predictor of extraprostatic extension at radical prostatectomy.17 Unlike acinar carcinoma, ductal cancers often spread to visceral organs such as the lungs and liver, and a number of patients with testicular or penile metastases have been reported.8, 16, 21, 22
There are a number of limitations to this study. First, although overall case ascertainment is near 100% in SEER, misclassified or missing data points introduce unmeasured bias. For example, SEER does not differentiate between mixed and pure ductal carcinoma, and therefore we were not able to separate these two entities. Central review of pathology was not possible and variation in pathologic interpretation may have introduced misclassification bias. It is also possible that ductal cancers were underreported in the SEER database, with some of the mixed ductal carcinomas being miscategorized as acinar adenocarcinomas. Second, comorbidity and secondary treatment data are not available. Third, tumor grade is reported as well, moderately, and poorly differentiated, limiting evaluation of histologic grade across the entire study cohort. However, analysis of patients with complete Gleason grade information — those diagnosed from 2004–2006 — showed that both grade and stage were higher in ductal relative to acinar tumors. Likely due to an insufficient number of events, no difference in PCSM was observed in this subgroup. Finally, as a retrospective study, there may have been unrecognized differences between the ductal and acinar groups for which we were unable to control.
CONCLUSION
In this large study of ductal carcinoma, we characterize a rare but important subtype of prostate cancer. The findings in this study lend support to the more aggressive natural history of ductal adenocarcinomas when compared to acinar adenocarcinomas. Ductal adenocarcinomas are more likely to be high grade and present with distant disease, and they carry a significantly increased mortality risk in those with locoregional disease independent of pathologic variables and treatment. We also found that the incidence of ductal carcinoma has statistically increased over the past 10 years. Whether this represents an increase in the recognition of this distinct tumor or an actual increase in this tumor histology cannot be determined in this analysis. In addition, serum PSA levels were found to be significantly lower in patients with ductal compared to acinar cancer, and this could adversely impact the detection of ductal carcinomas. Further prospective research will be needed to confirm the findings in this study and potentially help identify the factors responsible for the observed differences between ductal and acinar prostate cancers.
Footnotes
Disclosures: NIH Grants: T32 CA009168-30
References
- 1.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus) Cancer. 1967;20:1715. doi: 10.1002/1097-0142(196710)20:10<1715::aid-cncr2820201022>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Bock BJ, Bostwick DG. Does prostatic ductal adenocarcinoma exist? Am J Surg Pathol. 1999;23:781. doi: 10.1097/00000478-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer. 1986;57:111. doi: 10.1002/1097-0142(19860101)57:1<111::aid-cncr2820570123>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features. Clinical, pathologic, and ultrastructural findings. Am J Surg Pathol. 1985;9:595. doi: 10.1097/00000478-198508000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Greene LF, Farrow GM, Ravits JM, et al. Prostatic adenocarcinoma of ductal origin. J Urol. 1979;121:303. doi: 10.1016/s0022-5347(17)56763-4. [DOI] [PubMed] [Google Scholar]
- 7.Dube VE, Farrow GM, Greene LF. Prostatic adenocarcinoma of ductal origin. Cancer. 1973;32:402. doi: 10.1002/1097-0142(197308)32:2<402::aid-cncr2820320218>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Tu SM, Reyes A, Maa A, et al. Prostate carcinoma with testicular or penile metastases. Clinical, pathologic, and immunohistochemical features. Cancer. 2002;94:2610. doi: 10.1002/cncr.10546. [DOI] [PubMed] [Google Scholar]
- 9.Wernert N, Luchtrath H, Seeliger H, et al. Papillary carcinoma of the prostate, location, morphology, and immunohistochemistry: the histogenesis and entity of so-called endometrioid carcinoma. Prostate. 1987;10:123. doi: 10.1002/pros.2990100204. [DOI] [PubMed] [Google Scholar]
- 10.Brinker DA, Potter SR, Epstein JI. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol. 1999;23:1471. doi: 10.1097/00000478-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Christensen WN, Steinberg G, Walsh PC, et al. Prostatic duct adenocarcinoma. Findings at radical prostatectomy. Cancer. 1991;67:2118. doi: 10.1002/1097-0142(19910415)67:8<2118::aid-cncr2820670818>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Rubin MA, de la Taille A, Bagiella E, et al. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol. 1998;22:840. doi: 10.1097/00000478-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox G, Soh S, Chakraborty S, et al. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Human Pathology. 1998;29:1119. doi: 10.1016/s0046-8177(98)90423-3. [DOI] [PubMed] [Google Scholar]
- 14.Millar EK, Sharma NK, Lessells AM. Ductal (endometrioid) adenocarcinoma of the prostate: a clinicopathological study of 16 cases. Histopathology. 1996;29:11. doi: 10.1046/j.1365-2559.1996.d01-483.x. [DOI] [PubMed] [Google Scholar]
- 15.Leibovici D, Spiess PE, Agarwal PK, et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer. 2007;109:198. doi: 10.1002/cncr.22372. [DOI] [PubMed] [Google Scholar]
- 16.Orihuela E, Green JM. Ductal prostate cancer: contemporary management and outcomes. Urol Oncol. 2008;26:368. doi: 10.1016/j.urolonc.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Samaratunga H, Duffy D, Yaxley J, et al. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol. 2010;41:281. doi: 10.1016/j.humpath.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Aydin H, Zhang J, Samaratunga H, et al. Ductal adenocarcinoma of the prostate diagnosed on transurethral biopsy or resection is not always indicative of aggressive disease: implications for clinical management. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08812.x. [DOI] [PubMed] [Google Scholar]
- 19.Samaratunga H, Letizia B. Prostatic ductal adenocarcinoma presenting as a urethral polyp: a clinicopathological study of eight cases of a lesion with the potential to be misdiagnosed as a benign prostatic urethral polyp. Pathology. 2007;39:476. doi: 10.1080/00313020701570004. [DOI] [PubMed] [Google Scholar]
- 20.Tu SM, Lopez A, Leibovici D, et al. Ductal adenocarcinoma of the prostate: clinical features and implications after local therapy. Cancer. 2009;115:2872. doi: 10.1002/cncr.24326. [DOI] [PubMed] [Google Scholar]
- 21.Copeland JN, Amin MB, Humphrey PA, et al. The morphologic spectrum of metastatic prostatic adenocarcinoma to the lung: special emphasis on histologic features overlapping with other pulmonary neoplasms. Am J Clin Pathol. 2002;117:552. doi: 10.1309/PCND-T3N0-5K01-D454. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y, Caraway N, Stewart J, et al. Metastatic ductal adenocarcinoma of the prostate: cytologic features and clinical findings. Am J Clin Pathol. 2006;126:302. doi: 10.1309/4TT6-LVJP-QVFW-DB6P. [DOI] [PubMed] [Google Scholar]