Abstract
Objectives
The male reproductive axis is responsive to energetic deficits, including multi-day fasts, but little is known about brief periods of fasting (<24 hours). Reduced testosterone in low-energy balance situations is hypothesized to reflect redirection of resources from reproduction to survival. This study tests the hypothesis that testosterone levels decrease during a minor caloric deficiency by assessing the effects of a single missed (evening) meal on morning testosterone in 23 healthy male participants, age 19–36.
Methods
Participants provided daily saliva and urine samples for two baseline days and the morning following an evening fast (water only after 4PM). Testosterone, cortisol, and luteinizing hormone were measured with enzyme immunoassays.
Results
Fasting specimens had significantly lower overnight urinary luteinizing hormone (p=0.045) and morning salivary testosterone than baseline (p=0.037). In contrast to morning salivary testosterone, there was a significant increase in overnight urinary testosterone (p=0.000) following the evening fast, suggesting an increase in urinary clearance rates. There was a marginal increase in overnight urinary cortisol (p=0.100), but not morning salivary cortisol (p=0.589).
Conclusion
These results suggest the male reproductive axis may react more quickly to energetic imbalances than has been previously appreciated.
Keywords: testosterone, LH, fasting, cortisol
Introduction
There is a growing body of evidence suggesting that the effects of testosterone are energetically expensive, and may play an energy-allocating role in the life history trade off between reproduction and survival (Charnov 1993; Folstad and Karter 1992; Muehlenbein 2008; Muehlenbein and Bribiescas 2005). High testosterone levels are hypothesized to lead to increased investment in reproduction, including behavioral strategies and secondary sexual characteristics (Muehlenbein and Bribiescas 2005). Testosterone plays an important role in increasing and maintaining male muscle mass, which can enhance competitive ability and mate attraction (Bhasin and others 1996; Bribiescas 2001). This comes at a cost: not only is muscle energetically expensive (Lassek and Gaulin 2009), but high levels of testosterone also increase the catabolism of fat tissues, depleting reserves that could be essential during periods of food shortage (Bhasin and others 2005).
Studies of male human and non-human primates suggest that short-term fasts can have significant down-regulatory effects on the hypothalamic-pituitary-gonadal (HPG) axis, prior to any change in body weight or composition (Aloi and others 1997; Bergendahl and Veldhuis 1995; Cameron and others 1991). In human males, fasting appears to significantly reduce the number, and amplitude of gonadotropin releasing hormone (GnRH) pulses, thus decreasing luteinizing hormone (LH) pulsatility (Aloi and others 1997; Rojdmark 1987). It is difficult to directly measure GnRH in humans, as it exists solely within the brain, but administration of intravenous GnRH during caloric restriction restores LH pulses, suggesting that fasting down regulates GnRH production (Aloi and others 1997). Problems with direct testing for GnRH make it difficult to ascertain whether it is the frequency of GnRH pulses, or their amplitude that is affected by short term fasting (Aloi and others 1997). Administration of intravenous GnRH has also been shown to restore testosterone levels in fasting subjects (Rojdmark 1987).
Once a fast is broken, LH pulsatility rebounds in male subjects; both in human and non-human primates (Friedl and others 2000; Parfitt and others 1991; Schreihofer and others 1993a; Schreihofer and others 1993b). LH secretion begins within twenty-to-forty minutes of re-feeding, and is monotonically related to the size of the re-feeding meal (Parfitt and others 1991). Resumption of LH secretion is unrelated to the actual act of eating; macaques re-fed via gastric cannulae showed an LH rebound statistically identical to macaques that were re-fed naturally (Schreihofer and others 1993a). When normal weight primates were overfed just prior to the onset of fasting, there was no effect on LH secretion, suggesting that acute caloric deficit plays an important role in hormonal down regulation (Cameron 1996; Schreihofer and others 1993b).
Previous studies find that multi-day fasts cause a reduction in LH and testosterone. This project tests the responsiveness of the male HPG and HPA to a brief caloric deficit. We hypothesize that the male reproductive axis is sensitive to even minor energetic disruptions, with reductions in LH and testosterone the morning following a missed evening meal. We also hypothesize that testosterone will return to normal levels rapidly after re-feeding. Cortisol is hypothesized to rise due to HPA activation from the psychological and physiological stress of fasting. Understanding the effects of short term caloric reduction on the endocrine system will provide insight into the role of testosterone in the trade-off between survival and reproduction in human males.
Methods
Participants
Twenty four male volunteers were recruited from the University of Washington campus. Individuals who regularly chewed tobacco, reported taking prescription or non-prescription steroids, or other medications known to affect testosterone production were not eligible for the study (Bhasin and others 1996; Granger and others 2004). One participant did not complete the study protocol, leaving a total of 23 participants who ranged in age from 19 to 36 years (mean age 26.7), see Table 1. Self reported weight and height were used to calculate BMI (mean 23.7 kg/m2, range 19.5–29.0 kg/m2), see Table 1. All procedures were approved by the Institutional Review Board of the University of Washington.
Table 1.
Summary statistics for hormonal and anthropometric characteristics over two baseline days (averaged), and following a brief fast (n= 23 men)
| Time | Mean ±SE | Minimum | Maximum | 95% CI | |
|---|---|---|---|---|---|
| Age (yr) | Baseline | 26.7 ±5.3 | 19 | 36 | (25.2, 28.3) |
| BMI (kg/m2) | Baseline | 23.7 ±2.2 | 19.5 | 29.0 | (24.5, 29.1) |
| Waking Salivary Testosterone (pg/mL) | Baseline | 385.7 ±14.8 | 269.7 | 708.3 | (356.0, 415.3) |
| Fasting | 337.3 ±19.8 | 223.8 | 573.6 | (297.8, 376.9) | |
| Waking Salivary Cortisol (pg/mL) | Baseline | 3340.8 ±129.2 | 1859.4 | 6114.9 | (3082.5, 3599.1) |
| Fasting | 3062.4 ±158.7 | 1781.6 | 4983.5 | (2745.1, 3379.6) | |
| 11AM Salivary Testosterone (pg/mL) | Baseline | 283.1 ±10.2 | 190.9 | 490.2 | (262.6, 303.5) |
| Post re-feeding | 321.4 ±14.5 | 230.4 | 534.5 | (292.5, 350.3) | |
| 11AM Salivary Cortisol (pg/mL) | Baseline | 2241.8 ±140.2 | 791.8 | 4845.4 | (1961.5, 2522.1) |
| Post re-feeding | 2485.5 ±152.5 | 1507.3 | 4742.0 | (2180.6, 2790.4) | |
| Urinary Testosterone (pg/mL) | Baseline | 670.6 ±18.2 | 422.3 | 992.0 | (634.2, 706.9) |
| Fasting | 892.1 ±29.8 | 662.1 | 1217.3 | (832.5, 951.8) | |
| Urinary Cortisol (pg/mL) | Baseline | 2745.3 ±171.3 | 1034.4 | 5875.8 | (2403, 3087.7) |
| Fasting | 3581.8 ±240.1 | 1919.5 | 6633.2 | (3101.8, 4061.8) | |
| Urinary LH (pg/mL) | Baseline | 286 ±12.2 | 128.8 | 517.4 | (261.5, 310.5) |
| Fasting | 252.8 ±18 | 92.2 | 519.4 | (216.9, 288.7) |
Specimen Collection
Participants collected first void morning urine specimens as well as waking and late morning (11AM) passive drool saliva specimens each day for three days. The first two days of specimen collection were designed to establish baseline levels of testosterone, cortisol, LH. On the second day of specimen collection, participants were asked not to eat or drink anything aside from water after 4PM. The protocol stressed the importance of not eating extra throughout the day, and not simply eating dinner early was stressed. On the third morning, after providing waking saliva and urine specimens, participants resumed their normal diet and ate breakfast before taking a final late morning (11AM) saliva specimen.
Passive drool saliva was collected in polypropylene centrifuge tubes after participants arose from bed each morning, after participants rinsed their mouths with water, but before eating, drinking, or brushing their teeth. Participants also collected a mid-stream first void morning urine specimen (2–5mL). After going about their normal daily routine until 11 AM, participants provided a second specimen, regardless of their time of wake up. Asking all participants to take the sample at 11AM as opposed to 4 hours after wake-up simplified an already complex study protocol. Waking urine and saliva specimens were frozen immediately, and 11AM specimens were frozen as soon as possible. Specimens were stored in home freezers until the end of the study, at which point they were stored at −80° C in the laboratory for up to 26 days before assay.
To control for differences in morning testosterone caused by behavioral differences rather than fasting, participants were asked to refrain from major exercise, competitive sports, and sexual activity for the duration of the study (Burnham and others 2003; Gray 2003; Gray and others 2006). Weight lifting or jogging were considered major exercise, while walking the dog, or across campus were considered acceptable. Participants recorded the times they went to bed and woke up, and the time that each saliva specimen was taken. These data were later used to correct salivary hormone concentrations for time of wake up (Flinn and England 1997). The self-reported age, weight, and height of the participants was also collected to control for inter-individual differences in testosterone production (Burnham and others 2003; Gray 2003; Gray and others 2006).
Laboratory Methods
In the lab, saliva specimens was centrifuged at 2800 RPM for 20 minutes, and the aqueous layer was removed and stored at −80° C until assay. Urine specific gravity was measured with a hand refractometer (Model No. 2721 URC-PN, Atago Inc., Farmingdale, NY), and urine specimens were stored at −80° C until assay. All specimens had gone through two freeze thaw cycles when initially assayed. Enzyme immunoassays (EIA) were used to measure testosterone, and cortisol in saliva, and LH, testosterone and cortisol in urine; all specimens were run in duplicate. Urine hormone concentrations were corrected for hydration status using specimen specific gravity and a reference population mean specific gravity of 1.015 (Miller and others 2004).
The EIA for testosterone used a polyclonal antibody that cross reacts 100% with testosterone, 57.4% with 5α-dihydrotestosterone, 0.27% with androstenodione, and less than 0.05% with other androgens (Muir and others 2001). All saliva specimens were run neat, and all urine specimens were run at a 1:100 dilution in 0.1% bovine serum albumin (BSA) blocking buffer. The within and between assay coefficients of variation (CV) for this project (n=10 plates) were 3.76% and 8.37% respectively for the high (513.0pg/mL), 4.87% and 8.54% for the medium (412.7pg/mL), and 3.68% and 8.84% for the low (350.3pg/mL) in-house controls.
The EIA for cortisol used a polyclonal antibody that cross-reacted 100% with cortisol and less than 10% with other steroids tested (Munro and Stabenfeldt 1985). All saliva specimens were run at a 1:2 dilution in 0.1% BSA blocking buffer, and urine specimens were diluted to 1:60 in the same buffer. The within and between assay CVs for cortisol for this project (n=7 plates) were 4.27% and 2.44% for the high (1047.7pg/mL), 4.51% and 5.51% for the medium (553.8pg/mL), and 6.79% and 7.30% for the low (337.0pg/mL) in-house controls, respectively.
An immunoenzymometric assay (IEMA) was used to measure the beta (β) sub-unit of LH in urine (Brindle and others 2006). The cross reactivity was 100% for βLH, 7.60% for intact LH, and 0% for all other cross reactants tested. Specimens were heated to 100° C for 2 minutes before assay to disassociate the β sub-unit of LH, and run neat. The within and between assay CVs for this project (n=3 plates) were 16.56% and 27.03% for the high (116.8pg/mL) and 14.37% and 16.47% for the low (87.7pg/mL) in house controls, respectively.
Color reactions were quantified at 405nm (test) and 570nm (reference) for testosterone, cortisol, and LH assays using a Synergy HT microtiter plate reader (Bio Tek Instruments, Inc., Winooski, VT). Concentrations were estimated with a four parameter logistic calibration curve fit (Gen5, Bio Tek Instruments Inc.).
Statistical Methods
Linear mixed effects regression models with maximum likelihood estimation were run to determine the effects of a single skipped meal on individual 1) waking salivary testosterone and cortisol; 2) waking urinary LH, testosterone, and cortisol; and 3) 11 AM salivary testosterone and cortisol. All hormone data were log transformed and normality was graphically assessed. To control for the non-independence of repeated specimens from each participant, individuals were modeled as a random effects (West and others 2007). Analysis of waking saliva specimens controlled for the time of waking, hours of sleep, an interaction between the amount of sleep and time of waking, BMI, age, and an interaction term for age and BMI. The 11AM salivary analysis controlled for waking hormone concentration, time since waking, BMI, age, and an interaction term for age and BMI. The urinary analysis controlled for the number of hours of sleep, BMI, age, and an interaction term for age and BMI. The threshold for statistical significance was set at α =0.05, and all analyses were completed in STATA 10.1 (College Station, TX).
Results
Twenty four participants began the study; one subject did not complete study protocol, leaving a total of 23 participants. Two specimens were not analyzed due to improper collection time, and one participant missed collection of a single sample. All morning saliva and urine specimens were taken between 5:45AM and 10:40AM, and all 11AM saliva specimens were taken within one hour of 11 AM. Three LH specimens were below the limits of detection, and four cortisol specimens were above the limits of detection for the assay; these specimens were assigned the concentration determined to be the upper or lower limits of detection of the assay. Results are reported in Table 1 and Table 2.
Table 2.
Results of separate linear mixed effects models examining the response of logged endocrine measures to fasting (each hormone modeled separately) and the control variables for each model.
| Coefficient | Std. Error | p-value | 95% CI | |
|---|---|---|---|---|
| Waking Salivary Testosterone** | −0.1072 | 0.0514 | 0.037 | (−0.2079, −0.0064) |
| Waking Salivary Cortisol | −0.0561 | 0.1038 | 0.589 | (−0.2596, 0.1474) |
| Waking salivary specimen analysis controlled for time of wakening, hours of sleep, age, BMI, and interactions between time of wake and hours of sleepT as well as age and BMI. Individuals were treated as random effects. | ||||
|
11AM Salivary Testosterone** (Post re-feeding) |
0.1487 | 0.0589 | 0.012 | (0.0333, 0.2641) |
|
11AM Salivary Cortisol (Post re-feeding) |
0.1119 | 0.0916 | 0.222 | (−0.0677, 0.2915) |
| 11AM salivary specimen analysis controlled for waking salivary hormone levels (testosterone controlled for waking salivary testosteroneT, cortisol controlled for waking salivary cortisolC), hours since wake, ageT, BMIT, and an interaction between age and BMIT. Individuals were treated as random effects. | ||||
| Urinary Testosterone*** | 0.2368 | 0.0531 | 0.000 | (0.1328, 0.3409) |
| Urinary Cortisol* | 0.1802 | 0.1095 | 0.100 | (−0.0344, 0.3948) |
| Urinary LH** | −0.1524 | 0.0759 | 0.045 | (−0.3012, −0.0037) |
| First morning void urine specimens were corrected for specific gravity and the analysis controlled for time of wake, ageT, C, LH, BMIC, LH, and an interaction between age and BMIT, C, LH. Individuals were treated as random effects. | ||||
indicates suggestive result p≤.10,
indicates significance at the p<.05 level,
indicates significance at the p<.01 level
indicates significant (p<.05) control variable for Testosterone,
indicates significant control variable for cortisol,
indicates significant control variable for LH
There was a significant decrease in waking salivary testosterone on the morning following the fast, when compared to two baseline days combined (β=−0.107, p=0.037), and a significant increase in 11 AM salivary testosterone after participants fasted on the previous evening (β=0.149, p=0.012) (Figures1A and 2A). In contrast to salivary testosterone, morning urinary testosterone increased significantly (β=0.237, p<0.000) the morning following a single skipped meal (Figures 1B and 3B).
Figure 1. Daily Fitted Salivary and Urinary Testosterone.
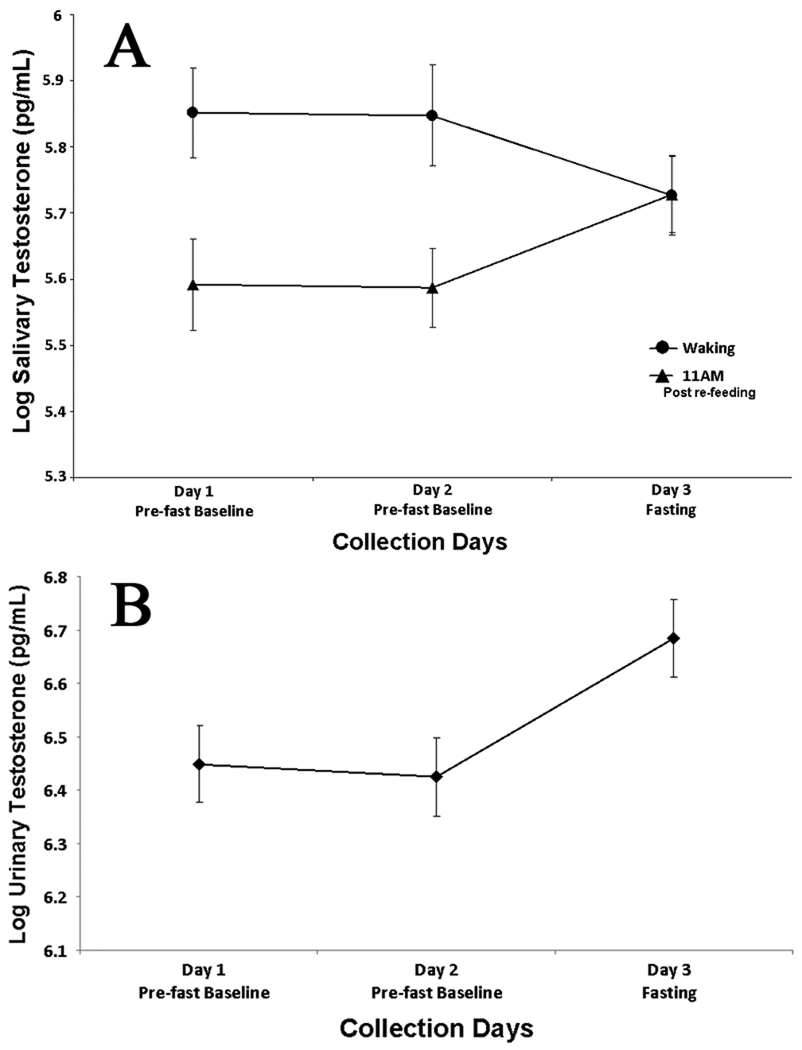
A: Two days of non-fasting baseline waking and 11AM fitted log salivary testosterone levels (mean ± SE), followed by a third day showing the effects of missing a single meal the previous evening. B: First morning fitted log urinary testosterone (specific gravity corrected) for two baseline days and the morning following a single missed meal (mean ± SE).
Figure 2. Individual Fitted Daily Salivary Testosterone and Cortisol.
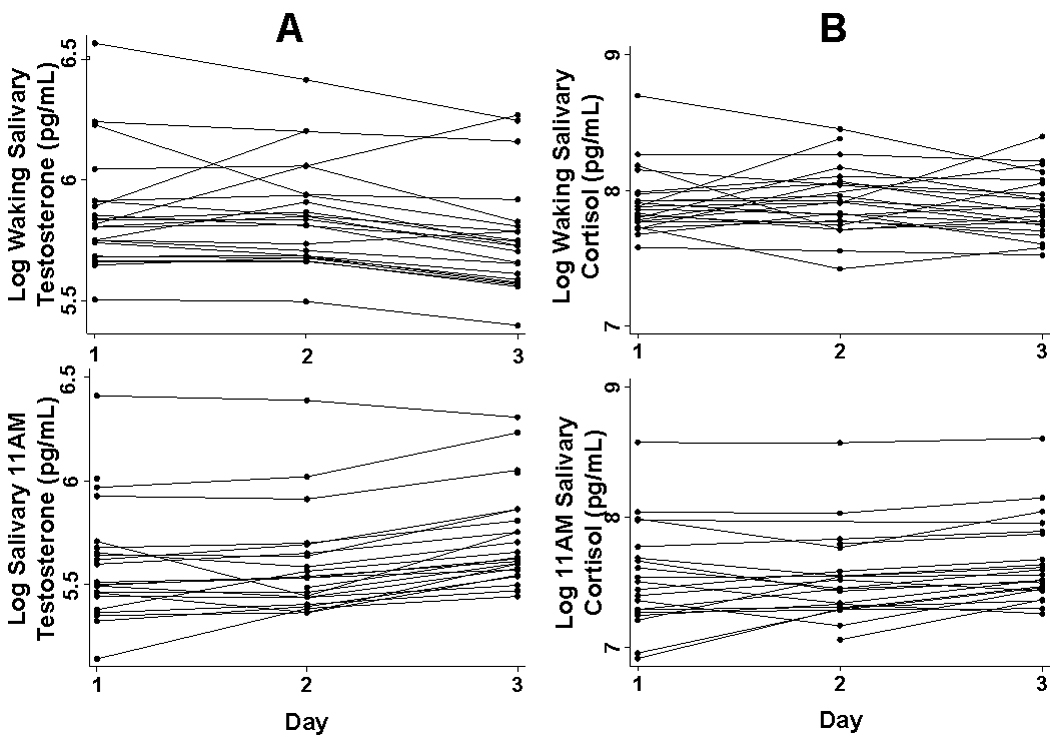
A: Two days of non-fasting baseline waking and 11AM fitted log salivary testosterone levels, followed by a third day showing the individual effects of missing a single meal the previous evening. B: Two days of non-fasting baseline waking and 11AM fitted log salivary cortisol levels, followed by a third day showing the individual effects of missing a single meal the previous evening.
Figure 3. Individual Fitted Daily Urinary Hormone Measures.
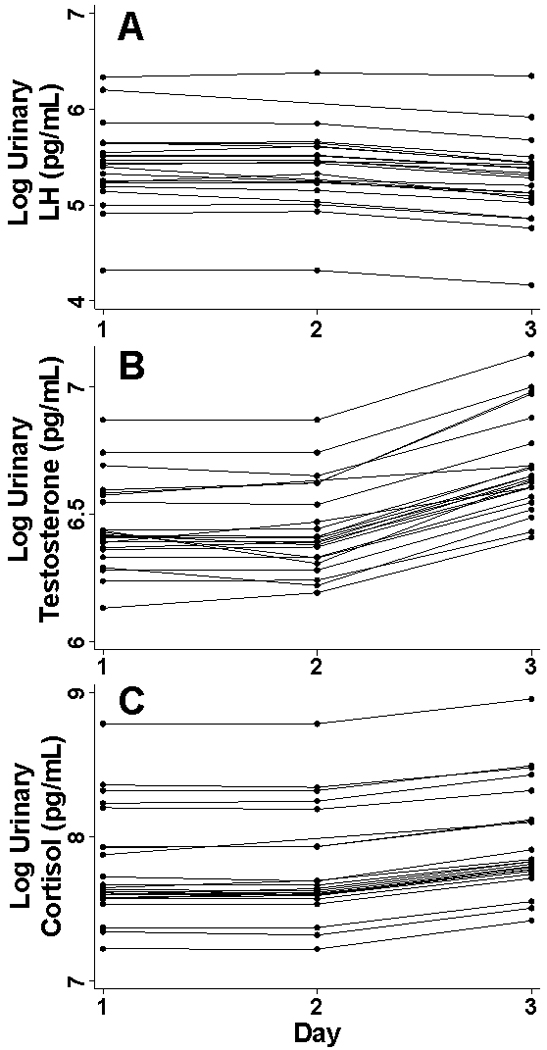
Daily first morning fitted log urinary LH (A) Testosterone (B) and Cortisol (C) for two days of baseline, and the morning following a single missed meal, corrected for specific gravity.
Salivary cortisol showed no significant change between baseline and fasting levels, either at waking (p=0.589) or 11 AM (p=0.222) (Figures 4A and 2B). Similar to urinary testosterone, overnight urinary cortisol increased (β=0.180, p=0.100) on the morning following a single missed meal, though the results did not reach statistical significance (Figures 4B and 3C). Urinary LH decreased significantly (β=−0.152, p=0.045) after a single skipped meal (Figures 5 and 3A). Due to the high LH CVs, an additional bootstrapped statistical analysis (1000 replications) was run building within plate variability into the model as a second random effect. This analysis also concluded that urinary LH decreased significantly (p=0.010) following an evening fast.
Figure 4. Daily Fitted Salivary and Urinary Cortisol.

A: Two days of non-fasting baseline waking and 11AM log salivary cortisol levels (mean ± SE), followed by a third day showing the effects of missing a single meal the previous evening. B: First morning fitted log urinary cortisol (specific gravity corrected) for two baseline days and the morning following a single missed meal (mean ± SE).
Figure 5. Daily Fitted Urinary LH.

Day 1 and 2 are non-fasting, baseline log urinary beta LH specimens (specific gravity corrected), while the third sample was taken the morning immediately following an evening fast (mean ± SE).
Discussion
This study examined the effects of a single missed evening meal on the male reproductive axis. As hypothesized, urinary LH and waking salivary testosterone decreased significantly following the fast. These results are consistent with previous research in humans and animals which suggest that even minor energetic disturbances from fasting down-regulate the male reproductive axis (Aloi and others 1997; Bergendahl and others 1998; Bergendahl and Veldhuis 1995; Cameron and others 1991; Klibanski and others 1981). Previous studies of energetic imbalances have reported decreases in serum LH (Aloi and others 1997; Bergendahl and Veldhuis 1995; Cameron and others 1991; Veldhuis and others 1993), this report extends these findings to urinary LH. These results are also consistent with other research demonstrating that mild energetic imbalances, either from increased exercise (Nindl and others 2001), or immune system activation (Muehlenbein and Bribiescas 2005; Simmons and Roney 2009) cause decreases in circulating testosterone in human males.
Research in animal models suggests that decreases in hypothalamic GnRH production are responsible for decreased LH and testosterone (Cameron and others 1985). Participants in this study showed significantly higher 11 AM testosterone (post-breakfast) levels the morning after fasting compared to baseline days. This is consistent with previous work showing that re-feeding is associated with immediate increases in GnRH, LH, and testosterone, regardless of the macronutrient content of the meal (Friedl and others 2000; Parfitt and others 1991; Schreihofer and others 1993a; Schreihofer and others 1993b). To assess if larger than normal breakfast sizes could have been responsible for the increase in 11AM salivary testosterone, an analysis of relative meal size was conducted. As a measure of fasting compliance, participants recorded the size of their meals compared to their average meal size (coding each meal as smaller then usual, normal, or larger then usual). This provided a crude measure of relative food intake, making it possible to examine whether the increases in 11AM salivary testosterone were caused by significantly larger breakfasts the morning following the fast. A mixed effects regression was conducted on self reported relative breakfast size. This revealed that breakfasts the morning following the fast did not differ significantly in size from breakfasts on other days (p=0.307). While it is not possible to rule out the possibility that individuals ate breakfasts of different caloric or macronutrient content the morning following the fast, participants reported no differences in relative breakfast sizes in this crude measure of dietary intake.
In contrast to the waking salivary testosterone decrease following the fast, we observed a significant increase in first morning urinary testosterone following the fast. Studies testing paired urine and blood specimens in fasting individuals found that while blood hormone concentrations of LH and FSH decreased (Klibanski and others 1981), or stayed stable (Beitins and others 1980), over the course of a multi-day fast, there were significant increases in urinary hormone concentrations during fasting. This suggested to the authors that clearance rates increased significantly during a fast. Similar increases in peripheral testosterone clearance have been shown in patients in the days following surgery or trauma, while serum testosterone decreased (Spratt and others 2006). Increased urinary clearance of testosterone may be an adaptive response to rapidly eliminate circulating testosterone during energetic stress. Future studies should examine energetic stress and changes in testosterone clearance rates in greater detail.
Morning and 11 AM salivary cortisol showed no increase the morning following a missed meal compared to baseline; however, there was an increase in urinary cortisol the morning of the fast. Previous studies have reported increased serum cortisol following brief (1–2 day) fasts in primates (Schreihofer and others 1993b). Increasing cortisol following fasting is hypothesized to result from both the psychological stress of missing a meal, as well as the metabolic actions of cortisol (Cameron 1996; Lukas and others 2005). In our study, increases in cortisol from both psychological and metabolic stress may have occurred close to the time of the fast, and were thus only partially observable in the pooled overnight urinary measure of cortisol. Because the increase in urinary cortisol was not accompanied by a decrease in salivary cortisol the following morning, we believe that there was a significant increase in circulating cortisol the previous evening and into the night, either from the psychological or metabolic stress of going to sleep without eating (Cameron 1996; Lukas and others 2005). It is not possible to rule out increased urinary clearance of cortisol, thought the authors are unaware of any previous literature showing increased cortisol excretion during caloric deficit. In addition, high morning levels due to the cortisol awakening response may have swamped any expected morning increase in salivary cortisol (Clow and others 2004)
Circulating glucose and insulin rise immediately after eating, and then gradually decline as the time from the meal increases (Van Cauter and others 1992). Insulin receptors are expressed at high levels in the hypothalamus, pituitary, and testis, and it has been suggested that changes in insulin concentration could play a role in reproductive downregulation during fasting (Bruning and others 2000). Previous research indicates that extreme hypoglycemia induced by glycemic clamps can cause an acute decrease in LH and testosterone (Oltmanns and others 2001; Oltmanns and others 2005). However, these studies generated severe hypoglycemia, unlike the very mild changes in glycemic levels seen during natural fasting in young adult males (Haymond and others 1982; Merimee and Tyson 1974), thus it is unlikely that missing a single meal caused severe enough hypoglycemia to downregulate LH and testosterone (Cameron 1996). Primate research on post-fast refeeding finds that different iso-caloric mixtures of protein, fats and carbohydrates make no difference in the LH rebound, suggesting that naturally circulating glucose levels are not responsible for changes in LH secretion (Cameron 1996; Schreihofer and others 1993a; Schreihofer and others 1993b; Schreihofer and others 1996). Further studies of natural changes in circulating glucose and insulin are necessary to understand the mechanism by which energetic status downregulates human male reproductive hormones.
There were several limitations to this study. Participants were not physically monitored during the study, and although no participants reported any food intake during the fast on daily meal logs, this self report was not verified. Nutrient content of the meals was not assessed. When providing saliva samples, participants took their first saliva specimen upon waking and a second sample at 11AM. For some participants, the time of wake up may have been closer to 11AM than others, thus there was less time between waking and specimen collection. That said, there was no association between fasting and hours of sleep (p=0.819), or time of wake (p=0.720).
Another limitation was the use of self reported BMI. Previous studies have shown that men tend to overestimate height, which can skew BMI (Bolton-Smith and others 2000; Gorber and others 2007). BMI in the study ranged from 19.5–29.0 kg/m2, with a mean of 23.7 kg/m2, and thus our results may not generalize to individuals with lower or higher BMI. The majority of the participants were well educated, white undergraduate and graduate students at the University of Washington, thus the results may not be generalizeable to all males. The young age distribution of our sample precludes generalization to older men who have lower levels of testosterone (Harman and others 2001; Travison and others 2007) from reduced hypothalamic-pituitary gonadotropin feedback and secretion (Mulligan and others 1997; Veldhuis and others 2001), as well as reduced testicular function (Harman and Tsitouras 1980; Midzak and others 2009).
In sum, these results support that the male reproductive axis is more responsive to very brief caloric deficiencies than has previously been shown. Not only do overnight LH, and morning salivary testosterone decrease, but our data suggests that clearance rates of testosterone increase, resulting in a rapid drop in circulating testosterone levels. These results are consistent with previous studies of multi-day fasting, as well as studies examining energetic stresses such as immune activation (Cameron 1996; Muehlenbein 2008; Muehlenbein and Bribiescas 2005). This study adds to a growing body of literature suggesting that testosterone is energetically costly, and may play a key role in regulating trade-offs between survival and reproduction.
Acknowledgements
The authors would like to thank the participants for their time, energy, and willingness to skip a meal. We also would like to thank Eric A. Smith, Richard Bribiescas, Jane Shofer, Amanda Guyton, and two anonymous reviewers for their comments and feedback.
Grant Sponsorship: Funded in part by NICHD 5 T32 HD007543-08 and NICHD R24 HD042828.
Work Cited
- Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gonadotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal weight men. J Clin Endocrinol Metab. 1997;82(5):1543–1548. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- Beitins I, Shah A, K OL, Johnson L, Ostrea T, Van Wart J, McArthur J. The effects of fasting on serum and urnary gonadotropins in obese postmenopausal women. J Clin Endocrinol Metab. 1980;51(1):26–34. doi: 10.1210/jcem-51-1-26. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Aloi JA, Iranmanesh A, Mulligan TM, Veldhuis JD. Fasting suppresses pulsatile luteinizing hormone (LH) secretion and enhances orderliness of LH release in young but not older men. J Clin Endocrinol Metab. 1998;83(6):1967–1975. doi: 10.1210/jcem.83.6.4856. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Veldhuis JD. Altered pulsatile gonadotropin signaling in nutritional deficiency in the male. Trends Endocrinol Metab. 1995;6(5):145–159. doi: 10.1016/1043-2760(95)00081-r. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Bolton-Smith C, Woodward M, Tunstall-Pedoe H, Morrison C. Accuracy of the estimated prevalence of obesity from self reported height and weight in an adult Scottish population. J Epidemiol Community Health. 2000;54(2):143–148. doi: 10.1136/jech.54.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bribiescas RG. Reproductive ecology and life history of the human male. Am J Phys Anthropol Suppl. 2001;33:148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- Brindle E, Miller RC, Shofer JB, Klein NA, Soules MR, O'Connor KA. Urinary beta-luteinizing hormone and beta-follicle stimulating hormone immunoenzymometric assays for population research. Clin Biochem. 2006;39(11):1071–1079. doi: 10.1016/j.clinbiochem.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning J, Gautam D, Burks D, Gillette J, Schubert M, Orban P, Klein R, Krone W, Muller-Wieland D, Kahn C. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burnham TC, Chapman JF, Gray PB, McIntyre MH, Lipson SF, Ellison PT. Men in committed, romantic relationships have lower testosterone. Horm Behav. 2003;44(2):119–122. doi: 10.1016/s0018-506x(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Reviews of Reproduction. 1996:117–116. doi: 10.1530/ror.0.0010117. [DOI] [PubMed] [Google Scholar]
- Cameron JL, McNeill TH, Fraser HM, Bremner WJ, Clifton DK, Steiner RA. The role of endogenous gonadotropin-releasing hormone in the control of luteinizing hormone and testosterone secretion in the juvenile male monkey, Macaca fascicularis. Biol Reprod. 1985;33(1):147–156. doi: 10.1095/biolreprod33.1.147. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab. 1991;73(1):35–41. doi: 10.1210/jcem-73-1-35. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Life history invariants : some explorations of symmetry in evolutionary ecology. Oxford [England] New York: Oxford University Press; 1993. p. xv.p. 167. p. p. [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. Stress: The International Journal on the Biology of Stress. Taylor & Francis Ltd; 2004. The Awakening Cortisol Response: Methodological Issues and Significance; pp. 29–37. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. Am J Phys Anthropol. 1997;102(1):33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, Bright Males, and the Immunocompetence Handicap. Am Nat. 1992;139(3):603. [Google Scholar]
- Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol. 2000;88(5):1820–1830. doi: 10.1152/jappl.2000.88.5.1820. [DOI] [PubMed] [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obesity Reviews. 2007;8(4):307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The "trouble" with salivary testosterone. Psychoneuroendocrinology. 2004;29(10):1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am J Phys Anthropol. 2003;122(3):279–286. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Gray PB, Yang CF, Pope HG., Jr Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc Biol Sci. 2006;273(1584):333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman S, Tsitouras P. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51(1):35–40. doi: 10.1210/jcem-51-1-35. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Haymond MW, Karl IE, Clarke WL, Pagliara AS, Santiago JV. Differences in circulating gluconeogenic substrates during short-term fasting in men, women, and children. Metabolism. 1982;31(1):33–42. [PubMed] [Google Scholar]
- Klibanski A, Beitins I, T B, Little R, McArthur J. Reproductive function during fasting in men. J Clin Endocrinol Metab. 1981;53(2):258–263. doi: 10.1210/jcem-53-2-258. [DOI] [PubMed] [Google Scholar]
- Lassek WD, Gaulin SJC. Costs and benefits of fat-free muscle mass in men: relationship to mating success, dietary requirements, and native immunity. Evolution and Human Behavior. 2009;30(5):322–328. [Google Scholar]
- Lukas WD, Campbell BC, Campbell KL. Urinary cortisol and muscle mass in Turkana men. American Journal of Human Biology. 2005;17(4):489–495. doi: 10.1002/ajhb.20402. [DOI] [PubMed] [Google Scholar]
- Merimee T, Tyson J. Stabilization of plasma glucose during fasting; Normal variations in two separate studies. N Engl J Med. 1974;291(24):1275. doi: 10.1056/NEJM197412122912404. [DOI] [PubMed] [Google Scholar]
- Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299(1):23–31. doi: 10.1016/j.mce.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O'Connor KA. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50(5):924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. Adaptive Variation in Testosterone Levels in Response to Immune Activation: Empirical and Theoretical Perspectives. Social Biology. 2008;53:13–23. doi: 10.1080/19485565.2006.9989113. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17(5):527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Muir C, Spironello-Vella E, Pisani N, deCatanzaro D. Enzyme immunoassay of 17 beta-estradiol, estrone conjugates, and testosterone in urinary and fecal samples from male and female mice. Horm Metab Res. 2001;33(11):653–658. doi: 10.1055/s-2001-18692. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol Regul Integr Comp Physiol. 1997;273(4):R1407–R1413. doi: 10.1152/ajpregu.1997.273.4.R1407. [DOI] [PubMed] [Google Scholar]
- Munro C, Stabenfeldt G. Development of a cortisol enzyme immunoassay in plasma. Clin Chem. 1985;31:956. [Google Scholar]
- Nindl BC, Kraemer WJ, Deaver DR, Peters JL, Marx JO, Heckman JT, Loomis GA. LH secretion and testosterone concentrations are blunted after resistance exercise in men. J Appl Physiol. 2001;91(3):1251–1258. doi: 10.1152/jappl.2001.91.3.1251. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86(10):4913–4919. doi: 10.1210/jcem.86.10.7892. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Peters A, Kern W, Fehm HL, Born J, Schultes B. Preserved inhibitory effect of recurrent hypoglycaemia on the male gonadotrophic axis. Clin Endocrinol (Oxf) 2005;62(2):217–222. doi: 10.1111/j.1365-2265.2005.02203.x. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Church KR, Cameron JL. Restoration of pulsatile luteinizing hormone secretion after fasting in rhesus monkeys (Macaca mulatta): dependence on size of the refeed meal. Endocrinology. 1991;129(2):749–756. doi: 10.1210/endo-129-2-749. [DOI] [PubMed] [Google Scholar]
- Rojdmark S. Influence of short-term fasting on the pituitary-testicular axis in normal men. Horm Res. 1987;25(3):140–146. doi: 10.1159/000180645. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Amico JA, Cameron JL. Reversal of fasting-induced suppression of luteinizing hormone (LH) secretion in male rhesus monkeys by intragastric nutrient infusion: evidence for rapid stimulation of LH by nutritional signals. Endocrinology. 1993a;132(5):1890–1897. doi: 10.1210/endo.132.5.8477642. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Parfitt DB, Cameron JL. Suppression of luteinizing hormone secretion during short-term fasting in male rhesus monkeys: the role of metabolic versus stress signals. Endocrinology. 1993b;132(5):1881–1889. doi: 10.1210/endo.132.5.8477641. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Renda F, Cameron JL. Feeding-induced stimulation of luteinizing hormone secretion in male rhesus monkeys is not dependent on a rise in blood glucose concentration. Endocrinology. 1996;137(9):3770–3776. doi: 10.1210/endo.137.9.8756545. [DOI] [PubMed] [Google Scholar]
- Simmons ZL, Roney JR. Androgens and energy allocation: quasi-experimental evidence for effects of influenza vaccination on men's testosterone. Am J Hum Biol. 2009;21(1):133–135. doi: 10.1002/ajhb.20837. [DOI] [PubMed] [Google Scholar]
- Spratt DI, Morton JR, Kramer RS, Mayo SW, Longcope C, Vary CPH. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006;291(3):E631–E638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol Endocrinol Metab. 1992;262(4):E467–E475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993;76(3):587–593. doi: 10.1210/jcem.76.3.8445014. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of Androgen Negative Feedback Unveils Impoverished Gonadotropin-Releasing Hormone/Luteinizing Hormone Secretory Reactivity in Healthy Older Men. J Clin Endocrinol Metab. 2001;86(2):529–535. doi: 10.1210/jcem.86.2.7200. [DOI] [PubMed] [Google Scholar]
- West B, Welch KB, Galecki AT. Linear mixed models : a practical guide using statistical software. Boca Raton: Chapman & Hall/CRC; 2007. pp. xix–353. p. p. [Google Scholar]


