Abstract
Morbidity and mortality in cystic fibrosis (CF) are due not only to abnormal epithelial cell function, but also to an abnormal immune response. We have shown previously that macrophages lacking CFTR, the gene mutated in CF, contribute significantly to the hyper-inflammatory response observed in CF. Here we show for the first time that lack of functional CFTR in murine macrophages causes abnormal Toll like receptor (TLR) 4 subcellular localization. Upon LPS stimulation, CFTR macrophages have prolonged TLR4 retention in the early endosome and reduced translocation into the lysosomal compartment. This abnormal TLR4 trafficking leads to increased LPS-induced activation of the NF-kB, MAPK and IRF-3 pathways, and to decreased TLR4 degradation, which affects downregulation of the proinflammatory state. In addition to primary murine cells, mononuclear cells isolated from CF patients demonstrate similar defects in response to LPS. Moreover, specific inhibition of CFTR function induces abnormal TLR4 trafficking and enhances the inflammatory response of wildtype murine cells to LPS. Thus, functional CFTR in macrophages influences the physiological TLR4 spatial and temporal localization and perturbs LPS-mediated signaling in both murine CF models and patients with CF.
INTRODUCTION
Airway obstruction, chronic bacterial infection and excessive inflammatory responses are major causes of morbidity and mortality in patients with cystic fibrosis (CF). CF is caused by homozygous mutation of the CFTR gene, which encodes a chloride channel that is expressed in airway epithelial cells and, at lower levels, in other cell types (1). Although the development of CF lung disease is not fully understood, it is clear that abnormal chloride transport on the apical membrane of airway epithelial cells leads to changes in the airway environment, such as water content, pH and ion concentrations resulting in airway obstruction by thick mucus and depletion of antimicrobial molecules (2). Together, these conditions may favor bacteria adaptation and chronic infection in the lungs, as observed with Pseudomonas aeruginosa (PA). Less clear is the etiology of the robust inflammatory response that characterizes CF lung disease. The excessive inflammatory response was thought to be a consequence of chronic infection, but evidence suggests that the etiology of this exaggerated response may be more complex. Macrophages and mast cells are present at higher levels in CF airways even during fetal development (3), and airway inflammation is already present in CF infants prior to establishment of chronic infection (4–6). In addition, young children with CF have an increased number of alveolar macrophages and CC chemokines even in the absence of pulmonary infection (7). These observations support the hypothesis that intrinsic abnormalities in the innate immune system may contribute to the disease process, and that CF lung pathology is due to intricate cross talk between dysfunctional epithelial and immune cells.
Recent data show that CFTR has a direct role in the normal function of immune cells including macrophages (8–12), neutrophils (13, 14), dendritic cells (15) and lymphocytes (16–18). By creating bone marrow (BM) chimeras, in which WT and CF mice were irradiated and transplanted with either WT BM or CF BM, we demonstrated that the increased levels of pro-inflammatory cytokines depends on lack of functional CFTR in immune rather than epithelial cells (11). We also found that, compared to WT, CFTR−/− BM-derived macrophages and alveolar macrophages have elevated LPS-induced transcription and secretion of many pro-inflammatory cytokines (11). The lack of functional CFTR in macrophages has been associated with abnormal acidification of cell organelles (9), abnormal lipid metabolism (19), and alteration of transcription factors (10) that can contribute to the hyper-inflammatory phenotype. Here we demonstrate that functional CFTR directly or indirectly affects the spatio-temporal compartmentalization of TLR4, which is necessary for well controlled TLR4 signaling and degradation. In addition, we show that macrophages from CF patients, as in mice, are hyper-responsive to acute LPS exposure. Thus, functional CFTR is necessary for controlling the innate immune response in macrophages and intrinsic defects of such early players in the innate immunity may directly influence the cascade of events leading to CF lung disease.
MATERIALS AND METHODS
Mouse Breeding
Transgenic CFTR−/− (B6.129P2-Cftr tm1Unc) mice from Jackson Laboratory were bred in the Yale University Animal Facility, and are completely backcrossed to C57Bl/6 mice. WT mice used in the experiments were littermates controls derived from breading of CFTR−/+ pairs. Mice were fed with a liquid diet (Peptamen, Nestle, Deerfield, Illinois) as previously described in (11). All procedures were performed in compliance with relevant laws and institutional guidelines, and approved by the Yale University Institutional Animal Care and Use Committee.
Isolation and culture of murine BM-derived macrophages (BMDM) and human peripheral blood progenitor cell-derived macrophages
Murine macrophages
After overnight culture of fresh BM cells (11), non-adherent cells were differentiated for 7 days in 20ng/ml recombinant M-CSF (PeproTech Inc., Rocky Hill, NJ). Approximately 1–3 ×107 macrophages were obtained per mouse. Cells were plated at a concentration of 1×106 cells/well in 6 well plates overnight and challenged with Pseudomonas aeruginosa (PA) lipopolysaccharide (LPS) (25ng/ml, Sigma) or Pam3CSK4 (50 ng/ml, Imagenex) for the times indicated. Supernatant, RNA or protein lysates were harvested for analysis at different time points. For time 0h, untreated cells were used.
Human macrophages
Blood was obtained from healthy donors (HD) or from patients (age range 3–18 yrs, all pancreatic insufficient) with CF carrying at least one deltaF508 allele during their annual check-up with informed consent in accordance with the Yale University Medical School Human Investigation Committee. Human mononuclear cells were isolated by Histopaque-1077 (Sigma) from 5–10 ml of blood, and seeded at 5×106 cells/well in 24 well plates in RPMI supplemented with 10% FBS and 40ng/ml recombinant human M-CSF (PeproTech). Cells were split 1:1 every 3–4 days. After 2–3 weeks, cells were characterized by flow cytometry (CD14+/CD45+) and morphology analyzed on cytospin (Fig. S4). We obtained approximately 1×106 macrophages from 5 ml of blood. Before LPS treatment, cells were washed extensively with PBS, detached with Accutase (Innovative Cell Technology), and seeded at 0.25×106 cells per well of 24 well plates. The day after, cells were challenged with LPS (50ng/ml) at the times indicated in the text, and the supernatant and cells were harvested for analysis. For time 0h, fresh media without LPS was harvested after 12h of incubation.
Flow cytometry
For plasma membrane flow cytometry, WT and CF macrophages were detached with Accutase and washed in wash buffer (PBS/ 2% serum). After 10 min of incubation with Fc-block, cells were stained on ice for 30 min with either rat monoclonal APC-F4-80 (eBioscience) and rat monoclonal FITC-TLR4 (Imgenex) or APC-F4-80 and FITC-TLR2 (Imgenex). Dead cells were excluded with either PI or dead/live staining (Invitrogen, L10119). Appropriate isotype controls were used. For experiments with CFTRinh172 (kind gift from Dr. Alan Verkman), cells were pre-treated with 20µM of inhibitor or vehicle alone (DMSO) overnight before the experiment.
Human cells were stained with the mouse monoclonal FITC-TLR4 (Imgenex), and run on a FACS Calibur (Becton Dickinson) instrument followed by analysis using FlowJo software.
Immunofluorescence and pulse chase experiments for TLR4 co-localization with endosomal vesicles by immunofluorescence (IF) analysis and Imagestream
IF
WT and CF macrophages were grown on poly-L-lysine-coated 12mm round cover slips at 80% confluence. Untreated and LPS (25ng/ml) treated cells were fixed for 15min in 4% PFA, permeabilized for 5 min in PBS/0.1% Triton and stained with mouse monoclonal anti-TLR4 Ab (Imgenex; 1:200) and rat monoclonal anti-LAMP1 Ab (Southern Biotech, 1:100) for 30 min at RT, following by staining with secondary Alexa 488 anti-mouse and Alexa 555 anti rat (Invitrogen: 1:400) for 30 min at RT. Pictures were taken with a confocal microscope (Leica TCS SP5 Spectral Confocal Microscope).
Pulse chase experiments
WT and CF macrophages were grown in 6 well plates for Imagestream or on poly-L-lysine-coated 12mm round cover slips at 80% confluence. Keeping the cells at 4–6°C, cells were washed in PBS, incubated with Fc block for 5 min, and labeled with rat anti-TLR4 antibody (Imgenex) while also binding to LPS. After washing in cold PBS, 37°C media was added and cells were incubated at 37°C still in the presence of LPS to allow internalization of TLR4 bound to antibodies for the time indicated in the text. For confocal IF analysis, after incubation at 37°C, cells were fixed, permeabilized and stained with anti-EEA-1 Ab (Affinity Bio Reagents, PA1063A, dilution 1:250), which recognizes endosomal compartments. TLR4 was detected with a biotin-anti rat antibody (Invitrogen, 1:200) followed by tyramide signal amplification according to the manufacturer's instructions (Invitrogen). Pictures were taken with a confocal microscope (Leica, TCS SP5). For ImageStream, after incubation at 37°C, cells were detached using PBS/2mM EDTA on ice for 15 min, fixed, permeabilized and stained with anti-EEA-1 Ab and FITC anti-rat Ab. We gated on single cells, and focused on cells that were positive for both FITC (TLR4) and PE (EEA1). Within this population, we assessed the location of the TLR4 Ab and its colocalization with the EEA1 compartment. Using the Imagestream software, we also assessed the median fluorescence intensity for FITC (TLR4) in the EEA-1 compartment (5000 cells/experiment) during LPS stimulation, and determined the number and the size of EEA-1 positive vesicles over time.
Protein Analysis
For TLR4 Western blots, 1×106 cells were lysed and an equal amount of protein was separated by electrophoresis on 12% Bis-Tris Gels, transferred to nitrocellulose membrane (Bio-Rad Laboratories, CA) and incubated with a mouse anti-TLR4 antibody (1:100, Imagenex) . Horseradish Peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5000, Santa Cruz) and Amersham ECL Plus Western Blotting System (GE Healthcare Bio-Sciences Corp., NJ) were used for detection. For the degradation experiments, cells were treated with 100µg/ml of Cycloheximide (Sigma) for 30 min before harvesting controls (no LPS) and LPS treated (1h, 3h) cell lysates.
Two different assays were performed to detect the phosphorylated proteins: western blot and a bead-based phospho protein assay (8-Plex Multi-Pathway Signaling Kit, Millipore) using a Luminex instrument. In all phosphoprotein experiments, cells were serum starved for 1h before LPS addition. Phosphorylated protein levels were normalized to total target and/or beta-actin. Quantification of the phosphorylated form was assessed using Image J software for western blot and calculating the median fluorescence intensity for the Multi-Pathway Signaling assay. All antibodies used for phosphoprotein western blot were purchased from Cell Signaling Technology, Inc.
Cytokine quantification
Cytokine concentrations in the media of cultured murine or human macrophages were assessed either by ELISA (R&D System, Inc) or by Milliplex following the manufacturer’s instructions (Millipore, MPXHCYTO-60K-16).
Expression analysis
Real-time PCR analysis was performed with a Bio-Rad iCycler using TaqMan technology. Copy numbers were normalized to 18S expression and the fold increase overtime was calculated by the ΔΔCt method. Primers were purchased from Applied Biosystems (data shown in supplemental files).
MQAE Indicator Studies
Macrophages are incubated at 37°C with N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide (MQAE-30mM) (Invitrogen) for 30 min. Subsequently, cells are examined using an Olympus IX-71 inverted microscope with a digital imaging system collecting data every 5 seconds in arbitrary fluorescent intensity units. MQAE was excited at 340 +/− 10 nm and emitted fluorescent light was measured at 460 +/− 10 nm. Data is analyzed using Metafluor 7.0R program in conjunction with Microsoft Excel. Cl− is secreted from the cell as [Cl−]IC exceeds [Cl-]EC resulting in an increase in the intensity of fluorescence of MQAE. Initially macrophages are perfused with chloride-containing buffer (Krebs Bicarbonate Ringers Solution- 125mM NaCl, 2.4 mM K2HPO4, KH2PO4, 1.2 mM MgCl2, 1.2 mM CaCl2, 25mM NaHCO3, pH 7.4, 295–305 mOsm) at a perfusion rate of 3–4 ml/min. Following plateau of background fluorescence, the perfusion buffer is changed to a chloride-free solution: 150mM NaCyclamate, 2mM MgSO4, 0.5 mM CaCyclamate, 1mM EGTA, 5mM HEPES pH 7.4, 298 mOsm). The CFTR contribution to the observed Cl− efflux was assessed by treating cells with forskolin (20µM) to activate CFTR with the subsequent addition of CFTRinh172 (50µM) to inhibit CFTR-dependent Cl− efflux. The subsequent decrease in intensity of fluorescence in the presence of CFTR inhibitor is representative of Cl− secretion likely due to CFTR. Lastly, CFTR-inhibitor is removed from the perfusate and the increase in efflux is assessed to determine if there is restoration of CFTR activity (data shown in supplemental files).
Statistical analysis
Statistical analysis of the results was analyzed using a one-sided two-sample t-tests. Data are expressed as means ± SE. A P value <0.05 was considered significant.
RESULTS
Abnormal TLR4 localization in untreated CFTR−/− macrophages
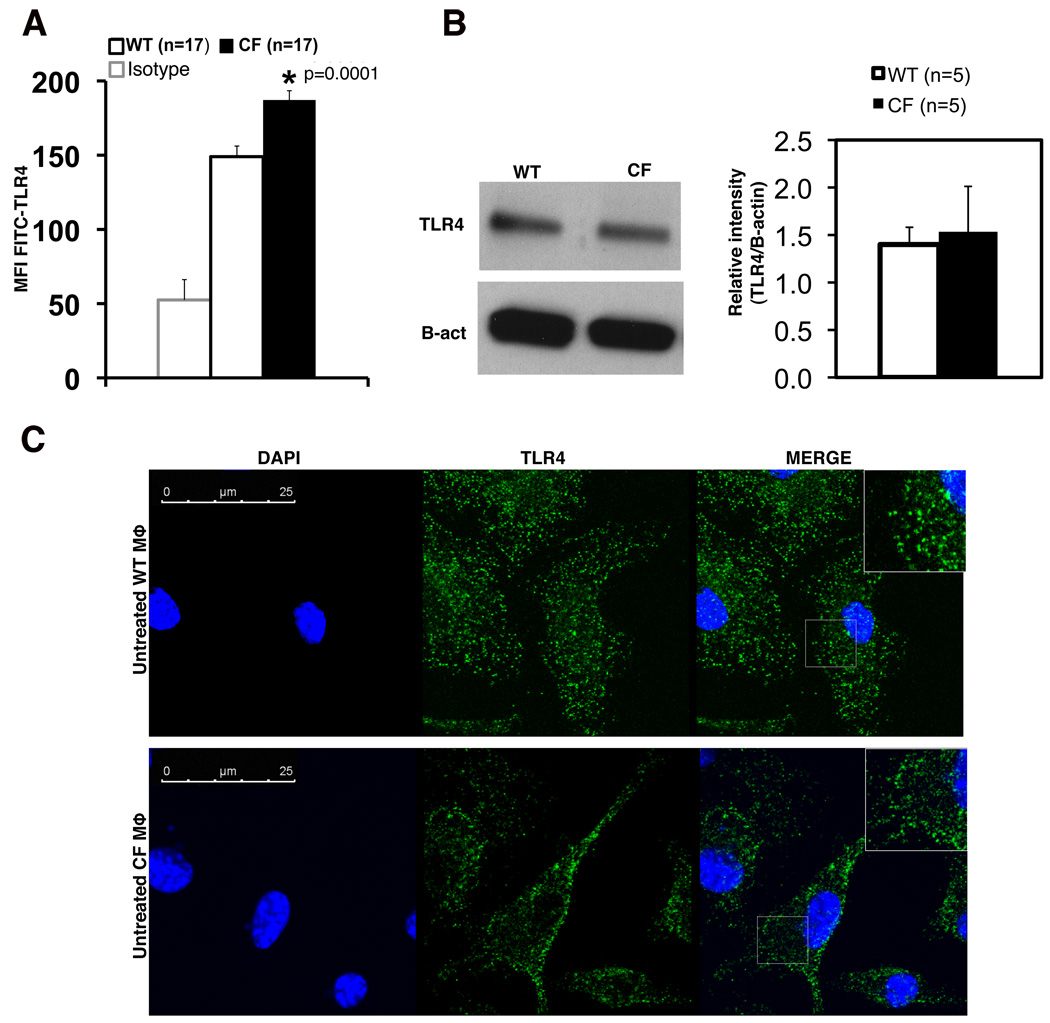
We have previously demonstrated that CFTR−/− bone marrow-derived (BMD) macrophages have increased transcription and secretion of pro-inflammatory cytokines in response to LPS (11). To investigate the nature of this hyper-inflammatory response, we examined extracellular TLR4 expression, the pattern recognition receptor for LPS, by flow cytometry, total TLR4 protein expression by western blot and TLR4 cell localization by immunofluorescence (IF) in WT and CF untreated macrophages.
CF macrophages displayed significantly higher levels of plasma membrane-associated TLR4 (Fig. 1A). Because forward and side scatter analysis did not reveal differences in macrophage size between genotypes (data not shown), the mean fluorescence intensity (MFI) for TLR4 assessed on 10,000 live F4/80+ cells can be used to compare the relative TLR4 per cell. Although these differences may appear to be modest, this degree of increase in TLR4 expression is known to cause a profound increase in the LPS response by immune cells (20, 21). There was no difference in TLR4 mRNA expression assessed by qPCR between WT and CF macrophages, (Fig. S1A) nor was there a difference in total TLR4 protein (Fig. 1B), suggesting that TLR4 may have abnormal post-translational regulation in CF macrophages. IF revealed that while in WT macrophages TLR4 is widely distributed in vesicular compartments throughout the cells, CF macrophages have a remarkably higher distribution of TLR4 in the peri-plasma membrane area (Fig. 1C). In contrast, no differences were observed in expression of plasma membrane TLR2 between WT and CF BM-derived macrophages in the absence (Fig. S1C) or presence of its specific ligand Pam3CSK (data not shown).
Fig. 1. CF macrophages have abnormal TLR4 localization compared to WT mouse macrophages.
(A) Flow cytometry of plasma membrane TLR4 on untreated WT (white bars) and CF (black bars) macrophages. The isotype control is also shown. Data are representative of 17 independent experiments. (B) Western blot of total TLR4 protein from untreated WT or CF macrophages; gel is representative of 5 independent experiments; mean ± SEM relative quantification normalized to beta-actin is shown on the right. Statistical analysis was performed by two-sample t-tests. (C) Representative IF for TLR4 on untreated WT (upper panels) and CF (lower panels) macrophages; nuclei were stained with DAPI. In the merged microphotographs, the inserts represent a higher magnification of specific cell regions (gray boxes). Confocal images were taken using a 63× oil immersion lens.
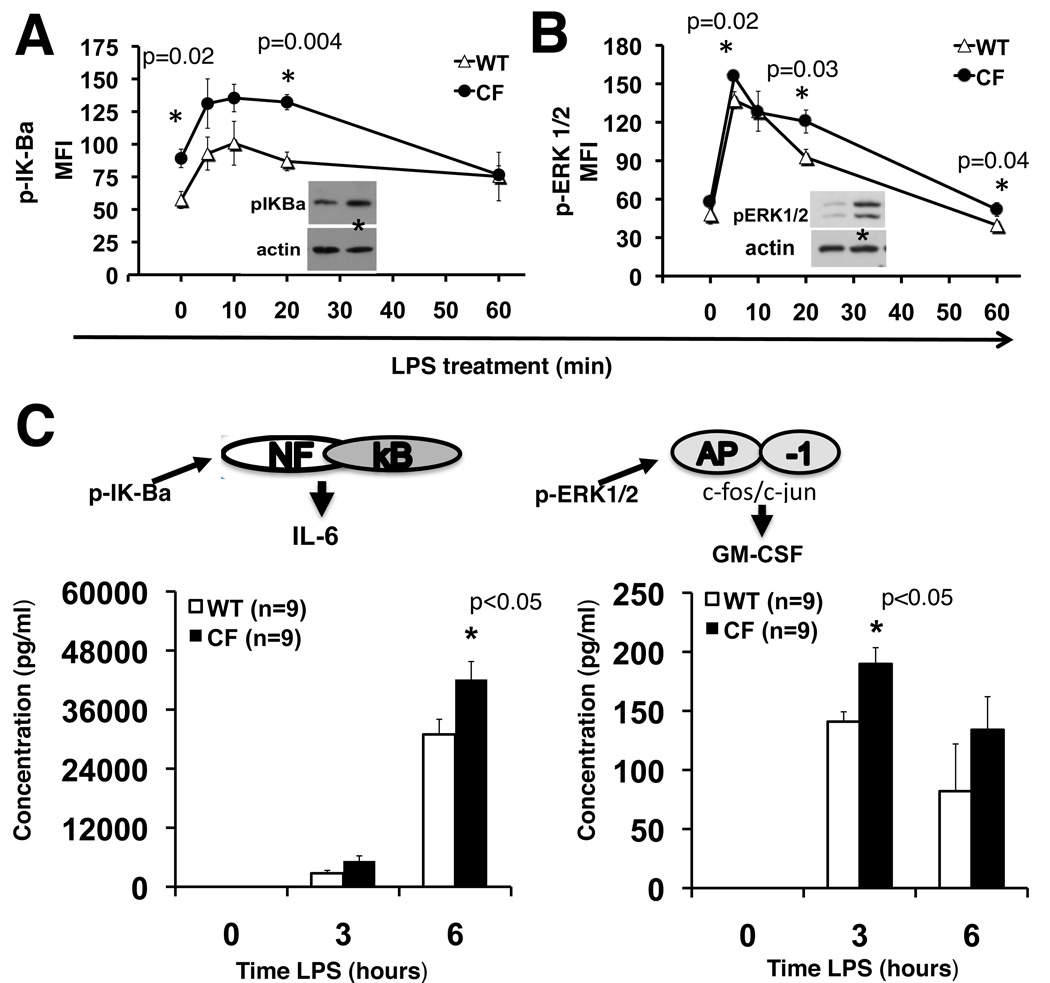
CF macrophages have more rapid and prolonged plasma membrane TLR4 signal transduction
As a consequence of abnormal TLR4 distribution, we expected to see differences in TLR4 signal transduction via the TIRAP-MyD88 pathway in CF cells (22, 23). BMD-macrophages were exposed to LPS, and phospho-proteins were analyzed by western blot (2–4 mice per genotype) and/or Luminex (4 mice per genotype) technology. Untreated WT and CF cells served as controls. Consistent with findings in CF airway epithelial cells (24) and CF alveolar macrophages (12), untreated CF macrophages had higher steady state levels of phosphorylated IkB alpha (pIk-Ba) than WT macrophages. After LPS activation, Ik-Ba phosphorylation was more robust and prolonged (20 min) in CF than WT macrophages (Fig. 2A). At the transcriptional level, LPS treatment led to a more than 10-fold increase in Ik-Ba mRNA expression in both CF and WT cells. However, from 6h to 16h of LPS stimulation, CF macrophages had lower Ik-Ba expression with a more rapid decline over time, suggesting slower NF-kb inactivation (Fig S1B). ERK1/2 phosphorylation, similar to Ik-Ba, phosphorylation, was robust and prolonged in CF compared to WT cells (Fig. 2B). After 20 minutes of LPS, pERK1/2 decreased in WT cells while it remained elevated in CF cells. These data were confirmed by western blot analysis for phosphorylated ERK1/2 (Fig. 2B, inset). Other MAPK’s analyzed, JNK and p38, did not show differences in the kinetics of LPS-induced activation in CF and WT macrophages (Fig S1C–F). To confirm that these phosphorylated proteins were functionally associated with an increase in signal transduction, we measured the expression and secretion of cytokines that are predominantly activated by the two specific pathways assessed. At the transcriptional level, CF macrophages had more LPS-induced IL-6 and CCL-2 mRNA than WT cells (data not shown). Consistent with the expression profile, LPS treated CF macrophages also secreted more pro-inflammatory cytokines, including IL-6 and GM-CSF than WT cells (Fig. 2C). In conclusion, these data suggest that untreated CF macrophages have higher TLR4 expression on the plasma membrane and in the peri-plasma membrane area compared to WT cells, and that this abnormal distribution may contribute to the pro-inflammatory state with more robust LPS-mediated TIRAP-MyD88 signal transduction. In contrast and consistent with the unchanged TLR2 plasma membrane expression in CF cell, there was no difference in Pam3CSK4-induced TIRAP/Myd88-dependent signal transduction between WT and CF cells, as assessed by IL-6 expression over time (Fig. S1D).
Fig. 2. CF macrophages have more robust and prolonged TLR4 signal transduction.
(A) pIK-Balpha and (B) p-ERK1/2 assessed in untreated (0) and LPS treated (5, 10, 20 and 60 minutes) WT (open triangles) and CF (close circles) macrophages by Luminex-based phosphoprotein assay (y-axis: mean fluorescence intensity); representative western blots are also showed (20min); (C) mean ± SEM of IL-6 and GM-CSF concentration (pg/ml) in the supernatant of LPS treated WT (white bars) and CF (black bars) macrophages untreated (0) or treated with LPS for 3 and 6 hours.
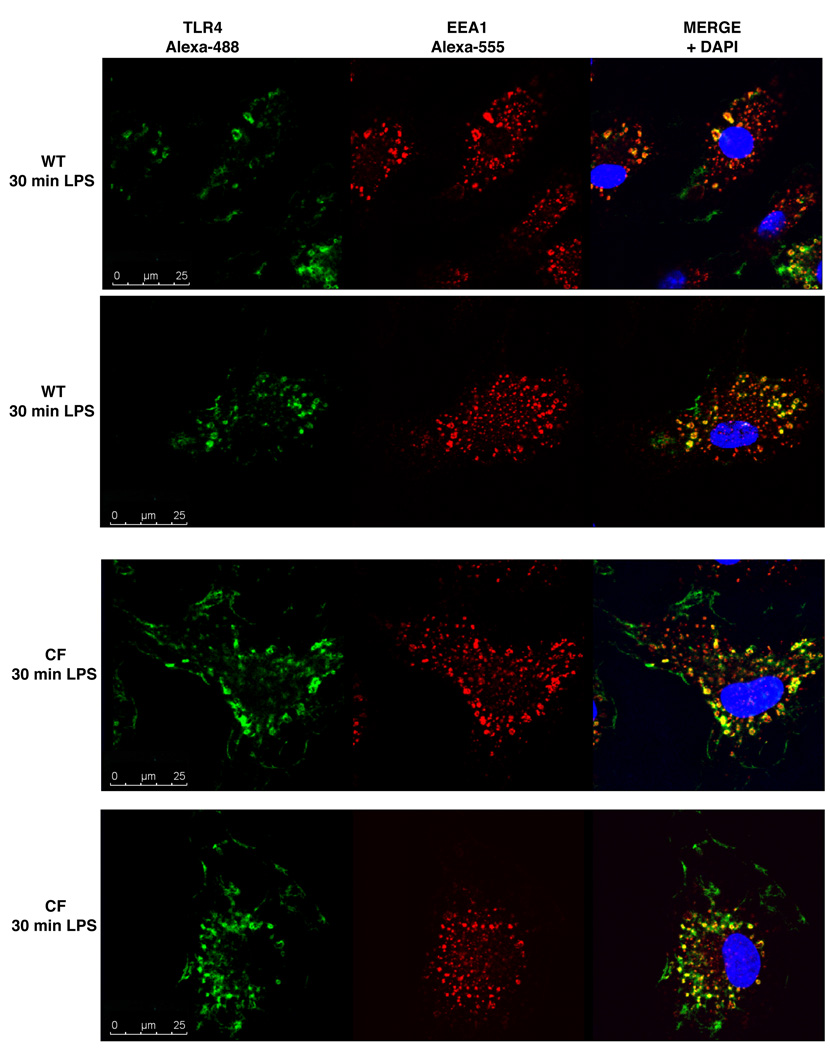
Increased and prolonged localization of active TLR4 in the endosomal compartment of CFTR−/− macrophages
Upon activation on the plasma membrane, TLR4 is internalized into endosomal compartments where it activates the TRAM/TRIF pathway (25). We hypothesized that the trafficking and endosomal signaling may be aberrant in CF cells as well. We performed pulse-chase studies using both confocal microscopy and Imagestream to follow the trafficking of LPS-activated plasma membrane-TLR4 into early endosomes (EEA1-positive). The experimental design is described in detail in material and methods. After labeling the cell membrane with fluorescent TLR4 antibody and inducing internalization in the presence of LPS, cells were stained with anti-EEA-1 antibody and anti-FITC. After 30 minutes of LPS stimulation, activated TLR4 was localized within EEA-1-positive endosomes predominantly in CF cells, and there was significantly less TLR4 colocalized with the EEA-1 positive compartment in WT macrophages (Fig. 3), suggesting that internalized TLR4 receptors are retained in CF endosomal compartments during LPS stimulation.
Fig. 3. Accumulation of activated TLR4 in the endosomal compartment of CF macrophages.
After labeling plasma membrane TLR4, cells were incubated at 37°C in the presence of LPS for 30 min. Internalized TLR4 was detected by immunofluorescence for TLR4 (green), EEA-1 (red) and DAPI (blue) in 2 representative WT (upper panels) and CF (bottom panels) cells.
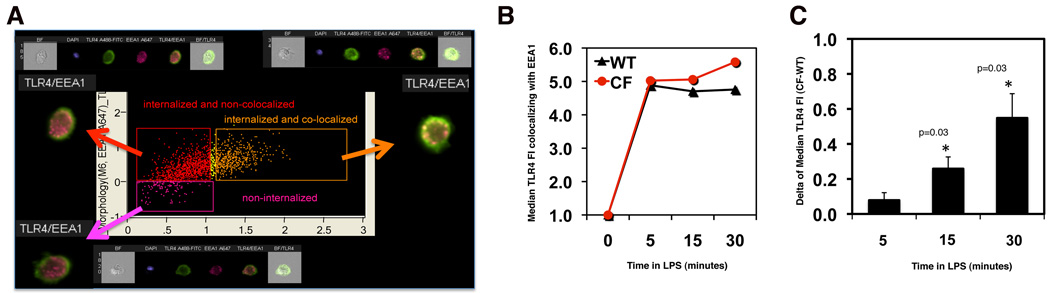
To accurately quantify the subcellular localization of TLR4, we used Imagestream technology in which flow cytometry technology is combined with imaging of single cells (26). Gating on single cells that were positive for both FITC (TLR4) and Alexa-568 (EEA1), we correlated the location of the TLR4 antibody (extra- vs. intra-cellular; y axis Fig. 4A) with the colocalization of TLR4 with the EEA1 compartment (x axis, Fig. 4A). There were three populations: 1) extracellular TLR4 shown in 4A in pink; 2) internalized TLR4 not colocalized with EEA1 in red; and 3) internalized TLR4 colocalized with EEA1 in orange. We assessed the median fluorescence intensity for FITC (TLR4) in the EEA-1 compartment (5000 cells/experiment) during LPS stimulation. The time course from a representative experiment is shown in Fig 4B. The mean difference (Δ=CF-WT) in median TLR4 FI between WT and CF during LPS stimulation of three independent experiments (Fig. 4C) revealed that, while accumulation of TLR4 in the endosomes was the same for WT and CF cells at 5 minutes, CF macrophages had statistically significantly greater accumulation of internalized TLR4 in the endosomal compartment than WT macrophages after 15 (p=0.03) and 30 minutes (p=0.03) of LPS treatment. Consistent with the finding that CF macrophages have retention of internalized TLR4 in the endosomal compartment, TRAM-TRIF signal transduction from the CF endosomal compartment, assessed by western blot for phosphorylated IRF-3 (25), was more robust in CF than WT cells (p=0.004, Fig. S1G). Secretion of RANTES, which is predominantly activated by this specific pathway, was also higher in LPS treated CF macrophages than WT cells (p=0.005, Fig. S1H). Together, these data suggest that in CF cells, TLR4 in endosomal compartments engages the TRIF adaptor and is actively signaling for a prolonged period compared to WT cells.
Fig. 4. TLR4 quantification in the endosomal compartment of CF macrophages.
(A) Representative Imagestream analysis, as described in the text; (B) graph from a representative experiment showing the median TLR4 fluorecence intensity (MFI) in EEA-1 positive vesicles normalized to time 0 for WT (black) and CF (red) macrophages; (C) mean ± SEM difference (Δ=CF-WT) in TLR4 MFI between WT and CF during LPS stimulation from three independent experiments. In each experiment, 5000 cells were analyzed. Statistical analysis was performed by two-sample t-tests.
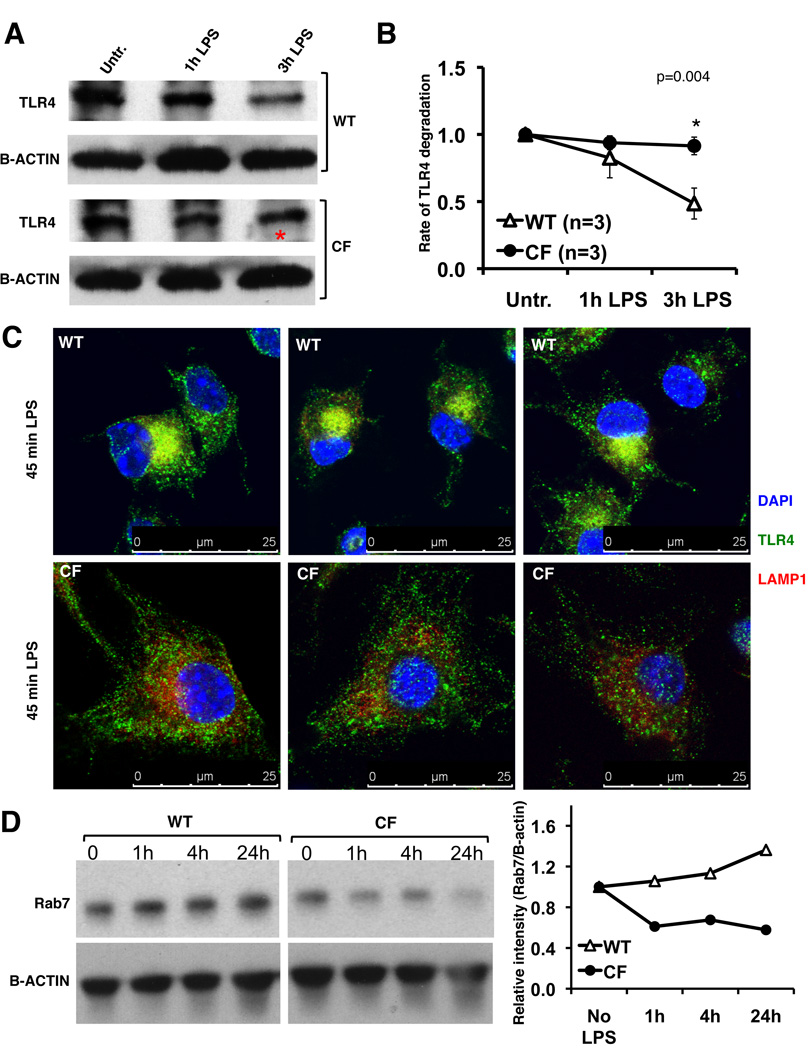
Decreased TLR4 degradation in CFTR−/− macrophages
Once in the endosomal compartment, TLR4 is targeted to the lysosomal compartment for degradation, which is fundamental for resolution of signaling (21, 27). One potential explanation for the prolonged retention of active TLR4 in endosomal compartments and the subsequent increase in signal transduction in CF cells is that TLR4 degradation is slower in CF cells. To test this hypothesis, WT and CF macrophages, treated with the protein synthesis inhibitor cycloheximide, were exposed to LPS (1h and 3h) and total TLR4 protein was assessed by western blot (Fig. 5A). Indeed, during LPS stimulation, at 3h post LPS treatment, TLR4 was decreased by more than 50% in WT cells. In contrast, in CF macrophages the rate of degradation was slower than in WT cells; after 3h of LPS exposure, only 20% of the protein was degraded (Fig. 5B).
Fig. 5. CF macrophages have reduced TLR4 protein degradation and translocation to the lysosomal compartment.
(A) Representative western blot of TLR4 from WT and CF macrophages treated with protein synthesis inhibitor and challenged with LPS. (B) Mean ± SEM relative quantification normalized to beta-actin with time zero is set at 1 for 3 independent experiments. Statistical analysis was performed by two-sample t-tests; (C) IF for TLR4 (green), LAMP-1 (red) in WT (top) and CF (bottom) macrophages treated with LPS for 45 minutes; nuclei were stained with DAPI; (D) representative western blot (left) and relative quantification normalized to b-actin (right) for Rab7 in WT and CF macrophages untreated or challenged with LPS as indicated.
To test whether decreased TLR4 degradation in CF macrophages was due to reduced translocation to the lysosomal compartment, WT and CF macrophages treated with LPS for 45 minutes were analyzed for colocalization of TLR4 with the lysosomal-associated membrane protein 1 (LAMP1) by immunofluorescence. While WT macrophages had a robust TLR4 colocalization in the lysosomal compartment, in CF cells there was minimal TLR4 associated with LAMP1 positive vesicles (Fig. 5C). LAMP-1 positive vesicles (red signal) were clustered in the nuclear area in WT cells while the signal was present diffusely throughout the cytoplasm in CF cells (Fig. 5C and additional samples and a representative z-stack in Fig. S2). Finally, the small GTPase Rab7, which is implicated in the transport from early to late endosomes (28) and a known key regulator for proper aggregation and fusion of late endocytic structures in the perinuclear region and consequently for the biogenesis and maintenance of functional lysosomal compartment (29), failed to increase during LPS stimulation in CF compared to WT macrophages (Fig. 5D). Together, these data suggest that lack of functional CFTR in macrophages leads to aberrant maturation of vesicles in the endosomal-lysosomal axis, which may explain why TLR4 is retained in the endosomal compartment in its active state.
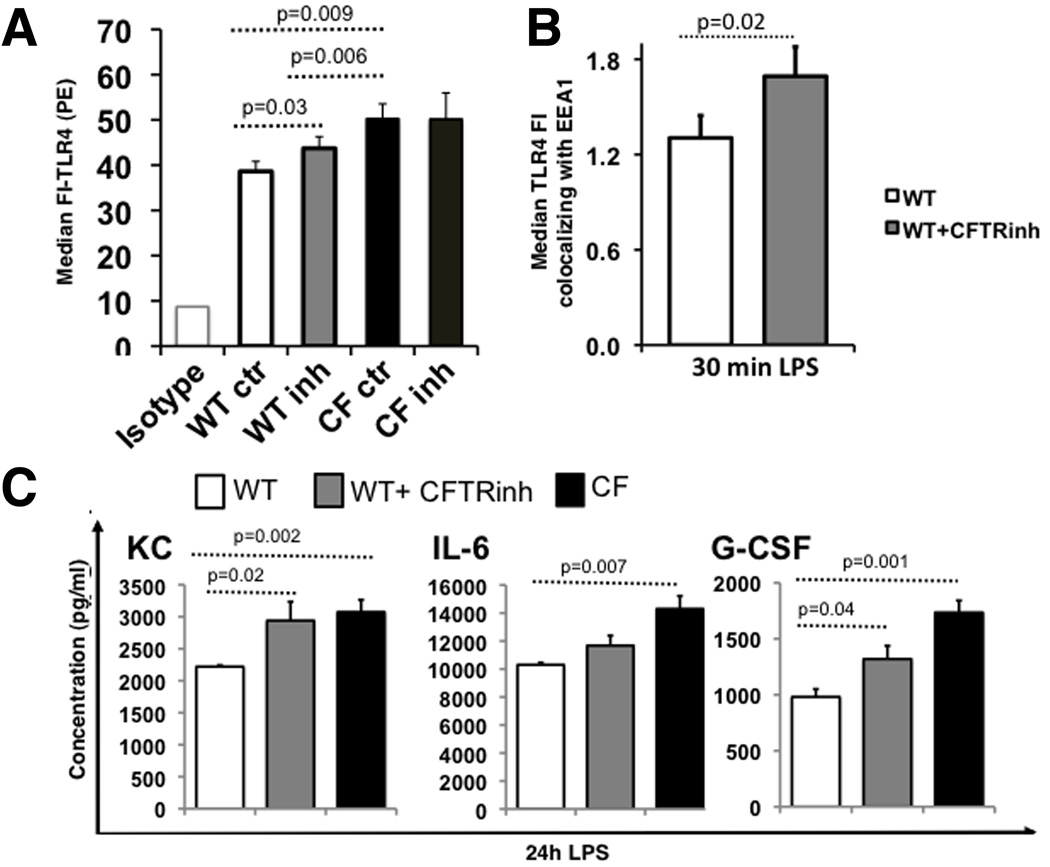
The abnormal trafficking and signaling in CFTR−/− macrophages is CFTR-dependent
In WT mice, though CFTR expression is ~100-fold lower in immune cells than lung (9, 15)(Fig. S3A), it is still functional. Function of CFTR in WT macrophages is shown by forskolin stimulated Cl− secretion that can be inhibited with the specific thiazolidinone CFTR inhibitor (CFTR inh172(30)) that is not detected in CF cells (Fig. S3B). To determine whether lack of CFTR function contributes directly to abnormal TLR4 signaling and trafficking, WT and CF macrophages were pretreated overnight with CFTRinh172, as described previously for airway epithelial cells (31). Plasma membrane TLR4 expression was significantly increased (p=0.03) on WT cells treated with inhibitor (WTinh) compared to WT macrophages, but remained less than CF macrophages. The expression of plasma membrane TLR4 in CF macrophages treated with the inhibitor (CFinh) was not different compared to CF cells treated with vehicle (DMS (Fig. 6A). Secretion of KC, IL-6 and G-CSF, which we previously demonstrated are hyper-secreted by CF macrophages (11), was assessed by ELISA (n=3 mice per genotype). For all cytokines analyzed, WTinh had increased secretion levels, which were greater than those of WT but less than those of CF macrophages. The secretion of KC and G-CSF was significantly increased in WTinh macrophages compared to WT cells while IL-6 showed an increasing trend (Fig. 6B). Lastly, using Imagestream technology, WTinh macrophages displayed an increased accumulation of TLR4 in the EEA-1 positive compartment after 30 minutes of LPS stimulation (p=0.02, Fig. 6C). These data confirm that the lack of functional CFTR affects TLR4 localization and trafficking, and has repercussions on the inflammatory response.
Fig. 6. The abnormal trafficking and signaling in CFTR−/− macrophages is CFTR-dependent.
(A) TLR4 plasma membrane expression as indicated for WT, WT treated with CFTRinh172, CF and CF treated with CFTRinh172 cells: average results for 3 independent experiments (isotype control at left); (B) KC, IL-6 and G-CSF concentration (pg/ml) in the supernatant of LPS treated WT (white bars), WT treated with CFTRinh172 (gray bars) and CF (black bars) macrophages; (C) Average ± SEM MFI of TLR4 localized to the endosomal compartment after 30 minutes of LPS stimulation as assessed by Imagestream for three independent experiments (WT is white, WT treated with CFTRinh172 is light gray). Statistical analysis was performed by two-sample t-tests.
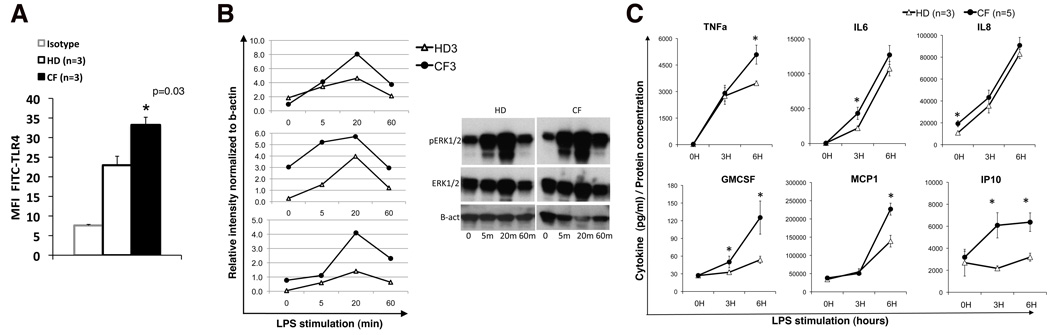
Human macrophages from CF patients are hyper-responsive to LPS
We next assessed whether primary CF human macrophages are also hyper-responsive to LPS. Human mononuclear cells from the peripheral blood of three healthy donors (HD) and five CF patients carrying at least one deltaF508 allele, were differentiated with M-CSF in vitro into adherent cells with macrophage-like morphology and immunophenotype (CD45;CD14 positive (Fig. S4A,B)). CFTR was detectable in cells from HD (Fig. S4C). Despite the fact that the human cells were derived from the peripheral blood and the murine cells that we have studies were matured in vitro from BM precursors, the human cells showed higher levels of TLR4 in CF compared to HD macrophages (Fig. 7A). As observed in murine cells, phosphorylation of ERK1/2 in response to LPS was more robust in CF cells compared to HD controls. Three independent experiments and a representative western blot are shown in Fig. 7B. Though there was donor variability in the kinetics of ERK1/2 activation, in all 3 experiments, CF patients had more robust ERK1/2 phosphorylation than HD after 20 and 60 min of LPS stimulation. In some of the experiments we observed higher pERK1/2 in untreated CF cells compared to WT cells. The detection of the other phosphoproteins was not possible due to the low number of human cells available for the assay. The supernatants of LPS treated and untreated human macrophages were analyzed for cytokine content by Luminex. Similar to our observations in murine cells (11), human CF macrophages had significantly (p<0.05) higher secretion of TNFa (6h), IL6 (3h), GMCSF (3h and 6h), MCP1 (6h), IP-10 (3h and 6h) (Fig. 7C). GCSF (6h, 24h) RANTES (6h), and IL1b (6h, 24h) were also elevated (data not shown). Consistent with the findings of Zaman et al. and Xu et al.(8, 12), the basal level of IL-8 in the supernatant of WT and CF macrophages was abundant and significantly higher for CF than HD cells. However, during activation, the secretion of IL-8 increased similarly in both CF and HD cells. No differences were observed in secretion of IL-1a and GRO (data not shown). These data suggest that, similar to murine cells, human CF macrophages are hyper-responsive to LPS, and further support the hypothesis that immune cells may contribute directly to the development of CF lung disease in humans.
Fig. 7. Human macrophages from CF patients are hyper-responsive to LPS.
(A) Mean ± standard deviation of TLR4 expression in macrophages from healthy donors and patients with CF; isotype control is also shown; (B) p-ERK1/2 assessed in untreated (0) and LPS treated (5, 20 and 60 minutes) HD and CF macrophages by western blot normalized to b-actin, data are from 3 separate experiments with different CF and HD samples; a representative blot is shown (right); (C) mean ± SEM cytokine concentration in the supernatant of HD (open triangle) and CF patient (close circles) macrophages untreated (0) and treated with LPS as indicated. Statistical analysis was performed by two-sample t-tests.
DISCUSSION
We previously reported that CF macrophages are hyper-responsive to LPS (11). Here we demonstrate that abnormal trafficking of TLR4 contributes to this exaggerated response. Tight control of active TLR4 signaling is critical for an adequate inflammatory response to LPS and is essential for preventing injury to the host (32). In resting cells, TLR4 is located both in the Golgi and at the plasma membrane, and TLR4–MD2-CD14 constantly cycles between the plasma membrane and the Golgi (27, 33). In response to LPS binding, the TLR4 complex composed of CD14, TLR4, and MD-2 assembles on lipid rafts and signals through the TIRAP-MyD88 adaptors(34, 35) activating the NF-kB and MAPKs pathways(22, 23). After LPS engagement, TLR4 is ubiquitinated and associates with the ubiquitin-binding endosomal sorting protein hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) which sorts TLR4 into EEA1 positive early endosomes (27, 36). In this location, TLR4-TRAM engages TRIF and activates the IRF-3 pathway leading to transcription of targets including RANTES, IP-10, and interferon genes (25). Translocation to endosomes is also associated with TLR4 de-dimerization, detachment from LPS (37), and engagement of the TAG protein, which is required for trafficking of TLR4 to Rab7 positive late endosomes (38). These events drive receptors to the degradation pathway (21, 27, 38) required for resolution of the inflammatory response. Minimal perturbation of any of these steps causes an abnormal inflammatory response (20, 21).
In this study, we have demonstrated that naive CF macrophages have abnormal TLR4 subcellular localization and increased TLR4 plasma membrane expression compared to WT cells. Since CF cells do not have more TLR4 mRNA or total TLR4 protein than WT cells, it is likely that trafficking regulation is responsible for the increased plasma membrane TLR4 protein levels. A possible explanation for the increased plasma membrane TLR4 of untreated CF macrophages could be abnormal vesicular sorting and trafficking between the Golgi and the plasma membrane. Although this hypothesis remains to be fully verified, this hypothesis may explain why untreated WT macrophages pretreated with CFTR inhibitor display increased plasma membrane TLR4, while this effect does not occur in CF cells pre-treated with the CFTR inhibitor.
These data differ from a recent study (39) in which TLR4 expression in CF epithelial cells was lower than WT airway epithelial cells. This is not surprising since epithelial cells and macrophages play unique roles during an inflammatory response. For instance, epithelial cells express TLR4 mainly on the basolateral membrane of the cell rather than on the apical membrane where TLR2 and TLR5 predominate (40). However, consistent with data from human CF airway epithelial cells (24, 31), we found that prior to LPS challenge, CF macrophages have increased phosphorylated IK-Ba suggesting that absence of functional CFTR activity leads to a pro-inflammatory state. The increase of plasma membrane TLR4 observed in untreated CF macrophages is likely responsible for the robust plasma membrane TIRAP-MyD88 mediated signal transduction that we observed after LPS stimulation. Increased phosphorylation of IK-Ba and ERK1/2 resulted in increased production of downstream inflammatory cytokines such as IL-6 and GM-CSF. This exuberant TLR4-dependent inflammatory response is CFTR dependent, based on data showing that WT cells pretreated with a CFTR inhibitor exhibit abnormalities analogous to those in CF cells. In contrast, there was no difference in plasma membrane TLR2 at rest or after stimulation with its ligand Pam3CSK4. TLR2 primarily signals from the plasma membrane and does not internalize in response to stimulation. These data provide support that CFTR affects TLR4 signaling/internalization in a specific manner, perhaps due to the fact that TLR4 regulation is highly dependent on its trafficking between the plasma membrane and intracellular compartments.
We also found that after activation and internalization, TLR4 was retained in early endosomes of CF macrophages. In the CF endosomes, TLR4 engages the TRIF adaptor, as shown by the robust phosphorylation of IRF-3 and RANTES production after LPS stimulation. TLR4 retention in the endosomes could also explain the prolonged phosphorylation of IK-Ba and ERK1/2 in CF macrophages, as a second wave of signaling. Translocation to endosomes is associated with TLR4 de-dimerization and detachment from LPS (37), which is required for the engagement of degradation adaptors (e.g. TAG) as well as TLR4 shuffling to Rab7-positive late endosomes for degradation (38). Importantly, Wang et al. (21), have shown that blocking the endosomal-lysosomal TLR4 trafficking in macrophages causes a decreased TLR4 degradation and an increased plasma membrane TLR4 expression, which leads to a more robust inflammatory response to LPS, similar to what we have observed in CF cells. In our studies Rab7 fails to increase during LPS stimulation in CF macrophages. Rab7 is a crucial GTPase protein implicated in the delivery of receptors from early to late endosomes (28). In addition, Rab7 is a key regulatory protein for proper aggregation and fusion of late endocytic structures in the perinuclear region and consequently for the biogenesis and maintenance of the lysosomal compartment (29). Therefore a decreased expression of Rab7 in CF cells may account for reduced trafficking of TLR4 to the degradation pathway. Consistent with this hypothesis, we found that CF macrophages after LPS stimulation (45 min) had a minimal TLR4 translocation in LAMP1 positive vesicles while WT macrophages had a robust trafficking of TLR4 to the lysosomal compartment. In addition, LAMP-1 positive vesicles were clustered in the nuclear area of WT cells while the signal was more diffusely distributed in the cytoplasm of CF cells (Fig. 5C and Fig. S2). Not surprisingly, we found that during LPS stimulation, the rate of TLR4 degradation was slower in CF cells compared to WT controls. These data suggest that, in the CF environment, there is reduced TLR4 translocation to lysosomes due to aberrant maturation of vesicles in the endosomal-lysosomal axis during LPS stimulation. Taken together, these data show that lack of functional CFTR affects TLR4 signaling and degradation and that it is related to the accumulation of active receptor in the endosomal compartments.
A potential alternative explanation for TLR4 retention in early endosomes in CF cells could be altered endosomal acidification as suggested by Saitoh et al. who found that impaired acidification was associated with retention of TLR4 in the endosomal compartment (37, 41). However, the role of CFTR in vesicle acidification is less than convincing and fairly controversial (42). Multiple studies from experienced investigators have resulted in conflicting conclusions with some reports providing evidence for such a role in airway epithelial cells (43) and macrophages (9, 44), and others finding no role for CFTR in organelle acidification (45–47). The data presented here examine that fate of TLR4 in a broader context than endosomal acidification and provide additional investigative lines of study which may lead to a better understand of the relationship between CFTR, lysosome formation, and TLR4 trafficking and degradation.
Finally, it has been reported that CF epithelial cells have abnormal trafficking and accumulation of unesterified cholesterol in endolysosomes (48). Importantly, perturbation of cholesterol trafficking is associated with accumulation of TLR4 in the endosomal compartment with subsequently increased signal transduction from this cell compartment (49). Therefore, cholesterol trafficking may be one of the causes of activated TLR4 retention in the endosomes of CF macrophages.
Lastly, in the current study, we demonstrate that human CF macrophages are intrinsically more responsive to LPS stimulation than HD cells, similar to our findings in murine CF macrophages. In 2004, Zaman and collaborators first reported that monocytes from CF patients (and from heterozygous subjects) were more sensitive to low dose LPS stimulation (8). They demonstrated that CF monocytes had elevated secretion of IL-8 and more robust activation of the MAPK pathway. More recently, Xu et al. obtained similar results by silencing CFTR expression in human alveolar macrophages (12). In order to determine if the findings we observe in the murine model are similar to what is observed in CF patients, we differentiated macrophages from peripheral blood cells, which allowed us to overcome the difficulties in previous studies with low monocyte numbers, and the heterogeneous immunophenotype of freshly isolated cells. We found that macrophages from CF patients respond to LPS in a manner similar to that observed in murine CF macrophages, including increased TLR4 and an hyper-responsiveness to LPS with more robust signal transduction and elevated secretion of several pro-inflammatory cytokines compared to healthy donor cells. During the preparation of this manuscript, Sturges et al. published a study in which the expression of TLR4 and TLR2 was assessed on peripheral blood monocytes of a cohort of 66 young children with CF and compared to both healthy controls and children without CF. TLR4 expression analyzed using flow cytometry was significantly higher in patients with CF compared to healthy controls (P = 0.017) and non-CF disease controls (P = 0.025), whereas no differences were seen in TLR2 expression (50). The similarities in the LPS response of murine and human macrophages lead us to hypothesize that the abnormal molecular mechanisms observed in CF murine macrophages can be extended to CF human cells.
Here we report that CF macrophages have intrinsically abnormal TLR4 signaling and trafficking. Thus, our findings further support the hypothesis that there is a primary role of immune cells in development of CF lung disease. Since inflammatory cells such macrophages are key to an appropriate innate immune response, abnormal activity of these cells due to the lack of CFTR may be one of the upstream causes of the chronic infection that characterizes CF lung disease. This study may help to identify new molecular targets for therapeutic intervention of the CF lung disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephanie Donaldson, Lesley Devine, Michael Caplan, John Geibel, Stephanie Halene, Stephanie Massaro, Kate Hahn, Sharon Lin, Simon Lai for their contributions to these studies. We also thank Richard De Marco and Tad George from Amnis for assistance with the Imagestream study and data analysis. Finally, we thank Alan Verkman and Nitin D. Sonawane for the kind gift of the CFTR inhibitor 172.
Source of support: This work was supported by the National Institutes of Health (R01 HL093004 to MEE and EMB; RO1 DK61846 and HL073742 to DSK; and P30 DK072442, the Yale Center of Excellence in Molecular Hematology) and Cystic Fibrosis Foundation (EGAN08G0 and C019-TDC to MEE).
Special abbreviations used
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CF
Cystic Fibrosis
- WT
wild-type
- HD
healthy donors
- PA
Pseudomonas aeruginosa
- IF
immunofluorescence
- BMDM
bone marrow derived macrophages
Footnotes
AUTHOR CONTRIBUTIONS
E.M.B. designed the study, developed assays, performed experiments, analyzed data and wrote the manuscript; P.Z. developed the culture of primary human macrophages, helped in developing assays and in data analysis and performed experiments; A. Satoh contributed to study design, data analysis and performed the confocal experiments, C.C. technical help; A. Shenoy assays the chloride efflux on primary macrophage; D.S.K and M.E.E and R.M. contributed to design of the study, interpretation of the data and writing the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol. 2001;108:524–529. doi: 10.1067/mai.2001.118516. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ. 1995;310:1571–1572. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 6.Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, Turner S, de Klerk N, Franklin P, Winfield KR, Balding E, Stick SM, Sly PD. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax. 2005;60:159–163. doi: 10.1136/thx.2004.026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009 doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 8.Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, Kelly C, O'Sullivan BP, Freedman SD. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol. 2004;11:819–824. doi: 10.1128/CDLI.11.5.819-824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 10.Andersson C, Zaman MM, Jones AB, Freedman SD. Alterations in immune response and PPAR/LXR regulation in cystic fibrosis macrophages. J Cyst Fibros. 2008;7:68–78. doi: 10.1016/j.jcf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Krause A, Hamai H, Harvey BG, Worgall TS, Worgall S. Proinflammatory phenotype and increased caveolin-1 in alveolar macrophages with silenced CFTR mRNA. PLoS One. 2010;5:e11004. doi: 10.1371/journal.pone.0011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris MR, Doull IJ, Dewitt S, Hallett MB. Reduced iC3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clin Exp Immunol. 2005;142:68–75. doi: 10.1111/j.1365-2249.2005.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Painter RG, Valentine VG, Lanson NA, Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Tertilt C, Krause A, Quadri LE, Crystal RG, Worgall S. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir Res. 2009;10:26. doi: 10.1186/1465-9921-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120:518–525. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubeau C, Le Naour R, Abely M, Hinnrasky J, Guenounou M, Gaillard D, Puchelle E. Dysregulation of IL-2 and IL-8 production in circulating T lymphocytes from young cystic fibrosis patients. Clin Exp Immunol. 2004;135:528–534. doi: 10.1111/j.1365-2249.2003.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of Cftr in CD3+ Lymphocytes Leads to Aberrant Cytokine Secretion and Hyper-inflammatory Adaptive Immune Responses. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Li X, Grassme H, Doring G, Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J Immunol. 2010;184:5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 20.Bihl F, Salez L, Beaubier M, Torres D, Lariviere L, Laroche L, Benedetto A, Martel D, Lapointe JM, Ryffel B, Malo D. Overexpression of Toll-like receptor 4 amplifies the host response to lipopolysaccharide and provides a survival advantage in transgenic mice. J Immunol. 2003;170:6141–6150. doi: 10.4049/jimmunol.170.12.6141. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Chen T, Han C, He D, Liu H, An H, Cai Z, Cao X. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood. 2007;110:962–971. doi: 10.1182/blood-2007-01-066027. [DOI] [PubMed] [Google Scholar]
- 22.Muzio M, Polntarutti N, Bosisio D, Prahladan MK, Mantovani A. Toll like receptor family (TLT) and signalling pathway. Eur Cytokine Netw. 2000;11:489–490. [PubMed] [Google Scholar]
- 23.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 24.Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS ONE. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653–670. doi: 10.1016/j.cll.2007.05.008. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. Embo J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonawane ND, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg Med Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol. 2007;292:L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 32.McGettrick AF, O'Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol. 2009 doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 35.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 36.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 37.Saitoh SI. Chaperones and transport proteins regulate TLR4 trafficking and activation. Immunobiology. 2009 doi: 10.1016/j.imbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Palsson-McDermott EM, Doyle SL, McGettrick AF, Hardy M, Husebye H, Banahan K, Gong M, Golenbock D, Espevik T, O'Neill LA. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat Immunol. 2009;10:579–586. doi: 10.1038/ni.1727. [DOI] [PubMed] [Google Scholar]
- 39.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Saitoh S, Tanimura N, Takahashi K, Kawasaki K, Nishijima M, Fujimoto Y, Fukase K, Akashi-Takamura S, Miyake K. Regulatory roles for MD-2 and TLR4 in ligand-induced receptor clustering. J Immunol. 2006;176:6211–6218. doi: 10.4049/jimmunol.176.10.6211. [DOI] [PubMed] [Google Scholar]
- 42.Haggie PM, Verkman AS. Defective organellar acidification as a cause of cystic fibrosis lung disease: reexamination of a recurring hypothesis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L859–L867. doi: 10.1152/ajplung.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 44.Deriy LV, Gomez EA, Zhang G, Beacham DW, Hopson JA, Gallan AJ, Shevchenko PD, Bindokas VP, Nelson DJ. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haggie PM, Verkman AS. CFTR-independent phagosomal acidification in macrophages. J Biol Chem. 2007 doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 46.Haggie PM, Verkman AS. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J Biol Chem. 2009;284:7681–7686. doi: 10.1074/jbc.M809161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barriere H, Bagdany M, Bossard F, Okiyoneda T, Wojewodka G, Gruenert D, Radzioch D, Lukacs GL. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–3141. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gentzsch M, Choudhury A, Chang XB, Pagano RE, Riordan JR. Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J Cell Sci. 2007;120:447–455. doi: 10.1242/jcs.03350. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT, Ohno K, Ninomiya H. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci. 2007;27:1879–1891. doi: 10.1523/JNEUROSCI.5282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturges NC, Wikstrom ME, Winfield KR, Gard SE, Brennan S, Sly PD, Upham JW. Monocytes from children with clinically stable cystic fibrosis show enhanced expression of Toll-like receptor 4. Pediatr Pulmonol. 2010;45:883–889. doi: 10.1002/ppul.21230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.